Study on the Flow Characteristics of Desulfurization Ash Fine Particles in a Circulating Fluidized Bed
Abstract
1. Introduction
2. Experimental Setup and Particle Properties
2.1. Experimental Setup
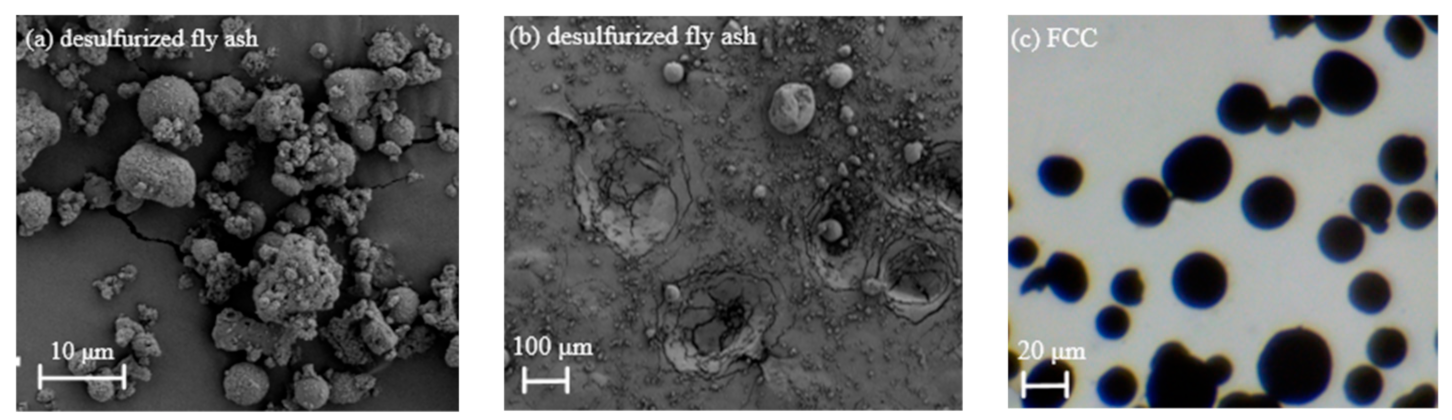
2.2. Particle Properties
3. Results
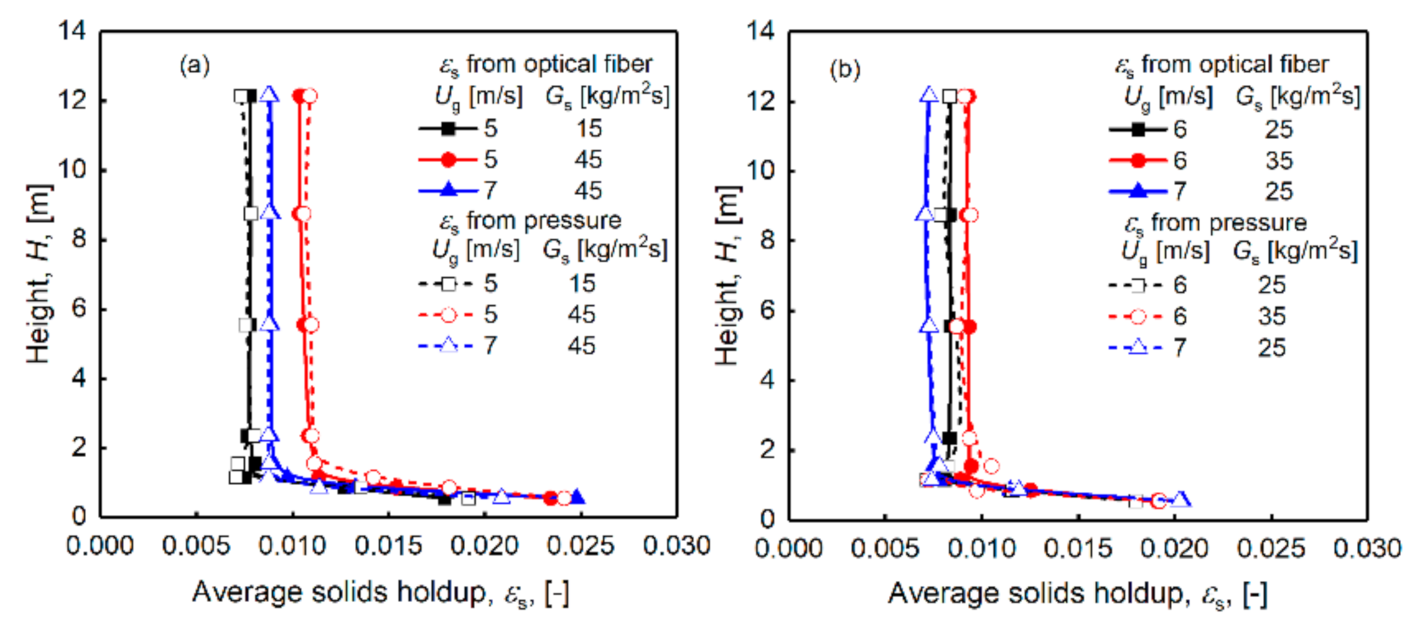
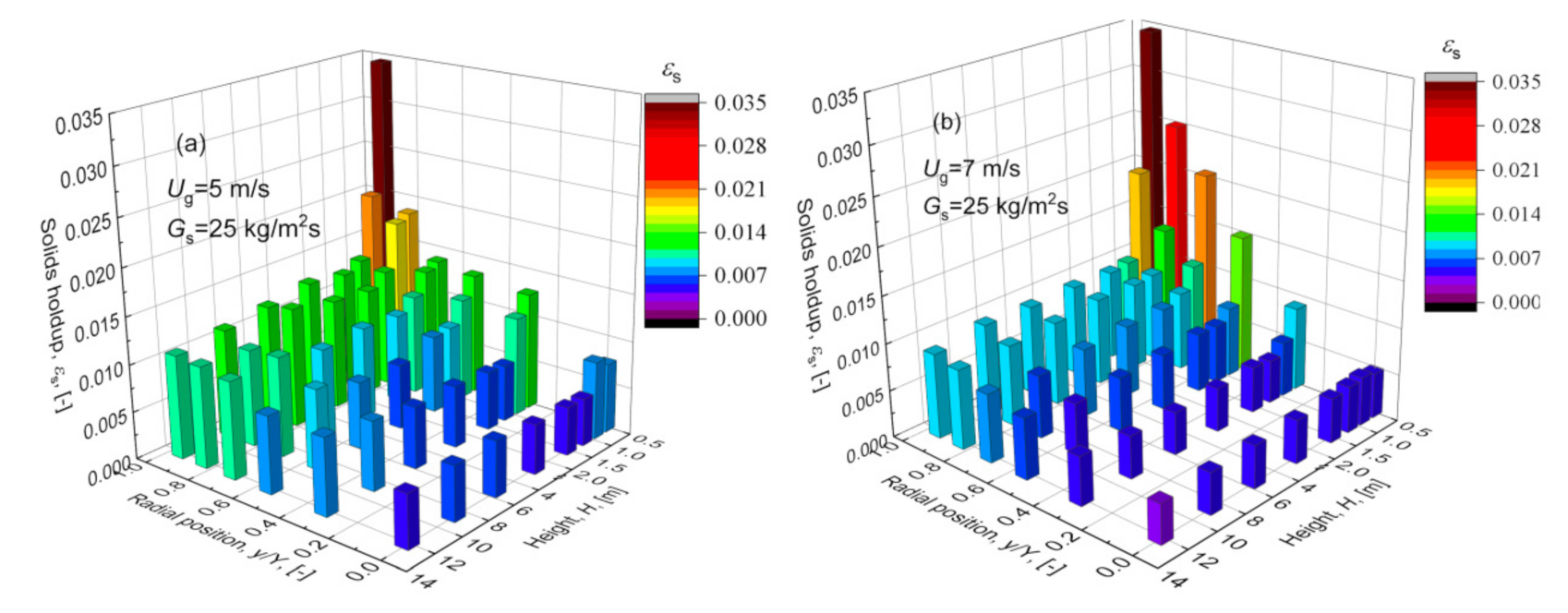
3.1. Distribution of Solids Holdup of Desulphurization Ash Particles
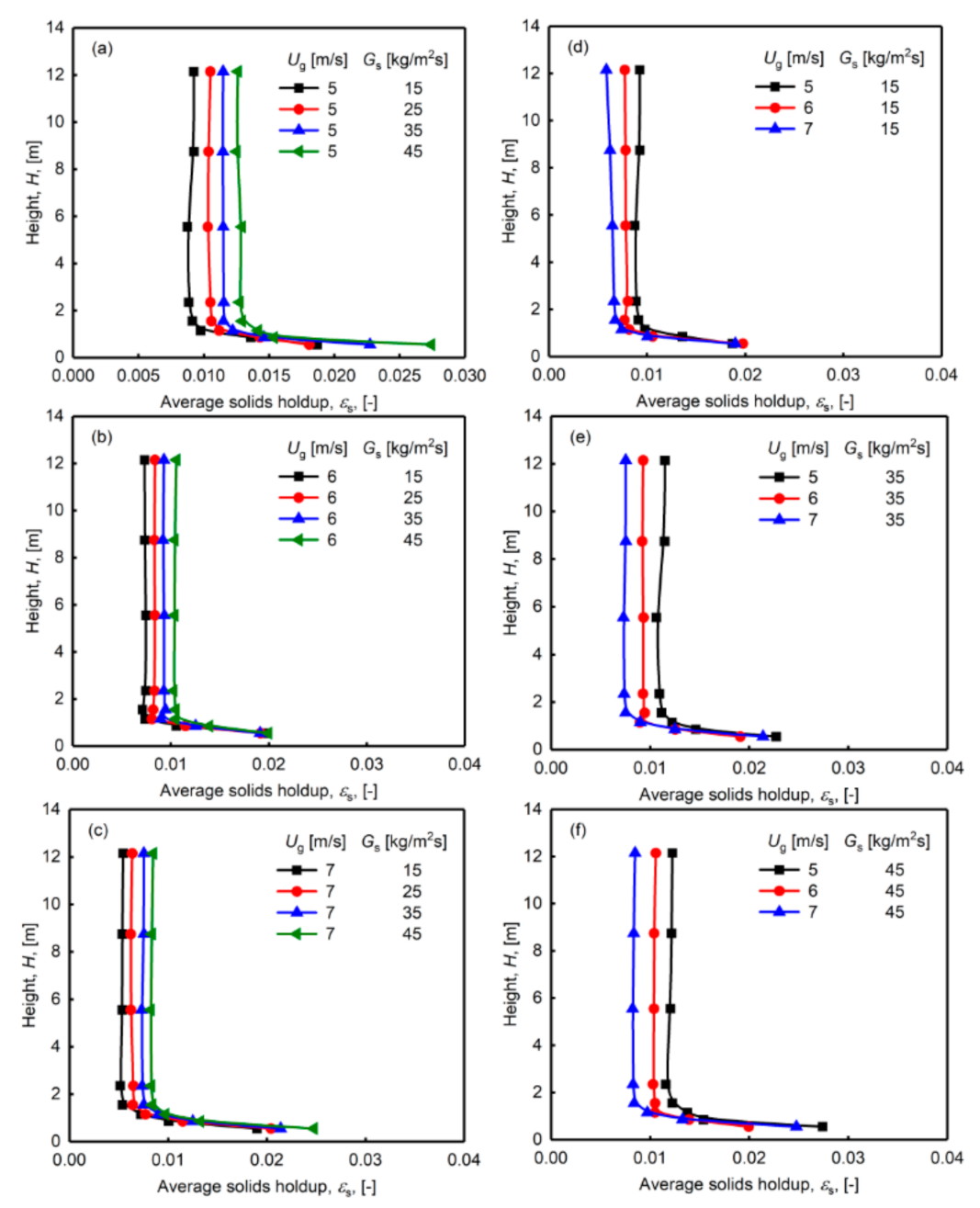
3.2. Influence of Operating Conditions on Solids Holdup of Desulphurization Ash Particles
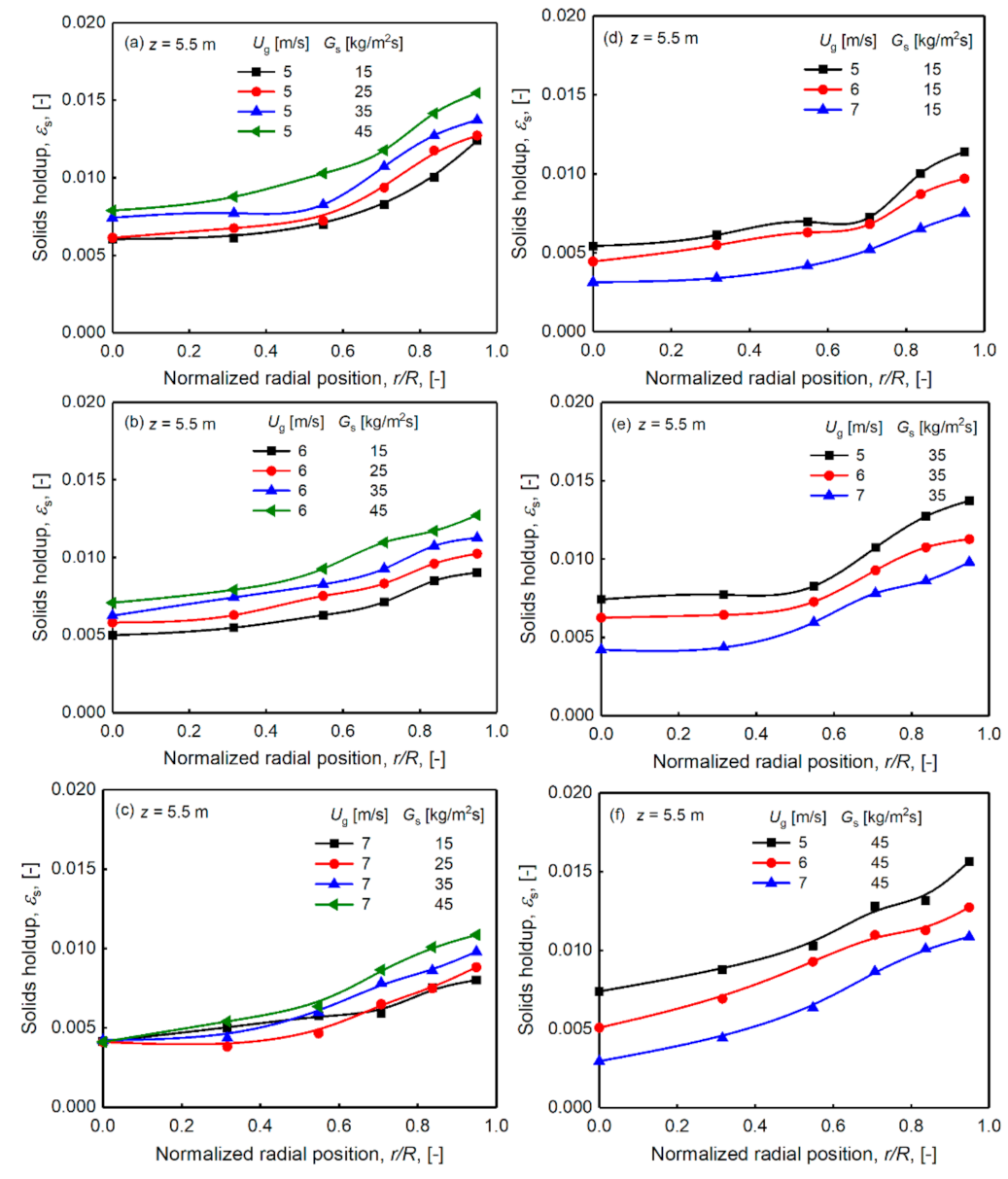
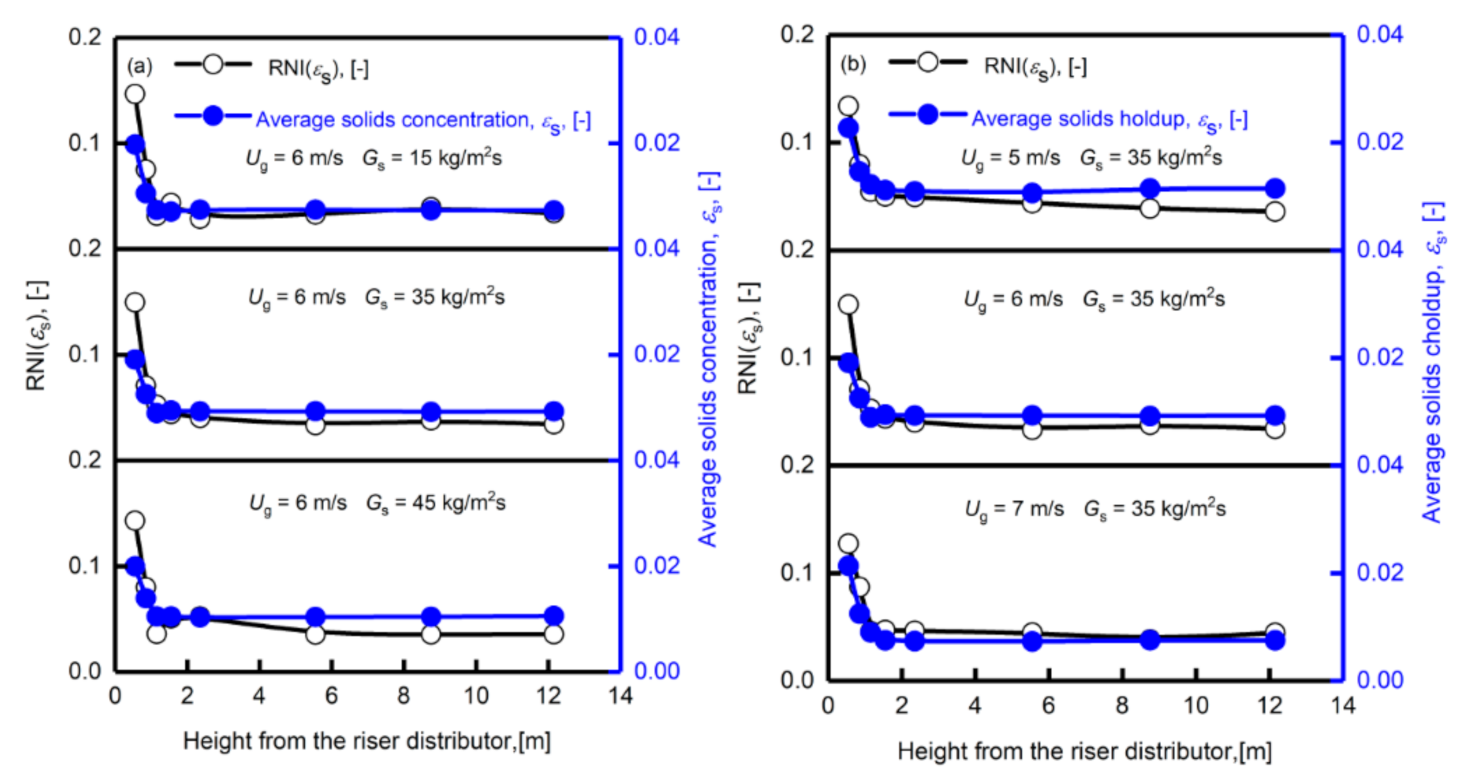
3.3. Radial Distribution of Particle Solids Holdup, Non-Uniform Distribution
3.4. The Relationship between Particle Flow Parameters and Particle Solids Holdup
4. Conclusions
- (1)
- The solids holdup is relatively high at the riser bottom, gradually decreases with the increase in the axial height, and has a stable value at the top of the riser outlet. The solids holdup is between 0.005 and 0.03. There is also an uneven gas–solids distribution in the radial direction, which may affect the gas–solids contact and thus the efficiency of the desulfurization process.
- (2)
- The superficial gas velocity and the solids circulation rate have different influences on the solids holdup distribution. Changes in the solids holdup in the axial direction are more obvious when the gas velocity is lower, which indicates that the solids holdup is more sensitive to the change in the solids circulation rate at a lower superficial gas velocity. The solids holdup values of different radial regions have different sensitivities to the circulation. At lower gas velocities, the changes in the center and wall regions of the riser are roughly the same. When the superficial gas velocity is higher, the solids holdup of the wall area is more prominently affected by the solids circulation rate than in the center area.
- (3)
- The particle-based Archimedes number has a linear relationship with the solids holdup under all operating conditions.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Ars | Archimedes number (-) |
| dp | Particle diameter (μm) |
| D | Device diameter (m) |
| H | Device height (m) |
| Gs | Solids circulation rate (kg/m2·s) |
| g | acceleration of gravity (m/s2) |
| Ug | Superficial gas velocity (m/s) |
| z | Height (m) |
| ρf | Gas density (kg/m3) |
| ρg | Particle density (kg/m3) |
| Averaged solids holdup (-) | |
| εs | Solids holdup (-) |
| μ | Dynamic viscosity of fluidizing air (Pas) |
References
- Gao, H.; Sun, B. Influence of Energy Saving and Emission Reduction on Innovation of Science, Technology, Industry and Enterprises in China. IOP Conf. Ser. Earth Environ. Sci. 2021, 770, 012067. [Google Scholar] [CrossRef]
- Cui, L.; Liu, M.; Yuan, X.; Wang, Q.; Ma, Q.; Wang, P.; Hong, J.; Liu, H. Environmental and economic impact assessment of three sintering flue gas treatment technologies in the iron and steel industry. J. Clean. Prod. 2021, 311, 127703. [Google Scholar] [CrossRef]
- Qi, H.; You, C.; Xu, X. Interaction between SO2 from flue gas and sorbent particles in dry FGD processes. China Particuol. 2005, 3, 114. [Google Scholar] [CrossRef]
- Lidong, W.; Juan, W.; Peiyao, X.; Qiangwei, L.; Wendi, Z.; Shuai, C. Selectivity of transition metal catalysts in promoting the oxidation of solid sulfites in flue gas desulfurization. Appl. Catal. A Gen. 2015, 508, 52–60. [Google Scholar] [CrossRef]
- Wang, J.; Zhong, W. Simultaneous desulfurization and denitrification of sintering flue gas via composite absorbent. Chin. J. Chem. Eng. 2016, 24, 1104–1111. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, H.; Zhuo, J.; You, C. Experimental and numerical study on effects of deflectors on flow field distribution and desulfurization efficiency in spray towers. Fuel Process. Technol. 2017, 162, 1–12. [Google Scholar] [CrossRef]
- Guo, J.; Li, Y.; Xiong, J.; Zhu, T. Coupling mechanism of activated carbon mixed with dust for flue gas desulfurization and denitrification. J. Environ. Sci. China 2020, 98, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.M.; Sun, B.S.; Qi, N.Z. Efficiency Prediction of Limestone-Gypsum Wet Desulphurization Based on Support Vector Machine. Adv. Mater. Res. 2014, 989–994, 913–917. [Google Scholar] [CrossRef]
- Nan, J.; Chen, X.; Wang, X.; Lashari, M.S.; Wang, Y.; Guo, Z.; Du, Z. Effects of applying flue gas desulfurization gypsum and humic acid on soil physicochemical properties and rapeseed yield of a saline-sodic cropland in the eastern coastal area of China. J. Soils Sediments 2015, 16, 38–50. [Google Scholar] [CrossRef]
- Pan, D.; Yu, R.; Bao, J.; Wu, H.; Huang, R.; Yang, L. Emission and Formation Characteristics of Aerosols from Ammonia-Based Wet Flue Gas Desulfurization. Energy Fuels 2015, 30, 666–673. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, C.; Wang, P.; Zhang, K. Effect of Fe3+ concentration and pH on arsenic removal, migration and speciation transformation in lab-scale wet flue gas desulfurization system. Fuel 2021, 295. [Google Scholar] [CrossRef]
- Li, Y.; You, C. Experimental and model investigation on the mass balance of a dry circulating fluidized bed for flue gas desulfurization system. Korean J. Chem. Eng. 2011, 28, 1956–1963. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, X. Effect of Humidification Water on Semi-Dry Flue Gas Desulfurization. Procedia Environ. Sci. 2011, 11, 1023–1028. [Google Scholar] [CrossRef][Green Version]
- Wang, X.; Li, Y.; Zhu, T.; Jing, P.; Wang, J. Simulation of the heterogeneous semi-dry flue gas desulfurization in a pilot CFB riser using the two-fluid model. Chem. Eng. J. 2015, 264, 479–486. [Google Scholar] [CrossRef]
- Wu, F.; Yue, K.; Gao, W.; Gong, M.; Ma, X.; Zhou, W. Numerical simulation of semi-dry flue gas desulfurization process in the powder-particle spouted bed. Adv. Powder Technol. 2020, 31, 323–331. [Google Scholar] [CrossRef]
- Sun, M.; Lou, Z.; Cheng, G.; Baig, S.A.; Fang, L.; Zhou, X.; Shen, Y.; Xu, X. Process migration and transformation of mercury in simulated wet flue gas desulfurization slurry system. Fuel 2015, 140, 136–142. [Google Scholar] [CrossRef]
- Jiang, L.; Mei, K.; Chen, K.; Dao, R.; Li, H.; Wang, C. Design and prediction for highly efficient SO2 capture from flue gas by imidazolium ionic liquids. Green Energy Environ. 2020. [Google Scholar] [CrossRef]
- Zhu, Z.; Ma, Y.; Qu, Z.; Fang, L.; Zhang, W.; Yan, N. Study on a new wet flue gas desulfurization method based on the Bunsen reaction of sulfur-iodine thermochemical cycle. Fuel 2017, 195, 33–37. [Google Scholar] [CrossRef]
- Zhang, Y.; Qian, W.; Zhou, P.; Liu, Y.; Lei, X.; Li, B.; Ning, P. Research on red mud-limestone modified desulfurization mechanism and engineering application. Sep. Purif. Technol. 2021, 272. [Google Scholar] [CrossRef]
- Cheng, T.; Zhou, X.; Yang, L.; Wu, H.; Fan, H. Transformation and removal of ammonium sulfate aerosols and ammonia slip from selective catalytic reduction in wet flue gas desulfurization system. J. Environ. Sci. China 2020, 88, 72–80. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, L.; Wu, H.; Yang, H. Promoting fine particle removal in double-tower cascade wet flue gas desulfurization system by flue gas temperature reduction. Powder Technol. 2020, 373, 581–589. [Google Scholar] [CrossRef]
- Li, H.; Zhang, H.; Li, L.; Ren, Q.; Yang, X.; Jiang, Z.; Zhang, Z. Utilization of low-quality desulfurized ash from semi-dry flue gas desulfurization by mixing with hemihydrate gypsum. Fuel 2019, 255. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, B.; Ye, Q.; Su, Y. Wet flue gas desulfurization using micro vortex flow scrubber: Characteristics, modeling and simulation. Sep. Purif. Technol. 2020, 247. [Google Scholar] [CrossRef]
- Zhou, D.; Wei, R.; Zhu, Y.; Long, H.; Huang, B.; Wang, Y.; Wu, S. Calcium sulfate whisker one-step preparation using semi-dry flue gas desulfurization ash and directional growth control. J. Clean. Prod. 2021, 290. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, H.; Chang, D.; You, C. Numerical Simulation of Moderate Temperature Desulfurization in Circulating Fluidized Bed Reactor Considering Sorbent Abrasion. Energy Fuels 2018, 33, 484–492. [Google Scholar] [CrossRef]
- Chen, B.; Sun, F.; Gao, M.; Shi, Y. A 1-D model of spraying performance for wet flue gas desulfurization scrubber based on predicted slurry temperature. Appl. Therm. Eng. 2019, 155, 259–266. [Google Scholar] [CrossRef]
- Chen, Z.; You, C.; Wang, H.; Liu, Q. Experimental study on the synergetic removal of fine particles by wet flue gas desulfurization tower with a flow pattern control device. Powder Technol. 2019, 343, 122–128. [Google Scholar] [CrossRef]
- Salehi, E.; Eidi, B.; Soleimani, Z. An integrated process consisting of Mg(OH)2—Impregnated ceramic foam filters as adsorbent and Mg(OH)2 as scrubbing solution for intensified desulfurization of flue gas. Sep. Purif. Technol. 2019, 216, 34–42. [Google Scholar] [CrossRef]
- Li, X.; Dong, M.; Li, S.; Feng, Z.; Zhang, Z.; Li, W.; Ren, Y.; Lu, J. A numerical study of the ammonia desulfurization in the spray scattering tower. Chem. Eng. Process. Process. Intensif. 2020, 155. [Google Scholar] [CrossRef]
- You, C.; Qi, H.; Wang, W.; Xu, X. A model for flue gas desulfurization with lime absorbent at middle temperature. Dev. Chem. Eng. Mineral. Process. 2001, 9, 289–300. [Google Scholar] [CrossRef]
- You, C.; Li, Y. Desulfurization characteristics of rapidly hydrated sorbents with various adhesive carrier particles for a semidry CFB-FGD system. Environ. Sci. Technol. 2013, 47, 2754–2759. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.Y.; Leu, L. Multiresolution analysis on identification and dynamics of clusters in a circulating fluidized bed. AIChE J. 2009, 55, 612–629. [Google Scholar] [CrossRef]
- Fan, B.; You, C.; Qi, H.; Xiang, G.; Xu, X. Influence of Chemical and Thermodynamic Parameters on the Flue Gas Desulphurization Efficiency in a Circulating Fluidized Bed. J. Therm. Sci. 2000, 10, 286–290. [Google Scholar] [CrossRef]
- Wang, F.; Ma, S.; Wen, J.; Lu, C. Gas hydrodynamics of a novel MTO high-speed loop reactor: The bypassing and backmixing along with average residence time. Powder Technol. 2020, 364, 1062–1075. [Google Scholar] [CrossRef]
- Li, Y.; Qi, H.; Wang, J. SO2 capture and attrition characteristics of a CaO/bio-based sorbent. Fuel 2012, 93, 258–263. [Google Scholar] [CrossRef]
- Zhou, G.; Zhong, W.; Zhou, Y.; Wang, J.; Wang, T. 3D simulation of sintering flue gas desulfurization and denitration in a bubbling gas absorbing tower. Powder Technol. 2017, 314, 412–426. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, Y.; Gao, W.; Baleta, J.; Liu, C.; Li, W.; Weng, W.; Dai, H.; Zheng, C.; Gao, X. Simulation of SO2 absorption and performance enhancement of wet flue gas desulfurization system. Process. Saf. Environ. Prot. 2021, 150, 453–463. [Google Scholar] [CrossRef]
- Li, Y.; Song, C.; You, C. Experimental Study on Abrasion Characteristics of Rapidly Hydrated Sorbent for Moderate Temperature Dry Flue Gas Desulfurization. Energy Fuels 2010, 24, 1682–1686. [Google Scholar] [CrossRef]
- Li, Y.; You, C.; Song, C. Adhesive carrier particles for rapidly hydrated sorbent for moderate-temperature dry flue gas desulfurization. Environ. Sci. Technol. 2010, 44, 4692–4696. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, T.; Yang, H.; Zhang, H.; Zhang, X. Experimental study on SO2 recovery using a sodium–zinc sorbent based flue gas desulfurization technology. Chin. J. Chem. Eng. 2015, 23, 241–246. [Google Scholar] [CrossRef]
- Chang, G.; Song, C.; Wang, L. A modeling and experimental study of flue gas desulfurization in a dense phase tower. J. Hazard. Mater. 2011, 189, 134–140. [Google Scholar] [CrossRef]
- Li, S.H.; Qi, G.C.; Tang, G.X.; Wang, H. Numerical Simulation of Venturi Device in Flue-Gas Circulating Fluidized Bed Desulphurization Tower. Adv. Mater. Res. 2011, 356–360, 1262–1266. [Google Scholar] [CrossRef]
- Montagnaro, F.; Salatino, P.; Scala, F.; Urciuolo, M. A Population Balance Model on Sorbent in CFB Combustors: The Influence of Particle Attrition. Ind. Eng. Chem. Res. 2011, 50, 9704–9711. [Google Scholar] [CrossRef]
- Zhang, H.; Johnston, P.M.; Zhu, J.X.; de Lasa, H.I.; Bergougnou, M.A. A novel calibration procedure for a fiber optic solids concentration probe. Powder Technol. 1998, 100, 260–272. [Google Scholar] [CrossRef]
- Wang, C.; Zhu, J.; Barghi, S.; Li, C. Axial and radial development of solids holdup in a high flux/density gas–solids circulating fluidized bed. Chem. Eng. Sci. 2014, 108, 233–243. [Google Scholar] [CrossRef]
- Wang, C.; Li, C.; Zhu, J. Axial solids flow structure in a high density gas–solids circulating fluidized bed downer. Powder Technol. 2015, 272, 153–164. [Google Scholar] [CrossRef]
- Geldart, D.; Harnby, N.; Wong, A. Fluidization of cohesive powders. Powder Technol. 1984, 25, 25–37. [Google Scholar] [CrossRef]
- Geldart, D.; Abdullah, E.C.; Hassanpour, A.; Nwoke, L.C.; Wouters, I. Characterization of powder flowability using measurement of angle of repose. China Particuol. 2006, 4, 104–107. [Google Scholar] [CrossRef]
- Werther, J. Measurement techniques in fluidized beds. Powder Technol. 1999, 102, 15–36. [Google Scholar] [CrossRef]
- Van Ommen, J.R.; Mudde, R.F. Measuring the gas–solids distribution in fluidized beds—A review. Int. J. Chem. React. Eng. 2008, 6, 1–32. [Google Scholar] [CrossRef]
- Chang, J.; Zhang, K.; Zhu, W.; Yang, Y. Gas–solid flow in a high-density circulating fluidized bed riser with Geldart A particles. Particuology 2016, 29, 103–109. [Google Scholar] [CrossRef]
- Wei, Y.; Cheng, L.; Wu, E.; Li, L. Experimental research on steady-state operation characteristics of gas–solid flow in a 15.5 m dual circulating fluidized bed system. Chin. J. Chem. Eng. 2021, 32, 70–76. [Google Scholar] [CrossRef]
- Zhu, J.; Manyele, S.V. Radial nonuniformity index (RNI) in fluidized beds and other multiphase flow systems. Can. J. Chem. Eng. 2001, 79, 203–214. [Google Scholar] [CrossRef]
- Xu, G.; Hartge, E.-U.; Werther, J.; Gao, S. Saturation carrying capacity at high archimedes number of vertical concurrent gas–particle flow. Chem. Eng. Sci. 2006, 61, 7115–7124. [Google Scholar] [CrossRef]




| Authors | Device Size, m | Ug, m/s Gs, kg/m2·s | Particle Type | Particle Size, μm |
|---|---|---|---|---|
| You et al., 2001 [30] | -- | -- | CaO | 282–560 |
| Qi et al., 2005 [3] | D: 0.15; H: 3 | 4–5.2 53–489 | FCC | 78 |
| Li et al., 2011 [12] | D: 0.305; H: 6 | 2.5 -- | ash sorbent | <10 |
| You et al., 2013 [31] | D: 0.305; H: 6 | 1.5 -- | fly ash; river sand | <10, <1000 |
| Zhang et al., 2018 [25] | D: 0.305; H: 6 | 2.5 -- | fresh sorbent | 116 |
| Chen et al., 2017 [6] | D: 1.2; H: 4.08 | 0.2–0.4 -- | Ca(OH)2 | -- |
| Chen et al., 2019 [27] | D: 1.2; H: 4.08 | 3–4 | -- | 0.05–4 |
| Yang et al., 2016 [8] | -- | -- | Ca(OH)2 | 154–280 |
| Yang et al., 2009 [32] | -- | <3.5 | Ash | 2 |
| Wang et al., 2016 [5] | -- | -- | Ca(OH)2 | -- |
| Fan et al., 2000 [33] | D: 0.28; H: 6 | 1.9 4.5 | Ca(OH)2 | -- |
| Wang et al., 2020 [34] | D: 0.05; H: 2.5 | 2.5 0–15 | Fine particle | 100 |
| Particle Size, μm | Volume Fraction, % | Particle Size, μm | Volume Fraction, % |
|---|---|---|---|
| 0–1 | 7.60 | 50–100 | 13.32 |
| 1–10 | 39.67 | 100–200 | 6.71 |
| 10–50 | 27.05 | 200–563 | 5.65 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Wang, C.; Lan, X.; Gao, J. Study on the Flow Characteristics of Desulfurization Ash Fine Particles in a Circulating Fluidized Bed. Processes 2021, 9, 1343. https://doi.org/10.3390/pr9081343
Yang X, Wang C, Lan X, Gao J. Study on the Flow Characteristics of Desulfurization Ash Fine Particles in a Circulating Fluidized Bed. Processes. 2021; 9(8):1343. https://doi.org/10.3390/pr9081343
Chicago/Turabian StyleYang, Xiao, Chengxiu Wang, Xingying Lan, and Jinsen Gao. 2021. "Study on the Flow Characteristics of Desulfurization Ash Fine Particles in a Circulating Fluidized Bed" Processes 9, no. 8: 1343. https://doi.org/10.3390/pr9081343
APA StyleYang, X., Wang, C., Lan, X., & Gao, J. (2021). Study on the Flow Characteristics of Desulfurization Ash Fine Particles in a Circulating Fluidized Bed. Processes, 9(8), 1343. https://doi.org/10.3390/pr9081343






