Energy Optimization and Effective Control of Reactive Distillation Process for the Production of High Purity Biodiesel
Abstract
:1. Introduction
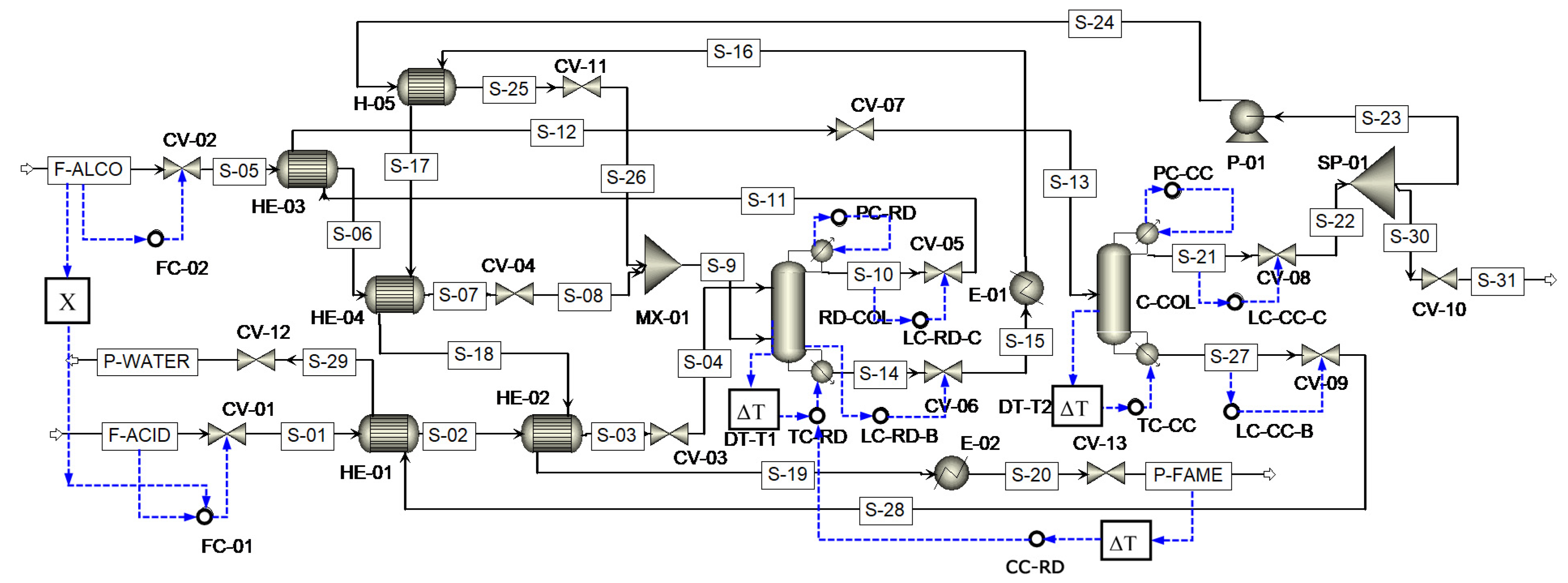
2. Process Description
2.1. Process Kinetics
2.2. VLE Thermodynamics
- The liquid and vapor in all the trays are completely mixed
- Both the liquid and vapor streams leaving the tray are in equilibrium.
- The vapor hold-up in the tray is negligible
- The liquid phase is homogenous in all trays.
2.3. Steady State RD Process
3. Results and Discussion
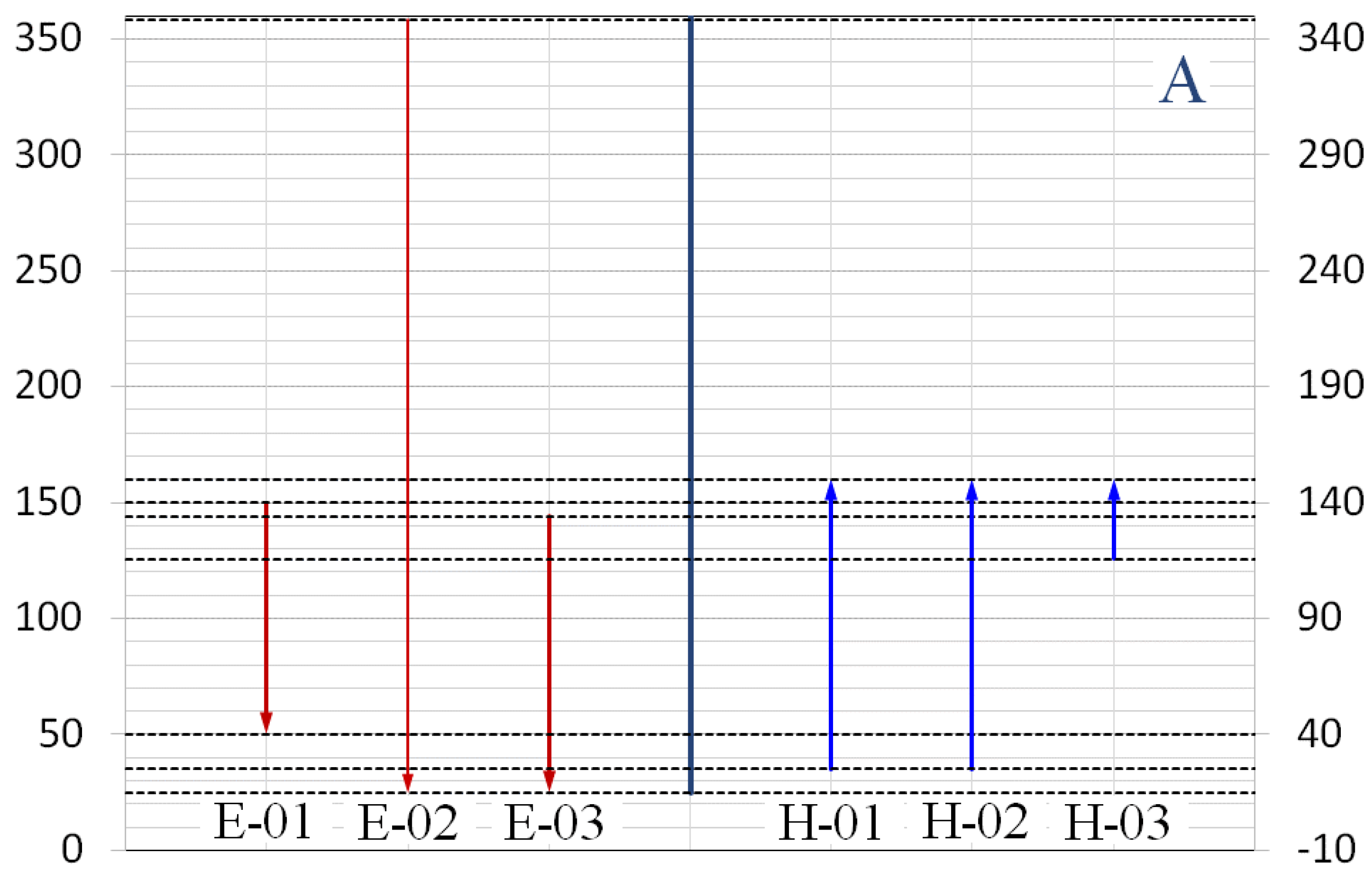
3.1. Heat Integration
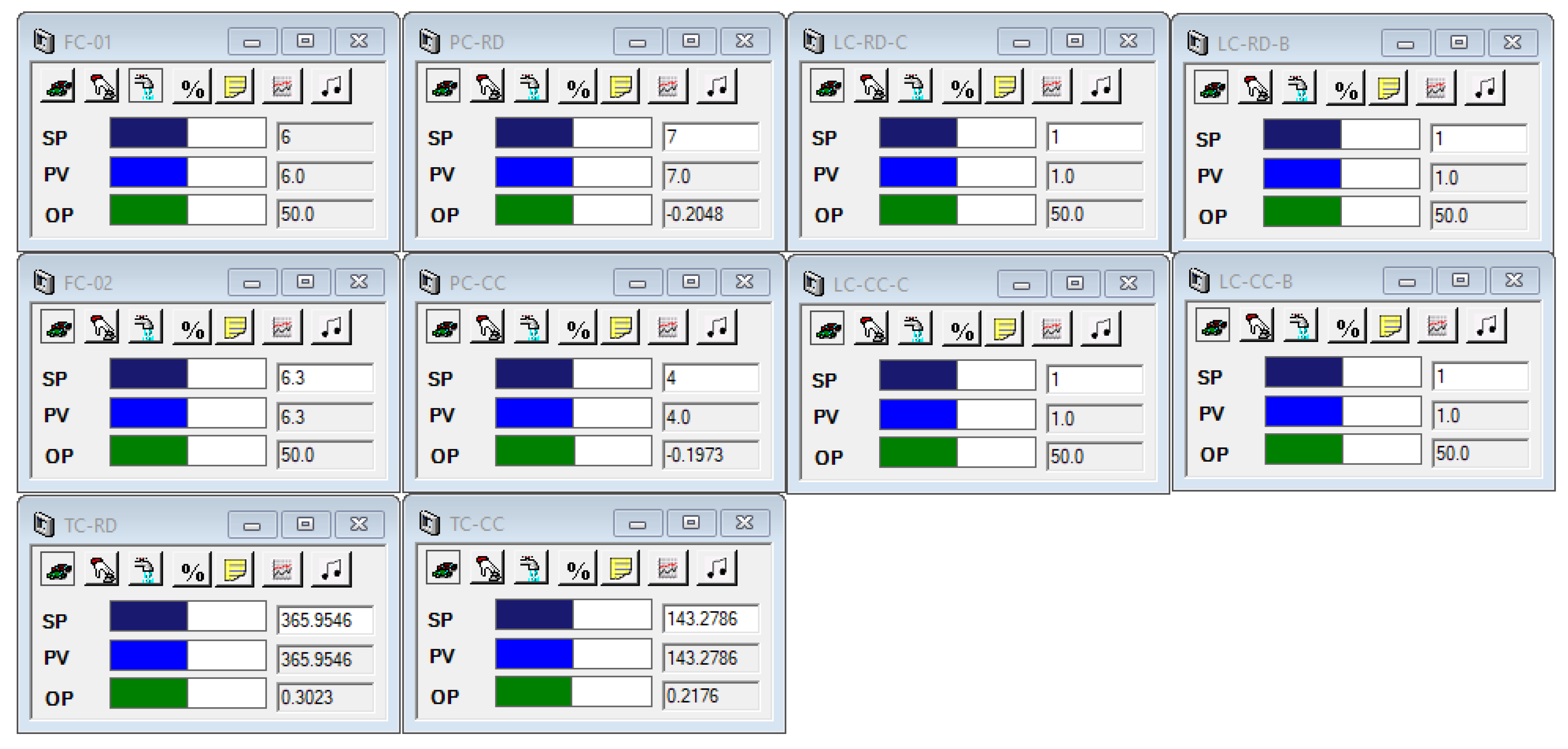
3.2. Process Control Structure
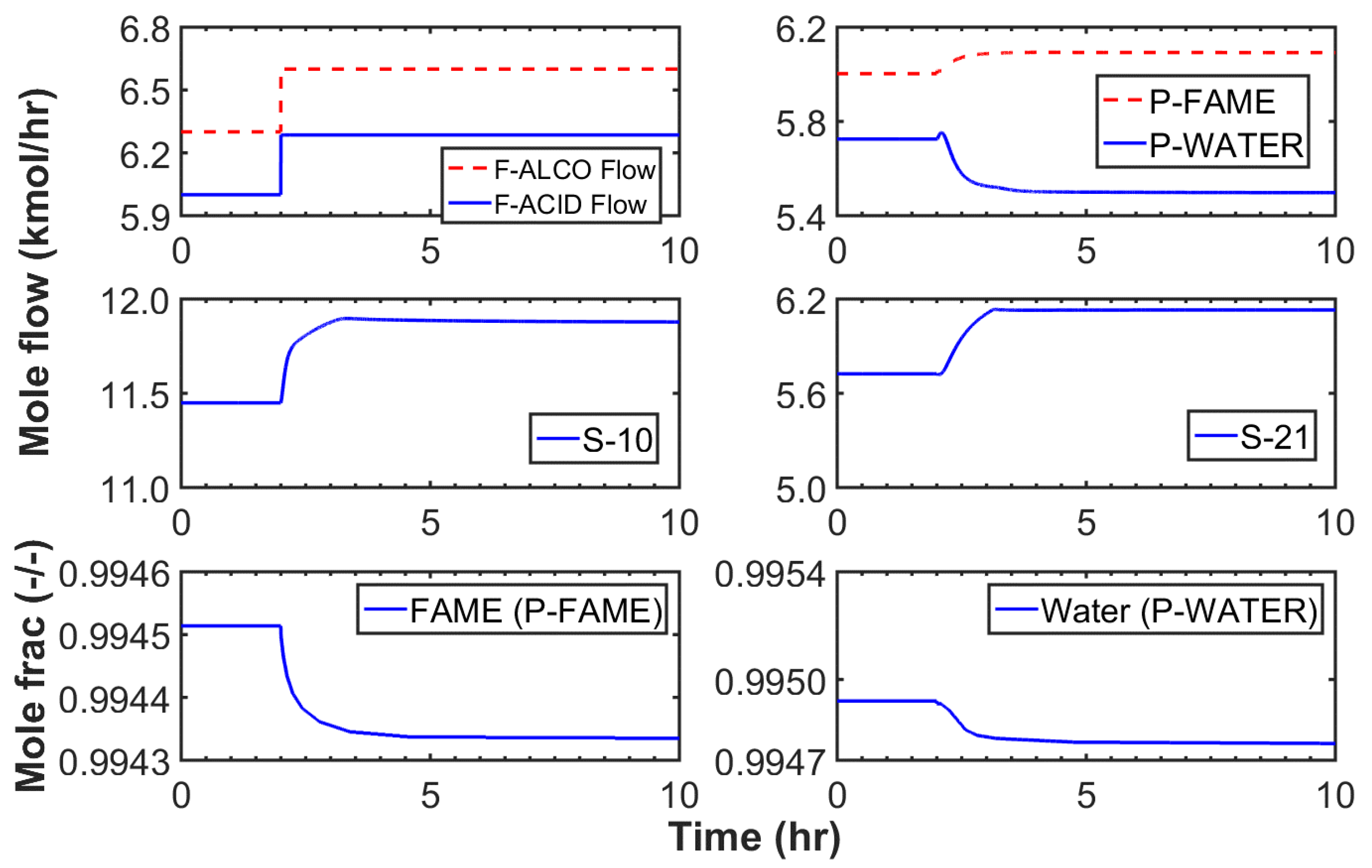
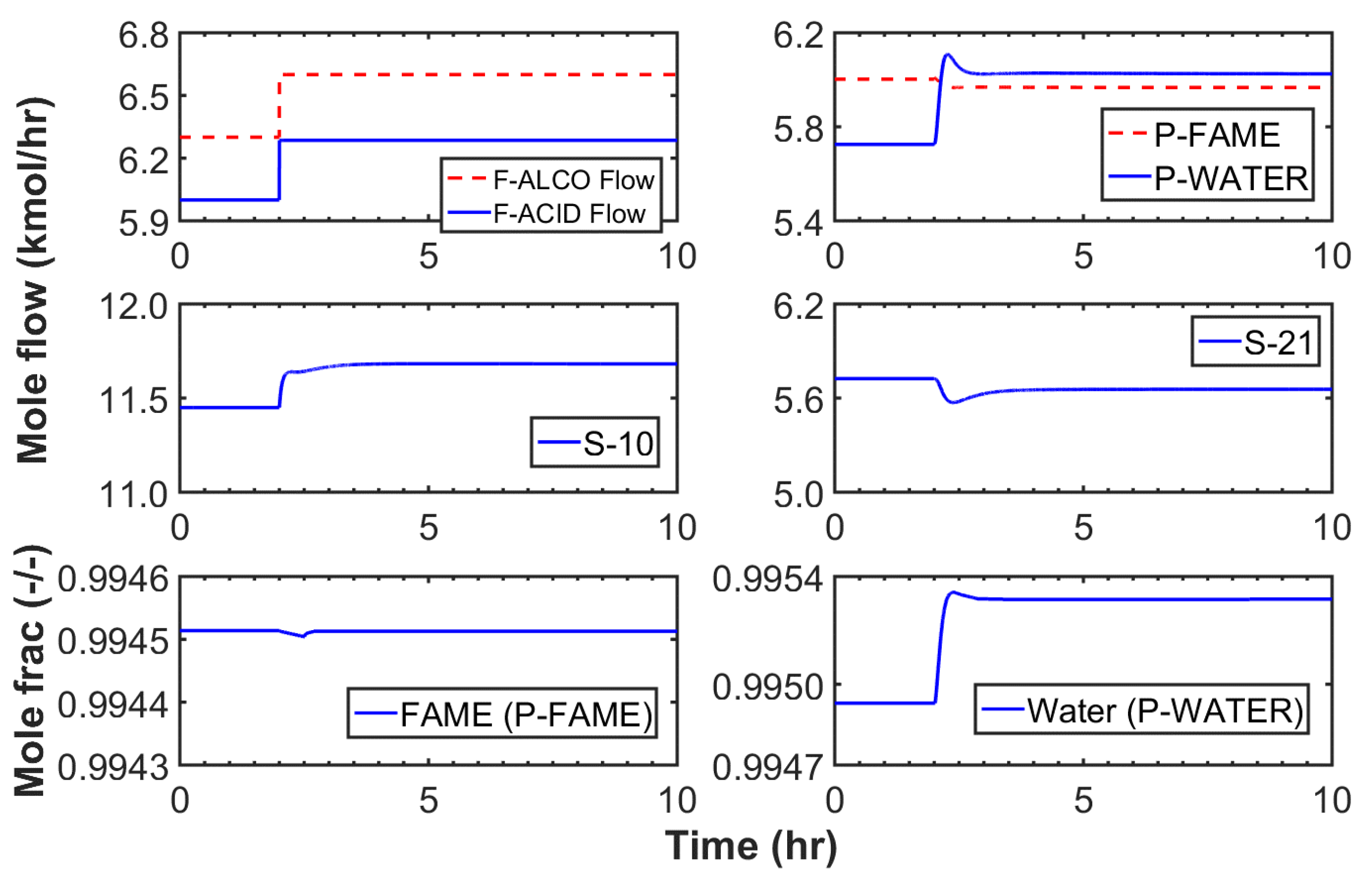
3.2.1. Case I
3.2.2. Case II
3.2.3. Case III
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Veljković, V.B.; Biberdžić, M.O.; Banković-Ilić, I.B.; Djalović, I.G.; Tasić, M.B.; Nježić, Z.B.; Stamenković, O.S. Biodiesel production from corn oil: A review. Renew. Sustain. Energy Rev. 2018, 91, 531–548. [Google Scholar] [CrossRef]
- Thangaraj, B.; Solomon, P.R.; Muniyandi, B.; Ranganathan, S.; Lin, L. Catalysis in biodiesel production—A review. Clean Energy 2018, 3, 2–23. [Google Scholar] [CrossRef] [Green Version]
- Pasha, M.K.; Dai, L.; Liu, D.; Guo, M.; Du, W. An overview to process design, simulation and sustainability evaluation of biodiesel production. Biotechnol. Biofuels 2021, 14, 129. [Google Scholar] [CrossRef] [PubMed]
- Atadashi, I.M.; Aroua, M.K.; Aziz, A.A. Biodiesel separation and purification: A review. Renew. Energy 2011, 36, 437–443. [Google Scholar] [CrossRef]
- Patel, A.D.; Zabeti, M.; Seshan, K.; Patel, M.K. Comparative Technical Process and Product Assessment of Catalytic and Thermal Pyrolysis of Lignocellulosic Biomass. Processes 2020, 8, 1600. [Google Scholar] [CrossRef]
- Chozhavendhan, S.; Vijay Pradhap Singh, M.; Fransila, B.; Praveen Kumar, R.; Karthiga Devi, G. A review on influencing parameters of biodiesel production and purification processes. Curr. Res. Green Sustain. Chem. 2020, 1–2, 1–6. [Google Scholar] [CrossRef]
- Castro, F.I.G.; Ramirez, V.R.; Hernandez, J.G.S.; Castro, S.H.; El-Halwagi, M.M. Simulation study on biodiesel production by reactive distillation with methanol at high pressure and temperature: Impact on costs and pollutant emissions. Comput. Chem. Eng. 2013, 52, 204–215. [Google Scholar] [CrossRef]
- Dimian, A.C.; Bildea, C.S.; Omota, F.; Kiss, A.A. Innovative process for fatty acid esters by dual reactive distillation. Comput. Chem. Eng. 2009, 33, 743–750. [Google Scholar] [CrossRef]
- Bildea, C.S.; Kiss, A.A. Dynamics and control of a biodiesel process by reactive absorption. Chem. Eng. Res. Des. 2011, 89, 187–196. [Google Scholar] [CrossRef]
- Abomohra, A.E.-F.; Elsayed, M.; Esakkimuthu, S.; El-Sheekh, M.; Hanelt, D. Potential of fat, oil and grease (FOG) for biodiesel production: A critical review on the recent progress and future perspectives. Prog. Energy Combust. Sci. 2020, 81, 100868. [Google Scholar] [CrossRef]
- Vargas, E.C.; Hernandez, S.; Hernandez, J.G.S.; Rodriguez, M.I.C. Simulation study of the production of biodiesel using feedstock mixtures of fatty acids in complex reactive distillation columns. Energy 2011, 36, 6289–6297. [Google Scholar] [CrossRef]
- Šulgan, B.; Labovský, J.; Labovská, Z. Multi-Aspect Comparison of Ethyl Acetate Production Pathways: Reactive Distillation Process Integration and Intensification via Mechanical and Chemical Approach. Processes 2020, 8, 1618. [Google Scholar] [CrossRef]
- Estrada-Villagrana, A.D.; Quiroz-Sosa, G.B.; Jiménez-Alarcón, M.L.; Alemán-Vázquez, L.O.; Cano-Domínguez, J.L. Comparison between a conventional process and reactive distillation for naphtha hydrodesulfurization. Chem. Eng. Process. Process Intensif. 2006, 45, 1036–1040. [Google Scholar] [CrossRef]
- Kiss, A.A.; Omota, F.; Dimian, A.C.; Rothenberg, G. The heterogeneous advantage: Biodiesel by catalytic reactive distillation. Top. Catal. 2006, 40, 141–150. [Google Scholar] [CrossRef]
- Segovia-Hernández, J.G.; Hernández, S.; Bonilla Petriciolet, A. Reactive distillation: A review of optimal design using deterministic and stochastic techniques. Chem. Eng. Process. Process Intensif. 2015, 97, 134–143. [Google Scholar] [CrossRef]
- Saha, B.; Teo, H.; Alqahtani, A. iso-Amyl Acetate Synthesis by Catalytic Distillation. Int. J. Chem. React. Eng. 2005, 3, A11. [Google Scholar] [CrossRef]
- Al-Arfaj, M.A.; Luyben, W.L. Plantwide control for TAME production using reactive distillation. AIChE J. 2004, 50, 1462–1473. [Google Scholar] [CrossRef]
- Ali, S.S.; Hossain, S.S.; Asif, M. Dynamic modeling of the isoamyl acetate reactive distillation process. Pol. J. Chem. Technol. 2017, 19, 59. [Google Scholar] [CrossRef] [Green Version]
- Hasabnis, A.; Mahajani, S. Acetalization of Glycerol with Formaldehyde by Reactive Distillation. Ind. Eng. Chem. Res. 2014, 53, 12279–12287. [Google Scholar] [CrossRef]
- Guo, B.; Li, Y. Analysis and simulation of reactive distillation for gasoline alkylation desulfurization. Chem. Eng. Sci. 2012, 72, 115–125. [Google Scholar] [CrossRef]
- Yamaki, T.; Matsuda, K.; Na-Ranong, D.; Matsumoto, H. Intensification of Reactive Distillation for TAME Synthesis Based on the Analysis of Multiple Steady-State Conditions. Processes 2018, 6, 241. [Google Scholar] [CrossRef] [Green Version]
- Wei, H.-Y.; Rokhmah, A.; Handogo, R.; Chien, I.L. Design and control of reactive-distillation process for the production of diethyl carbonate via two consecutive trans-esterification reactions. J. Process Control 2011, 21, 1193–1207. [Google Scholar] [CrossRef]
- Kiss, A.A. Heat-integrated reactive distillation process for synthesis of fatty esters. Fuel Process. Technol. 2011, 92, 1288–1296. [Google Scholar] [CrossRef]
- Agarwal, M.; Singh, K.; Chaurasia, S.P. Simulation and sensitivity analysis for biodiesel production in a reactive distillation column. Pol. J. Chem. Technol. 2012, 14, 59. [Google Scholar] [CrossRef] [Green Version]
- Kianimanesh, H.R.; Abbaspour-Aghdam, F.; Derakhshan, M.V. Biodiesel production from vegetable oil: Process design, evaluation and optimization. Pol. J. Chem. Technol. 2017, 19, 49. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Zhou, H.; Sun, L.; Zhang, N. Design and control of different pressure thermally coupled reactive distillation for synthesis of isoamyl acetate. Chem. Eng. Process. Process Intensif. 2019, 139, 51–67. [Google Scholar] [CrossRef] [Green Version]
- Zhao, T.; Li, J.; Zhou, H.; Ma, Z.; Sun, L. A thermally coupled reactive distillation process to intensify the synthesis of isopropyl acetate. Chem. Eng. Process. Process Intensif. 2018, 124, 97–108. [Google Scholar] [CrossRef]
- Kiss, A.A.; Bildea, C.S. Integrated reactive absorption process for synthesis of fatty esters. Bioresour. Technol. 2011, 102, 490–498. [Google Scholar] [CrossRef]
- Nguyen, N.; Demirel, Y. Using thermally coupled reactive distillation columns in biodiesel production. Energy 2011, 36, 4838–4847. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Cisneros, E.S.; Mena-Espino, X.; Rodríguez-López, V.; Sales-Cruz, M.; Viveros-García, T.; Lobo-Oehmichen, R. An integrated reactive distillation process for biodiesel production. Comput. Chem. Eng. 2016, 91, 233–246. [Google Scholar] [CrossRef]
- Poddar, T.; Jagannath, A.; Almansoori, A. Use of reactive distillation in biodiesel production: A simulation-based comparison of energy requirements and profitability indicators. Appl. Energy 2017, 185, 985–997. [Google Scholar] [CrossRef]
- Seborg, D.E.; Edgar, T.F.; Mellichamp, D.A.; Doyle, F.J.D., III. Process Dynamics and Control, 4th ed.; John Wiley and Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Hung, S.-B.; Chen, J.-H.; Lin, Y.-D.; Huang, H.-P.; Lee, M.-J.; Ward, J.D.; Yu, C.-C. Control of plantwide reactive distillation processes: Hydrolysis, transesterification and two-stage esterification. J. Taiwan Inst. Chem. Eng. 2010, 41, 382–402. [Google Scholar] [CrossRef]
- Luyben, W.L.; Yu, C.C. Reactive Distillation Design and Control, 1st ed.; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar] [CrossRef]
- Luyben, W.L. Economic and Dynamic Impact of the Use of Excess Reactant in Reactive Distillation Systems. Ind. Eng. Chem. Res. 2000, 39, 2935–2946. [Google Scholar] [CrossRef]
- Lee, H.-Y.; Lai, I.K.; Huang, H.-P.; Chien, I.L. Design and Control of Thermally Coupled Reactive Distillation for the Production of Isopropyl Acetate. Ind. Eng. Chem. Res. 2012, 51, 11753–11763. [Google Scholar] [CrossRef]
- Omota, F.; Dimian, A.C.; Bliek, A. Fatty acid esterification by reactive distillation. Part 1: Equilibrium-based design. Chem. Eng. Sci. 2003, 58, 3159–3174. [Google Scholar] [CrossRef]
- Kiss, A.A.; Dimian, A.C.; Rothenberg, G. Biodiesel by Catalytic Reactive Distillation Powered by Metal Oxides. Energy Fuels 2008, 22, 598–604. [Google Scholar] [CrossRef]
- Elliott, J.R.; Lira, C.T. Introductory Chemical Engineering Thermodynamics, 2nd ed.; Prentice Hall: Hoboken NJ, USA, 2012. [Google Scholar]
- Ali, S.S.; Asif, M.; Basu, A. Design and simulation of high purity biodiesel reactive distillation process. Pol. J. Chem. Technol. 2019, 21, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Simon Araya, S.; Liso, V.; Cui, X.; Li, N.; Zhu, J.; Sahlin, S.L.; Jensen, S.H.; Nielsen, M.P.; Kær, S.K. A Review of The Methanol Economy: The Fuel Cell Route. Energies 2020, 13, 596. [Google Scholar] [CrossRef] [Green Version]
- Luyben, W.L. Distillation Design and Control Using Aspen Simulation, 2nd ed.; John Wiley and Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
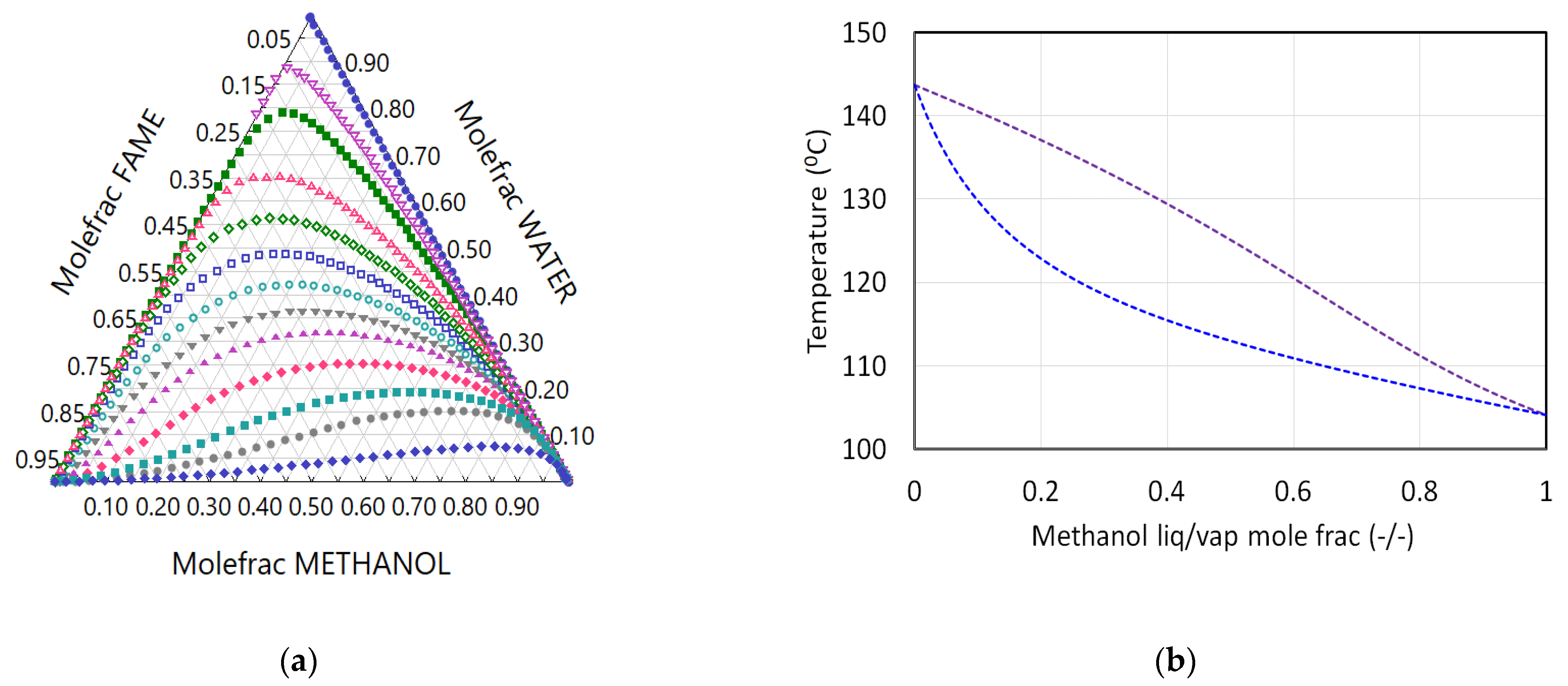
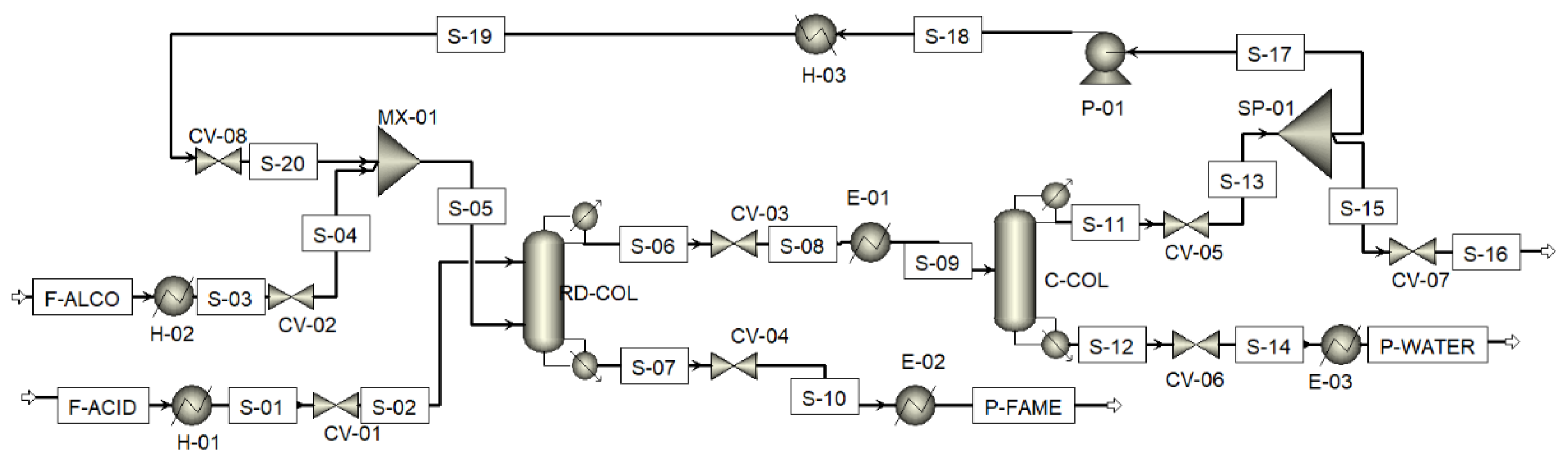
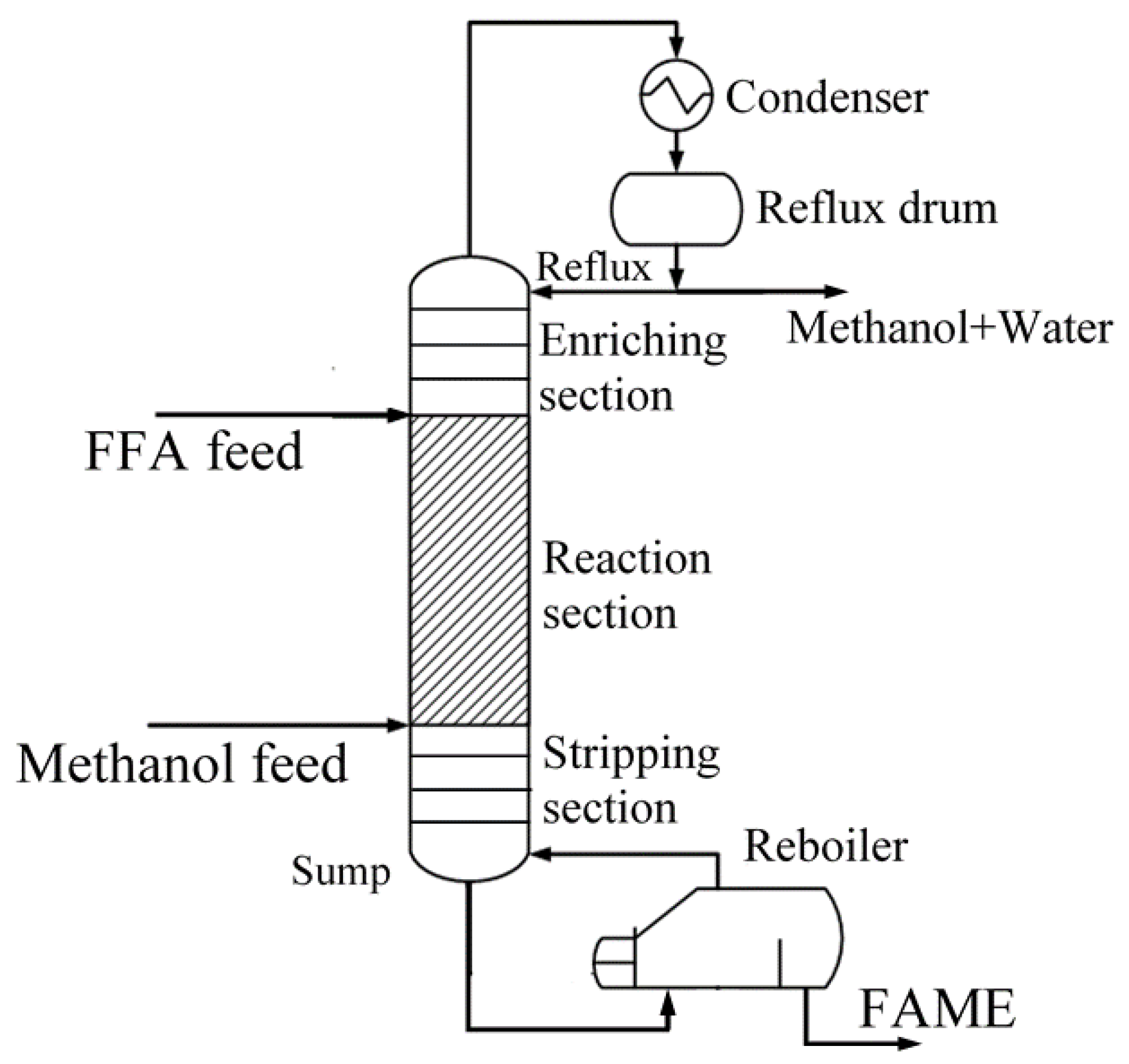
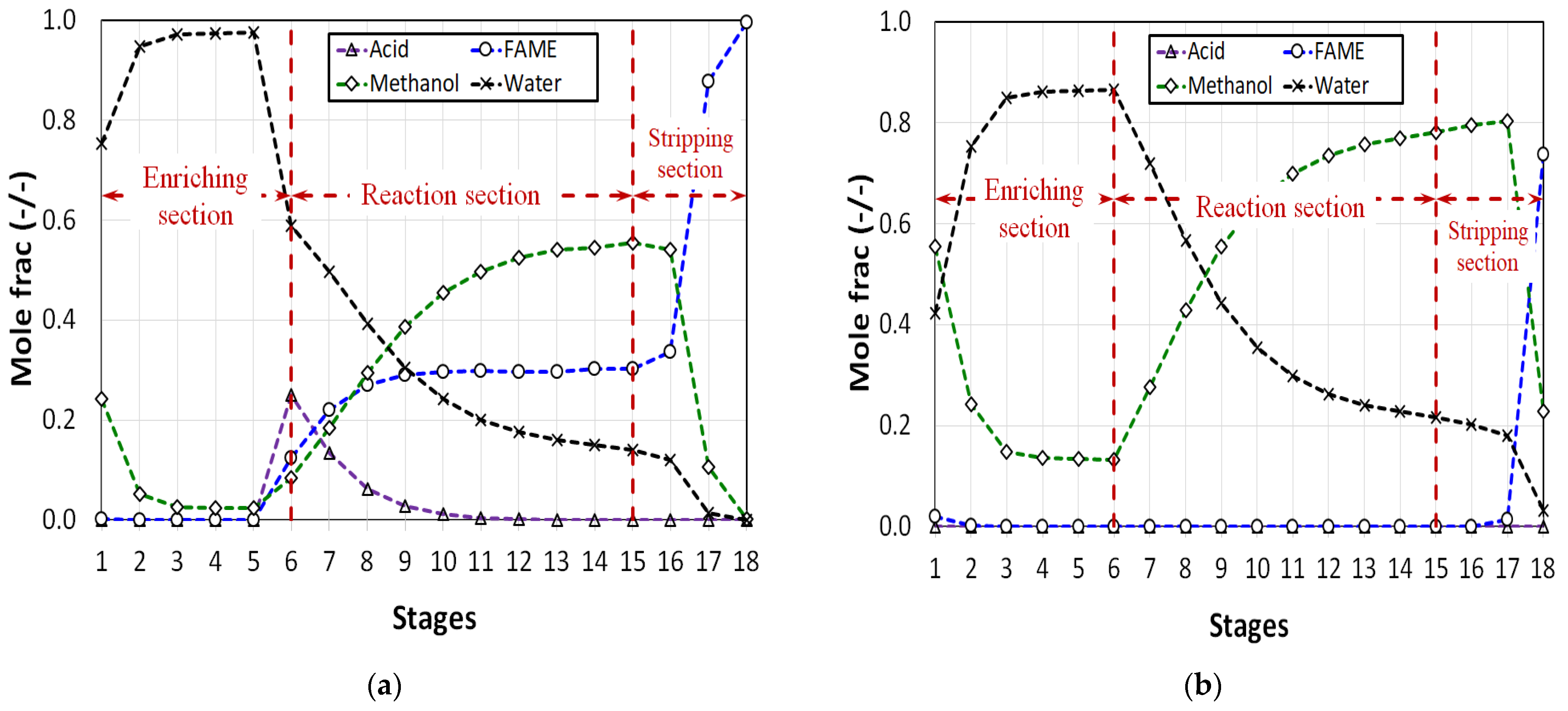



| Comp. i | Acid | Methanol | Methanol | Methanol | Acid | Water |
|---|---|---|---|---|---|---|
| Comp. j | FAME | Water | Acid | FAME | Water | FAME |
| (K) | 0 | −1.0662 | 0 | 0 | −0.29924 | 0 |
| (K) | 0 | 0.6437 | 0 | 0 | −0.38437 | 0 |
| (K2) | 238.8469 | 432.8785 | 48.3493 | 31.789 | −195.44 | −216.733 |
| (K2) | −369.561 | −322.1312 | −309.554 | −539.979 | −107.62 | −658.816 |
| RD Column | Column 2 | |
|---|---|---|
| Number of stages | 18 | 10 |
| Feed stage | Above 6 and 16 | 7 |
| Reactive stages | 6–15 | - |
| Operating pressure | 9 bar | 5 bar |
| Reflux ratio | 0.8 | 3 |
| Reboiler duty | 360 kW | 258.2 kW |
| Condenser duty | −208.5 kW | −234.8 kW |
| Reactive stages liquid hold-up | 50 L | - |
| Stream Name | F-ACID | F-ALCO | S-05 | P-FAME | P-WATER | S-16 |
|---|---|---|---|---|---|---|
| Temperature (°C) | 25 | 25 | 150 | 375.9 | 144.22 | 114.16 |
| Pressure (bar) | 13.03 | 13.1 | 9.1 | 4.1 | 2.06 | 1.0 |
| Vapor Fraction | 0 | 0 | 1 | 0 | 0 | 0 |
| Mole Flows (kmol/h) | 6 | 6.3 | 9.84 | 6.03 | 5.69 | 0.57 |
| Mole Fractions (-/-) | ||||||
| ACID | 1 | 0 | 0 | 0.0006 | 0.000 | 0.000 |
| FAME | 0 | 0 | 0 | 0.9945 | 0.000 | 0.000 |
| METHANOL | 0 | 1 | 0.92 | 0 | 0.0048 | 0.47 |
| WATER | 0 | 0 | 0.08 | 0.0049 | 0.9952 | 0.53 |
| Streams | Condition | Tin (°C) | Tout (°C) | Qavailable (kW) |
|---|---|---|---|---|
| F-ALCO | Cold | 25 | 150 | −76.2 |
| F-ACID | Cold | 25 | 150 | −99.1 |
| S-08 | Hot | 150 | 50 | 26.6 |
| S-10 | Hot | 358.4 | 25 | 333.8 |
| S-14 | Hot | 144.2 | 25 | 16.2 |
| S-18 | Cold | 115.7 | 150 | −35.3 |
| Process without Heat Integration | Heat Integrated Process | |
|---|---|---|
| Capital cost (USD) | 5,910,470 | 5,901,860 |
| Annual utility cost (USD) | 173,599 | 115,256 |
| Total hot utilities (kW) | 799.5 | 618.2 |
| Total cold utilities (kW) | −801.1 | −602.5 |
| Energy savings | - | 33.6% |
| Controller ID | Controller Type | Controlled Variable | Manipulated Variable | Tuning Parameters |
|---|---|---|---|---|
| FC-01 | PI | Acid feed flow | Acid feed flow | |
| FC-02 | PI | Methanol feed flow | Methanol feed flow | |
| LC-RD-C | P-only | RD-reflux drum level | RD-distillate flow | |
| LC-RD-B | P-only | RD-sump level | RD-bottom flow | |
| LC-CC-C | P-only | CC-reflux drum level | CC-distillate flow | |
| LC-CC-B | P-only | CC-sump level | CC-bottom flow | |
| PC-RD | PI | RD-condenser pressure | RD-condenser duty | |
| PC-CC | PI | CC-condenser pressure | CC-condenser duty | |
| TC-RD | PI | RD-tray 17 temperature | RD-reboiler duty | |
| TC-CC | PI | CC-tray 9 temperature | CC-reboiler duty | |
| CC-RD | PI | RD-bottom composition | RD-TC set point |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, S.S.; Arsad, A.; Hossain, S.S.; Basu, A.; Asif, M. Energy Optimization and Effective Control of Reactive Distillation Process for the Production of High Purity Biodiesel. Processes 2021, 9, 1340. https://doi.org/10.3390/pr9081340
Ali SS, Arsad A, Hossain SS, Basu A, Asif M. Energy Optimization and Effective Control of Reactive Distillation Process for the Production of High Purity Biodiesel. Processes. 2021; 9(8):1340. https://doi.org/10.3390/pr9081340
Chicago/Turabian StyleAli, Syed Sadiq, Agus Arsad, SK Safdar Hossain, Avijit Basu, and Mohammad Asif. 2021. "Energy Optimization and Effective Control of Reactive Distillation Process for the Production of High Purity Biodiesel" Processes 9, no. 8: 1340. https://doi.org/10.3390/pr9081340
APA StyleAli, S. S., Arsad, A., Hossain, S. S., Basu, A., & Asif, M. (2021). Energy Optimization and Effective Control of Reactive Distillation Process for the Production of High Purity Biodiesel. Processes, 9(8), 1340. https://doi.org/10.3390/pr9081340








