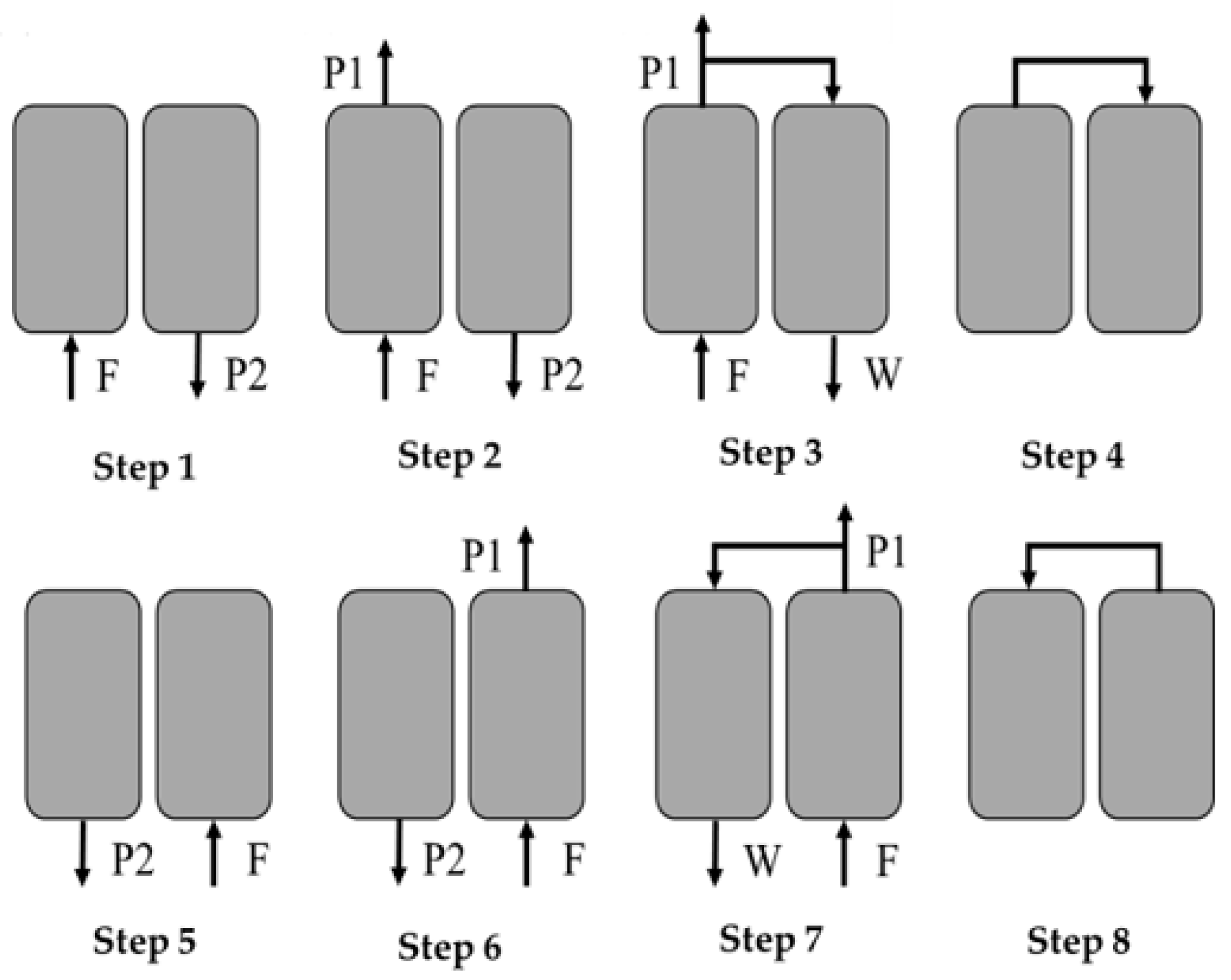
1. Introduction
Methane (CH
4) is a valuable renewable energy source and one of the leading gases responsible for the greenhouse effect. CO
2 has a global warming potential (GWP) of 1 regardless of the time period used, whereas CH
4 is estimated to have a GWP of 28–36 over 100 years [
1]. After CO
2, CH
4 is the second most abundant anthropogenic greenhouse gas, accounting for approximately 20 percent of global emissions [
2]. The main components of biogas are 60–70% CH
4, 30–40% CO
2, and other trace gas compounds, such as 0–4000 ppm hydrogen sulfide (H
2S) produced by anaerobic decomposition [
3]. In order to mitigate global warming, biogas upgrading technologies have been promoted in various countries worldwide in recent years.
Gas separation technologies can not only recover CH
4 and capture CO
2 in order to reduce greenhouse gas emissions, but can also produce high-purity natural gas for use in industrial applications, such as heating and power generation, or as biofuel for vehicles. After removing excess components from biogas in order to achieve ≥97% CH
4 purity, the purified biogas can be injected into the natural gas grid [
4] or compressed into bio-compressed natural gas (bio-CNG) at a high pressure range of 20~25 MPa, which can be used as vehicular fuel [
5]. In comparison to gasoline and diesel, bio-natural gas can reduce air pollution caused by automobile exhaust emissions and net greenhouse gas emissions. In addition, the content of H
2S must be less than 4 ppm for the natural gas pipeline standards in Taiwan.
The main sulfide in biomass biogas is H
2S, and because it is toxic and causes corrosion in compressors, gas storage tanks, and engines, and forms more toxic gases (SO
2 and SO
3) during biogas combustion [
6,
7], biogas must be desulfurized before entering the PSA process, unless the concentration of H
2S is less than 300 ppm [
8].
In 2017, thermal power generation was Taiwan’s primary source of energy. For example, the proportion of coal-fired power generation is 39.2%, followed by natural gas power generation at 38.6%. Taiwan is dependent on imports for more than 97% of its natural gas, so it is critical to developing Taiwan’s natural gas production. Among them was the Danish continuous stirred tank reactor technology installed at the Shi-An farm in Kaohsiung. The chicken manure and wastewater are mixed and stirred together to produce biogas during the anaerobic fermentation process. Following desulfurization, the biogas is burned to generate electricity, and the electricity generator is connected in parallel with the Taiwan Power Company (TPC) grid to directly sell green power to TPC, saving waste disposal and reducing carbon emissions by 93%, thus, reflecting circular economy practice [
9].
As the cost of petroleum rises, so do the associated greenhouse gas emissions caused by the extensive use of fuel combustion; many researchers are committed to using modern technology to obtain energy from renewable energy sources. As a result, it is important to implement biogas upgrading techniques. There are several significant technologies for CH
4/CO
2 separation in industrial practice, including organic physical scrubbing, water scrubbing, chemical scrubbing, PSA, membrane separation methods, and cryogenic technology [
4,
5,
10,
11]. PSA benefits include low energy requirements, low capital cost, safety, and ease of operation [
12]. PSA has enormous potential for gas separation and purification. Therefore, it is widely used in the industry for gas drying, solvent vapor recovery, air separation, hydrogen purification, etc. [
13]. Many researchers use the PSA process to recover greenhouse gases, sulfur dioxide, and nitrogen oxides.
Heck et al. [
14] compared the separation performance of zeolites 13X, 5A, and 4A for biogas upgrading. The results show that zeolites 13X and 5A can produce 98% pure CH
4 with a 60–70% recovery rate. Gholipour and Mofarahi [
15] measured the pure and binary adsorption isotherm data on zeolite 13X using a volumetric method, then calculated the selectivity and evaluated the optimal separation conditions at a pressure of 4 bar and a temperature of 303 K. Augelletti et al. [
12] used zeolite 5A as an adsorbent to separate biogas (60% CH
4/40% CO
2) at a feed flow rate of 100 Nm
3/h, and they simulated a two-stage PSA process and obtained 99% pure CH
4 with 81% recovery.
Liu and Ritter [
16] used a fractional factorial design to investigate factor interaction effects on the PSA process performance. They discussed the influence of seven factors: purge feed ratio, pressure level, pressure ratio, heat transfer coefficient, feed concentration, feed volumetric flow rate, and bed length to diameter ratio. The results showed that the light product purity was affected by all seven factors, and the bed capacity factor was affected mainly by the purge to feed ratio, the heat transfer coefficient, and the feed concentration. In Grande’s study [
17], the results showed that PSA is an efficient technology for biogas upgrading under different operating conditions. Better results of PSA can be achieved through process improvements, and the factors affecting product purity include pressure, purge ratio, purge and blowdown volumetric flow rate, etc.
Design of experiment (DOE) and factorial analysis were frequently used to evaluate the performance of different systems. For example, Qian et al. [
18] used a factorial design method to investigate and evaluate the effects of air circulation strategies and operating conditions on the cooling performance, and then observed the significant factors that can achieve the research purpose. Alvarez et al. [
19] discussed the methane production from llama and cow manures from the Bolivian high plateau, and used a fractional factorial design for the two raw materials. Llama manure was found to be the best raw material of the two for biogas production. Furthermore, it was found that the temperature factor significantly affected the production. Qian et al. [
20] used a factorial design to discuss the safety of stair ascent and descent, and used an analysis of variance (ANOVA) table to observe whether the main effect and the interaction effect between two factors are significant or not. After the statistical analysis, moderately adjusting the factor can reduce the possibility of injuries and fatalities during stair climbing at both the home and the workplace. DOE and factorial analysis can also be effective and innovative in analyzing the PSA process.
Most articles in the literature studying biogas upgrading by PSA mainly only discussed the separation of CH4 and CO2 to produce the CH4 product, but the content of H2S in the produced product is less discussed. Due to the fact that Taiwan’s natural gas pipeline standards require >95% CH4 with H2S content < 4 ppm, the purpose of this research is to use DOE and factorial analysis to analyze the PSA process, and design a full scale PSA process with CH4 product purity greater than 97% (or at least not less than 95%) and content of H2S less than 4 ppm. The CH4 recovery is expected to be more than 90%.
This study compared various adsorbents and selected the one with the best separation performance based on three selectivity parameters. Then, biogas from Taiwan’s Institute of Nuclear Energy Research after desulphurization and water removal was used as the feed (64% CH
4, 36% CO
2, and 100 ppm H
2S) for the PSA. Afterwards, we designed laboratory-scale PSA processes in order to generate high-purity CH
4 product. Next, to find the optimal operating conditions that will yield optimal results, the DOE method was used. Lastly, a full scale PSA process was developed with adsorption towers dealing with 500 m
3/h biogas feed for possible commercial applications.
Figure 1 is the schematic diagram of the research design.
3. Materials and Experimental Procedure
In this study, biogas is produced from anaerobic fermentation, and the main components are CO2, CH4, and H2S. Measurements of amount of equilibrium adsorbed were performed on three different types of adsorbents at temperatures of 298 and 333 K. Afterwards, we compared the adsorbents’ performance based on the experimental results.
3.1. Materials
Three types of commercial adsorbents were used to perform the CO2 and CH4 equilibrium adsorption experiments. Zeolite 13X, zeolite 5A (produced by COSMO), and activated carbon (AC) were used. Furthermore, according to the literature, zeolite 13X was frequently used as an adsorbent in the biogas separation process. As a result, we conducted experiments with three different brands of zeolite 13X produced by COSMO, UOP, and EIKME.
3.2. Experimental Apparatus
In this study, the adsorption isotherm experiment was measured using a Micro-balance Thermo D-200.
Figure 5 shows that the pressure can be operated up to 6.89 × 10
6 Pa, the maximum temperature is 673 K, and the maximum load capacity is 1.5 × 10
−3 kg with a sensitivity of 1 × 10
−10 kg. The weight of the adsorbent was determined by allowing the gas to pass via various pressures while maintaining a constant temperature. The adsorbent’s weight change under different pressures is recorded, and its isotherm curve is plotted.
3.3. Selectivity Parameters
Due to the fact that the adsorbent has a significant impact on the separation results, we compared the performance of each adsorbent by calculating three selectivity parameters.
The term “equilibrium selectivity” (
) is used in the following expression [
24]:
In Equation (7), and represent the mole fractions of CO2 and CH4 on the adsorbent, respectively, and and represent the mole fractions in the gas phase.
The second parameter is the working capacity selectivity ratio (
) and is defined as [
24]:
In Equation (8), represents the adsorbent’s working capacity, which is typically defined as the difference between the adsorption amount at adsorption and desorption pressures for component i.
The selection parameter (
) can be calculated from the two parameters mentioned above and is defined as follows [
24]:
The higher the selection parameter, the more effectively the adsorbent can separate different gases under the specific gas composition.
5. Conclusions
Equilibrium adsorption experiments were performed for three types of commercial adsorbents: zeolite 13X, zeolite 5A, and AC. As a result of its excellent effect on separating CH4 from biogas, the COSMO zeolite 13X was chosen as the adsorbent for the dual-bed eight-step PSA process to upgrade biogas after calculating the three selectivity parameters. The isotherm parameters were obtained for the COSMO zeolite 13X by fitting the CO2, CH4, and H2S adsorption data from the experiments and literature.
After water removal and desulfurization, the feed composition of PSA’s biogas inlet provided by Taiwan’s Institute of Nuclear Energy Research is 64% CH4, 35% CO2, and 100 ppm H2S. The simulation results for the basic case of the laboratory-scale PSA process with 2 m3/d biogas feed showed that the purity and recovery of CH4 in the top product could reach 95.47% and 91.27%, respectively, and the content of H2S was 1.06 ppm, all of which meet the standards of natural gas pipelines in Taiwan, but don’t reach the target (>97% CH4). Therefore, we used DOE’s full factorial design to find the optimal operating conditions. The simulation results showed that the purity and recovery of CH4 could reach 99.28% and 91.44%, respectively, which meet the research target. In addition, the concentration of H2S in the top product was reduced to 0.015 ppm and could greatly reduce the damage of pipeline corrosion. The energy consumption increased relatively to 0.86 GJ/ton-CH4.
In the last part, we used the same feed compositions and dual-bed eight-step process as the laboratory scale to carry out the full scale PSA process design with 500 m3/h biogas feed. The simulation results showed that the purity and recovery of CH4 in the top product could reach 96.1% and 91.39%, respectively, and the content of H2S was 1.14 ppm. Although slightly lower than the target of 97% CH4 purity, it was still higher than the pipeline standard of 95% purity in Taiwan and the process effectively reduced the content of H2S. For the future study, we plan to improve the full scale process in order to have a CH4 product purity of more than 97%, and to study the economic feasibility of the process.