Biolubricant Production through Double Transesterification: Reactor Design for the Implementation of a Biorefinery Based on Rapeseed
Abstract
:1. Introduction
- Use of natural and renewable sources.
- Production of valuable products (like biodiesel and biolubricant).
- Generation of exploitable by-products (such as glycerol, with a wide range of uses depending on its purity [9]).
- Reutilization of by-products (like methanol).
2. Materials and Methods
2.1. Biodiesel and Biolubricant Production
- First transesterification with methanol. For fatty acid methyl ester production (biodiesel), the chemical conditions were based on previous studies [11].
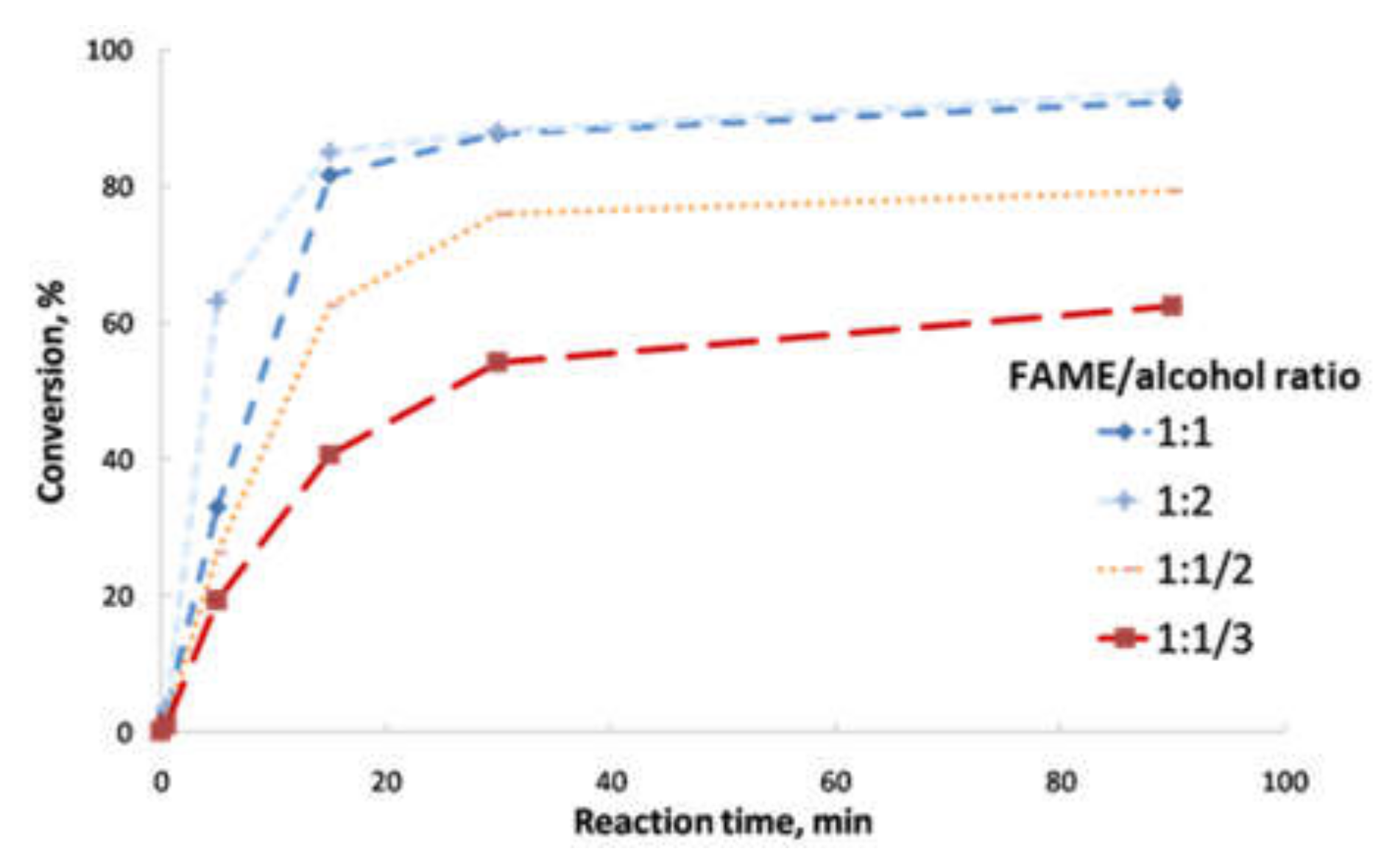
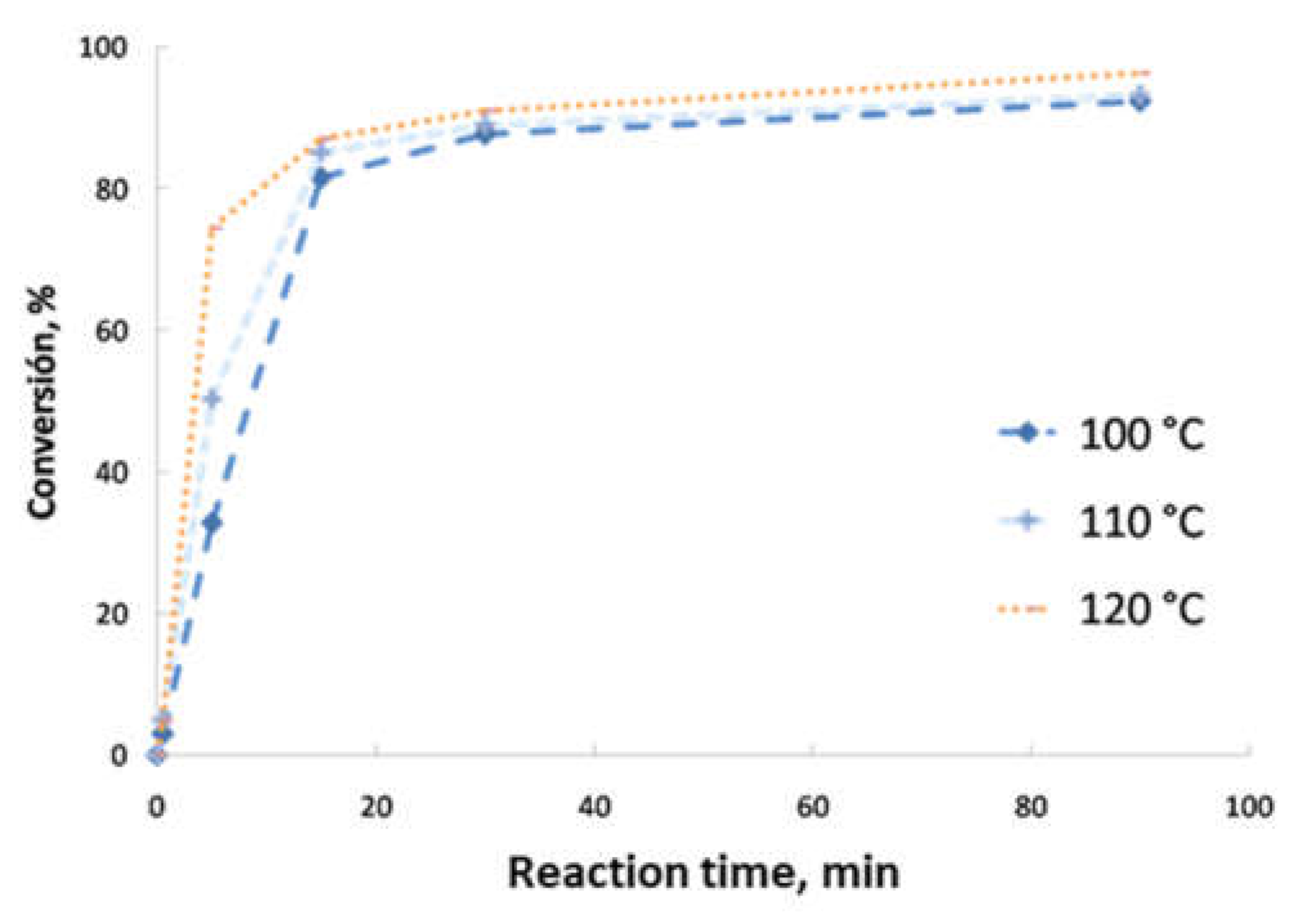
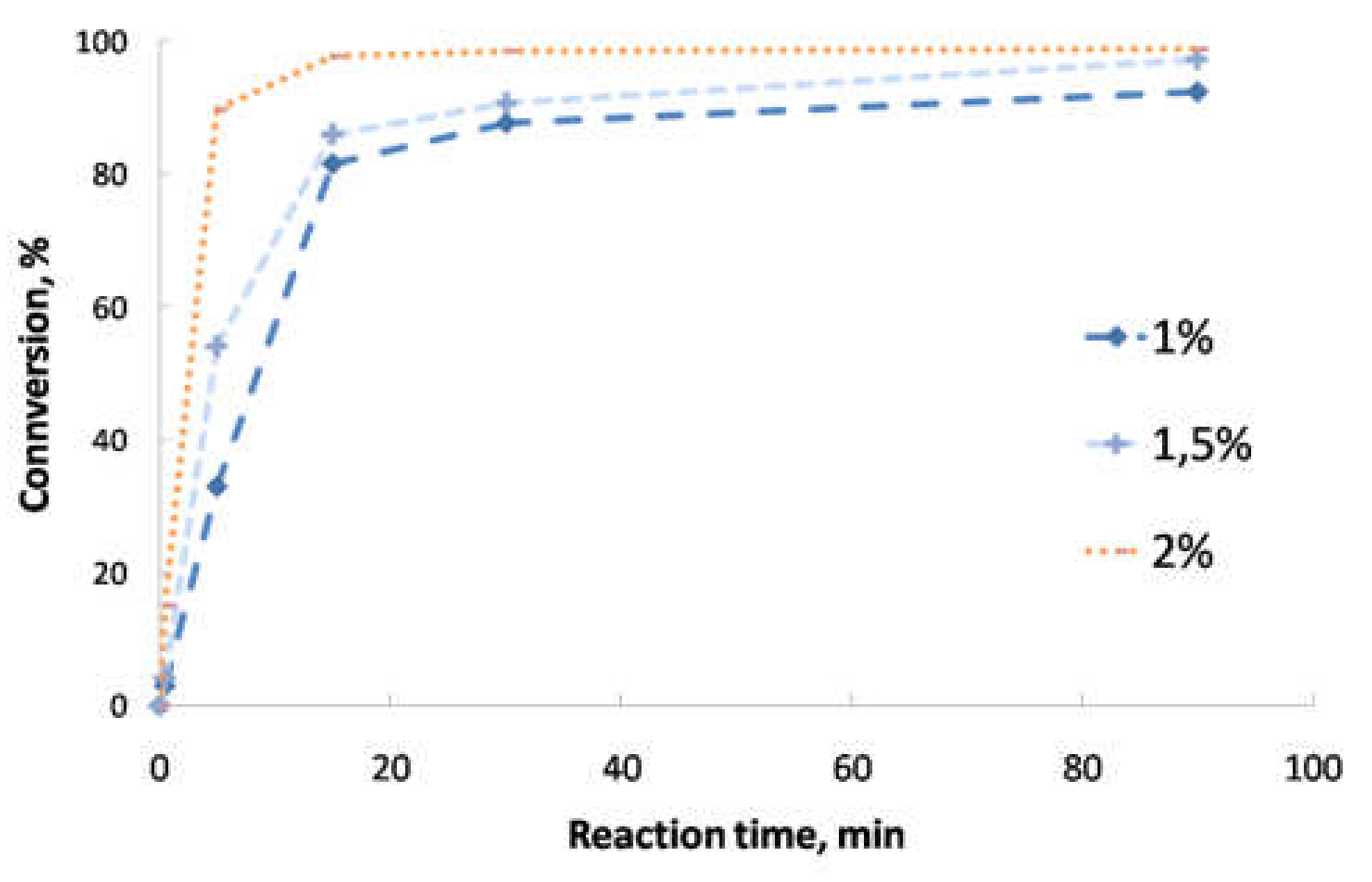
- Second transesterification with 2-ethyl-2-hydroxymethyl-1,3-propanediol (Sigma-Aldrich, Saint Louis, MO, USA; solid state; molar mass, 134.18 g·mol−1; density, 1.08 g·cm−3; melting point, 56–58 °C; vapor pressure, 67 hPa at 200 °C; flash and combustion points, 180 and 367 °C, respectively). The biolubricant production was optimized in order to obtain high yields, exceeding 95%. For that purpose, experimental sets at different FAME/alcohol ratios (1:1/3; 1:1/2, 1:1 and 1:2), temperatures (100, 110 and 120 °C) and catalyst concentrations (1.0, 1.5 and 2.0% w/w) were carried out. These experiments were also used for the kinetic determination and reactor design. Once the reaction took place, the sample was purified by vacuum filtration, separating the surplus alcohol.
2.2. Biodiesel and Biolubricant Characterization
2.3. Kinetic Study and Volume Reactor Determination
- Characterization of the biolubricant, which determines its use in industry, paying special attention to viscosity at 40 and 100 °C and viscosity index and comparing with traditional lubricants.
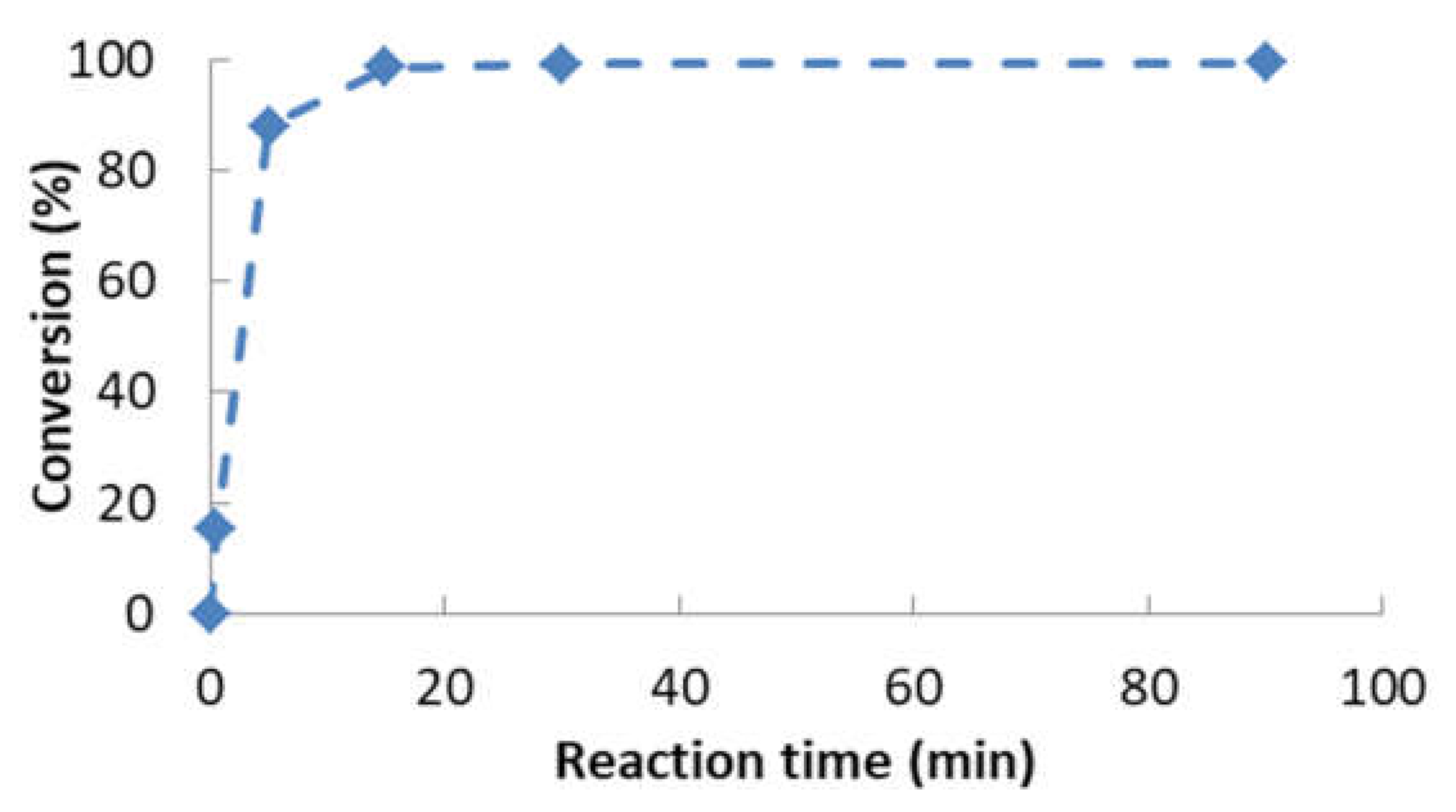
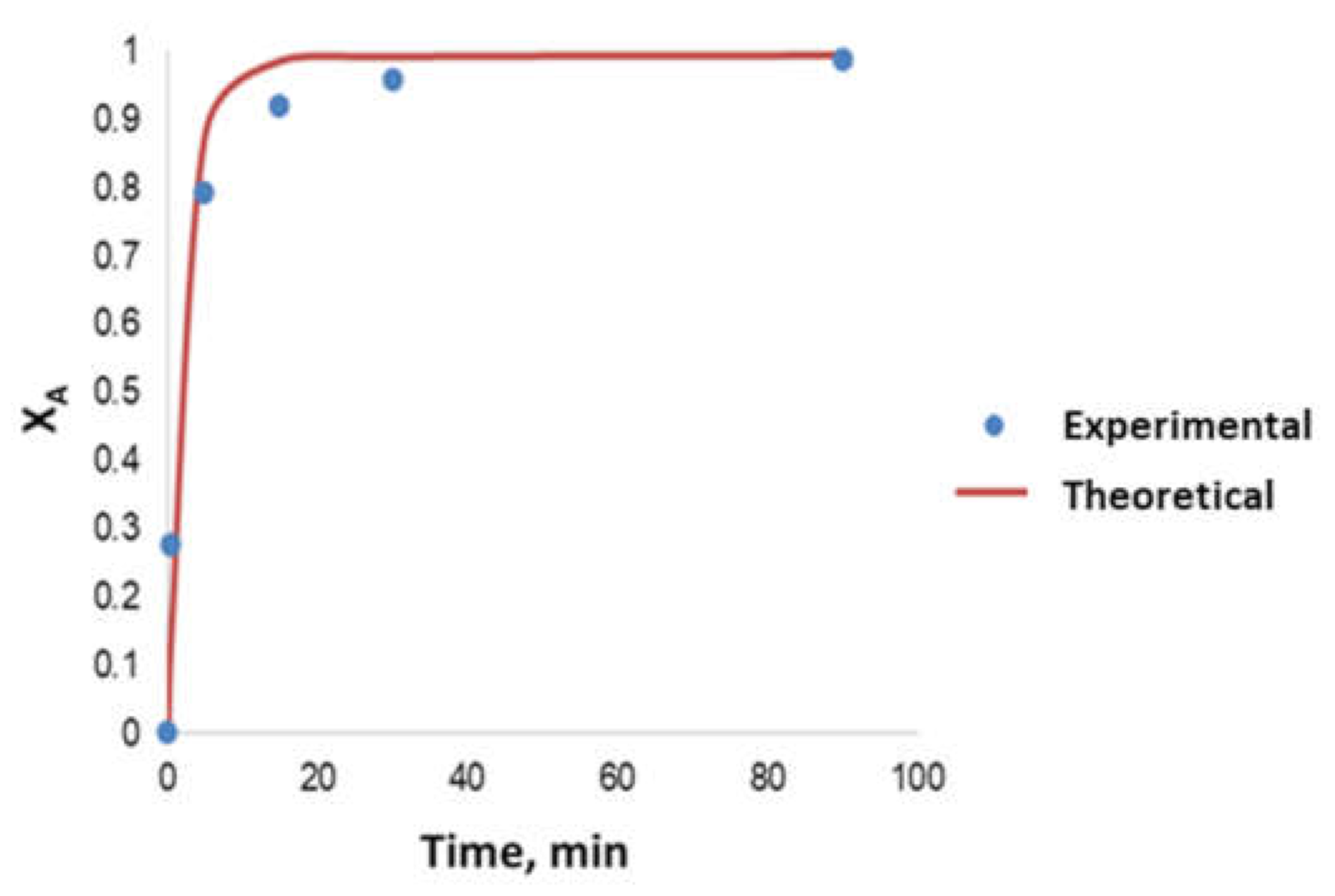
- Determination of the order of reaction of the second transesterification process, obtaining the main kinetic parameters. For that purpose, the decrease in FAMEs from biodiesel to biolubricant production at different reaction times (0, 5, 15, 25 and 90 min) was measured to determine the reaction evolution. Different parameters like temperature and catalyst addition were studied.
- Calculation of biolubricant production at a national level (in this case, Spain), comparing with the equivalent lubricant production at the same level.
- Selection of the suitable reactor depending on production, obtaining the design equation by a mass balance.
- Reactor volume determination.
3. Results and Discussion
3.1. Biodiesel Characterization
3.2. Reaction Optimization
3.3. Biolubricant Characterization
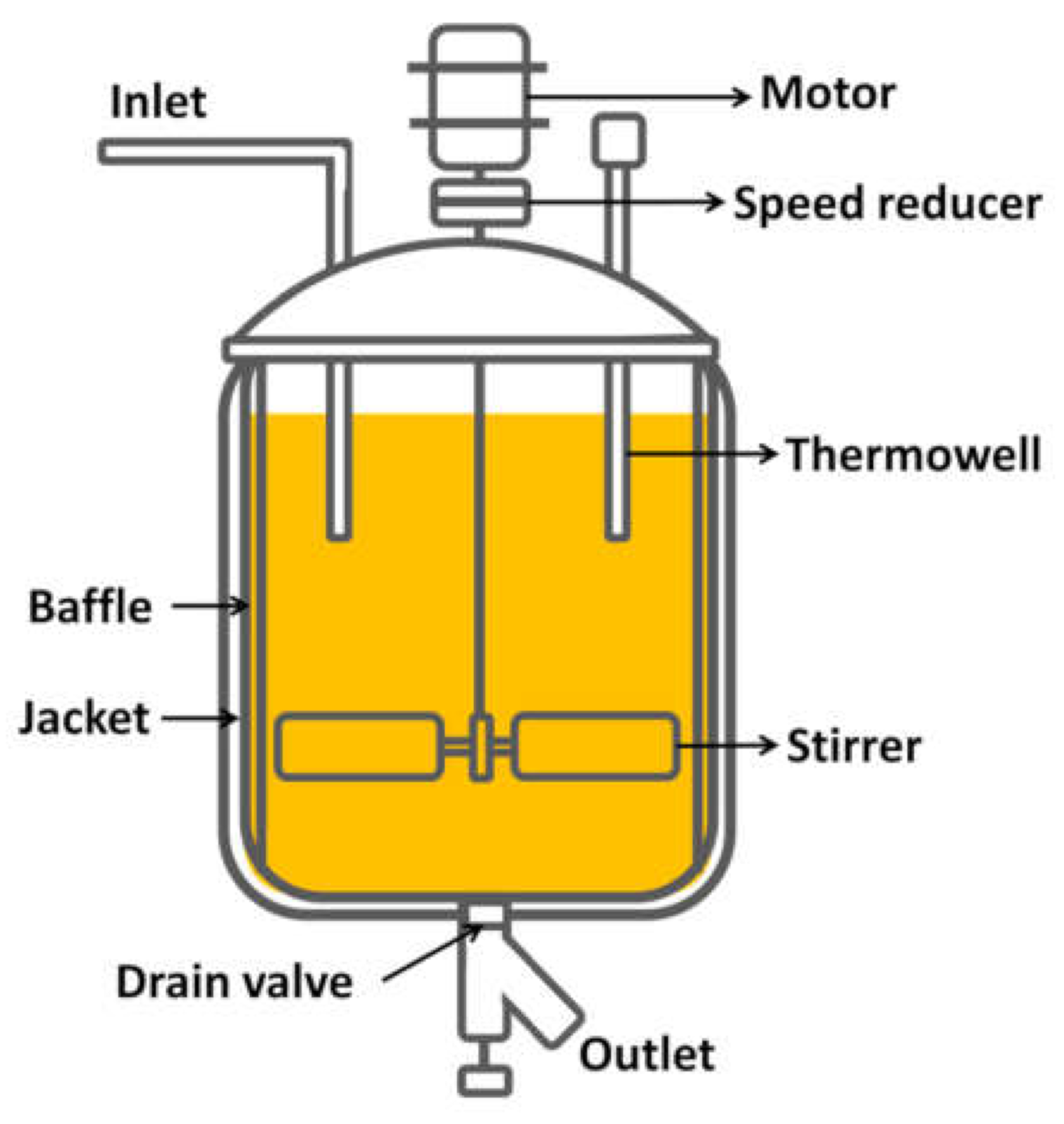
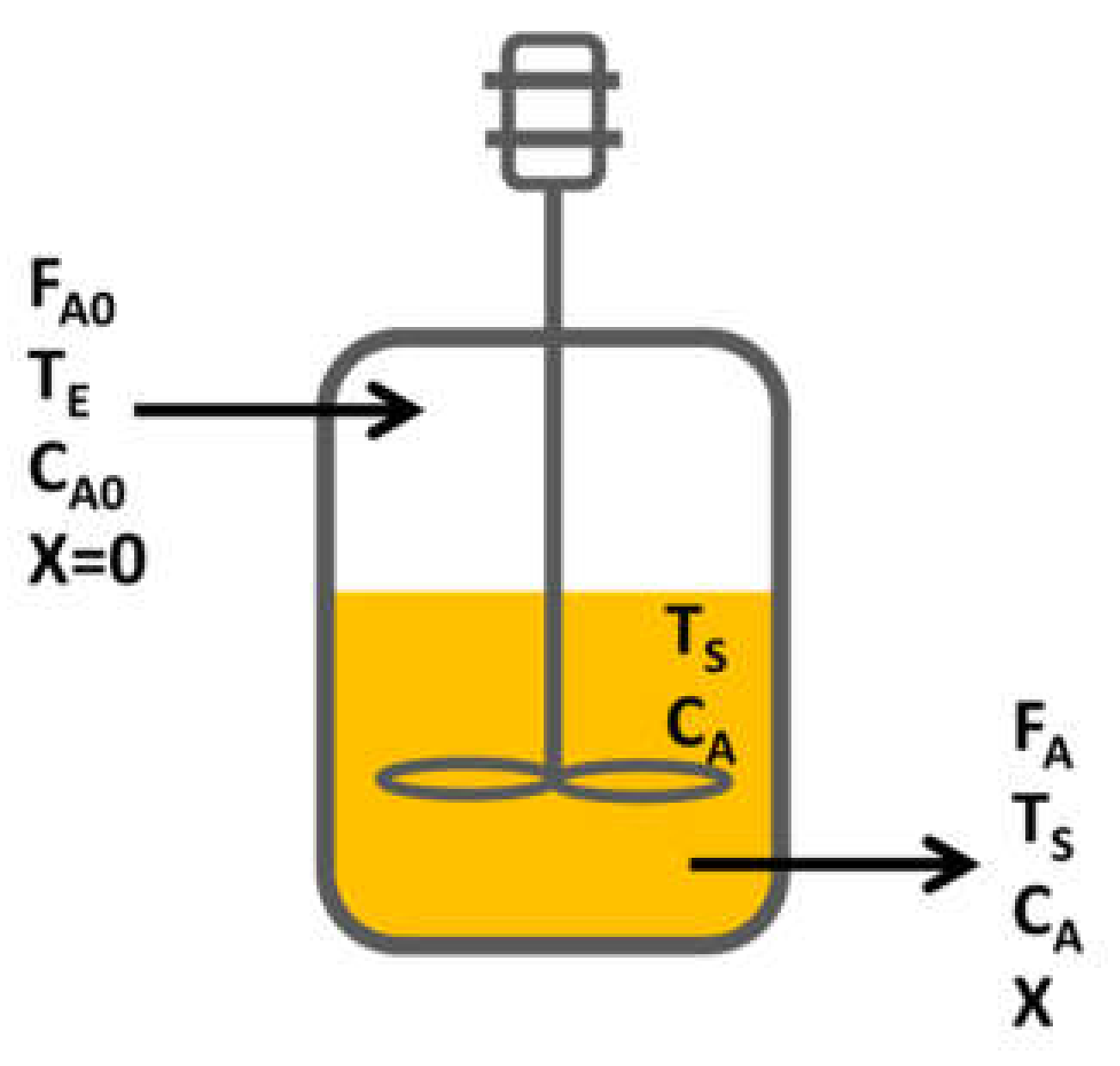
3.4. Reactor Design
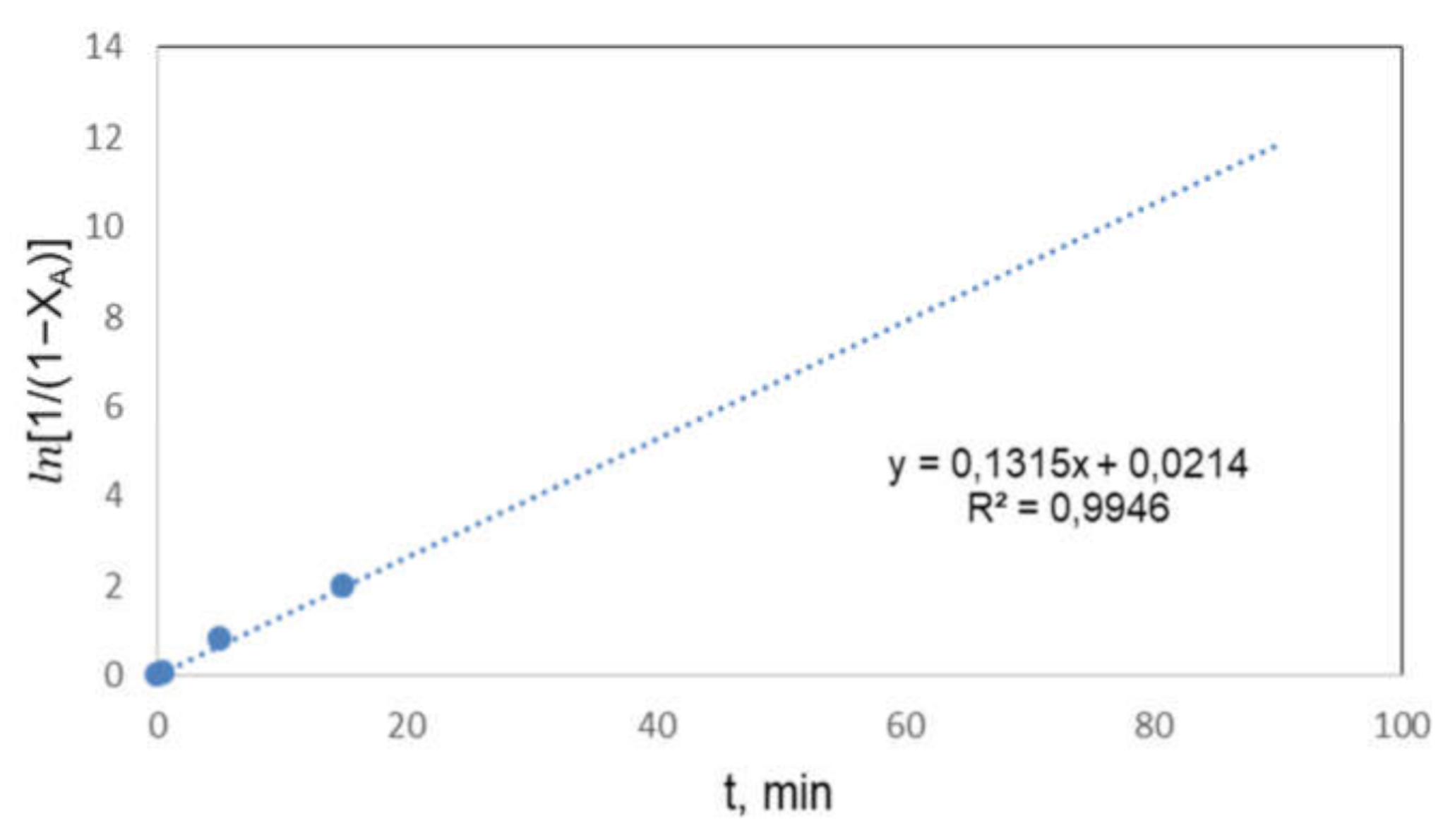
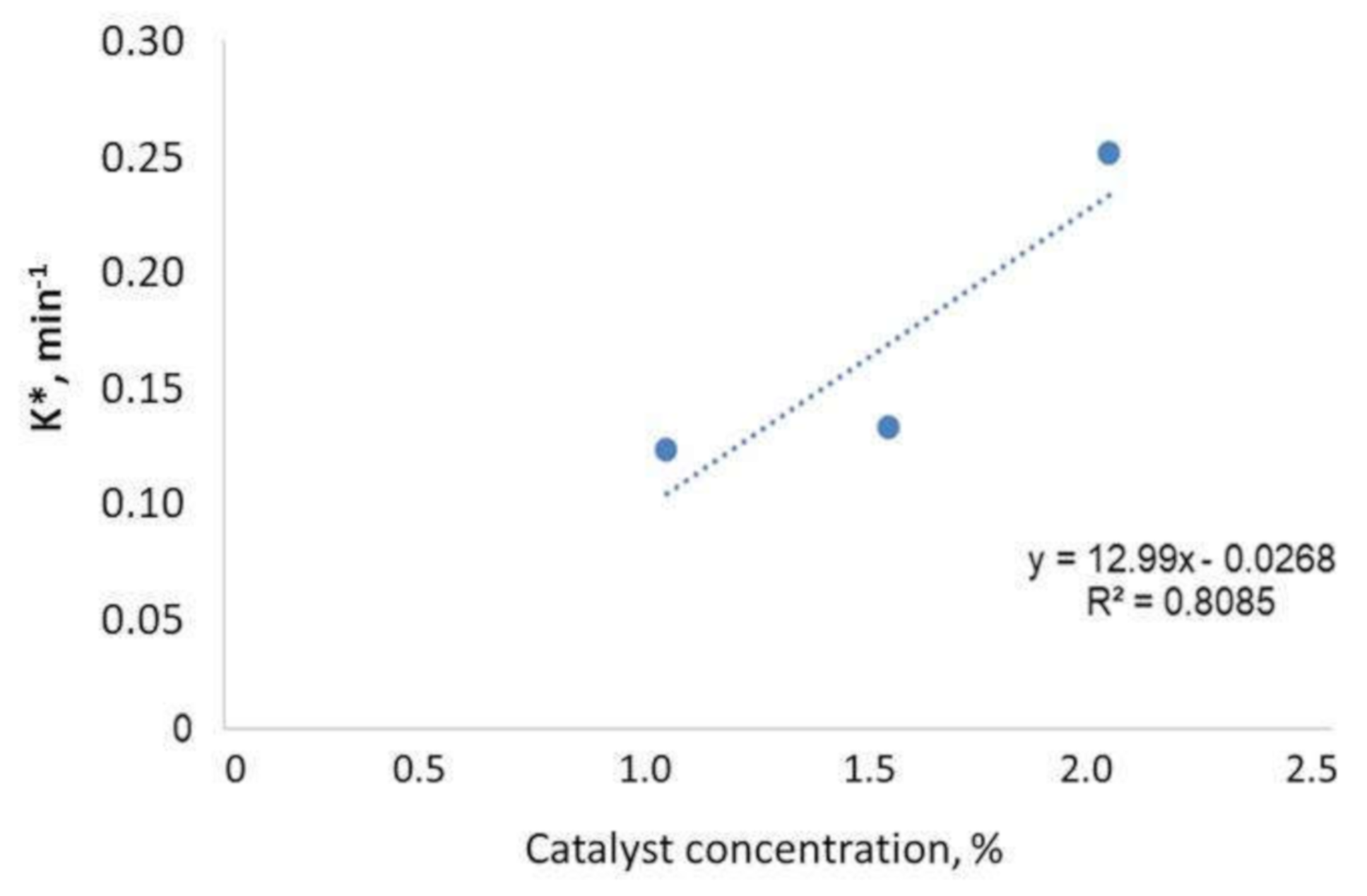
3.5. Kinetic Study
3.6. Reactor Volume
4. Conclusions
- Rapeseed biodiesel complied with almost all the standard requirements, highlighting the high flash and combustion points (exceeding 190 °C). However, oxidative stability did not comply with the standard (5.37 h), although it was relatively high compared to other equivalent samples found in the literature.
- The optimum chemical conditions to obtain high yields of rapeseed biolubricant were biodiesel/alcohol (2-ethyl-2-hydroxymethyl-1,3-propanediol) mole ratio: 1/1; catalyst (sodium methoxide) concentration, 1.5% w/w; reaction temperature, 120 °C; reaction time: 90 min. Under these circumstances, high yields (exceeding 99%) were obtained.
- The characteristics of the biolubricant were calculated, being equivalent to a SAE 10W30 lubricant, suitable for Diesel engines.
- For the reactor design, due to the operating conditions, the kind of reaction and desired production, a continuous reactor was selected.
- The kinetics of the process could be considered a pseudo-first order reaction, obtaining an activation energy of 6.91 kJ·mol−1.
- According to the annual production (once the kinetics was known), a reactor volume of 9.66 m3 was obtained (12 m3 if oversizing is considered).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| CAo | Inlet concentration |
| CA | Outlet concentration |
| FAo | Inlet mole flow |
| FA | Outlet mole flow |
| k* | Pseudo-first reaction order constant |
| −rA | Reaction rate |
| TE | Inlet temperature |
| Ts | Outlet temperature |
| VR | Reactor volume |
| XA | Conversion |
References
- Salimon, J.; Salih, N.; Yousif, E. Industrial development and applications of plant oils and their biobased oleochemicals. Arab. J. Chem. 2012, 5, 135–145. [Google Scholar] [CrossRef] [Green Version]
- Encinar, J.M.; Nogales-Delgado, S.; Sánchez, N.; González, J.F. Biolubricants from rapeseed and castor oil transesterification by using titanium isopropoxide as a catalyst: Production and characterization. Catalysts 2020, 10, 366. [Google Scholar] [CrossRef] [Green Version]
- Cecilia, J.A.; Plata, D.B.; Saboya, R.M.A.; de Luna, F.M.T.; Cavalcante, C.L.; Rodríguez-Castellón, E. An overview of the biolubricant production process: Challenges and future perspectives. Processes 2020, 8, 257. [Google Scholar] [CrossRef] [Green Version]
- Beyzi, E.; Gunes, A.; Buyukkilic Beyzi, S.; Konca, Y. Changes in fatty acid and mineral composition of rapeseed (Brassica napus ssp. oleifera L.) oil with seed sizes. Ind. Crop. Prod. 2019, 129, 10–14. [Google Scholar] [CrossRef]
- Chozhavendhan, S.; Vijay Pradhap Singh, M.; Fransila, B.; Praveen Kumar, R.; Karthiga Devi, G. A review on influencing parameters of biodiesel production and purification processes. Curr. Res. Green Sustain. Chem. 2020, 1–2, 1–6. [Google Scholar] [CrossRef]
- Martínez, G.; Sánchez, N.; Encinar, J.M.; González, J.F. Fuel properties of biodiesel from vegetable oils and oil mixtures. Influence of methyl esters distribution. Biomass Bioenergy 2014, 63, 22–32. [Google Scholar] [CrossRef]
- McNutt, J.; He, Q.S. Development of biolubricants from vegetable oils via chemical modification. J. Ind. Eng. Chem. 2016, 36, 1–12. [Google Scholar] [CrossRef]
- Moncada, B.J.; Aristizábal, M.V.; Cardona, A.C.A. Design strategies for sustainable biorefineries. Biochem. Eng. J. 2016, 116, 122–134. [Google Scholar] [CrossRef]
- Checa, M.; Nogales-Delgado, S.; Montes, V.; Encinar, J.M. Recent advances in glycerol catalytic valorization: A review. Catalysts 2020, 10, 1279. [Google Scholar] [CrossRef]
- Nogales-Delgado, S.; Sánchez, N.; Encinar, J.M. Valorization of Cynara Cardunculus L. Oil as the Basis of a Biorefinery for Biodiesel and Biolubricant Production. Energies 2020, 13, 5085. [Google Scholar] [CrossRef]
- Nogales-Delgado, S.; Encinar, J.M.; Guiberteau, A.; Márquez, S. The Effect of Antioxidants on Corn and Sunflower Biodiesel Properties under Extreme Oxidation Conditions. J. Am. Oil Chem. Soc. 2019, 97, 201–212. [Google Scholar] [CrossRef]
- ISO. UNE-EN 14214:2013 V2+A1:2018 Liquid Petroleum Products—Fatty acid Methyl Esters (FAME) for Use in Diesel Engines and Heating Applications—Requirements and Test Methods; International Organization of Standardization: Geneva, Switzerland, 2018. [Google Scholar]
- ISO. UNE-EN-12634:1999 Productos Petrolíferos y Lubricantes. Determinación del Índice de Ácido. Método de Valoración Potenciométrica en un Medio no Acuoso; International Organization of Standardization: Geneva, Switzerland, 1999. [Google Scholar]
- ISO. UNE-EN 14111:2003 Fat and Oil Derivatives. Fatty Acid Methyl Esters (FAME); Determination of Iodine Value; International Organization of Standardization: Geneva, Switzerland, 2003. [Google Scholar]
- ISO. UNE-EN 116:2015 Diesel and Domestic Heating Fuels—Determination of Cold Filter Plugging Point—Stepwise Cooling Bath Method; International Organization of Standardization: Geneva, Switzerland, 2015. [Google Scholar]
- ISO. UNE-EN ISO 12966-2:2011 Animal and Vegetable Fats and Oils—Gas Chromatography of Fatty Acid Methyl Esters—Part 2: Preparation of Methyl Esters of Fatty Acids; International Organization of Standardization: Geneva, Switzerland, 2011. [Google Scholar]
- Focke, W.W.; Van Der Westhuizen, I.; Oosthuysen, X. Biodiesel oxidative stability from Rancimat data. Thermochim. Acta 2016, 633, 116–121. [Google Scholar] [CrossRef] [Green Version]
- Encinar, J.M.; Nogales, S.; González, J.F. Biodiesel and biolubricant production from different vegetable oils through transesterification. Eng. Rep. 2020, 1–10. [Google Scholar] [CrossRef]
- Varatharajan, K.; Pushparani, D.S. Screening of antioxidant additives for biodiesel fuels. Renew. Sustain. Energy Rev. 2018, 82, 2017–2028. [Google Scholar] [CrossRef]
- Dunn, R.O. Antioxidants for improving storage stability of biodiesel. Biofuels Bioprod. Biorefin. 2008. [Google Scholar] [CrossRef]
- Da Silva, J.A.C.; Soares, V.F.; Fernandez-Lafuente, R.; Habert, A.C.; Freire, D.M.G. Enzymatic production and characterization of potential biolubricants from castor bean biodiesel. J. Mol. Catal. B Enzym. 2015, 122, 323–329. [Google Scholar] [CrossRef]
- Encinar, J.M.; Nogales, S.; González, J.F. Biorefinery based on different vegetable oils: Characterization of biodiesel and biolubricants. In Proceedings of the 3rd International Conference in Engineering Applications (ICEA), Ponta Delgada, Portugal, 8–11 July 2019. [Google Scholar]
- Salimon, J.; Salih, N.; Yousif, E. Biolubricant basestocks from chemically modified ricinoleic acid. J. King Saud Univ. Sci. 2012, 24, 11–17. [Google Scholar] [CrossRef] [Green Version]
- Kania, D.; Yunus, R.; Omar, R.; Abdul Rashid, S.; Mohamad Jan, B. A review of biolubricants in drilling fluids: Recent research, performance, and applications. J. Pet. Sci. Eng. 2015, 135, 177–184. [Google Scholar] [CrossRef]
- ASELUBE Asociación Española de Lubricantes. Available online: http://aselube.net (accessed on 31 May 2020).
- Statista.com. Lubricant Sales in Spain. Available online: https://www-statista-com.ezproxy.unex.es (accessed on 31 May 2021).

| Reaction | Reaction Time, min | Reaction Temperature, °C | Alcohol/Oil Ratio 1 | Catalyst Concentration 2, % |
|---|---|---|---|---|
| First transesterification | 150 | 60 | 6:1 | 1.5 |
| Second transesterification | 90 | 120 | 1:1 | 1.5 |
| Property | Units | Rapeseed Biodiesel | EN-14214 Limit | Compliance with the Standard | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| FAME content | % | 98.4 | 96.5 | - |  |
| Moisture | mg/kg | 250 | - | 500 |  |
| Density at 15 °C | kg/m3 | 868 | 860 | 900 |  |
| Viscosity at 40 °C | mm2/s | 4.90 | 3.50 | 5.00 |  |
| Flash point | °C | 194 | 101 | - |  |
| Oxidative stability | h | 5.37 | 8 | - |  |
| Acid number | mg KOH/g | 0.35 | - | 0.50 |  |
| Iodine number | g I2/100g | 105 | - | 120 |  |
| Methyl linolenate | % (m/m) | 9.1 | - | 12 |  |
| CFPP | °C | −4 | −10 | 0 |  |
| Feature 1 | Units | Rapeseed Biolubricant | Commercial Lubricant 1 (SAE 10W30) | Commercial Lubricant 2 (SAE 10W30) |
|---|---|---|---|---|
| Conversion | % | 99.41 | n.d. 2 | n.d. |
| Density at 15 °C | kg/m3 | 949 | 859 | 869 |
| Moisture | % | 0.03 | n.d. | n.d. |
| Acid number | mg KOH/g | 0.28 | n.d. | n.d. |
| Viscosity at 40 °C | mm2/s | 75.5 | 66 | 69.8 |
| Viscosity at 100 °C | mm2/s | 10.7 | 10.4 | 10.5 |
| Viscosity index | Dimensionless | 128 | 145 | 137 |
| Flash point | °C | 210 | 232 | 228 |
| Combustion point | °C | 222 | n.d. | n.d. |
| Oxidative stability | h | 4.94 | n.d. | n.d. |
| Methyl Ester | Percentage (%) | Molecular Weight (g/mol) | Weighted Molecular Weight (g/mol) |
|---|---|---|---|
| Oleate | 66.4 | 296.49 | 196.87 |
| Linoleate | 16.6 | 294.48 | 48.88 |
| Linolenate | 9.10 | 292.50 | 26.62 |
| Palmitate | 3.80 | 270.46 | 10.28 |
| Stearate | 0.55 | 298.50 | 1.64 |
| Total | 96.45 | - | 284.29 |
| Reactives | Mass Flow(tm/Year) | Volumetric Flow (m3/Year) | Mole Flow (kmol/Year) |
|---|---|---|---|
| Biodiesel | 5293.12 | 6098.07 | 18,618.75 |
| Alcohol | 2498.26 | 2313.21 | 18,618.75 |
| Catalyst | 116.87 | 120.49 | 2163.07 |
| Total | 7908.26 | 8531.76 | 39,400.56 |
| Units | 1% | 1.5% | 2% |
|---|---|---|---|
| k*,min−1 | 0.1213 | 0.1315 | 0.2512 |
| R2 | 0.9869 | 0.9946 | 0.9242 |
| Units | 100 °C | 110 °C | 120 °C |
|---|---|---|---|
| k*,min−1 | 0.1213 | 0.1269 | 0.1359 |
| R2 | 0.9869 | 0.9984 | 0.8819 |
| Slope (−E/R) | Intercept | Activation Energy | R2 |
|---|---|---|---|
| −831.7 | 0.1151 | 6.91 kJ/mol | 0.9821 |
| Variable | Variable Calculation, Units |
|---|---|
| Inlet mole flow, FAo | FAo = 18,618.75 kmol·year−1= 0.5904 mol·s−1 |
| Conversion, XA | XA = 0.9941 (obtained from final experiment) |
| Inlet concentration, CAo | CAo = FAo/FVtotal = 2182.3 mol/m3 |
| Outlet concentration, CA | CA = CAo(1- XA) = 12.9 mol/m3 |
| Pseudo-first reaction order constant, k* | k* = 0.2827 min−1 = 4.7117.10−3·s−1 |
| Reaction rate, −rA | −rA = k* CA = 4.7117·10−3·12.9 = 6.078·10−2 mol/m3.s |
| Reactor volume, VR | VR= FAo·XA/−rA = 0.5904·0.9941/6.078·10−2 = 9.6564 m3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Encinar, J.M.; Nogales-Delgado, S.; Pinilla, A. Biolubricant Production through Double Transesterification: Reactor Design for the Implementation of a Biorefinery Based on Rapeseed. Processes 2021, 9, 1224. https://doi.org/10.3390/pr9071224
Encinar JM, Nogales-Delgado S, Pinilla A. Biolubricant Production through Double Transesterification: Reactor Design for the Implementation of a Biorefinery Based on Rapeseed. Processes. 2021; 9(7):1224. https://doi.org/10.3390/pr9071224
Chicago/Turabian StyleEncinar, José María, Sergio Nogales-Delgado, and Antonio Pinilla. 2021. "Biolubricant Production through Double Transesterification: Reactor Design for the Implementation of a Biorefinery Based on Rapeseed" Processes 9, no. 7: 1224. https://doi.org/10.3390/pr9071224
APA StyleEncinar, J. M., Nogales-Delgado, S., & Pinilla, A. (2021). Biolubricant Production through Double Transesterification: Reactor Design for the Implementation of a Biorefinery Based on Rapeseed. Processes, 9(7), 1224. https://doi.org/10.3390/pr9071224








