Towards Full Utilization of Biomass Resources: A Case Study on Industrial Hemp Residue and Spent Mushroom Substrate
Abstract
:1. Introduction
2. Experimental Section
2.1. Raw Materials and Chemicals
2.2. Pretreatment
2.3. Analytical Studies
2.4. FTIR and SEM Analysis
3. Results and Discussion
3.1. Hydrolysates and Solid Residues
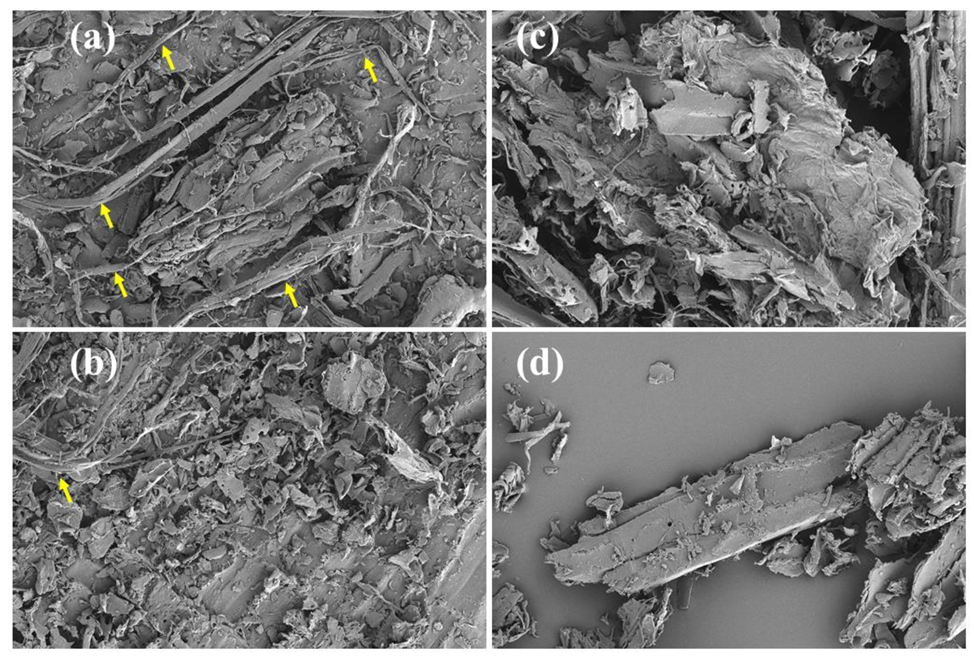
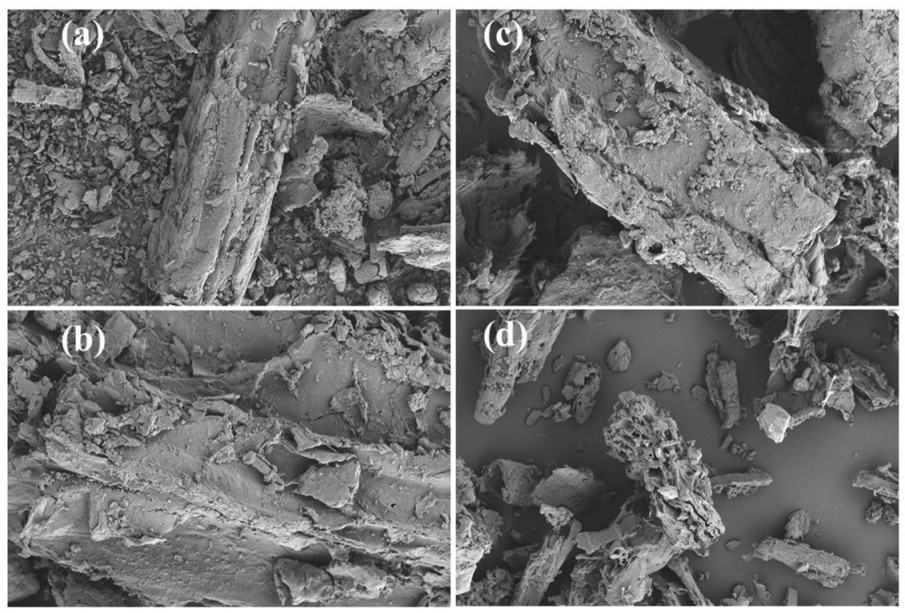
3.2. SEM Analysis
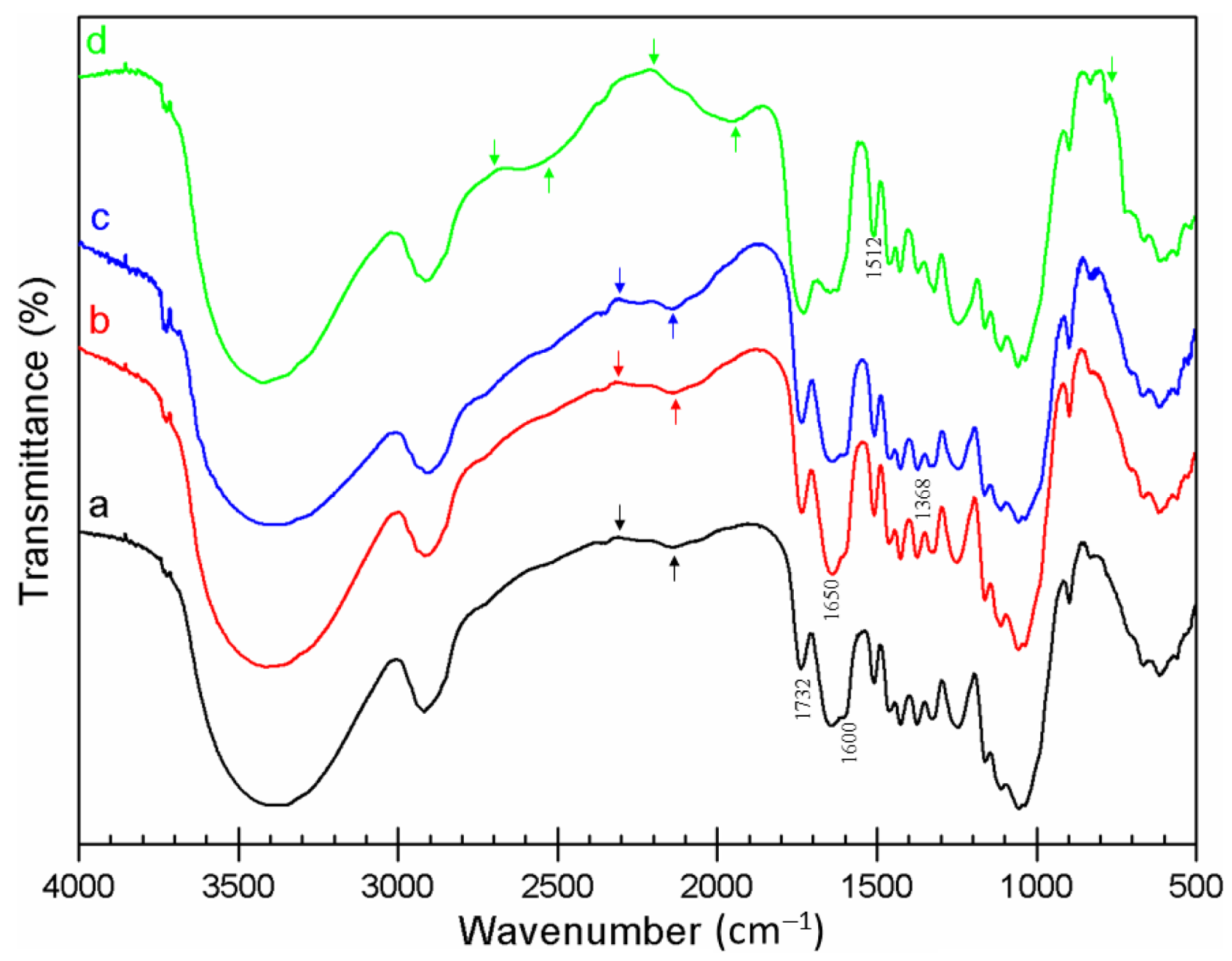
3.3. FTIR Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, D.P.; Dwevedi, A. Chapter 2—Production of clean energy by green ways. In Solutions to Environmental Problems Involving Nanotechnology and Enzyme Technology; Dwevedi, A., Ed.; Academic Press: Waltham, MA, USA, 2019; pp. 49–90. [Google Scholar] [CrossRef]
- Dessie, W.; Luo, X.; Wang, M.; Feng, L.; Liao, Y.; Wang, Z.; Yong, Z.; Qin, Z. Current advances on waste biomass transformation into value-added products. Appl. Microbiol. Biotechnol. 2020, 104, 4757–4770. [Google Scholar] [CrossRef]
- Godfray, H.C.J.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food Security: The Challenge of Feeding 9 Billion People. Science 2010, 327, 812–818. [Google Scholar] [CrossRef] [Green Version]
- Calabrò, P.; Catalán, E.; Folino, A.; Sánchez, A.; Komilis, D. Effect of three pretreatment techniques on the chemical composition and on the methane yields of Opuntia ficus-indica (prickly pear) biomass. Waste Manag. Res. 2018, 36, 17–29. [Google Scholar] [CrossRef]
- Nguyen, Q.A.; Smith, W.A.; Wahlen, B.D.; Wendt, L.M. Total and Sustainable Utilization of Biomass Resources: A Perspective. Front. Bioeng. Biotechnol. 2020, 8, 546. [Google Scholar] [CrossRef]
- Fazzino, F.; Mauriello, F.; Paone, E.; Sidari, R.; Calabrò, P.S. Integral valorization of orange peel waste through optimized ensiling: Lactic acid and bioethanol production. Chemosphere 2021, 271, 129602. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, Y.; Wang, W.; Griffin, J.; Wang, D. Conversion of liquid hot water, acid and alkali pretreated industrial hemp biomasses to bioethanol. Bioresour. Technol. 2020, 309, 123383. [Google Scholar] [CrossRef] [PubMed]
- Ingrao, C.; Lo Giudice, A.; Bacenetti, J.; Tricase, C.; Dotelli, G.; Fiala, M.; Siracusa, V.; Mbohwa, C. Energy and environmental assessment of industrial hemp for building applications: A review. Renew. Sustain. Energy Rev. 2015, 51, 29–42. [Google Scholar] [CrossRef]
- Das, L.; Li, W.; Dodge, L.A.; Stevens, J.C.; Williams, D.W.; Hu, H.; Li, C.; Ray, A.E.; Shi, J. Comparative Evaluation of Industrial Hemp Cultivars: Agronomical Practices, Feedstock Characterization, and Potential for Biofuels and Bioproducts. ACS Sustain. Chem. Eng. 2020, 8, 6200–6210. [Google Scholar] [CrossRef]
- Wan-Mahari, W.A.; Peng, W.; Nam, W.L.; Yang, H.; Lee, X.Y.; Lee, Y.K.; Keey, R.L.; Nyuk, L.M.; Aqilah, M.; Sonne, C.; et al. A review on valorization of oyster mushroom and waste generated in the mushroom cultivation industry. J. Hazard Mater. 2020, 400, 123156. [Google Scholar] [CrossRef] [PubMed]
- Li, T.-H.; Che, P.-F.; Zhang, C.-R.; Zhang, B.; Ali, A.; Zang, L.-S. Recycling of spent mushroom substrate: Utilization as feed material for the larvae of the yellow mealworm Tenebrio molitor (Coleoptera: Tenebrionidae). PLoS ONE 2020, 15, e0237259. [Google Scholar] [CrossRef]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Luo, X.; Dessie, W.; Wang, M.; Duns, G.J.; Rong, N.; Feng, L.; Zeng, J.; Qin, Z.; Tan, Y. Comprehensive utilization of residues of Magnolia officinalis based on fiber characteristics. J. Mater. Cycles Waste Manag. 2021, 23, 548–556. [Google Scholar] [CrossRef]
- Koutrotsios, G.; Mountzouris, K.C.; Chatzipavlidis, I.; Zervakis, G.I. Bioconversion of lignocellulosic residues by Agrocybe cylindracea and Pleurotus ostreatus mushroom fungi—Assessment of their effect on the final product and spent substrate properties. Food Chem. 2014, 161, 127–135. [Google Scholar] [CrossRef]
- Stevulova, N.; Cigasova, J.; Estokova, A.; Terpakova, E.; Geffert, A.; Kacik, F.; Singovszka, E.; Holub, M. Properties Characterization of Chemically Modified Hemp Hurds. Materials 2014, 7, 8131–8150. [Google Scholar] [CrossRef]
- Cheng, B.; Zhang, X.; Lin, Q.; Xin, F.; Sun, R.; Wang, X.; Ren, J. A new approach to recycle oxalic acid during lignocellulose pretreatment for xylose production. Biotechnol. Biofuels 2018, 11, 324. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Huang, C.; Zhao, Y.; Zheng, C.; Su, H.; Zhang, L.; Luo, W.; Zhao, H.; Wang, S.; Huang, L.-J. Effect of Choline-Based Deep Eutectic Solvent Pretreatment on the Structure of Cellulose and Lignin in Bagasse. Processes 2021, 9, 384. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhang, Z.; Song, J.; Meng, Q.; Zhou, H.; He, Z.; Han, B. Metal-Oxide-Catalyzed Efficient Conversion of Cellulose to Oxalic Acid in Alkaline Solution under Low Oxygen Pressure. ACS Sustain. Chem. Eng. 2016, 4, 305–311. [Google Scholar] [CrossRef]
- Vom Stein, T.; Grande, P.; Sibilla, F.; Commandeur, U.; Fischer, R.; Leitner, W.; Domínguez de María, P. Salt-assisted organic-acid-catalyzed depolymerization of cellulose. Green Chem. 2010, 12, 1844–1849. [Google Scholar] [CrossRef]
- Lin, Y.; Ge, X.; Li, Y. Solid-state anaerobic co-digestion of spent mushroom substrate with yard trimmings and wheat straw for biogas production. Bioresour. Technol. 2014, 169, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, D.; Venkata-Mohan, S. Efficient resource valorization by co-digestion of food and vegetable waste using three stage integrated bioprocess. Bioresour. Technol. 2019, 284, 373–380. [Google Scholar] [CrossRef]
- Zhang, H.; Bai, Y.; Yu, B.; Liu, X.; Chen, F. A practicable process for lignin color reduction: Fractionation of lignin using methanol/water as a solvent. Green Chem. 2017, 19, 5152–5162. [Google Scholar] [CrossRef]
- Musiał, I.; Cibis, E.; Rymowicz, W. Designing a process of kaolin bleaching in an oxalic acid enriched medium by Aspergillus niger cultivated on biodiesel-derived waste composed of glycerol and fatty acids. Appl. Clay Sci. 2011, 52, 277–284. [Google Scholar] [CrossRef]
- Zürner, P.; Frisch, G. Leaching and Selective Extraction of Indium and Tin from Zinc Flue Dust Using an Oxalic Acid-Based Deep Eutectic Solvent. ACS Sustain. Chem. Eng. 2019, 7, 5300–5308. [Google Scholar] [CrossRef]
- Salmani Nuri, O.; Irannajad, M.; Mehdilo, A. Effect of surface dissolution by oxalic acid on flotation behavior of minerals. J. Mater Res. Technol. 2019, 8, 2336–2349. [Google Scholar] [CrossRef]
- Arimi, M.M.; Zhang, Y.; Götz, G.; Kiriamiti, K.; Geißen, S.-U. Antimicrobial colorants in molasses distillery wastewater and their removal technologies. Int. Biodeterior. Biodegrad. 2014, 87, 34–43. [Google Scholar] [CrossRef]
- Korniłłowicz-Kowalska, T.; Rybczyńska-Tkaczyk, K. Decolorization and biodegradation of melanoidin contained in beet molasses by an anamorphic strain of Bjerkandera adusta CCBAS930 and its mutants. World J. Microbiol. Biotechnol. 2020, 37, 1. [Google Scholar] [CrossRef] [PubMed]
- Semhaoui, I.; Maugard, T.; Zarguili, I.; Rezzoug, S.-A.; Zhao, J.-M.Q.; Toyir, J.; Nawdali, M.; Maache-Rezzoug, Z. Eco-friendly process combining acid-catalyst and thermomechanical pretreatment for improving enzymatic hydrolysis of hemp hurds. Bioresour. Technol. 2018, 257, 192–200. [Google Scholar] [CrossRef]
- Magro, P.; Marciano, P.; Di Lenna, P. Oxalic acid production and its role in pathogenesis of Sclerotinia sclerotiorum. FEMS Microbiol. Lett. 1984, 24, 9–12. [Google Scholar] [CrossRef]
- Arantes, V.; Qian, Y.; Milagres, A.M.F.; Jellison, J.; Goodell, B. Effect of pH and oxalic acid on the reduction of Fe3+ by a biomimetic chelator and on Fe3+ desorption/adsorption onto wood: Implications for brown-rot decay. Int. Biodeterior. Biodegrad. 2009, 63, 478–483. [Google Scholar] [CrossRef]
- Beckers, S.J.; Dallo, I.A.; Del Campo, I.; Rosenauer, C.; Klein, K.; Wurm, F.R. From Compost to Colloids—Valorization of Spent Mushroom Substrate. ACS Sustain. Chem. Eng. 2019, 7, 6991–6998. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, M.A.; Jaafar, J.; Ismail, A.F.; Othman, M.H.D.; Rahman, M.A. Chapter 1—Fourier Transform Infrared (FTIR) Spectroscopy. In Membrane Characterization; Hilal, N., Ismail, A.F., Matsuura, T., Oatley-Radcliffe, D., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 3–29. [Google Scholar] [CrossRef]
- Lin, Y.-S.; Liang, S.-H.; Lai, W.-L.; Lee, J.-X.; Wang, Y.-P.; Liu, Y.-T.; Wang, S.-H.; Lee, M.-H. Sustainable Extraction of Chitin from Spent Pupal Shell of Black Soldier Fly. Processes 2021, 9, 976. [Google Scholar] [CrossRef]
- Yan, T.; Wang, P.; Wang, L. Utilization of oxalic acid-modified spent mushroom substrate for removal of methylene blue from aqueous solution. Desalin. Water Treat. 2015, 55, 1007–1017. [Google Scholar] [CrossRef]
- Singh, B.; Kumar, P.; Yadav, A.; Datta, S. Degradation of fermentation inhibitors from lignocellulosic hydrolysate liquor using immobilized bacterium, Bordetella sp. BTIITR. Chem. Eng. J. 2019, 361, 1152–1160. [Google Scholar] [CrossRef]
- Bari, E.; Mohebby, B.; Naji, H.R.; Oladi, R.; Yilgor, N.; Nazarnezhad, N.; Ohno, K.M.; Nicholas, D.D. Monitoring the cell wall characteristics of degraded beech wood by white-rot fungi: Anatomical, chemical, and photochemical study. Maderas Cienc. Tecnol. 2018, 20, 35–56. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, G.; Wang, S.; Zhang, H.; Xu, F. TG-FTIR and Py-GC/MS study of the pyrolysis mechanism and composition of volatiles from flash pyrolysis of PVC. J. Energy Inst. 2020, 93, 2362–2370. [Google Scholar] [CrossRef]
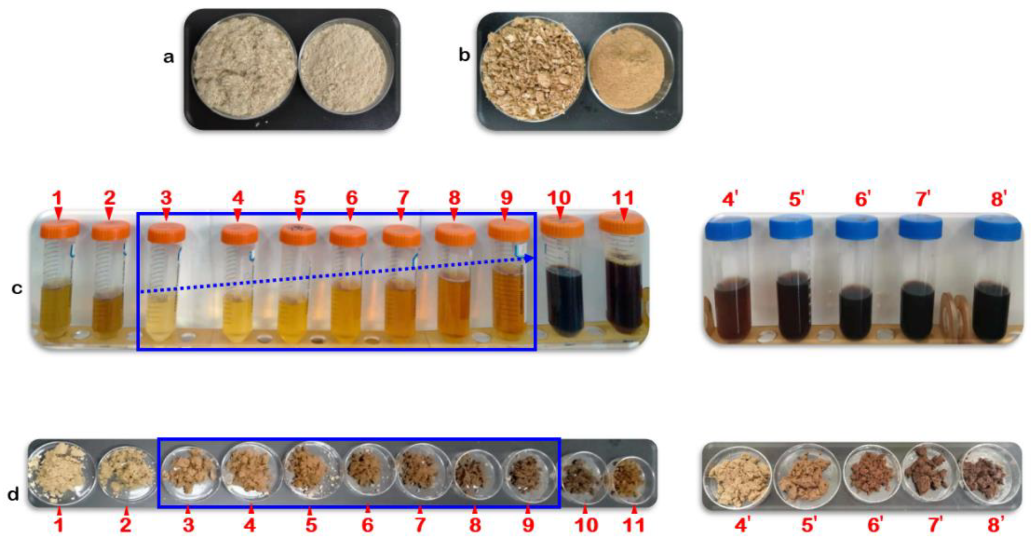

| SN | IHR (%) | SMS (%) | Pretreatment | pH | Solid Residue (%) |
|---|---|---|---|---|---|
| 1 | 100 | 0 | AH | 7.56 ± 0.31 | 88.40 ± 1.45 |
| 2 | 100 | 0 | TH | 6.85 ± 0.25 | 82.80 ± 1.08 |
| 3 | 100 | 0 | TCH | 0.48 ± 0.01 | 94.00 ± 1.07 |
| 4 | 90 | 10 | TCH | 0.52 ± 0.02 | 97.20 ± 1.08 |
| 4’ | 90 | 10 | TH | 6.24 ± 0.15 | 99.10 ± 0.51 |
| 5 | 75 | 25 | TCH | 0.51 ± 0.01 | 93.20 ± 1.04 |
| 5’ | 75 | 25 | TH | 6.30 ± 0.11 | 98.40 ± 0.97 |
| 6 | 50 | 50 | TCH | 0.57 ± 0.02 | 89.80 ± 1.62 |
| 6’ | 50 | 50 | TH | 6.50 ± 0.30 | 87.80 ± 0.88 |
| 7 | 25 | 75 | TCH | 0.55 ± 0.02 | 84.80 ± 1.52 |
| 7’ | 25 | 75 | TH | 6.70 ± 0.23 | 75.80 ± 1.73 |
| 8 | 10 | 90 | TCH | 0.62 ± 0.02 | 82.20 ± 1.18 |
| 8’ | 10 | 90 | TH | 7.10 ± 0.32 | 74.00 ± 1.63 |
| 9 | 0 | 100 | TCH | 0.65 ± 0.02 | 78.40 ± 1.19 |
| 10 | 0 | 100 | TH | 6.69 ± 0.34 | 74.20 ± 1.63 |
| 11 | 0 | 100 | AH | 6.86± 0.33 | 78.60 ± 1.38 |
| Wavenumber (cm−1) | Band Assignment | Remark | Ref. |
|---|---|---|---|
| 3150–3050 | Aromatic C–H stretch | SMS-TCH | [32] |
| 2250–2100 | Alkyne C≡O vibration | IHR, SMS-TCH | [32] |
| 1732 | Carbonyl C=O stretching vibration in hemicelluloses | Enhanced in IHR-raw, AH, and TH | [28] |
| 1650 | Primary amides | Enhanced in IHR-raw, AH, and TH | [33] |
| 1630 | C=C stretching vibration | SMS-TCH | [34] |
| 1600 | OH bending related to water in hemicelluloses | IHR-raw, AH, and TH | [28] |
| 1512 | Aromatic skeletal vibration in lignin | All IHR, enhanced in SMS-AH, TH, and TCH | [16] |
| 1430 | C–H bending of cellulose | All IHR, enhanced in SMS-AH, TH, and TCH | [28] |
| 1400 | –CH2 stretching | All IHR, enhanced in SMS-TCH | [35] |
| 1368 | C–OH stretching of the hydrogen bond in cellulose | All IHR, enhanced in SMS-raw | [28] |
| 1320 | C–H2 wagging in cellulose | All IHR, enhanced in SMS-TCH | [36] |
| 900–670 | Aromatic C–H out-of-plane bending | New peaks in SMS-TCH | [37] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dessie, W.; Luo, X.; Tang, J.; Tang, W.; Wang, M.; Qin, Z.; Tan, Y. Towards Full Utilization of Biomass Resources: A Case Study on Industrial Hemp Residue and Spent Mushroom Substrate. Processes 2021, 9, 1200. https://doi.org/10.3390/pr9071200
Dessie W, Luo X, Tang J, Tang W, Wang M, Qin Z, Tan Y. Towards Full Utilization of Biomass Resources: A Case Study on Industrial Hemp Residue and Spent Mushroom Substrate. Processes. 2021; 9(7):1200. https://doi.org/10.3390/pr9071200
Chicago/Turabian StyleDessie, Wubliker, Xiaofang Luo, Jiachen Tang, Wufei Tang, Meifeng Wang, Zuodong Qin, and Yimin Tan. 2021. "Towards Full Utilization of Biomass Resources: A Case Study on Industrial Hemp Residue and Spent Mushroom Substrate" Processes 9, no. 7: 1200. https://doi.org/10.3390/pr9071200
APA StyleDessie, W., Luo, X., Tang, J., Tang, W., Wang, M., Qin, Z., & Tan, Y. (2021). Towards Full Utilization of Biomass Resources: A Case Study on Industrial Hemp Residue and Spent Mushroom Substrate. Processes, 9(7), 1200. https://doi.org/10.3390/pr9071200







