Long-Term Effects of Calcium-Based Liming Materials on Soil Fertility Sustainability and Rye Production as Soil Quality Indicators on a Typic Palexerult
Abstract
:1. Introduction
2. Materials and Methods
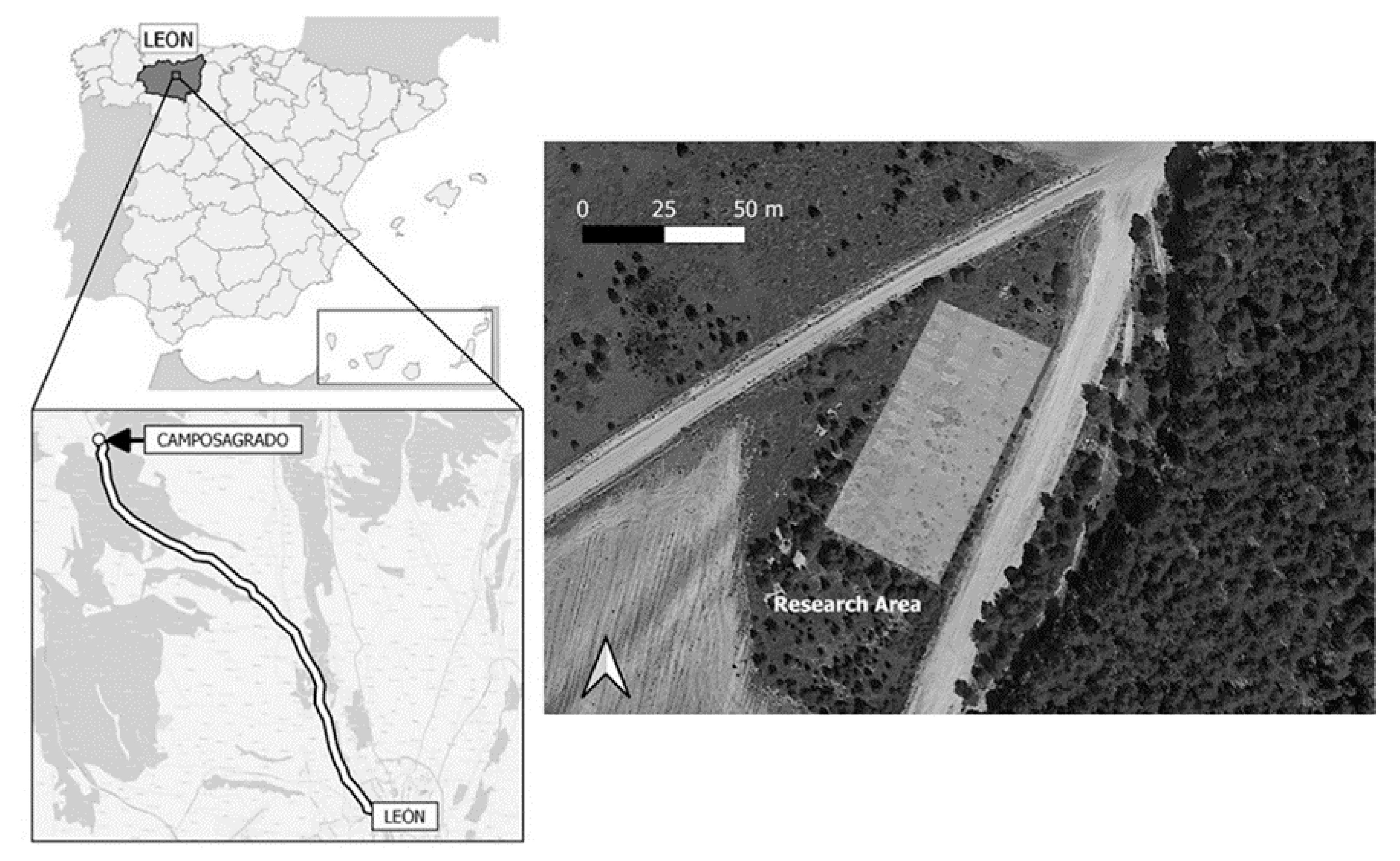
2.1. Study Site
2.2. Characterisation of the Liming Materials and Doses
2.3. Experimental Design and Statistical Analyses
2.4. Soil and Biomass Analyses
3. Results
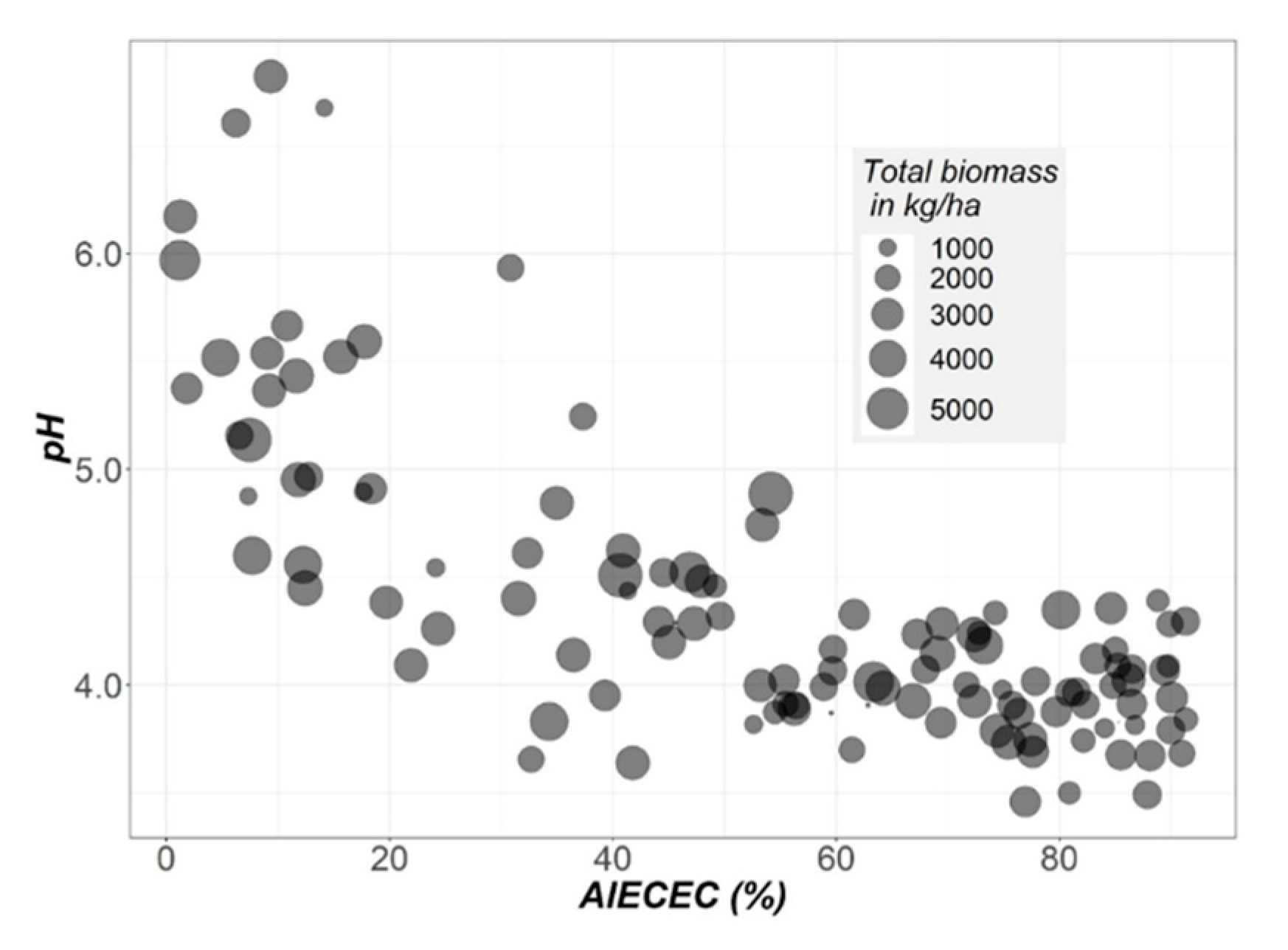
3.1. Initial Soil Characterisation before Liming
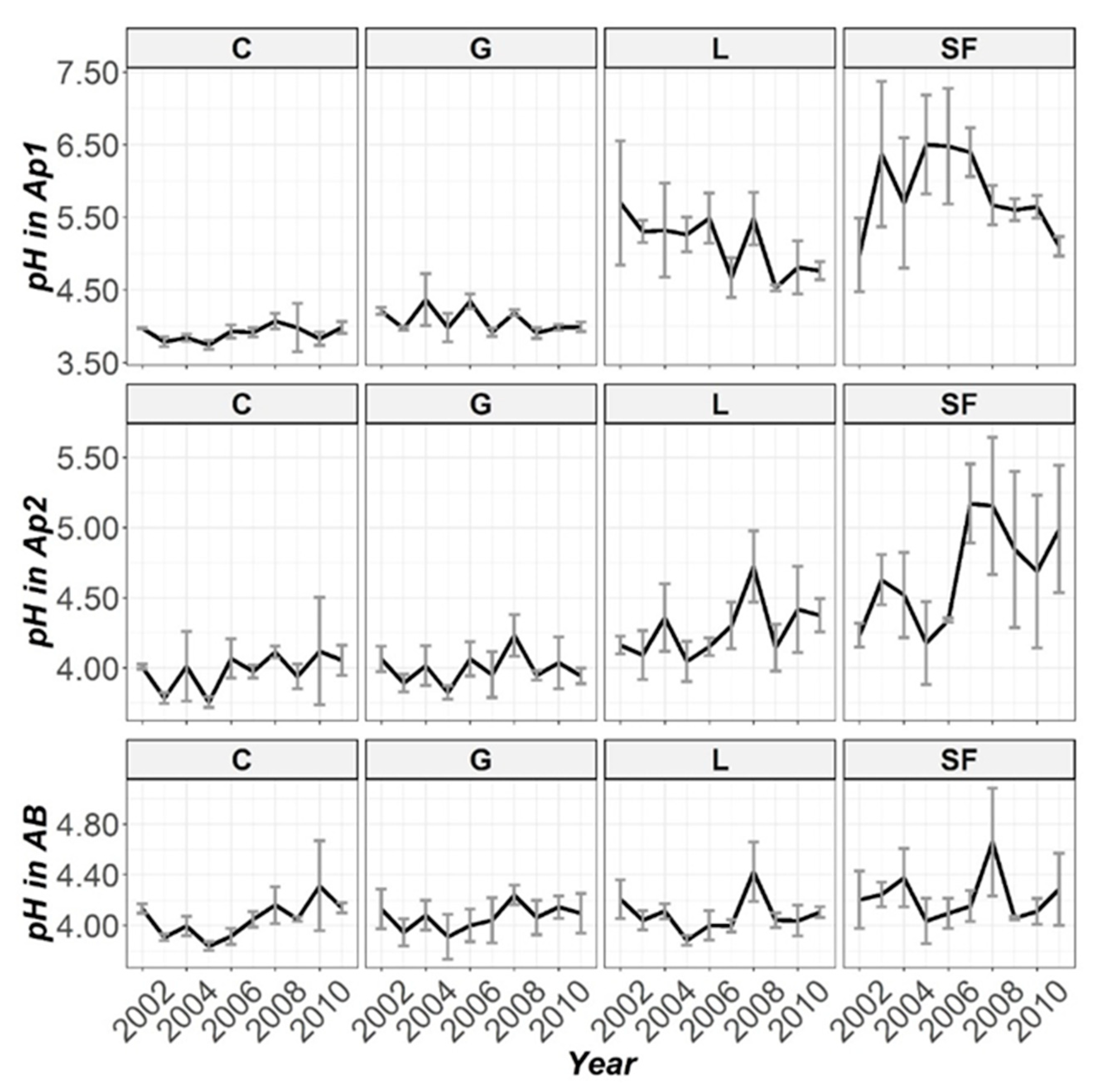
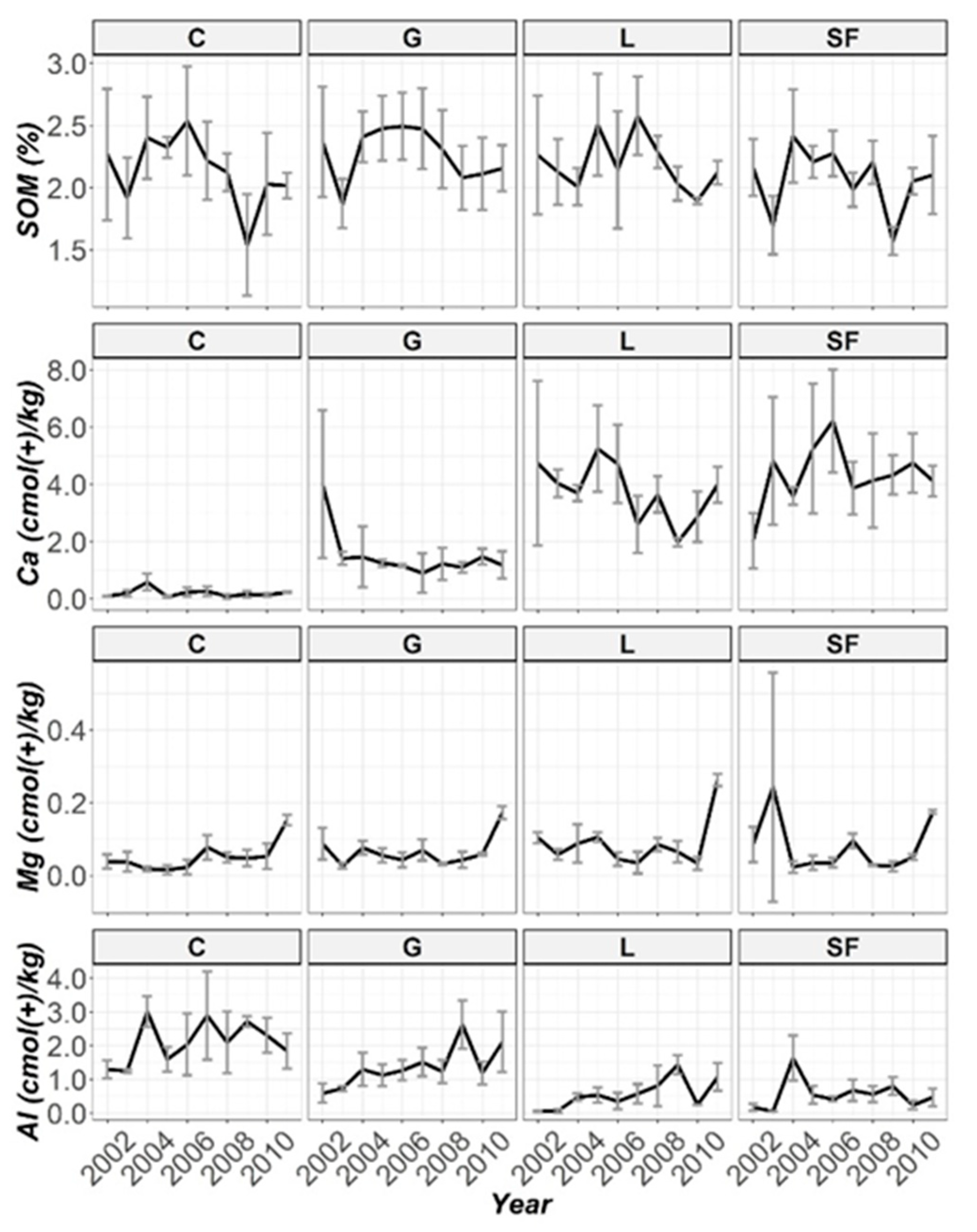
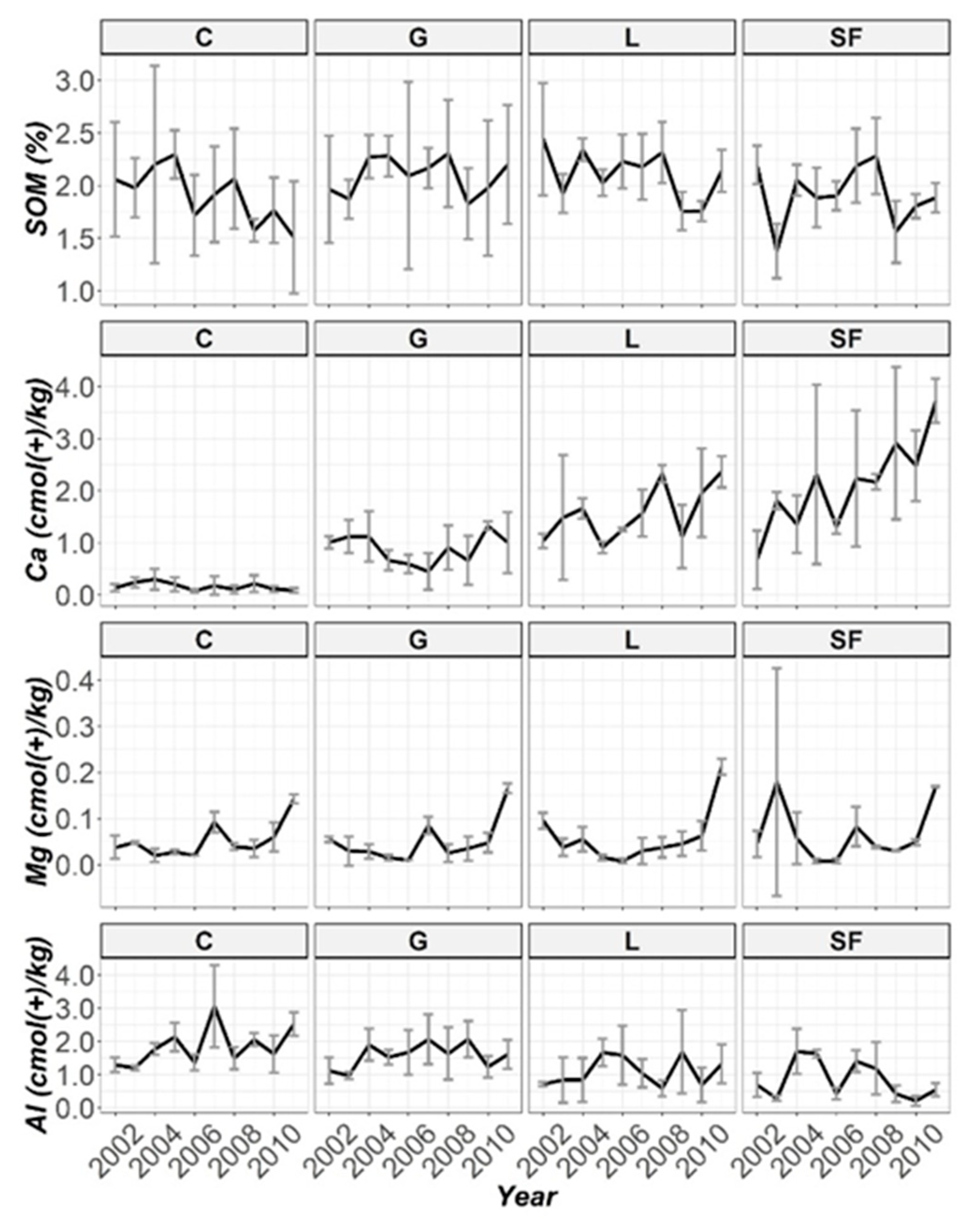
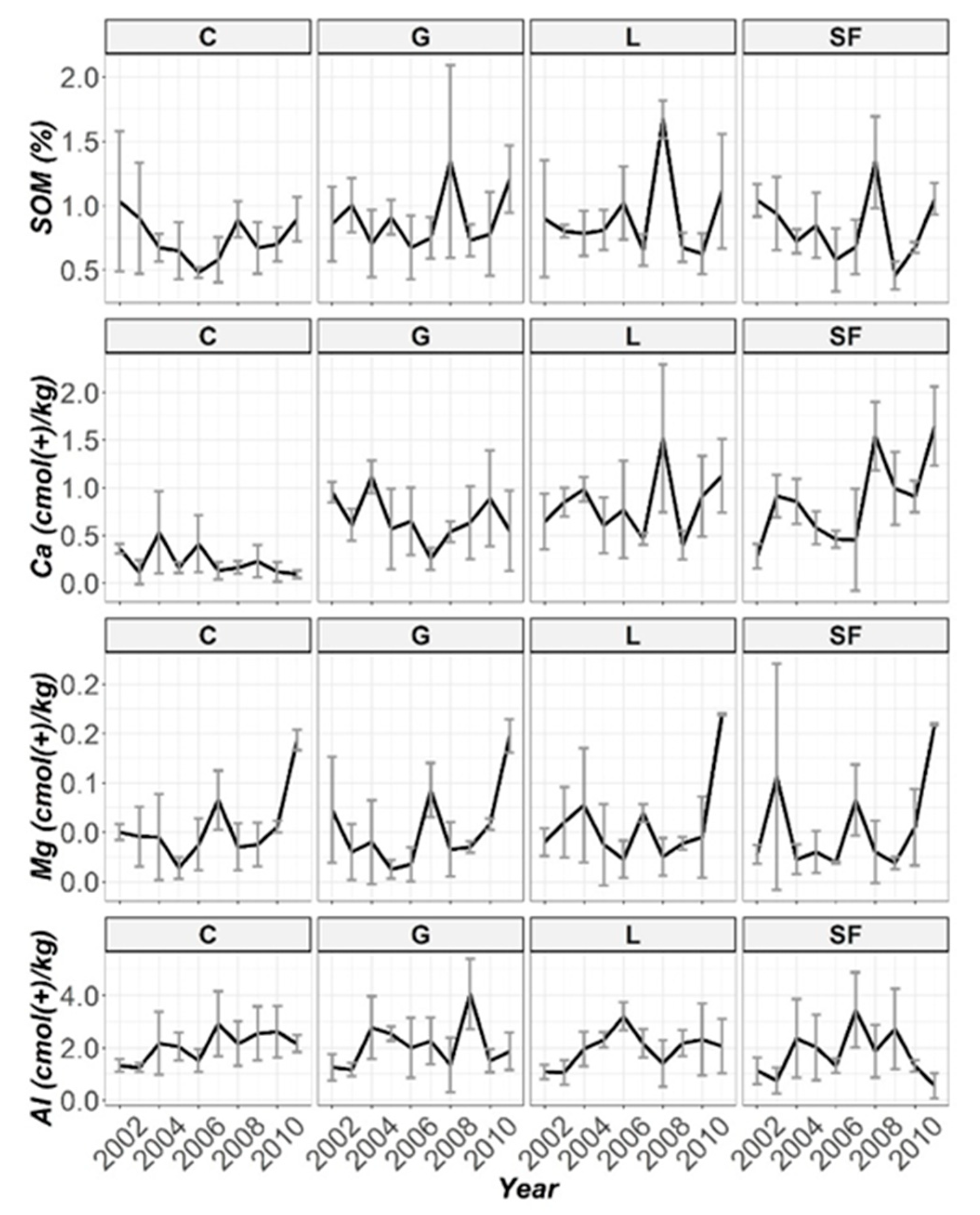
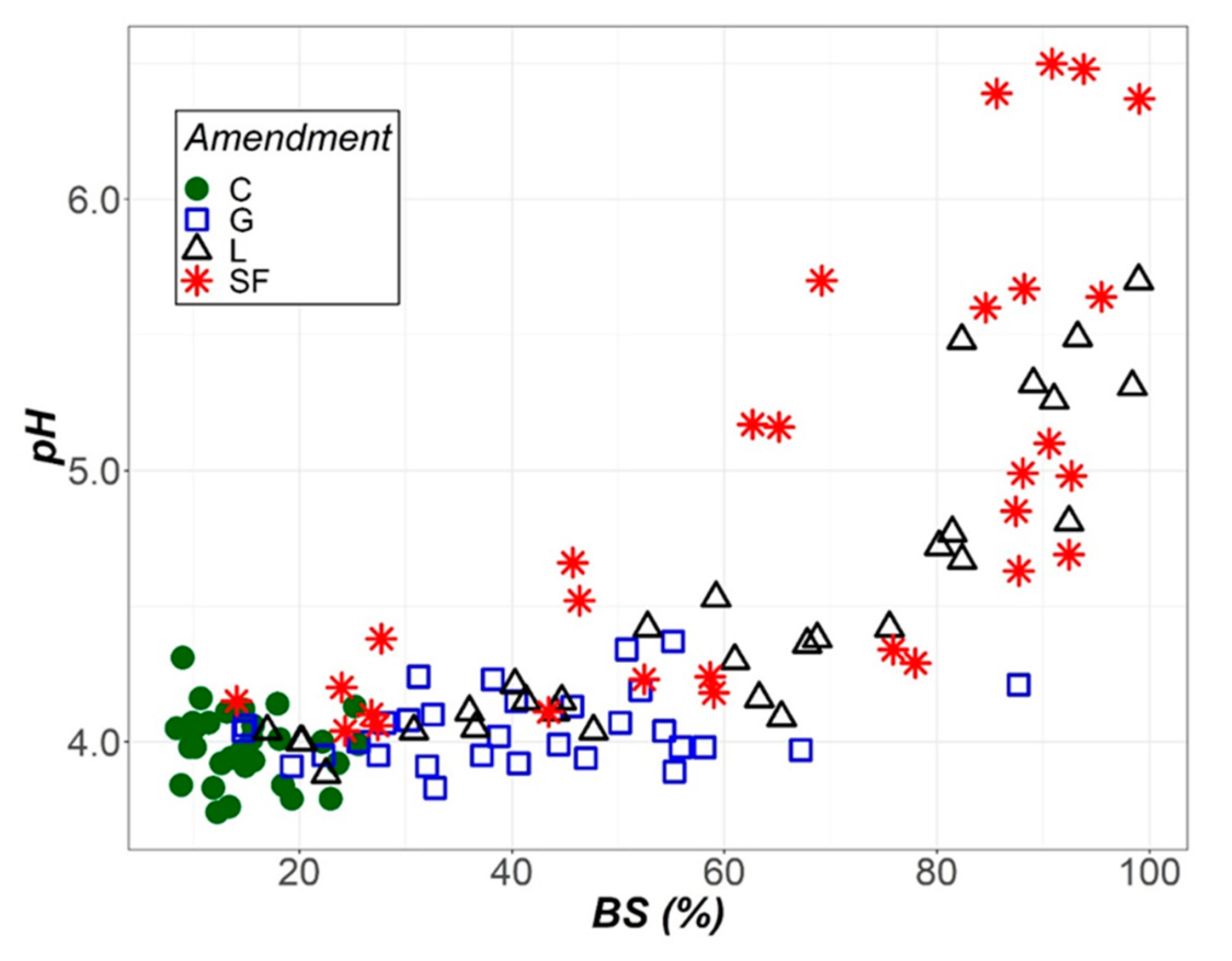
3.2. Temporal Evolution of Soil Parameters
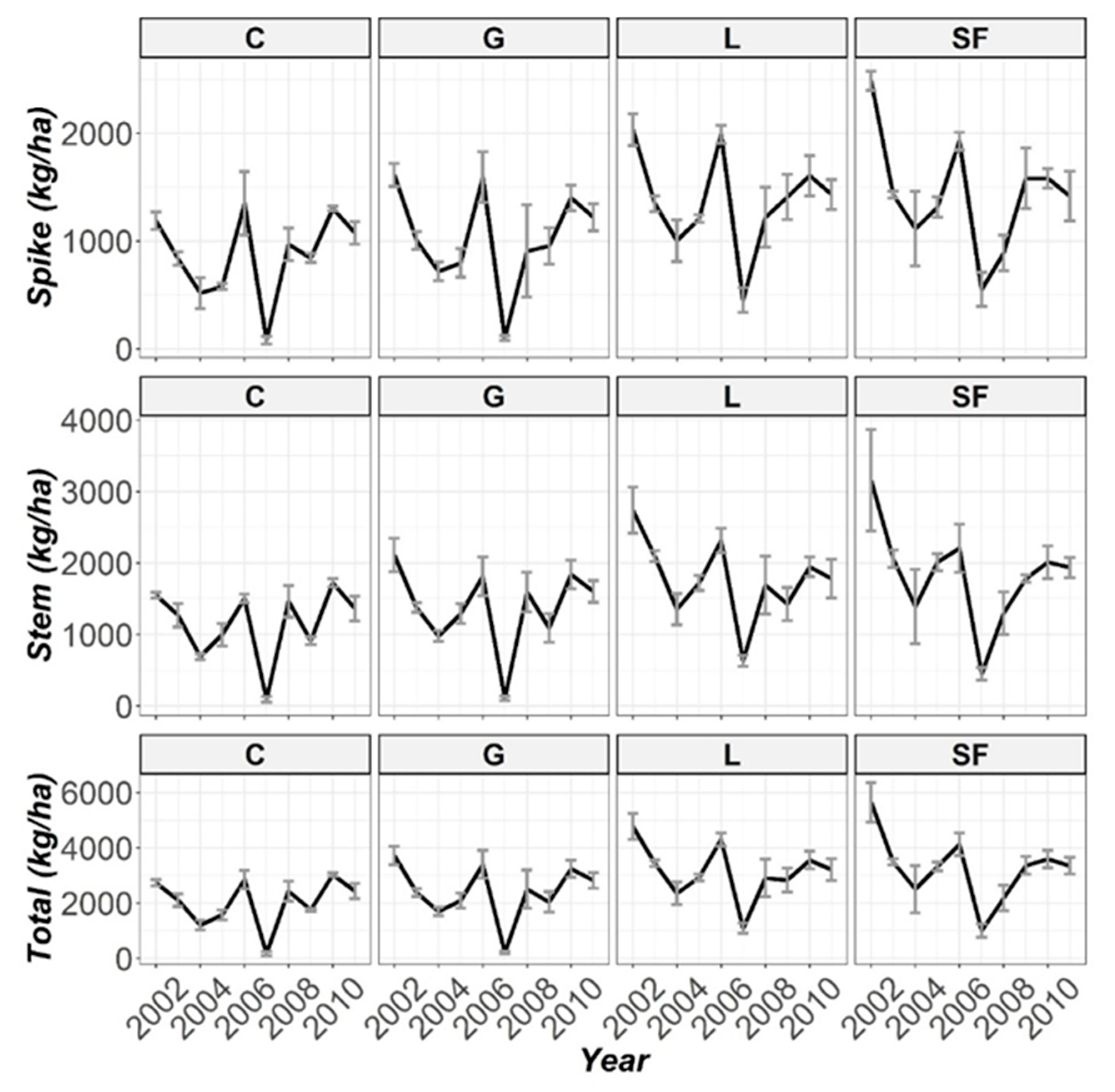
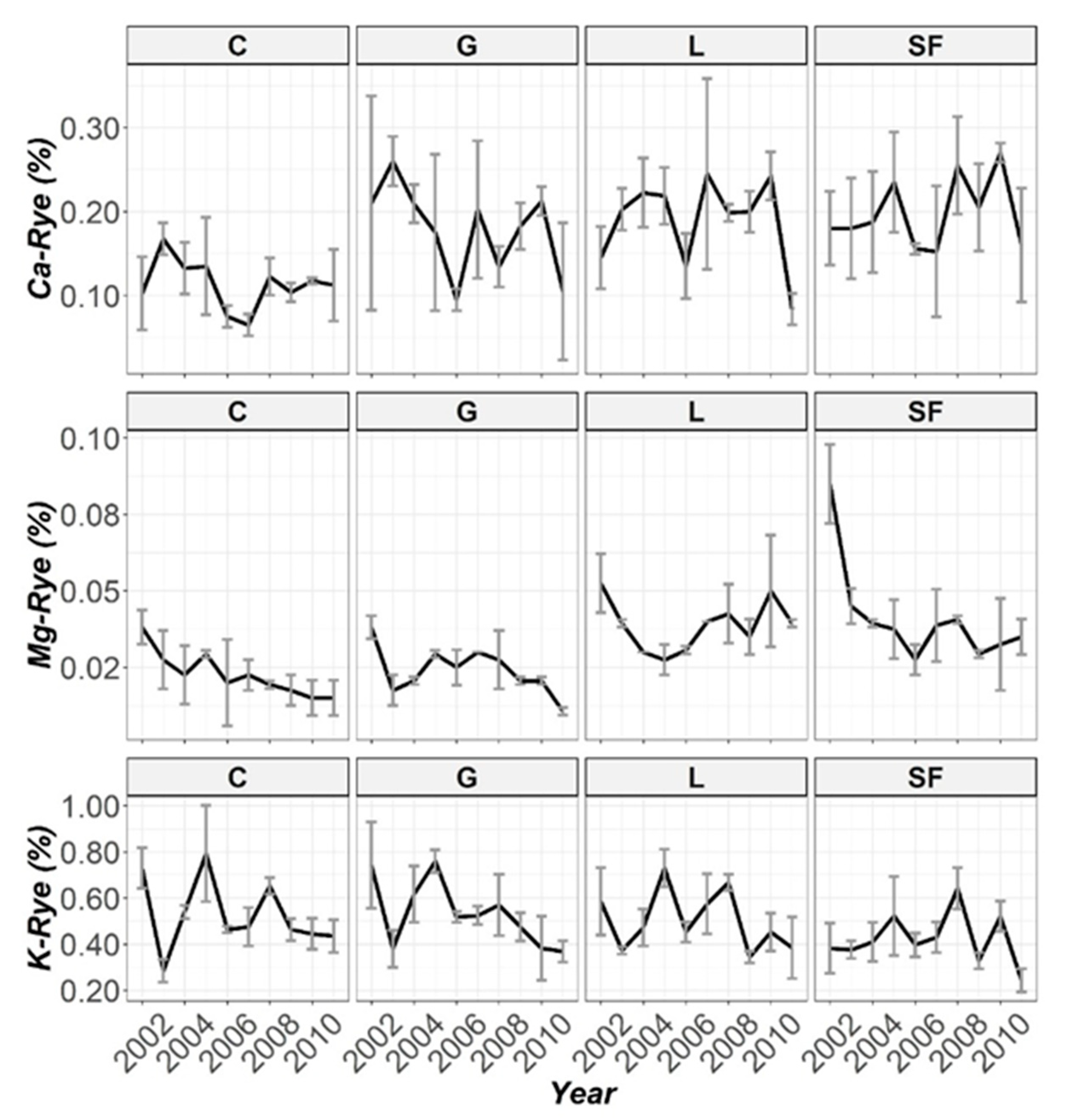
3.3. Temporal Evolution Biomass
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Voltr, V.; Menšik, L.; Hlisnikovský, L.; Hruška, M.; Pokorný, E.; Pospíšilová, L. The soil organic matter in connection with soil properties and soil inputs. Agronomy 2021, 11, 779. [Google Scholar] [CrossRef]
- Drever, J.I.; Stillings, L.L. The role of organic acids in mineral weathering. Colloids Surf. A 1997, 120, 167–181. [Google Scholar] [CrossRef]
- Baldock, J.A.; Broos, K. Soil organic matter. In Handbook of Soil Sciences: Properties and Processes. Part II. Soil Chemistry, 2nd ed.; Huang, P.M., Li, Y., Sumner, M.E., Eds.; CRC Press: Boca Raton, FL, USA, 2012; pp. 1–52. [Google Scholar]
- Brejda, J.J.; Karlen, D.L.; Smith, J.L.; Allan, D.L. Identification of regional soil quality factors and indicators: II. Northern Mississippi Loess Hills and Palouse Prairie. Soil Sci. Soc. Am. J. 2000, 64, 2125–2135. [Google Scholar] [CrossRef] [Green Version]
- Rees, R.M.; Ball, B.C.; Campbell, C.D.; Watson, C.A. Introduction. In Sustainable Management of Soil Organic Matter; Rees, R.M., Ball, B.C., Campbell, C.D., Watson, C.A., Eds.; CABI Publishing: Wallingford, UK, 2001; pp. 1–7. [Google Scholar]
- Vidal, M.; Garzón, E.; García, V.; Villa, E. Differentiating the amending effects of calcareous materials applied to acid soils by use of optimal scaling procedures. Agrochimica 2006, 50, 132–147. [Google Scholar]
- Kochian, L.V.; Piñeros, M.A.; Hoekenga, O.A. The physiology, genetics and molecular biology of plant aluminum resistance and toxicity. Plant Soil 2005, 274, 175–195. [Google Scholar] [CrossRef]
- Strawn, D.G.; Bohn, H.; O’Connor, G.A. Soil Chemistry, 5th ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2020. [Google Scholar]
- Garzón, E.; González-Andrés, F.; García-Martínez, V.; de Paz, J.M. Mineralization and nutrient release of an organic fertilizer made by flour, meat, and crop residues in two vineyard soils with different pH levels. Commun. Soil Sci. Plant Anal. 2011, 42, 1485–1496. [Google Scholar] [CrossRef]
- Havlin, J.L.; Tisdale, S.L.; Nelson, W.L.; Beaton, J.D. Soil Fertility and Fertilizers: An Introduction to Nutrient Management, 8th ed.; Pearson: Upper Saddle River, NJ, USA, 2014. [Google Scholar]
- Murphy, B.W. Soil Organic Matter and Soil Function. Review of the Literature and Underlying Data; Department of the Environment: Canberra, Australia, 2014. [Google Scholar]
- Ritchie, G.S.P.; Dolling, P.J. The role of organic matter in soil acidification. Aust. J. Soil Res. 1985, 23, 569–576. [Google Scholar] [CrossRef]
- Fageria, N.K.; Baligar, V.C. Ameliorating soil acidity of tropical Oxisols by liming for sustainable crop production. Adv. Agron. 2008, 99, 345–399. [Google Scholar]
- Paradelo, R.; Virto, I.; Chenu, C. Net effect of liming on soil organic carbon stocks: A review. Agric. Ecosyst. Environ. 2015, 202, 98–107. [Google Scholar] [CrossRef]
- González-Fernández, P.; Espejo-Serrano, R.; Ordóñez-Fernández, R.; Peregrina-Alonso, F. Comparative studies of the efficiency of lime refuse from sugar beet factories as an agricultural liming material. In Sustainable Organic Waste Management for Enviromental Protection and Food Safety: Nutrient and Carbon Cycling in Sustainable Plant-Soil Systems; Bernal, M.P., Moral, R., Clement, R., Paredes, C., Eds.; FAO and CSIC: Murcia, Spain, 2005; Volume I, pp. 157–160. [Google Scholar]
- Navarro, F.J.G.; Amorós, J.A.; Sánchez, C.J.; Bravo, S.; Márquez, E.; Jiménez, R. Application of sugar foam to red soils in a semiarid Mediterranean environment. Environ. Earth Sci. 2009, 59, 603–611. [Google Scholar] [CrossRef]
- Belardi, G.; Piga, L. Influence of calcium carbonate on the decomposition of asbestos contained in end-of-life products. Termochimica Acta 2013, 573, 220–228. [Google Scholar] [CrossRef]
- Wang, X.; Tang, C.; Baldock, J.A.; Butterly, C.R.; Gazey, C. Long-term effect of lime application on the chemical composition of soil organic carbon in acid soils varying in texture and liming history. Biol. Fertil. Soils 2016, 52, 295–306. [Google Scholar] [CrossRef]
- Soil Survey Staff-USDA. Claves para la Taxonomía de Suelos, 11th ed.; Soil Survey Staff-USDA, Natural Resources Conservation Service: Washington, DC, USA, 2010. [Google Scholar]
- Nafría, D.A.; Garrido, N.; Álvarez, M.V.; Cubero, D.; Fernández, M.; Villarino, I.; Gutiérrez, A.; Abia, I. Atlas Agroclimático Castilla y León, 1st ed.; ITACYL-AEMET: Valladolid, Spain, 2013. [Google Scholar]
- Villa, E. Incidencia de la Aplicación de Espumas de Azucarería y Otras Enmiendas calizas Sobre la Producción de Biomasa. Mejora a Corto plazo de Los Condicionantes Agronómicos de Los suelos Ácidos de Raña del Norte de León. Ph.D. Thesis, Universidad de León, León, Spain, 2005. [Google Scholar]
- Cochrane, T.T.; Salinas, J.G.; Sánchez, P.A. An equation for liming acid mineral soils to compensate crop aluminium tolerance. Trop. Agric. 1980, 57, 133–140. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing). 2019. Available online: http://www.R-project.org/ (accessed on 1 March 2021).
- Field, A.; Miles, J.; Field, Z. Discovering Statistics Using R, 1st ed.; SAGE Publications Ltd.: Newbury Park, CA, USA, 2012. [Google Scholar]
- Ministerio de Agricultura, Pesca y Alimentación. Métodos Oficiales de Análisis; Ministerio de Agricultura, Pesca y Alimentación, Secretaría General Técnica: Madrid, Spain, 1993; Volume III.
- Benton, J. Laboratory Guide for Conducting Soil Tests and Plant Analysis; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Little, I. The determination of exchangeable aluminium in soils. Aust. J. Soil Res. 1964, 2, 76–82. [Google Scholar] [CrossRef]
- González-Fernandez, P.; Ordóñez-Fernández, R.; Espejo-Serrano, R.; Peregrini-Alonso, F. Efectos a medio plazo de la espuma de azucarería, caliza magnesiana y yeso sobre las bases intercambiables y el aluminio en el perfil de un suelo ácido. Estudios de la Zona No Saturada del Suelo 2005, VII, 185–189. [Google Scholar]
- Smith, C.J.; Peoples, M.B.; Keerthisinghe, G.; James, T.R.; Garden, D.L.; Tuomi, S.S. Effect of surface applications of lime, gypsum and phosphogypsum on the alleviating of surface and subsurface acidity in a soil under pasture. Aust. J. Soil Res. 1994, 32, 995–1008. [Google Scholar] [CrossRef]
- Meriño-Gergichevich, C.; Alberdi, M.; Ivanov, A.G.; Reyes-Díaz, M. Al3+- Ca2+ interaction in plants growing in acid soils: Al-phytotoxicity response to calcareous amendments. J. Soil Sci. Plant Nutr. 2010, 10, 217–243. [Google Scholar]
- Goulding, K.W.T. Soil acidification and the importance of liming agricultural soils with particular reference to the United Kingdom. Soil Use Manag. 2016, 32, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Illera, V.; Garrido, F.; Vizcayno, C.; García-González, M.T. Field applications of industrial by-products as Al toxicity amendments: Chemical and mineralogical implications. Eur. J. Soil Sci. 2004, 55, 681–692. [Google Scholar] [CrossRef] [Green Version]
- Anderson, G.C.; Pathan, S.; Easton, J.; Hall, D.J.M.; Sharma, R. Short- and Long-term effects of lime and gypsum applications on acid soils in a water-limited environment: 2. Soil chemical properties. Agronomy 2020, 10, 1987. [Google Scholar] [CrossRef]
- Siepel, H.; Bobbink, R.; van de Riet, B.P.; van den Burg, A.B.; Jongejans, E. Long-term effects of liming on soil physico-chemical properties and micro-arthropod communities in Scotch pine forest. Biol. Fertil. Soils 2019, 55, 675–683. [Google Scholar] [CrossRef] [Green Version]
- Da Costa, C.H.M.; Crusciol, C.A.C. Long-term effects of lime and phosphogypsum application on tropical no-till soybean–oat–sorghum rotation and soil chemical properties. Europ. J. Agron. 2016, 74, 119–132. [Google Scholar] [CrossRef] [Green Version]
- Cifu, M.; Xiaonan, L.; Zhihong, C.; Zhengyi, H.; Wanzhu, M. Long-term effects of lime application on soil acidity and crop yields on a red soil in Central Zhejiang. Plant Soil 2004, 265, 101–109. [Google Scholar] [CrossRef]
- Jaskulska, I.; Jaskulski, D.; Kobierski, M. Effect of liming on the change of some agrochemical soil properties in a long-term fertilization experiment. Plant Soil Environ. 2014, 60, 146–150. [Google Scholar] [CrossRef] [Green Version]
- Haynes, R.J.; Naidu, R. Influence of lime, fertilizer and manure applications on soil organic matter content and soil physical conditions: A review. Nutr. Cycl. Agroecosyst. 1998, 51, 123–137. [Google Scholar] [CrossRef]
- Condron, L.M.; Tiessen, H.; Trasar-Cepeda, C.; Moir, J.O.; Stewart, J.W.B. Effect of liming on organic matter decomposition and phosphorus extractability in an acid humic Ranker soil from northwest Spain. Biol. Fertil. Soils 1993, 15, 279–284. [Google Scholar] [CrossRef]
- Yagi, R.; Ferreira, M.E.; Pessôa da Cruz, M.C.; Barbosa, J.C. Organic matter fractions and soil fertility under the influence of liming, vermicompost and cattle manure. Sci. Agric. 2003, 60, 549–557. [Google Scholar] [CrossRef]
- Chan, K.Y.; Heenan, D.P. Lime-induced loss of soil organic carbon and effect on aggregate stability. Soil Sci. Soc. Am. J. 1999, 63, 1841–1844. [Google Scholar] [CrossRef]
- Caires, E.F.; Churka, S.; Garbuio, F.J.; Ferrari, R.A.; Morgano, M.A. Soybean yield and quality as a function of lime and gypsum applications. Sci. Agric. 2006, 63, 370–379. [Google Scholar] [CrossRef]
- Silva, C.A.; Anderson, S.J.; Guilherme, L.R. Uso da cromatografía de exclusão por tamanho na caracterização de substâncias húmicas de Latossolo Vermelho-Escuro sob efeito da calagem. Rev. Bras. Ciênc. Solo 2000, 24, 495–503. [Google Scholar] [CrossRef]
- Grover, S.P.; Butterly, C.R.; Wang, X.; Tang, C. The short-term effects of liming on organic carbon mineralisation in two acidic soils as affected by different rates and application depths of lime. Biol. Fertil. Soils 2017, 53, 431–443. [Google Scholar] [CrossRef]
- Fuentes, J.P.; Bezdicek, D.F.; Flury, M.; Albrecht, S.; Smith, J.L. Microbial activity affected by lime in a long-term no-till soil. Soil Tillage Res. 2006, 88, 123–131. [Google Scholar] [CrossRef]
- Yan, N.D.; Dillon, P.J. Experimental neutralization of lakes near Sudbury, Ontario. In Studies of Lakes and Watersheds Near Sudbury, Ontario. Final Limnological Report; Report SES 009/82; Canadian Ministry of the Environment: Rexdale, ON, Canada, 1982; pp. 6–60. [Google Scholar]
- Karlik, B. Liming effect on dissolved organic matter leaching. Water Air Soil Pollut. 1995, 85, 949–954. [Google Scholar] [CrossRef]
- Nilsson, S.I.; Andersson, S.; Valeur, I.; Persson, T.; Bergholm, J.; Wirén, A. Influence of dolomite lime on leaching and storage of C, N and S in a Spodosol under Norway spruce (Picea abies (L.) Karst). For. Ecol. Manag. 2001, 146, 55–73. [Google Scholar] [CrossRef]
- Chen, L.; Dick, W.A. Gypsum as an Agricultural Amendment: General Use Guidelines; Ohio State University Extension: Columbus, OH, USA, 2011. [Google Scholar]
- Kostic, L.; Nikolic, N.; Samardzic, J.; Milisavljevic, M.; Maksimović, V.; Cakmak, D.; Manojlovic, D.; Nikolic, M. Liming of anthropogenically acidified soil promotes phosphorus acquisition in the rhizosphere of wheat. Biol. Fertil. Soils 2015, 51, 289–298. [Google Scholar] [CrossRef]
- Anikwe, M.A.N.; Eze, J.C.; Ibudialo, A.N. Influence of lime and gypsum application on soil properties and yield of cassava (Manihot esculenta Crantz.) in a degraded Ultisol in Agbani, Enugu Southeastern Nigeria. Soil Tillage Res. 2016, 158, 32–38. [Google Scholar] [CrossRef]
- Olego, M.A.; Quiroga, M.J.; Sánchez-García, M.; Cuesta, M.; Cara-Jiménez, J.; Garzón-Jimeno, J.E. Effects of overliming on the nutritional status of grapevines with special reference to micronutrient content. OENO One 2021, 55, 57–73. [Google Scholar] [CrossRef]
| Treatment | CaO a | MgO a | K2O a | Aluminum b | CCE c | OM c |
|---|---|---|---|---|---|---|
| Limestone (L) | 437 | 20.8 | 3.50 | 7870 | 0.83 | 0.00 |
| Sugar foam (SF) | 404 | 14.5 | 0.90 | 2470 | 0.76 | 0.08 |
| Gypsum (G) | 332 | 17.5 | 1.50 | 3350 | 0.64 | 0.00 |
| Factor | Levels |
|---|---|
| Treatments (T) | Four levels: control (C), gypsum (G), limestone (L) and sugar foam (SF) |
| Soil depth (D) | Three levels: Ap1, Ap2 and AB |
| Sampling year (Y) | Ten levels: 2002–2011 |
| Soil Depth | Textural Class a | pH | EC b | SOM c | Ca d | Mg d | K d | Al d | AlECEC e |
|---|---|---|---|---|---|---|---|---|---|
| Ap1 | Sandy loam | 3.99 | 0.05 | 2.27 | 0.10 | 0.06 | 0.09 | 1.30 | 84.1 |
| Ap2 | Sandy loam | 4.03 | 0.04 | 2.06 | 0.13 | 0.04 | 0.06 | 1.29 | 84.6 |
| AB | Sandy clay loam | 4.13 | 0.03 | 1.03 | 0.30 | 0.04 | 0.04 | 1.33 | 78.6 |
| Soil Parameter | ML Ratio (T) | ML Ratio (D) | ML Ratio (Y) | ML Ratio (T × D × Y) |
|---|---|---|---|---|
| pH | 195 (***) | 151 (***) | 16.9 (0.05) | 636 (***) |
| SOM | 5.49 (0.14) | 681 (***) | 33.3 (***) | 172 (***) |
| Ca | 182 (***) | 203 (***) | 10.5 (0.31) | 494 (***) |
| Mg | 9.61 (*) | 10.5 (***) | 45.7 (***) | 191 (***) |
| K | 34.0 (***) | 46.5 (***) | 52.1 (***) | 149 (***) |
| Al | 93.6 (***) | 98.0 (***) | 37.1 (***) | 265 (***) |
| Biomass Parameter | ML Ratio (T) | ML Ratio (Y) | ML Ratio (T × Y) |
|---|---|---|---|
| Spike | 135 (***) | 53.2 (***) | 92.9 (***) |
| Stem | 110 (***) | 51.0 (***) | 96.8 (***) |
| Total | 130 (***) | 52.9 (***) | 105 (***) |
| Ca-Rye | 50.8 (***) | 26.9 (***) | 59.3 (***) |
| Mg-Rye | 96.2 (***) | 35.1 (***) | 115 (***) |
| K-Rye | 25.6 (***) | 39.6 (***) | 77.9 (***) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olego, M.Á.; Quiroga, M.J.; Mendaña-Cuervo, C.; Cara-Jiménez, J.; López, R.; Garzón-Jimeno, E. Long-Term Effects of Calcium-Based Liming Materials on Soil Fertility Sustainability and Rye Production as Soil Quality Indicators on a Typic Palexerult. Processes 2021, 9, 1181. https://doi.org/10.3390/pr9071181
Olego MÁ, Quiroga MJ, Mendaña-Cuervo C, Cara-Jiménez J, López R, Garzón-Jimeno E. Long-Term Effects of Calcium-Based Liming Materials on Soil Fertility Sustainability and Rye Production as Soil Quality Indicators on a Typic Palexerult. Processes. 2021; 9(7):1181. https://doi.org/10.3390/pr9071181
Chicago/Turabian StyleOlego, Miguel Ángel, Miguel Javier Quiroga, Cristina Mendaña-Cuervo, Jorge Cara-Jiménez, Roberto López, and Enrique Garzón-Jimeno. 2021. "Long-Term Effects of Calcium-Based Liming Materials on Soil Fertility Sustainability and Rye Production as Soil Quality Indicators on a Typic Palexerult" Processes 9, no. 7: 1181. https://doi.org/10.3390/pr9071181
APA StyleOlego, M. Á., Quiroga, M. J., Mendaña-Cuervo, C., Cara-Jiménez, J., López, R., & Garzón-Jimeno, E. (2021). Long-Term Effects of Calcium-Based Liming Materials on Soil Fertility Sustainability and Rye Production as Soil Quality Indicators on a Typic Palexerult. Processes, 9(7), 1181. https://doi.org/10.3390/pr9071181








