Optimization of Spray-Drying Process with Response Surface Methodology (RSM) for Preparing High Quality Graphene Oxide Slurry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Experimental Design
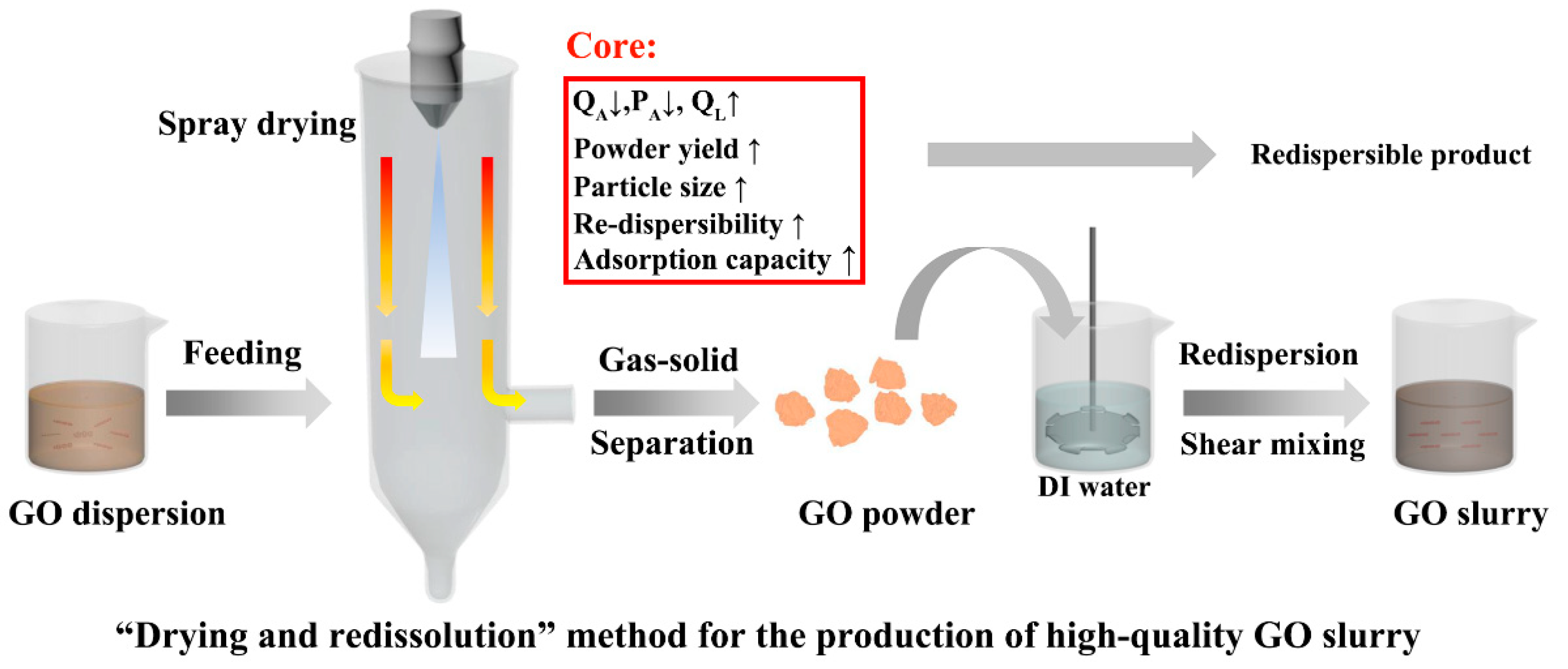
2.3. Sample Preparation and Spray Drying
2.4. Characterizations
3. Results and Discussions
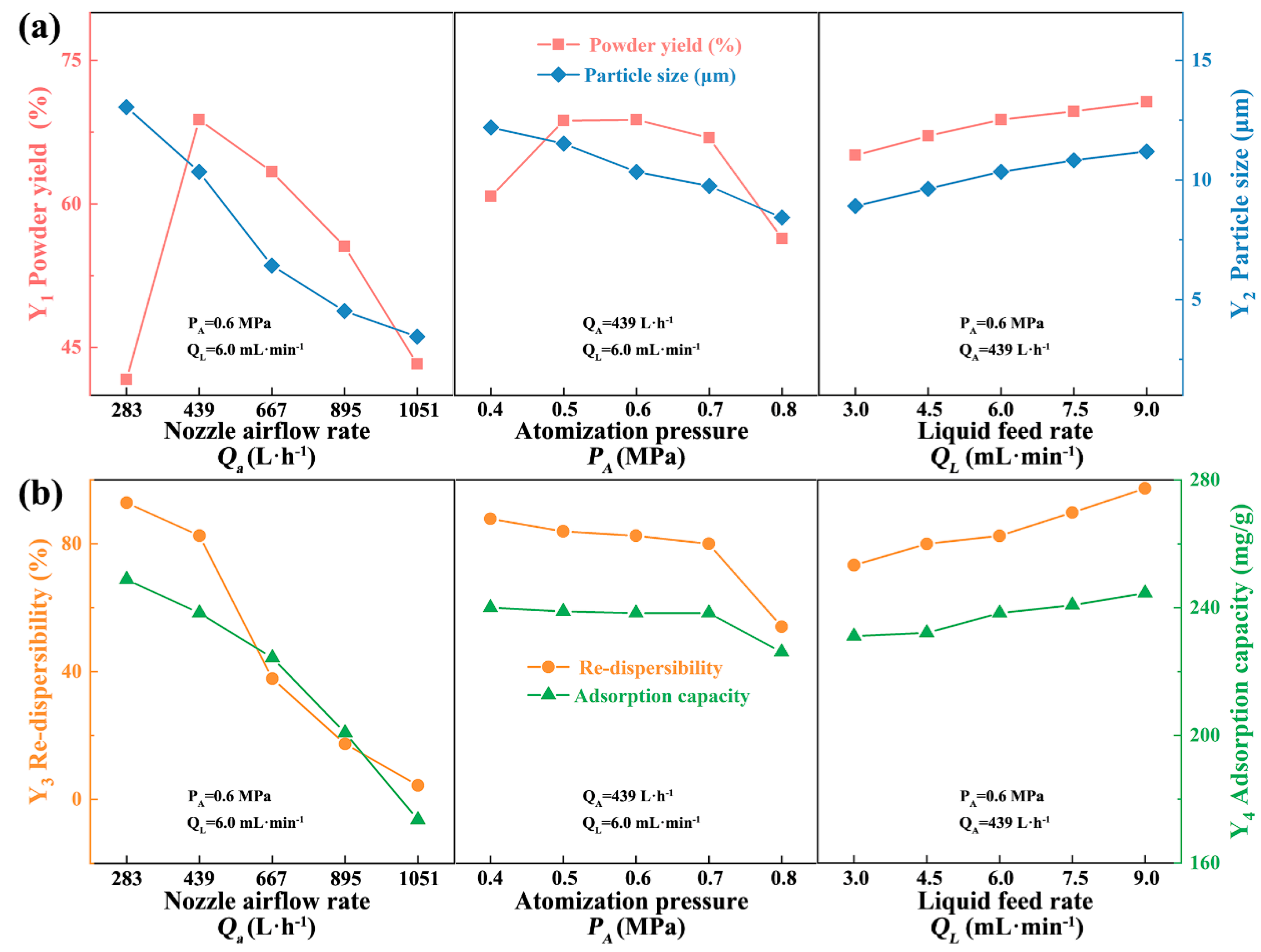
3.1. Single-Factor Experiments
3.2. RSM Analysis
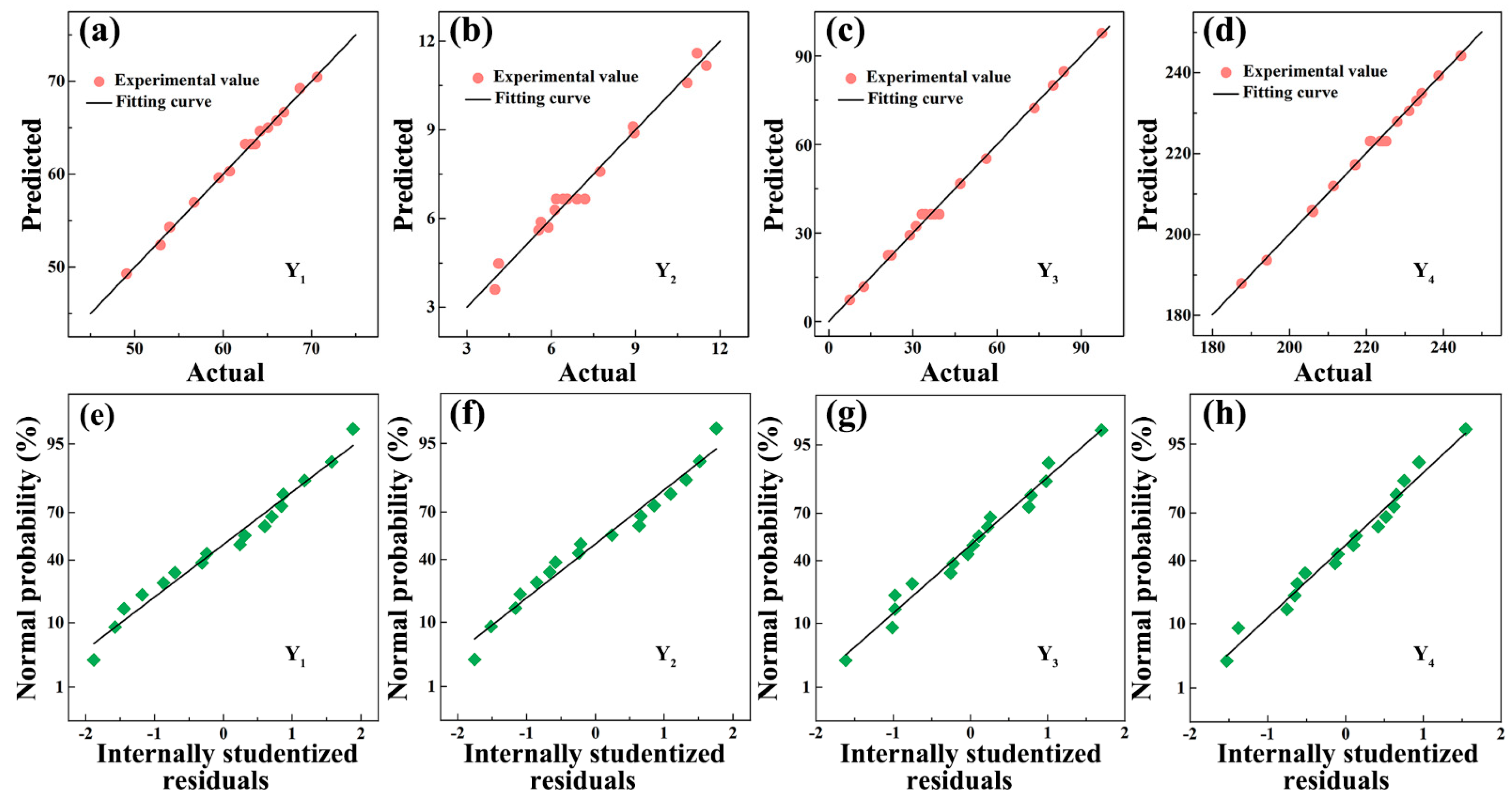
3.2.1. Model Fitting
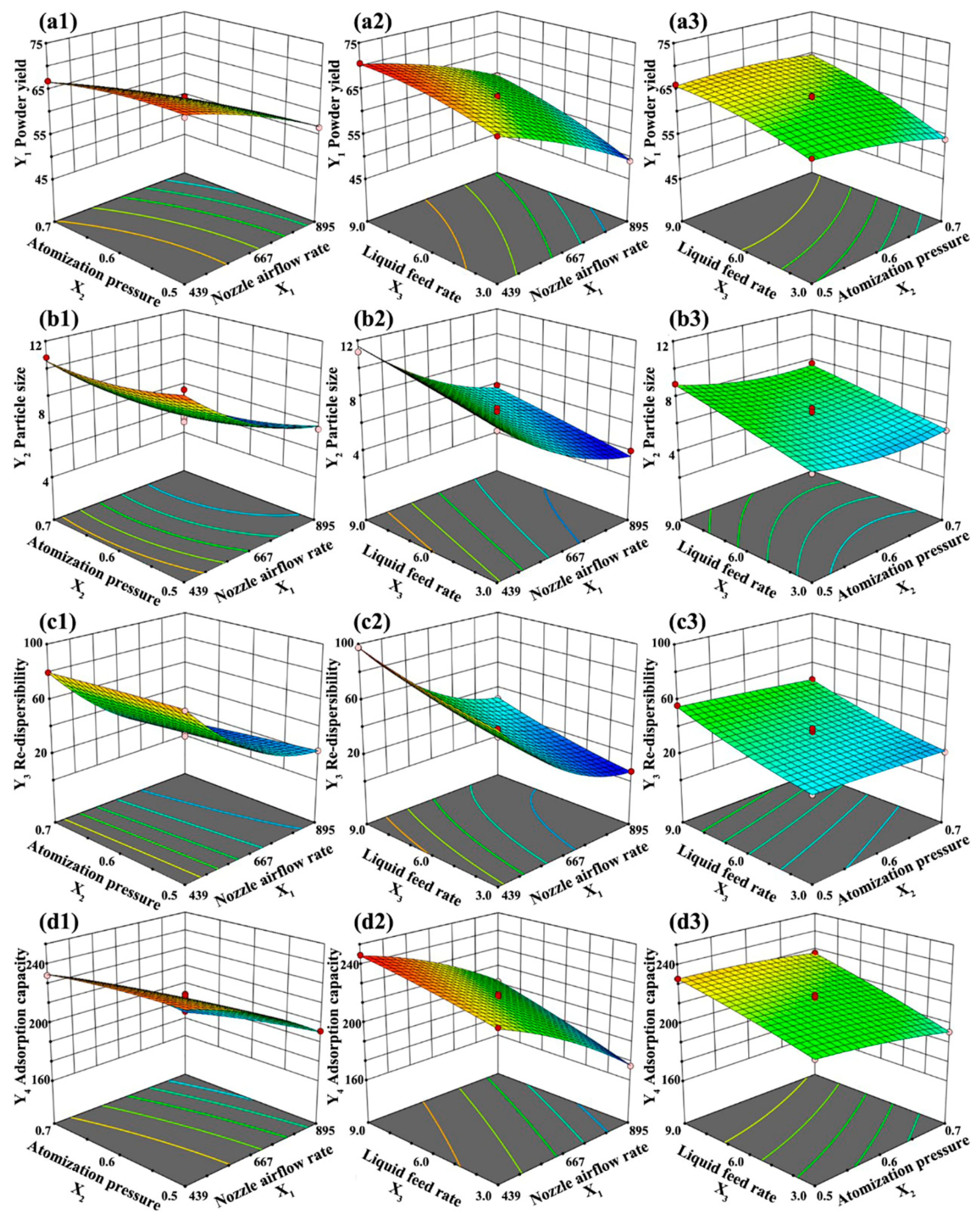
3.2.2. Response Surfaces and Contour Plots
3.2.3. Optimization of the Operating Parameters
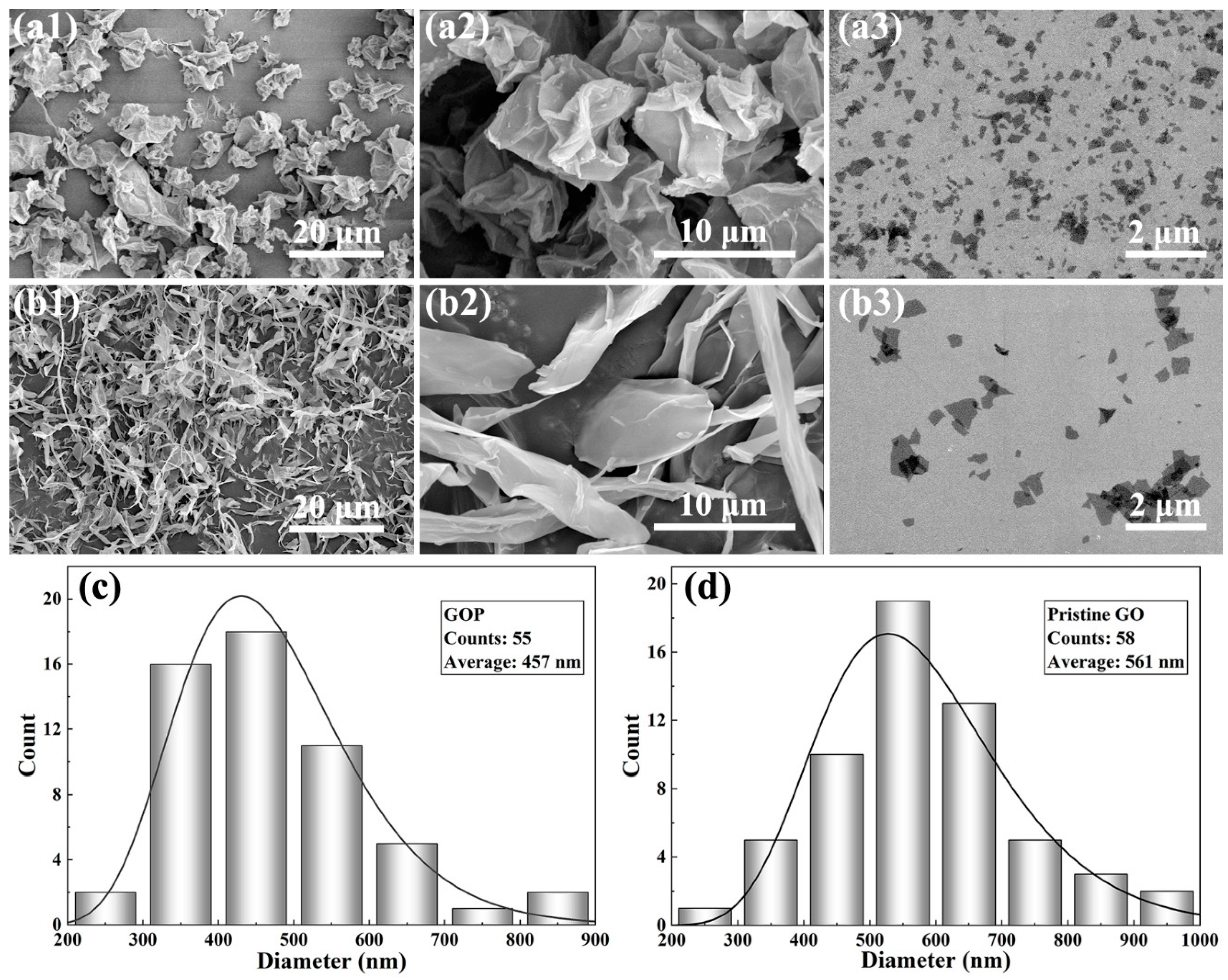
3.3. Analysis of the Optimized Spray-Dried GOP
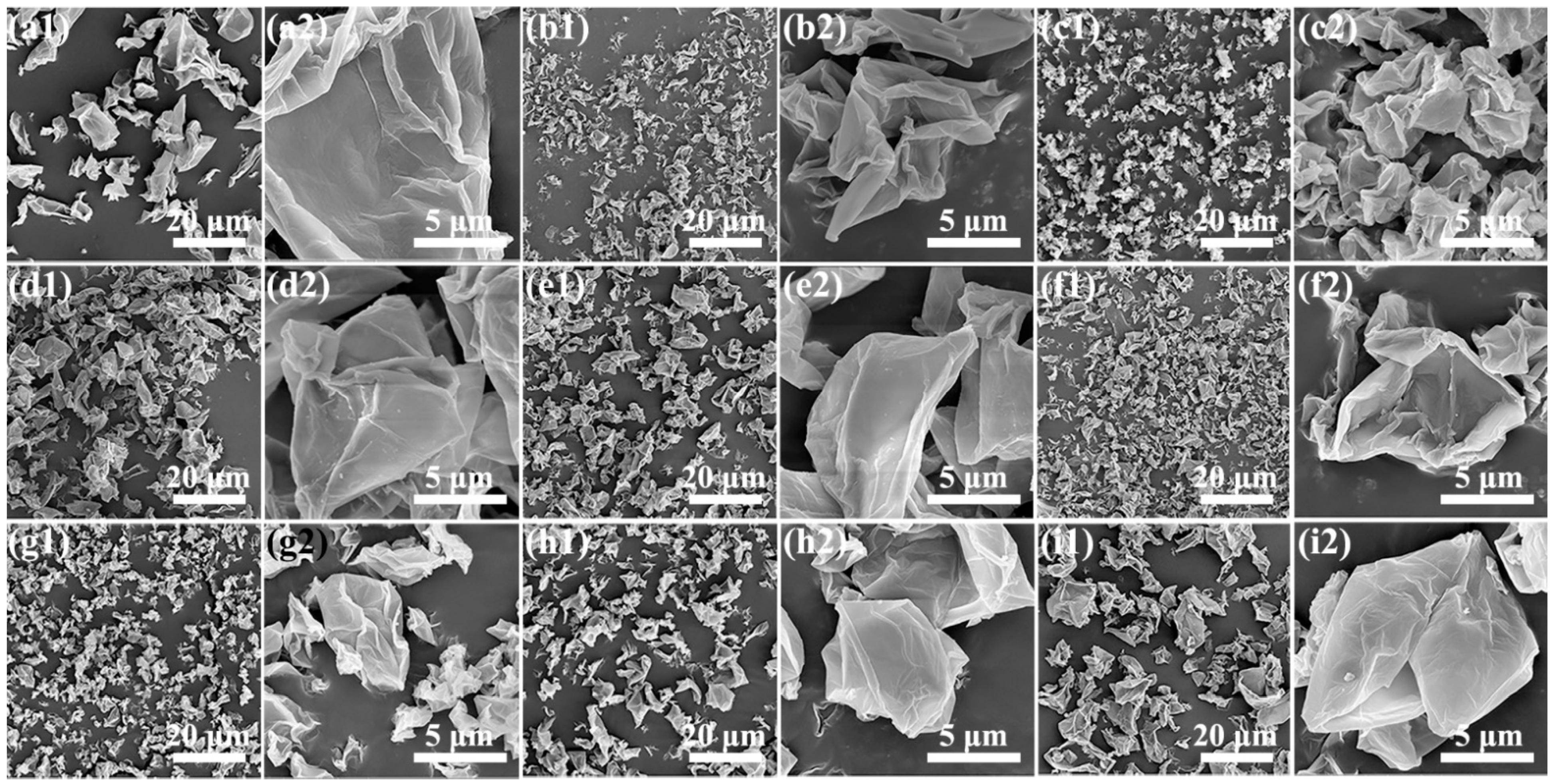
3.3.1. Morphology and Chemical Properties of GOP
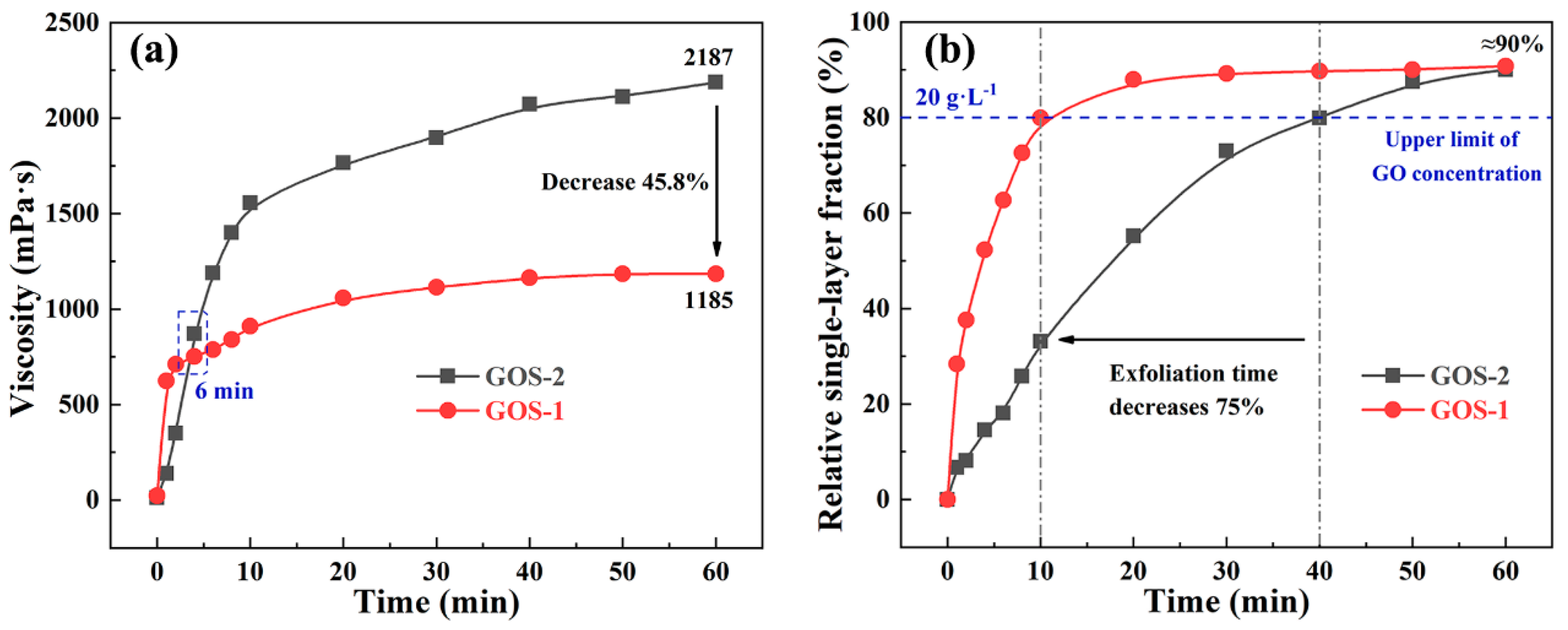
3.3.2. Re-Dispersity and Single-Layer Fraction of GOS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lin, L.; Peng, H.; Liu, Z. Synthesis Challenges for Graphene Industry. Nat. Mater. 2019, 18, 520–524. [Google Scholar] [CrossRef]
- Wang, B.; Ruan, T.; Chen, Y.; Jin, F.; Peng, L.; Zhou, Y.; Wang, D.; Dou, S. Graphene-Based Composites for Electrochemical Energy Storage. Energy Storage Mater. 2020, 24, 22–51. [Google Scholar] [CrossRef]
- Wu, G.; Tang, K.; Wu, D.; Li, Y.; Li, Y. Experimental Evaluation on the Catalytic Activity of a Novel CeZrK/rGO Nanocomposite for Soot Oxidation in Catalyzed Diesel Particulate Filter. Processes 2021, 9, 674. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, Z.; Baucom, J.; Wang, D.; Dai, L.; Chen, J.F. Nitrogen-Doped Graphene Foam as a Metal-Free Catalyst for Reduction Reactions under a High Gravity Field. Engineering 2020, 6, 680–687. [Google Scholar] [CrossRef]
- Ali, I.; Basheer, A.A.; Mbianda, X.Y.; Burakov, A.; Galunin, E.; Burakova, I.; Mkrtchyan, E.; Tkachev, A.; Grachev, V. Graphene Based Adsorbents for Remediation of Noxious Pollutants from Wastewater. Environ. Int. 2019, 127, 160–180. [Google Scholar] [CrossRef] [PubMed]
- Mohan, V.B.; Lau, K.-T.; Hui, D.; Bhattacharyya, D. Graphene-Based Materials and Their Composites: A Review on Production, Applications and Product Limitations. Compos. Part B Eng. 2018, 142, 200–220. [Google Scholar] [CrossRef]
- Wu, Y.; Zhu, J.; Huang, L. A Review of Three-Dimensional Graphene-Based Materials: Synthesis and Applications to Energy Conversion/Storage and Environment. Carbon N. Y. 2019, 143, 610–640. [Google Scholar] [CrossRef]
- Rajabathar, J.R.; Periyasamy, G.; Alanazi, A.M.; Govindasamy, M.; Arunachalam, P. Review on Carbon Nanotube Varieties for Healthcare Application: Effect of Preparation Methods and Mechanism Insight. Processes 2020, 8, 1654. [Google Scholar] [CrossRef]
- Li, C.; Guo, Y.; Shen, L.; Ji, C.; Bao, N. Scalable Concentration Process of Graphene Oxide Dispersions via Cross-Flow Membrane Filtration. Chem. Eng. Sci. 2019, 200, 127–137. [Google Scholar] [CrossRef]
- Dong, Z.; Jiang, C.; Cheng, H.; Zhao, Y.; Shi, G.; Jiang, L.; Qu, L. Facile Fabrication of Light, Flexible and Multifunctional Graphene Fibers. Adv. Mater. 2012, 24, 1856–1861. [Google Scholar] [CrossRef]
- Xiang, C.; Behabtu, N.; Liu, Y.; Chae, H.G.; Young, C.C.; Genorio, B.; Tsentalovich, D.E.; Zhang, C.; Kosynkin, D.V.; Lomeda, J.R.; et al. Graphene Nanoribbons as an Advanced Precursor for Making Carbon Fiber. ACS Nano 2013, 7, 1628–1637. [Google Scholar] [CrossRef] [PubMed]
- Kou, L.; Huang, T.; Zheng, B.; Han, Y.; Zhao, X.; Gopalsamy, K.; Sun, H.; Gao, C. Coaxial Wet-sSpun Yarn Supercapacitors for High-Energy Density and Safe Wearable Electronics. Nat. Commun. 2014, 5, 3754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parviz, D.; Metzler, S.D.; Das, S.; Irin, F.; Green, M.J. Tailored Crumpling and Unfolding of Spray-Dried Pristine Graphene and Graphene Oxide Sheets. Small 2015, 11, 2661–2668. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Jang, H.D.; Sun, T.; Xiao, L.; He, Z.; Katsoulidis, A.P.; Kanatzidis, M.G.; Gibson, J.M.; Huang, J. Compression and Aggregation-Resistant Particles of Crumpled Soft Sheets. ACS Nano 2011, 5, 8943–8949. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zachariah, M.R.; Zangmeister, C.D. Crumpled Nanopaper from Graphene Oxide. Nano Lett. 2012, 12, 486–489. [Google Scholar] [CrossRef]
- Shi, Y.; Li, C.; Shen, L.; Bao, N. Structure-Dependent Re-dispersibility of Graphene Oxide Powders Prepared by Fast Spray Drying. Chinese J. Chem. Eng. 2020, 32, 485–492. [Google Scholar] [CrossRef]
- Peng, H.; Wang, F.; Li, G.; Guo, J.; Li, B. Highly Efficient Recovery of Vanadium and Chromium: Optimized by Response Surface Methodology. ACS Omega 2019, 4, 904–910. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, F.; Xu, J.; Gu, C.; Zhou, K. Sensitive Carbon Monoxide Gas Sensor Based on Chemiluminescence on Nano-Au/Nd2O3-Ca3Nd2O6: Working Condition Optimization by Response Surface Methodology. ACS Omega 2020, 5, 20034–20041. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Xu, Q.; Hu, X.; Zhang, S.; Zhang, P.; You, Y. Optimization of Process Parameters of Rhamnolipid Treatment of Oily Sludge Based on Response Surface Methodology. ACS Omega 2020, 5, 29333–29341. [Google Scholar] [CrossRef]
- Thakur, M.; Pant, K.; Naik, R.R.; Nanda, V. Optimization of Spray Drying Operating Conditions for Production of Functional Milk Powder Encapsulating Bee Pollen. Dry. Technol. 2021, 39, 777–790. [Google Scholar] [CrossRef]
- Xiong, K.; Chen, Y.; Shen, S. Experimental Optimization and Mathematical Modeling of Supercritical Carbon Dioxide Extraction of Essential Oil from Pogostemon Cablin. Chin. J. Chem. Eng. 2019, 27, 2407–2417. [Google Scholar] [CrossRef]
- He, D.; Shen, L.; Zhang, X.; Wang, Y.; Bao, N.; Kung, H.H. An Efficient and Eco-Friendly Solution-Chemical Route for Preparation of Ultrastable Reduced Graphene Oxide Suspensions. AIChE J. 2014, 60, 2757–2764. [Google Scholar] [CrossRef]
- Shi, Y.; Li, C.; He, D.; Shen, L.; Bao, N. Preparation of Graphene Oxide–Cellulose Acetate Nanocomposite Membrane for High-Flux Desalination. J. Mater. Sci. 2017, 52, 13296–13306. [Google Scholar] [CrossRef]
- Iskandar, F.; Gradon, L.; Okuyama, K. Control of the Morphology of Nanostructured Particles Prepared by the Spray Drying of a Nanoparticle Sol. J. Colloid Interface Sci. 2003, 265, 296–303. [Google Scholar] [CrossRef]
- Poozesh, S.; Setiawan, N.; Akafuah, N.K.; Saito, K.; Marsac, P.J. Assessment of predictive models for characterizing the atomization process in a spray dryer’s bi-fluid nozzle. Chem. Eng. Sci. 2018, 180, 42–51. [Google Scholar] [CrossRef]
- Amaro, M.I.; Tajber, L.; Corrigan, O.I.; Healy, A.M. Optimisation of spray drying process conditions for sugar nanoporous microparticles (NPMPs) intended for inhalation. Int. J. Pharm. 2011, 421, 99–109. [Google Scholar] [CrossRef]
- Lisboa, H.M.; Duarte, M.E.; Cavalcanti-Mata, M.E. Modeling of food drying processes in industrial spray dryers. Food Bioprod. Process. 2018, 107, 49–60. [Google Scholar] [CrossRef]
- Yang, K.; Tan, F.; Wang, F.; Long, Y.; Wen, Y. Response surface optimization for process parameters of LiFePO 4/C preparation by carbothermal reduction technology. Chin. J. Chem. Eng. 2012, 20, 793–802. [Google Scholar] [CrossRef]
- LeClair, D.A.; Cranston, E.D.; Xing, Z.; Thompson, M.R. Optimization of Spray Drying Conditions for Yield, Particle Size and Biological Activity of Thermally Stable Viral Vectors. Pharm. Res. 2016, 33, 2763–2776. [Google Scholar] [CrossRef] [Green Version]
- Elversson, J.; Millqvist-Fureby, A. Particle Size and Density in Spray Drying-Effects of Carbohydrate Properties. J. Pharm. Sci. 2005, 94, 2049–2060. [Google Scholar] [CrossRef]
- Al-Asheh, S.; Jumah, R.; Banat, F.; Hammad, S. The Use of Experimental Factorial Design for Analysing the Effect of Spray Dryer Operating Variables on the Production of Tomato Powder. Food Bioprod. Process. Trans. Inst. Chem. Eng. Part C 2003, 81, 81–88. [Google Scholar] [CrossRef]
- Goula, A.M.; Adamopoulos, K.G. Spray Drying Performance of a Laboratory Spray Dryer for Tomato Powder Preparation. Dry. Technol. 2003, 21, 1273–1289. [Google Scholar] [CrossRef]
- Liu, T.; Li, Y.; Du, Q.; Sun, J.; Jiao, Y.; Yang, G.; Wang, Z.; Xia, Y.; Zhang, W.; Wang, K.; et al. Adsorption of Methylene Blue from Aqueous Solution by Graphene. Colloids Surf. B Biointerfaces 2012, 90, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Xu, Z.; Han, Y.; Sun, H.; Gao, C. Redissolution of Flower-Shaped Graphene Oxide Powder with High Density. ACS Appl. Mater. Interfaces 2016, 8, 8000–8007. [Google Scholar] [CrossRef] [PubMed]
- Saleem, H.; Haneef, M.; Abbasi, H.Y. Synthesis Route of Reduced Graphene Oxide via Thermal Reduction of Chemically Exfoliated Graphene Oxide. Mater. Chem. Phys. 2018, 204, 1–7. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Huang, L.; Jia, N.; Li, C.; Shi, G. Size Fractionation of Graphene Oxide Sheets via Filtration through Track-Etched Membranes. Adv. Mater. 2015, 27, 3654–3660. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Shi, Y.; Chen, X.; He, D.; Shen, L.; Bao, N. Controlled Synthesis of Graphite Oxide: Formation Process, Oxidation Kinetics, and Optimized Conditions. Chem. Eng. Sci. 2018, 176, 319–328. [Google Scholar] [CrossRef]

| Independent Variables | Levels of Coded Variables (xi) | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| X1: nozzle airflow rate/L·h−1 | 439 | 667 | 895 |
| X2: atomization pressure/MPa | 0.5 | 0.6 | 0.7 |
| X3: liquid feed rate/mL·min−1 | 3.0 | 6.0 | 9.0 |
| Test No. | x1 | x2 | x3 | Y1 (%) | Y2 (μm) | Y3 (%) | Y4 (mg·g−1) |
|---|---|---|---|---|---|---|---|
| 1 | 1 | 0 | 1 | 59.53 | 5.90 | 31.21 | 211.4 |
| 2 | 1 | 0 | −1 | 49.09 | 4.00 | 7.52 | 187.5 |
| 3 | 1 | 1 | 0 | 52.91 | 4.13 | 12.52 | 194.1 |
| 4 | 0 | −1 | 1 | 66.08 | 8.95 | 56.21 | 233.1 |
| 5 | 0 | 0 | 0 | 62.52 | 6.57 | 37.77 | 224.3 |
| 6 | 0 | 0 | 0 | 63.66 | 6.18 | 33.32 | 221.0 |
| 7 | 0 | 0 | 0 | 63.54 | 6.92 | 39.46 | 221.2 |
| 8 | 0 | 0 | 0 | 63.12 | 7.20 | 36.52 | 223.6 |
| 9 | 1 | −1 | 0 | 56.72 | 5.63 | 22.35 | 206.1 |
| 10 | 0 | 1 | −1 | 53.96 | 5.55 | 21.30 | 205.9 |
| 11 | 0 | 0 | 0 | 63.36 | 6.42 | 34.50 | 225.1 |
| 12 | 0 | −1 | −1 | 60.75 | 6.13 | 28.96 | 217.1 |
| 13 | 0 | 1 | 1 | 64.19 | 7.74 | 46.91 | 228.0 |
| 14 | −1 | 0 | −1 | 65.09 | 8.91 | 73.32 | 231.1 |
| 15 | −1 | 0 | 1 | 70.65 | 11.19 | 97.37 | 244.6 |
| 16 | −1 | −1 | 0 | 68.71 | 11.52 | 83.85 | 238.8 |
| 17 | −1 | 1 | 0 | 66.91 | 10.84 | 79.99 | 234.4 |
| Source | df | Y1 | Y2 | Y3 | Y4 | ||||
|---|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | F | p | ||
| Model | 9 | 189.53 | <0.0001 ** | 43.25 | <0.0001 ** | 277.84 | <0.0001 ** | 188.32 | <0.0001 ** |
| x11 | 1 | 1134.48 | <0.0001 ** | 307.25 | <0.0001 ** | 1982.81 | <0.0001 ** | 1261.93 | <0.0001 ** |
| x22 | 1 | 82.13 | <0.0001 ** | 9.32 | 0.0185 * | 27.36 | 0.0012 ** | 60.13 | 0.0001 ** |
| x33 | 1 | 400.61 | <0.0001 ** | 49.92 | 0.0002 ** | 294.73 | <0.0001 ** | 320.56 | <0.0001 ** |
| x1·x2 | 1 | 3.25 | - | 0.79 | - | 2.08 | - | 6.50 | 0.0382 * |
| x1·x3 | 1 | 19.16 | 0.0032 ** | 0.17 | - | 0.01 | - | 12.16 | 0.0102 * |
| x2·x3 | 1 | 19.31 | 0.0032 ** | 0.47 | - | 0.16 | - | 4.19 | - |
| x12 | 1 | 14.69 | 0.0064 ** | 15.76 | 0.0054 ** | 183.76 | <0.0001 ** | 23.64 | 0.0018 * |
| x22 | 1 | 10.64 | 0.0138 * | 4.63 | - | 0.10 | - | 2.54 | - |
| x32 | 1 | 16.65 | 0.0047 ** | 0.05 | - | 5.44 | - | 1.39 | - |
| Lack of fit | 1.36 | 0.2268 ns | 0.83 | 0.3076 ns | 0.3143 | 0.8156 ns | 1.95 | 0.9035 ns | |
| R2 | 0.996 | 0.982 | 0.997 | 0.996 | |||||
| C.V. (%) | 0.90 | 6.32 | 4.74 | 0.68 | |||||
| Responses | Predicted Value | Actual Value | Deviation |
|---|---|---|---|
| Y1 (%) | 70.45 | 71.96 ± 0.41 | +2.14% |
| Y2 (μm) | 11.65 | 11.06 ± 0.22 | −5.06% |
| Y3 (%) | 97.95 | 96.92 ± 0.79 | −1.05% |
| Y4 (mg/g) | 244.1 | 242.1 ± 0.9 | −0.89% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, X.; Shi, Y.; Shen, L.; Su, P.; Bao, N. Optimization of Spray-Drying Process with Response Surface Methodology (RSM) for Preparing High Quality Graphene Oxide Slurry. Processes 2021, 9, 1116. https://doi.org/10.3390/pr9071116
Ye X, Shi Y, Shen L, Su P, Bao N. Optimization of Spray-Drying Process with Response Surface Methodology (RSM) for Preparing High Quality Graphene Oxide Slurry. Processes. 2021; 9(7):1116. https://doi.org/10.3390/pr9071116
Chicago/Turabian StyleYe, Xingxing, Yexun Shi, Liming Shen, Peng Su, and Ningzhong Bao. 2021. "Optimization of Spray-Drying Process with Response Surface Methodology (RSM) for Preparing High Quality Graphene Oxide Slurry" Processes 9, no. 7: 1116. https://doi.org/10.3390/pr9071116
APA StyleYe, X., Shi, Y., Shen, L., Su, P., & Bao, N. (2021). Optimization of Spray-Drying Process with Response Surface Methodology (RSM) for Preparing High Quality Graphene Oxide Slurry. Processes, 9(7), 1116. https://doi.org/10.3390/pr9071116






