Abstract
An electron-donating−accepting (D−A) molecule, namely, 4-(1-(4-(9H-carbazol-9-yl)phenyl)-1H-1,2,3-triazol-4-yl)benzo[c][1,2,5]thiadiazole (BT-SCC) containing carbazole as the donor moiety and benzothiadiazole as the acceptor moiety is prepared. Single-crystal X-ray structure analysis elucidated the multiple intermolecular interactions, such as hydrogen bonds, CH…π, and π…π interplays. Interestingly, the aggregation-induced emission phenomenon is observed for BT-SCC featured with enhanced fluorescent quantum yield from diluted solution of CH2Cl2 (Φ = ca. 0.1) to CH2Cl2/hexane mixed solutions or solid states (Φ = ca. 0.8). Finally, aggregates of BT-SCC are obtained through precipitating from hot and saturated solutions or solvent-vapor methods and the aggregating morphologies could be easily controlled through different preparation methods. Fabulous cube-like micro-crystals and nanospherical structures are obtained, which is established by the synergistic effects of the multiple non-covalent interactions, endowing potential utility in the field of optoelectronic devices.
1. Introduction
Intramolecular charge-transfer (ICT) molecules, which are usually featured with an electron-donating (D) and an electron-accepting (A) moiety linked through π-conjugation [1], have been drawing considerable attention for their potential use in photoelectric devices, such as fluorescent sensors [2], dye-sensitized solar cells [3], organic light emitting diodes [4] and nonlinear optical materials [5,6]. The D or A units are the critical building blocks for ICT molecules. 2,1,3-benzothiadiazole is an typical electron-accepting building block with fused and π-extended structure. Due to its excellent optoelectronic properties, it has been widely applied to construct various organic semiconductors for different purposes, e.g., organic photovoltaic materials, organic light emission materials, organic field effect transistor materials, and covalent organic frameworks [7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22]. The successes of these benzothiadiazole-based materials are partially beneficial from the electron-accepting properties of benzothiadiazole and the resultant ICT effects. Therefore, synthesis of benzothiadiazole-based ICT molecules has been an interesting topic for a long time. In another aspect, copper(I)-catalyzed 1,3-dipolar cycloaddition of the terminal azides and alkynes, namely click reaction, is an efficient and feasible route toward triazole bridges. The afforded five-membered 1,2,3-triazole ring is a versatile and readily available linkage that has been applied to extend the conjugation of diverse aromatic systems [23,24]. Meanwhile, the triazole is a suitable functional group to form hydrogen bond for self-assembly [25]. In this work, a kind of ICT molecule, namely, 4-(1-(4-(9H-carbazol-9-yl)phenyl)-1H-1,2,3-triazol-4-yl)benzo[c][1,2,5]thiadiazole (BT-SCC) was synthesized, in which the electron-donating carbazole unit and the electron-accepting benzothiadiazole are linked with a triazole ring forming through click reaction to accomplish a D−A molecule with extended conjugation. BT-SCC could be easily driven to aggregate in highly uniform cube-like micro-crystals and spherical architectures with high morphological and chemical purity. Interestingly, this compound exhibits a significant aggregation-induced emission (AIE) effect. We expect that these constructed microstructures will be potentially useful in electronic and photonic devices.
2. Materials and Methods
2.1. Experimental Equipment and Theoretical Calculation
UV-vis spectra were performed on Hitachi U-3010 spectrophotometer (Chiyoda-ku, Tokyo, Japan). The fluorescence spectra were measured on Hitachi F-4500 spectrophotometer. Single crystal structures were characterized on ST-Saturn724+ spectrophotometer. SEM images were taken from Hitachi S-4800 microscopes at an accelerating voltage of 10 kV or 15 kV. Density functional theory (DFT) calculations were performed using the Gaussian 09 program [26] with the B3LYP exchange-correlation functional. All-electron triple-ξ valence basis sets with polarization functions (6-31G (d)) are used for all atoms [27,28]. Geometry optimizations were performed with full relaxation of all atoms.
2.2. Synthesis
The solution of compound 4-(trimethylsilylethynyl)benzo[c][1,2,5]thiadiazole (232 mg, 1.0 mmol) in 20 mL THF was added to 2 mL n-Bu4NF, (1 M soln. in THF) at 0 °C under N2 atmosphere. After 10 min, 9-(4-azidophenyl)-9H-carbazole (284 mg, 1.0 mmol), sodium ascorbate (40 mg, 0.2 mmol), and CuSO4 (16 mg, in 5 mL H2O) were added to the mixture. The mixture was stirred at room temperature (rt) under N2 atmosphere for 6 h. The solvent was concentrated under vacuum, and the residue was then diluted with CH2Cl2. The resultant solution was washed with brine, and dried over anhydrous MgSO4, filtered and concentrated under vacuum. The crude product was then purified by column chromatography on silica gel with CH2Cl2/PE (1:2) to afford BT-SCC as a solid (176 mg, 52%). 1H NMR (400 MHz, CDCl3): δ 7.34 (m, 2 H), 7.46 (m, 4 H), 7.78 (d, J = 7.5 Hz, 1 H), 7.81 (d, J = 8.4 Hz, 2 H), 8.05 (d, J = 8.8 Hz, 1 H).8.16 (d, J = 8.7 Hz, 4 H), 8.70 (d, J = 7.0 Hz, 1 H), 9.34 (s,1 H); 13C NMR (100 MHz, CDCl3): δ 154.5, 151.0, 143.1, 139.7, 137.3, 134.8, 129.1, 127.4, 125.3, 125.0, 122.8, 122.1, 121.0, 119.6, 119.5, 108.6; HRMS (EI, m/z): [M+] calcd. for C26H16N6S: 444.1157; found: 444.1163.
3. Results and Discussion
Starting compounds 1 and 2 were subjected to classic Sonogashira coupling reaction to afford compound 3 (Scheme 1). Then, target compound BT-SCC was obtained by copper (I) catalyzed 1,3-dipolar cycloaddition. High-quality bulk single crystals of BT-SCC were obtained by slow diffusion of n-hexane into a CH2Cl2 solution at rt. The crystal structure of BT-SCC was found to be triclinic with space group P-1 (a = 7.2734 Å, b = 12.313 Å, c = 14.076 Å, α = 68.5°, β = 76.3°, γ = 73.4°). The single crystal structures and packing diagrams of BT-SCC are shown in Figure 1. The phenyl group is twisted 47.7° from the plane of the carbazole unit, while the carbazole unit is nearly coplanar to 1,2,3-triazole ring (Figure 1a). As is well-known, the hydrogen bond is one of the most important interactions influencing the crystal architecture and supramolecular self-assembly. In BT-SCC, a number of intermolecular C8-H8…S1 (2.99 Å), C10-H10…S1 (2.95 Å), C14-H14…N4 (2.56 Å) interactions are found (Figure 1b). These are short intermolecular contacts with distances smaller than the sum of van der Waals radii. Meanwhile, the aromatic CH…π interaction was observed between benzothiadiazole moiety and neighboring hydrogen atoms of phenyl ring with the distances 2.87 Å. Furthermore, π…π stacking from the electron-rich carbazole ring to nearby 1,2,5-thiadiazole played a significant role for the formation of this head-to-tail crystal packing structure (Figure 1c).
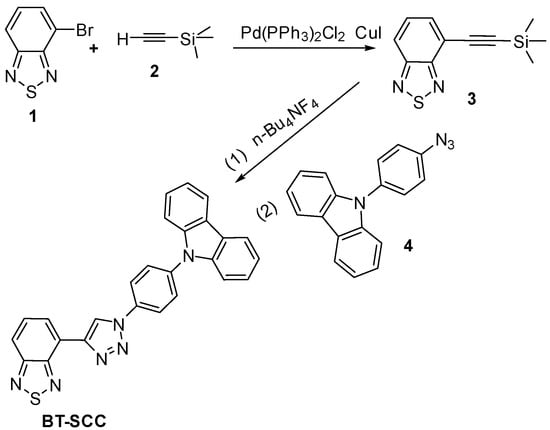
Scheme 1.
Synthetic route of BT-SCC.
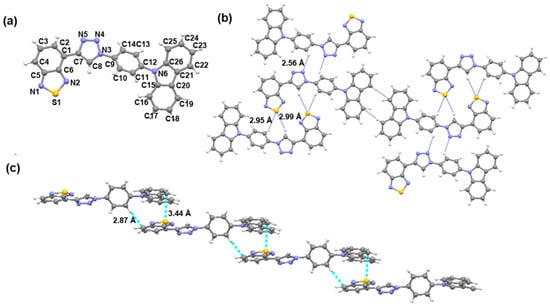
Figure 1.
Crystal structures of BT-SCC (a); hydrogen bonds contacts in adjacent molecular (b); and intermolecular C-H…π, π…π interaction (c).
The photophysical properties of BT-SCC in CH2Cl2 solution were shown in Figure 2. The absorption spectrum (λabs = ~326 nm) of BT-SCC is attributable to the π→π* transition of the benzothiadiazole unit [29] and a featured absorbance band at 360 nm, indicating the ICT from the carbazole moiety to the benzothiadiazole group [30]. It exhibited a weak fluorescent quantum yield (Φ) of ca. 0.10 (reference to quinine sulfate in 1 M H2SO4) with maxima emission at 546 nm. The observed large Stokes shift (Δλ = 220 nm) implies a possible conformational change upon photoexcitation. Due to the D−A structure of BT-SCC, emission from a photoexcited ICT can also account for this evident Stokes shift [11].
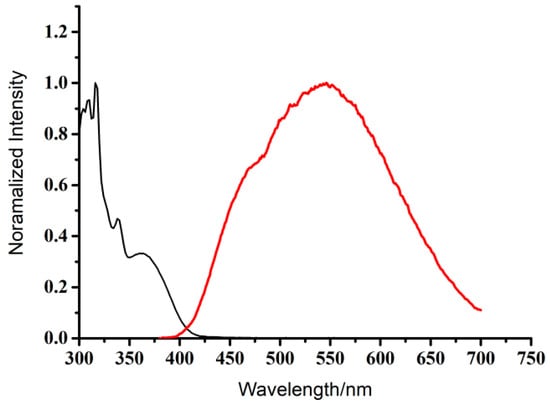
Figure 2.
Absorption spectra (left, black line) and fluorescence (right, red line) of BT-SCC in CH2Cl2 solution (2.0 × 10−5 mol L−1).
To further understand the absorption spectrum, a time-dependent density functional theory (TD-DFT) calculation was carried out to BT-SCC at the B3LYP/6-31G (d) level of theory. As shown in Figure 3, the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) of BT-SCC were separated. The molecular orbital density of the HOMO is mainly located on the carbazole subunit, whereas the LUMO level is primarily localized on the benzothiadiazole moiety, as shown in Figure S1, Supplementary Materials. The major absorption band in the solution peaked at 326 nm can be assigned to the HOMO to LUMO+1 with the highest oscillator strength of 0.288. The weak absorption in the solution at 350–400 nm can be assigned to HOMO−2 to LUMO with smaller oscillator strength of 0.171, indicating the good consistency between experimental and theoretical results.
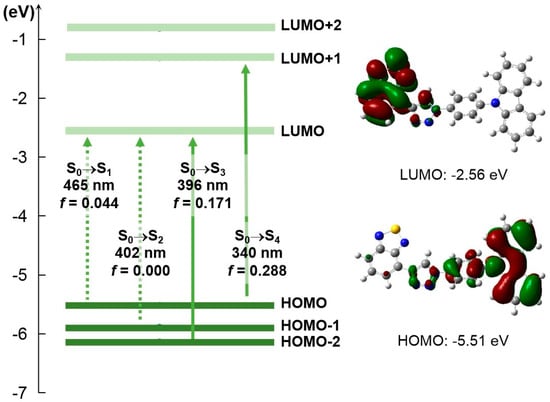
Figure 3.
Energy diagrams and frontier molecular orbitals of BT-SCC calculated at the B3LYP/6-31G(d) level of theory.
Similar to the ICT compounds previously reported [31], BT-SCC in the good solvents (CH2Cl2, CHCl3, and THF etc.) gave weak photoluminescence signal. However, the nanostructure suspensions in hexane or solid state were good emitters. Using a poor solvent of hexane and a good solvent of CH2Cl2 and based on the same operation parameters and the same concentration, we studied the relationship of the fluorescence emission intensity and the ratio of hexane/CH2Cl2 mixture to gain an insight into the fluorescence enhancement process (Figure 4a). Hexane was added to the BT-SCC/CH2Cl2 solution continually and the concentration (2.0 × 10−5 M) was kept unchanged. The photoluminescence intensity increased gradually by raising hexane content. At the same time, the emission peak shows a blue shift with the increase in the hexane, while their absorption spectra were fairly insensitive to the change of the fractions of mixture solution.
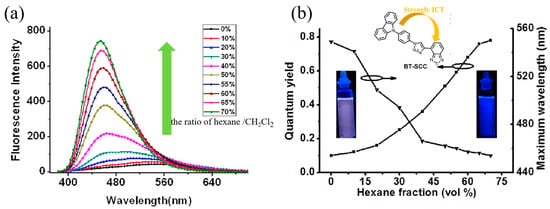
Figure 4.
Fluorescence spectra of BT-SCC suspended in different fractions of hexane with excitation wavelength of 360 nm (a); and the evolution of quantum yields and emission peaks depending on the hexane fractions (b). Insert is the solution pictures of BT-SCC exposed to UV light (254 nm).
The gradually enhanced fluorescence emission of BT-SCC/CH2Cl2 solution upon adding a poor solvent of hexane could be explained in terms of the intermolecular effect. BT-SCC consists of carbazole moiety as the donor and benzothiadiazole as the acceptor. In the good solution state, the carbazole moiety can rotate freely [32] and charge transfer may occur, leading to the low radiative relaxation processes [33]. In the poor solution state or solid state, their intramolecular rotations are greatly restricted by surrounding molecules. The excited species will be less deactivated by the intramolecular rotations [34]. Since the hexane solution is a poor solvent for BT-SCC, the molecules must have been aggregated when the content of hexane is high in the mixture solution. Thus, when the content of hexane increased, the molecules resided in a less polar environment, but their intramolecular rotations are physically restricted to lead to a blue shift color in emission (from light yellow to blue) and enhancement in emission intensity, which is a typical AIE system [35], as shown in Figure 4b.
Assemblies of well-defined supramolecules with different sizes, shapes, morphologies and functions are the results of cooperative intermolecular interactions, for example, hydrogen bond, π-π stacking, van der Waals contact, and dipole–dipole attraction [36]. Meanwhile, the self-assembly process is affected by many parameters, particularly the concentration and temperature [37]. The crystal growth inhibitors include surfactants and macromolecules, and they can also be added to alter the nanostructure morphology and crystal size [38]. In this work, well defined micro-crystals of BT-SCC were obtained by refluxing saturated solution of BT-SCC in THF and cooling at rt. The precipitates were observed to be “cubic” superstructure assemblies (Figure 5a,b). Beyond the solution processing methods, a solvent-vapor annealing technique was applied to construct different morphology on substrates. A drop of BT-SCC in THF/hexane (1:1) solution (4.0 × 10−3 M) was drop-casted on the Si substrate. After evaporation of the solution at rt, BT-SCC molecule tends to assemble into 0-D nanosphere (Figure 5c,d). By these simple methods, BT-SCC molecules were constructed into well-ordered and stable micro-sized superstructures without any additives, such as surfactant, catalyst, or template, indicating good self-assembly properties, which could be mainly attributed to the strong intermolecular interactions.
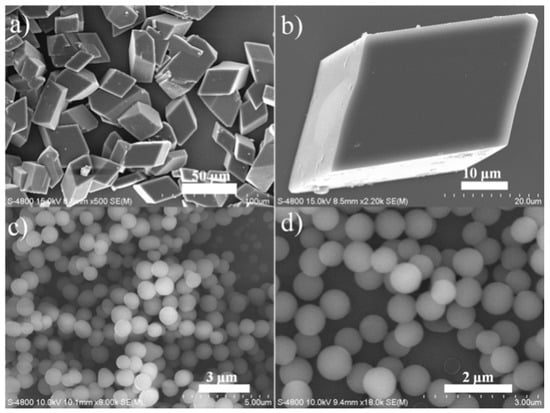
Figure 5.
Typical morphologies of BT-SCC self-assemblies. Cubic-like superstructure assemblies (a,b); and nanosphere (c,d).
4. Conclusions
An intramolecular charge transfer (ICT) molecule named BT-SCC was synthesized and utilized to tune molecular aggregate structures by self-assembly technology. The resulting controlling aggregate structures show uniform microcrystals and nanosphere. The single crystal structure was also studied, indicating multi-intermolecular interactions, such as hydrogen bond and π-π stacking. Another notable phenomenon is that this compound becomes a good emitter in solid or molecular aggregations, leading it to potential uses in optical devices.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/pr9071094/s1, Figure S1: Frontier molecular orbitals of BT-SCC calculated by B3LYP/6-31G (d) level of theory.
Author Contributions
Conceptualization, S.C. and J.H.; methodology, S.C.; validation, S.C., Y.L. and M.H.; formal analysis, Y.L. and M.H.; investigation, S.C., Y.L. and M.H.; data curation, S.C. and J.H.; writing—original draft preparation, S.C.; writing—review and editing, J.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Foundation of Longyan National Economic & Technological Development Zone and Scientific Starts Foundation of Longyan University (No. LB2018016).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li, Y.; Liu, T.; Liu, H.; Tian, M.-Z.; Li, Y. Self-assembly of intramolecular charge-transfer compounds into functional molecular systems. Acc. Chem. Res. 2014, 47, 1186–1198. [Google Scholar] [CrossRef]
- Dong, B.; Song, X.; Wang, C.; Kong, X.; Tang, Y.; Lin, W. Dual site-controlled and lysosome-targeted intramolecular charge transfer–photoinduced electron transfer–fluorescence resonance energy transfer fluorescent probe for monitoring pH changes in living cells. Anal. Chem. 2016, 88, 4085–4091. [Google Scholar] [CrossRef]
- Tian, H.; Yang, X.; Pan, J.; Chen, R.; Liu, M.; Zhang, Q.; Hagfeldt, A.; Sun, L. A triphenylamine dye model for the study of intramolecular energy transfer and charge transfer in dye-sensitized solar cells. Adv. Funct. Mater. 2008, 18, 3461–3468. [Google Scholar] [CrossRef]
- Yuan, W.Z.; Gong, Y.; Chen, S.; Shen, X.Y.; Lam, J.W.Y.; Lu, P.; Lu, Y.; Wang, Z.; Hu, R.; Xie, N.; et al. Efficient Solid Emitters with Aggregation-induced emission and intramolecular charge transfer characteristics: Molecular design, synthesis, photophysical behaviors, and OLED application. Chem. Mater. 2012, 24, 1518–1528. [Google Scholar] [CrossRef]
- Chen, S.; Li, Y.; Yang, W.; Chen, N.; Liu, H.; Li, Y. Synthesis and tuning optical nonlinear properties of molecular crystals of benzothiadiazole. J. Phys. Chem. C 2010, 114, 15109–15115. [Google Scholar] [CrossRef]
- Jiang, D.; Chen, S.; Xue, Z.; Li, Y.; Liu, H.; Yang, W.; Li, Y. Donor–acceptor molecules based on benzothiadiazole: Synthesis, X-ray crystal structures, linear and third-order nonlinear optical properties. Dye. Pigment. 2016, 125, 100–105. [Google Scholar] [CrossRef]
- Chen, S.; Qin, Z.; Liu, T.; Wu, X.; Li, Y.; Liu, H.; Song, Y.; Li, Y. Aggregation-induced emission on benzothiadiazole dyads with large third-order optical nonlinearity. Phys. Chem. Chem. Phys. 2013, 15, 12660–12666. [Google Scholar] [CrossRef]
- Chen, W.; Yang, Z.; Xie, Z.; Li, Y.; Yu, X.; Lu, F.; Chen, L. Benzothiadiazole functionalized D–A type covalent organic frameworks for effective photocatalytic reduction of aqueous chromium(vi). J. Mater. Chem. A 2019, 7, 998–1004. [Google Scholar] [CrossRef]
- Dogru, M.; Sonnauer, A.; Zimdars, S.; Döblinger, M.; Knochel, P.; Bein, T. Facile synthesis of a mesoporous benzothiadiazole-COF based on a transesterification process. CrystEngComm 2013, 15, 1500–1502. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; An, C.; Pisula, W.; Müllen, K. Cyclopentadithiophene–benzothiadiazole donor–acceptor polymers as prototypical semiconductors for high-performance field-effect transistors. Acc. Chem. Res. 2018, 51, 1196–1205. [Google Scholar] [CrossRef] [Green Version]
- Omer, K.M.; Ku, S.-Y.; Cheng, J.-Z.; Chou, S.-H.; Wong, K.-T.; Bard, A.J. Electrochemistry and electrogenerated chemiluminescence of a spirobifluorene-based donor (triphenylamine)−acceptor (2,1,3-Benzothiadiazole) molecule and its organic nanoparticles. J. Am. Chem. Soc. 2011, 133, 5492–5499. [Google Scholar] [CrossRef]
- Pati, P.B.; Senanayak, S.P.; Narayan, K.S.; Zade, S.S. Solution processable benzooxadiazole and benzothiadiazole based D-A-D molecules with chalcogenophene: Field effect transistor study and structure property relationship. ACS Appl. Mater. Interfaces 2013, 5, 12460–12468. [Google Scholar] [CrossRef]
- Peng, Z.; Zhang, K.; Huang, Z.; Wang, Z.; Duttwyler, S.; Wang, Y.; Lu, P. Emissions from a triphenylamine–benzothiadiazole–monocarbaborane triad and its applications as a fluorescent chemosensor and a white OLED component. J. Mater. Chem. C 2019, 7, 2430–2435. [Google Scholar] [CrossRef]
- Shang, H.; Fan, H.; Liu, Y.; Hu, W.; Li, Y.; Zhan, X. A Solution-processable star-shaped molecule for high-performance organic solar cells. Adv. Mater. 2011, 23, 1554–1557. [Google Scholar] [CrossRef]
- Wang, G.-B.; Li, S.; Yan, C.-X.; Lin, Q.-Q.; Zhu, F.-C.; Geng, Y.; Dong, Y.-B. A benzothiadiazole-based covalent organic framework for highly efficient visible-light driven hydrogen evolution. Chem. Commun. 2020, 56, 12612–12615. [Google Scholar] [CrossRef]
- Wang, J.-L.; Xiao, Q.; Pei, J. Benzothiadiazole-based D−π-A−π-D organic dyes with tunable band gap: Synthesis and photophysical properties. Org. Lett. 2010, 12, 4164–4167. [Google Scholar] [CrossRef]
- Wang, Z.; Peng, Z.; Huang, K.; Lu, P.; Wang, Y. Butterfly-shaped π-extended benzothiadiazoles as promising emitting materials for white OLEDs. J. Mater. Chem. C 2019, 7, 6706–6713. [Google Scholar] [CrossRef]
- Yuan, J.; Zhang, Y.; Zhou, L.; Zhang, C.; Lau, T.-K.; Zhang, G.; Lu, X.; Yip, H.-L.; So, S.K.; Beaupre, S.; et al. Fused benzothiadiazole: A building block for n-type organic acceptor to achieve high-performance organic solar cells. Adv. Mater. 2019, 31, 1807577. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Yang, T.; Xu, H.; Miao, Y.; Chen, R.; Shinar, R.; Shinar, J.; Wang, H.; Xu, B.; Yu, J. Triphenylamine/benzothiadiazole-based compounds for non-doped orange and red fluorescent OLEDs with high efficiencies and low efficiency roll-off. J. Mater. Chem. C 2021, 9, 4921–4926. [Google Scholar] [CrossRef]
- Zhang, Y.; Kim, C.; Lin, J.; Nguyen, T.-Q. Solution-processed ambipolar field-effect transistor based on diketopyrrolopyrrole functionalized with benzothiadiazole. Adv. Funct. Mater. 2012, 22, 97–105. [Google Scholar] [CrossRef]
- Zhou, H.; Yang, L.; Stuart, A.C.; Price, S.C.; Liu, S.; You, W. Development of fluorinated benzothiadiazole as a structural unit for a polymer solar cell of 7% Efficiency. Angew. Chem. Int. Ed. 2011, 50, 2995–2998. [Google Scholar] [CrossRef]
- Zhou, P.; Zhang, Z.-G.; Li, Y.; Chen, X.; Qin, J. Thiophene-fused benzothiadiazole: A strong electron-acceptor unit to build D–A copolymer for highly efficient polymer solar cells. Chem. Mater. 2014, 26, 3495–3501. [Google Scholar] [CrossRef]
- Dheer, D.; Singh, V.; Shankar, R. Medicinal attributes of 1,2,3-triazoles: Current developments. Bioorg. Chem. 2017, 71, 30–54. [Google Scholar] [CrossRef]
- Li, Y.; Flood, A.H. Pure C-H Hydrogen bonding to chloride ions: A preorganized and rigid macrocyclic receptor. Angew. Chem. Int. Ed. 2008, 47, 2649–2652. [Google Scholar] [CrossRef]
- Debnath, S.; Yang, J.; Ortoleva, P.; Raghavachari, K. Understanding the origin of 2D self-assembly of tricarbazole macrocycles: An integrated quantum mechanical/molecular dynamics study. J. Phys. Chem. C 2019, 123, 17616–17623. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian, Version 16, Revision A.03; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [Green Version]
- Anant, P.; Lucas, N.T.; Jacob, J. A simple route toward the synthesis of bisbenzothiadiazole derivatives. Org. Lett. 2008, 10, 5533–5536. [Google Scholar] [CrossRef]
- Liu, W.; Zheng, C.-J.; Wang, K.; Chen, Z.; Chen, D.-Y.; Li, F.; Ou, X.-M.; Dong, Y.-P.; Zhang, X.-H. Novel carbazol-pyridine-carbonitrile derivative as excellent blue thermally activated delayed fluorescence emitter for highly efficient organic light-emitting devices. ACS Appl. Mater. Interfaces 2015, 7, 18930–18936. [Google Scholar] [CrossRef]
- Gu, J.; Li, X.; Zhou, Z.; Liao, R.; Gao, J.; Tang, Y.; Wang, Q. Synergistic regulation of effective detection for hypochlorite based on a dual-mode probe by employing aggregation induced emission (AIE) and intramolecular charge transfer (ICT) effects. Chem. Eng. J. 2019, 368, 157–164. [Google Scholar] [CrossRef]
- Wiethaus, G.; Toldo, J.M.; da Silveira Santos, F.; da Costa Duarte, R.; Gonçalves, P.F.B.; Rodembusch, F.S. Experimental and theoretical investigation of long-wavelength fluorescence emission in push–pull benzazoles: Intramolecular proton transfer or charge transfer in the excited state? Phys. Chem. Chem. Phys. 2019, 21, 4408–4420. [Google Scholar] [CrossRef]
- Lee, K.J.; Lee, Y.U.; Fages, F.; Ribierre, J.-C.; Wu, J.W.; D’Aléo, A. Blue-shifting intramolecular charge transfer emission by nonlocal effect of hyperbolic metamaterials. Nano. Lett. 2018, 18, 1476–1482. [Google Scholar] [CrossRef]
- Ravindran, E.; Somanathan, N. Efficient white-light emission from a single polymer system with “spring-like” self-assemblies induced emission enhancement and intramolecular charge transfer characteristics. J. Mater. Chem. C 2017, 5, 4763–4774. [Google Scholar] [CrossRef]
- Sun, X.; Wang, Y.; Lei, Y. Fluorescence based explosive detection: From mechanisms to sensory materials. Chem. Soc. Rev. 2015, 44, 8019–8061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Xu, J.; Li, Y.; Li, Y. Aggregate nanostructures of organic, molecular materials. Acc. Chem. Res. 2010, 43, 1496–1508. [Google Scholar] [CrossRef]
- Walther, A.; Müller, A.H.E. Janus Particles: Synthesis, self-assembly, physical properties, and applications. Chem. Rev. 2013, 113, 5194–5261. [Google Scholar] [CrossRef]
- Mai, Y.; Eisenberg, A. Self-assembly of block copolymers. Chem. Soc. Rev. 2012, 41, 5969–5985. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).