Rapid Formation of Stem Cell Spheroids Using Two-Dimensional MXene Particles
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Preparation
2.2. MXene-Induced Stem Cell-Spheroid Formation and Evaluation
2.3. Testing for Cell Viability
2.4. Osteogenic Differentiation
2.5. Statistical Analysis
3. Results and Discussion
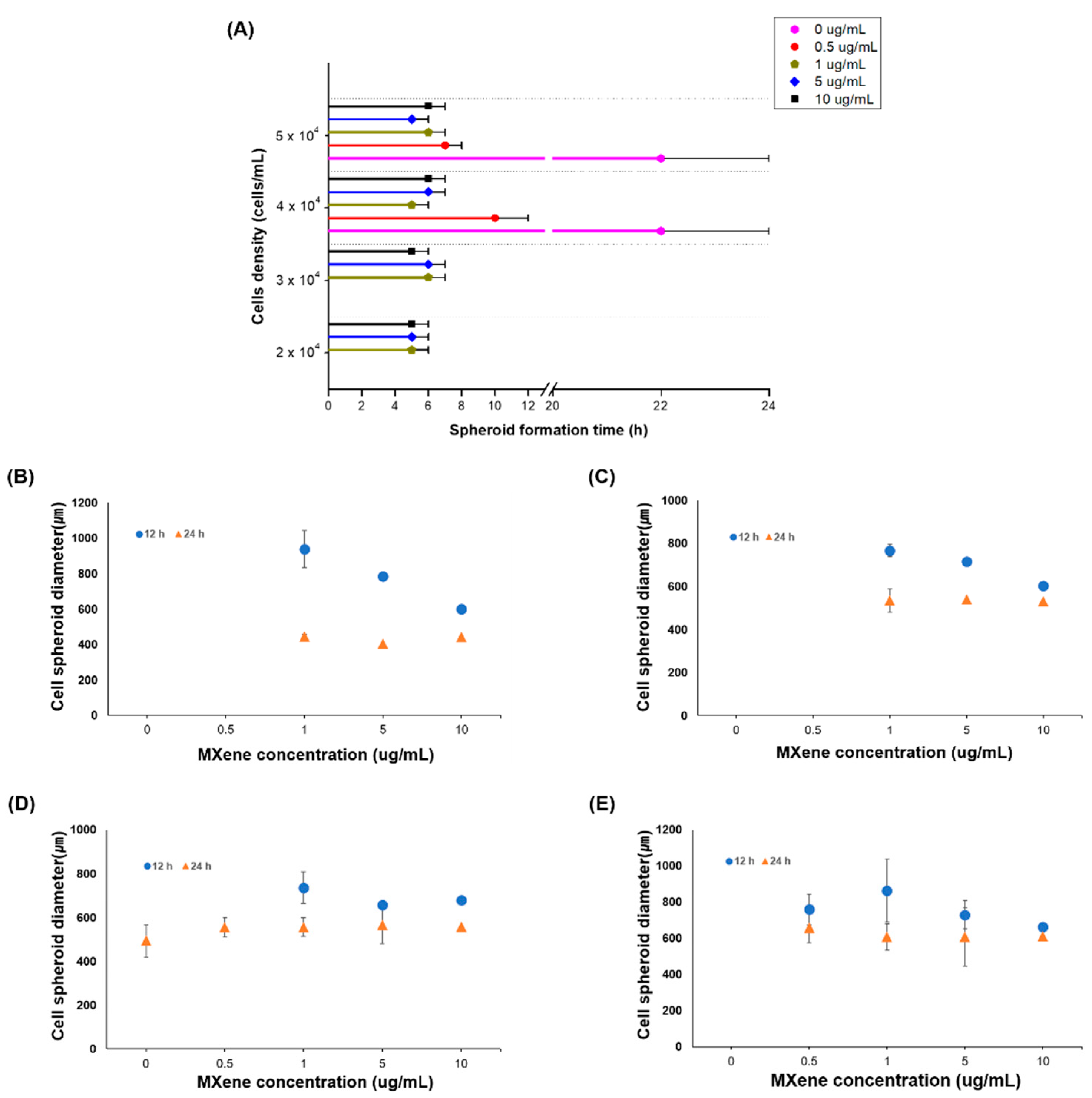
3.1. Cell Spheroids Induced by MXene Adhesive
3.2. Cell Spheroids Formation Time
3.3. Cell Spheroid Compaction
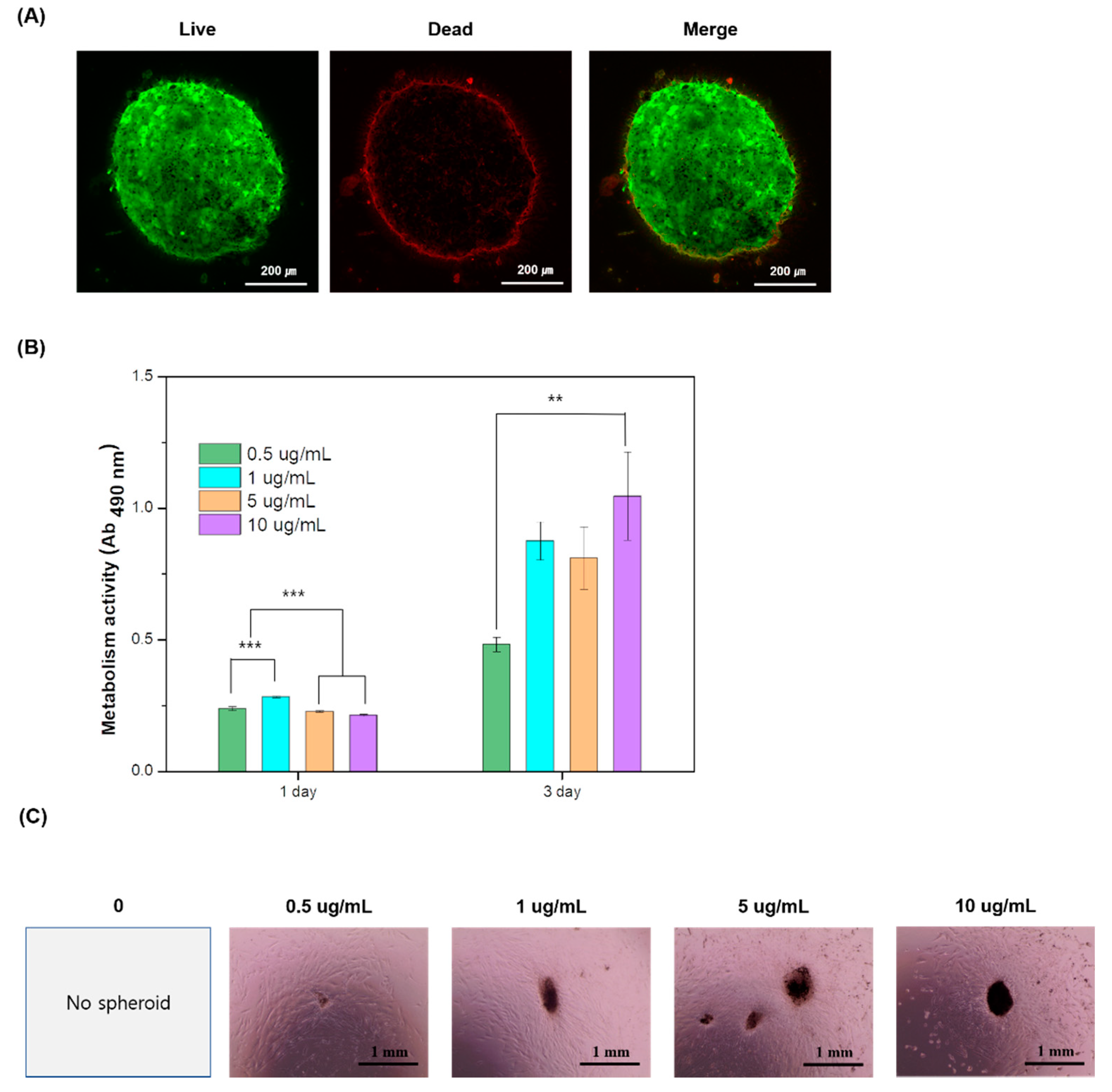
3.4. Cytotoxicity and Proliferation
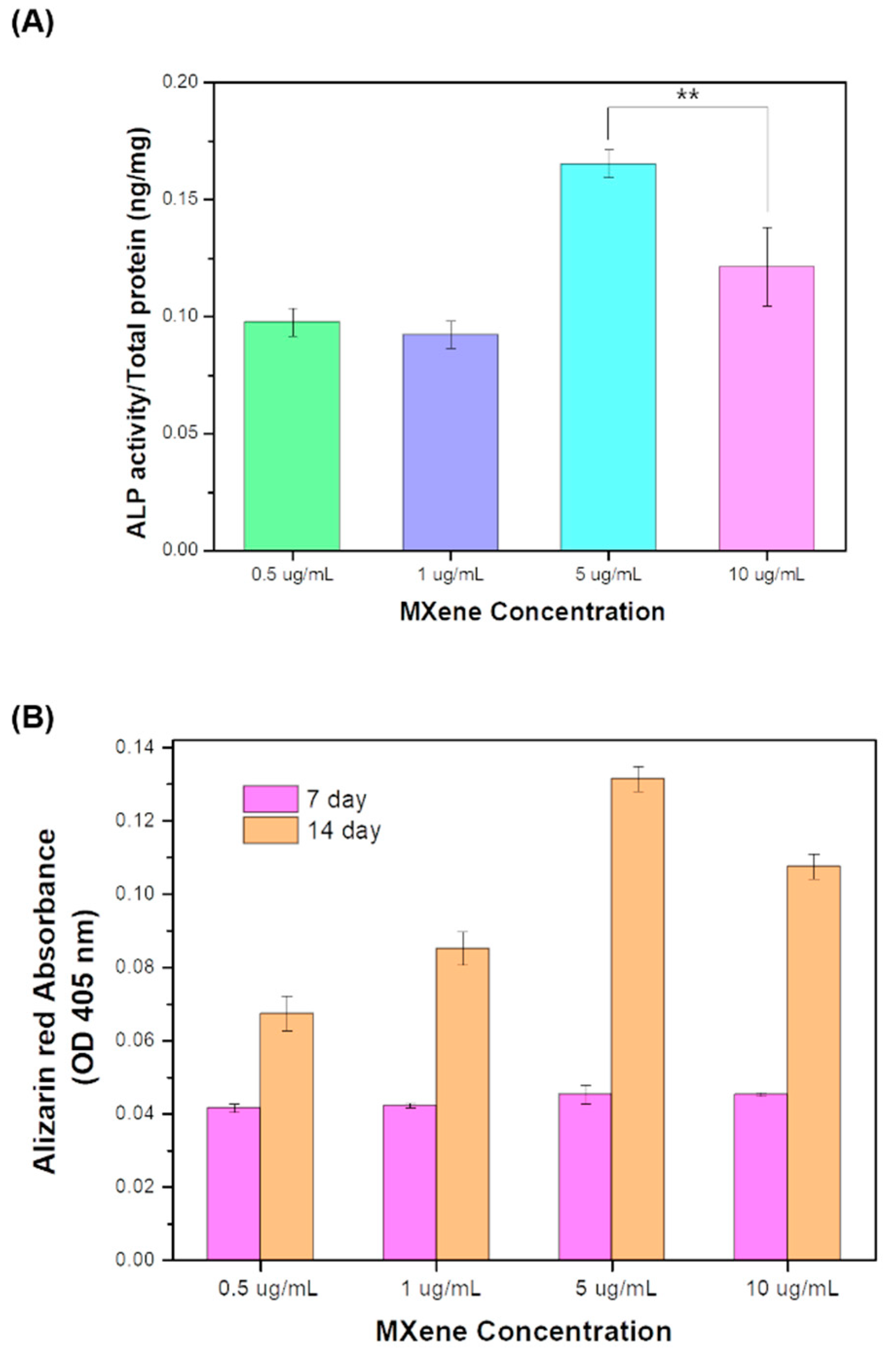
3.5. Osteogenic Differentiation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Lee, Y.B.; Kim, E.M.; Byun, H.; Chang, H.-K.; Jeong, K.; Aman, Z.M.; Choi, Y.S.; Park, J.; Shin, H. Engineering spheroids potentiating cell-cell and cell-ECM interactions by self-assembly of stem cell microlayer. Biomaterials 2018, 165, 105–120. [Google Scholar] [CrossRef] [PubMed]
- Rezende, R.A.; Pereira, F.D.A.S.; Kasyanov, V.; Kemmoku, D.T.; Maia, I.; da Silva, J.V.L.; Mironov, V. Scalable Biofabrication of Tissue Spheroids for Organ Printing. Procedia CIRP 2013, 5, 276–281. [Google Scholar] [CrossRef][Green Version]
- Petrenko, Y.; Syková, E.; Kubinová, Š. The therapeutic potential of three-dimensional multipotent mesenchymal stromal cell spheroids. Stem Cell Res. Ther. 2017, 8, 94. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, T.; Kundu, B.; Silva-Correia, J.; Kundu, S.C.; Oliveira, J.M.; Reis, R.L.; Correlo, V.M. Emerging tumor spheroids technologies for 3D in vitro cancer modeling. Pharmacol. Ther. 2018, 184, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Zanoni, M.; Piccinini, F.; Arienti, C.; Zamagni, A.; Santi, S.; Polico, R.; Bevilacqua, A.; Tesei, A. 3D tumor spheroid models for in vitro therapeutic screening: A systematic approach to enhance the biological relevance of data obtained. Sci. Rep. 2016, 6, 19103. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.-W.; Gao, J.-Q. Application of 3D cultured multicellular spheroid tumor models in tumor-targeted drug delivery system research. J. Control. Release 2018, 270, 246–259. [Google Scholar] [CrossRef] [PubMed]
- Nath, S.; Devi, G.R. Three-dimensional culture systems in cancer research: Focus on tumor spheroid model. Pharmacol. Ther. 2016, 163, 94–108. [Google Scholar] [CrossRef]
- Ryu, N.-E.; Lee, S.-H.; Park, H. Spheroid Culture System Methods and Applications for Mesenchymal Stem Cells. Cells 2019, 8, 1620. [Google Scholar] [CrossRef]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar] [CrossRef]
- Ge, Z.; Zhao, J.; Yu, H.; Yang, W.; Zhou, P.; Wang, Z.; Liu, L. Biomimetic construction of peritoneum to imitate peritoneal metastasis using digital micromirror device-based optical projection lithography. Lab Chip 2020, 20, 3109–3119. [Google Scholar] [CrossRef]
- Yang, W.; Yu, H.; Li, G.; Wei, F.; Wang, Y.; Liu, L. Mask-free fabrication of a versatile microwell chip for multidimensional cellular analysis and drug screening. Lab Chip 2017, 17, 4243–4252. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.R.; Li, Y.-C.; Jang, H.L.; Khoshakhlagh, P.; Akbari, M.; Nasajpour, A.; Zhang, Y.S.; Tamayol, A.; Khademhosseini, A. Graphene-based materials for tissue engineering. Adv. Drug Deliv. Rev. 2016, 105, 255–274. [Google Scholar] [CrossRef]
- Lou, Y.-R.; Kanninen, L.; Kaehr, B.; Townson, J.L.; Niklander, J.; Harjumäki, R.; Jeffrey Brinker, C.; Yliperttula, M. Silica bioreplication preserves three-dimensional spheroid structures of human pluripotent stem cells and HepG2 cells. Sci. Rep. 2015, 5, 13635. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Deng, D.; Sato, Y.; Hou, Y.T.; Watanabe, R.; Sasaki, K.; Kawabe, M.; Hirano, E.; Morinaga, T. Silicate fiber-based 3D cell culture system for anticancer drug screening. Anticancer Res. 2013, 33, 5301–5309. [Google Scholar] [PubMed]
- Kim, C.-H.; Suhito, I.R.; Angeline, N.; Han, Y.; Son, H.; Luo, Z.; Kim, T.-H. Vertically Coated Graphene Oxide Micro-Well Arrays for Highly Efficient Cancer Spheroid Formation and Drug Screening. Adv. Healthc. Mater. 2020, 9, 1901751. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.-H.; Kim, T.-H. Graphene Hybrid Materials for Controlling Cellular Microenvironments. Materials 2020, 13, 4008. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Nanocrystals Produced by Exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef]
- Huang, Z.; Cui, X.; Li, S.; Wei, J.; Li, P.; Wang, Y.; Lee, C.-S. Two-dimensional MXene-based materials for photothermal therapy. Nanophotonics 2020, 9, 2233–2249. [Google Scholar] [CrossRef]
- Yang, Q.; Yin, H.; Xu, T.; Zhu, D.; Yin, J.; Chen, Y.; Yu, X.; Gao, J.; Zhang, C.; Chen, Y.; et al. Engineering 2D Mesoporous Silica@MXene-Integrated 3D-Printing Scaffolds for Combinatory Osteosarcoma Therapy and NO-Augmented Bone Regeneration. Small 2020, 16, 1906814. [Google Scholar] [CrossRef]
- Rasool, K.; Helal, M.; Ali, A.; Ren, C.E.; Gogotsi, Y.; Mahmoud, K.A. Antibacterial Activity of Ti3C2Tx MXene. ACS Nano 2016, 10, 3674–3684. [Google Scholar] [CrossRef]
- George, S.M.; Kandasubramanian, B. Advancements in MXene-Polymer composites for various biomedical applications. Ceram. Int. 2020, 46, 8522–8535. [Google Scholar] [CrossRef]
- Cesarz, Z.; Tamama, K. Spheroid Culture of Mesenchymal Stem Cells. Stem Cells Int. 2016, 2016, 9176357. [Google Scholar] [CrossRef] [PubMed]
- Gregory, C.A.; Grady Gunn, W.; Peister, A.; Prockop, D.J. An Alizarin red-based assay of mineralization by adherent cells in culture: Comparison with cetylpyridinium chloride extraction. Anal. Biochem. 2004, 329, 77–84. [Google Scholar] [CrossRef]
- Conacci-Sorrell, M.; Simcha, I.; Ben-Yedidia, T.; Blechman, J.; Savagner, P.; Ben-Ze’ev, A. Autoregulation of E-cadherin expression by cadherin-cadherin interactions: The roles of beta-catenin signaling, Slug, and mapk. J. Cell Biol. 2003, 163, 847–857. [Google Scholar] [CrossRef] [PubMed]
- Kubota, S.; Johkura, K.; Asanuma, K.; Okouchi, Y.; Ogiwara, N.; Sasaki, K.; Kasuga, T. Titanium oxide nanotubes for bone regeneration. J. Mater. Sci. Mater. Med. 2004, 15, 1031–1035. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, J.; Lee, E.-J. Rapid Formation of Stem Cell Spheroids Using Two-Dimensional MXene Particles. Processes 2021, 9, 957. https://doi.org/10.3390/pr9060957
Jang J, Lee E-J. Rapid Formation of Stem Cell Spheroids Using Two-Dimensional MXene Particles. Processes. 2021; 9(6):957. https://doi.org/10.3390/pr9060957
Chicago/Turabian StyleJang, JunHwee, and Eun-Jung Lee. 2021. "Rapid Formation of Stem Cell Spheroids Using Two-Dimensional MXene Particles" Processes 9, no. 6: 957. https://doi.org/10.3390/pr9060957
APA StyleJang, J., & Lee, E.-J. (2021). Rapid Formation of Stem Cell Spheroids Using Two-Dimensional MXene Particles. Processes, 9(6), 957. https://doi.org/10.3390/pr9060957





