Degradation of Landfill Leachate Using UV-TiO2 Photocatalysis Combination with Aged Waste Reactors
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. TiO2 Nanoparticle Synthesis
2.3. Characterization of TiO2 Nanoparticles
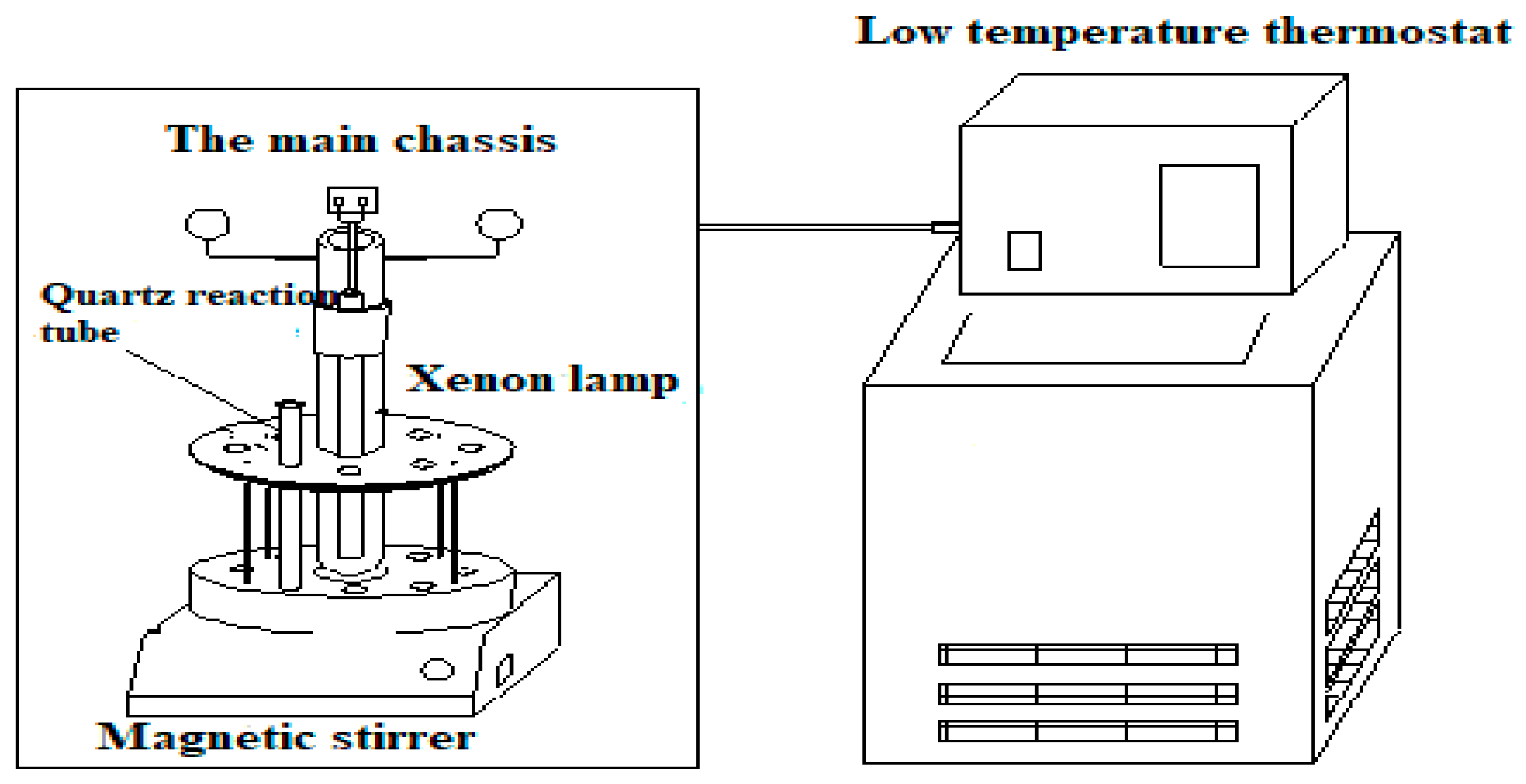
2.4. Photocatalytic Experiments
2.5. Cooperative Degradation Experiments
3. Results and Discussion
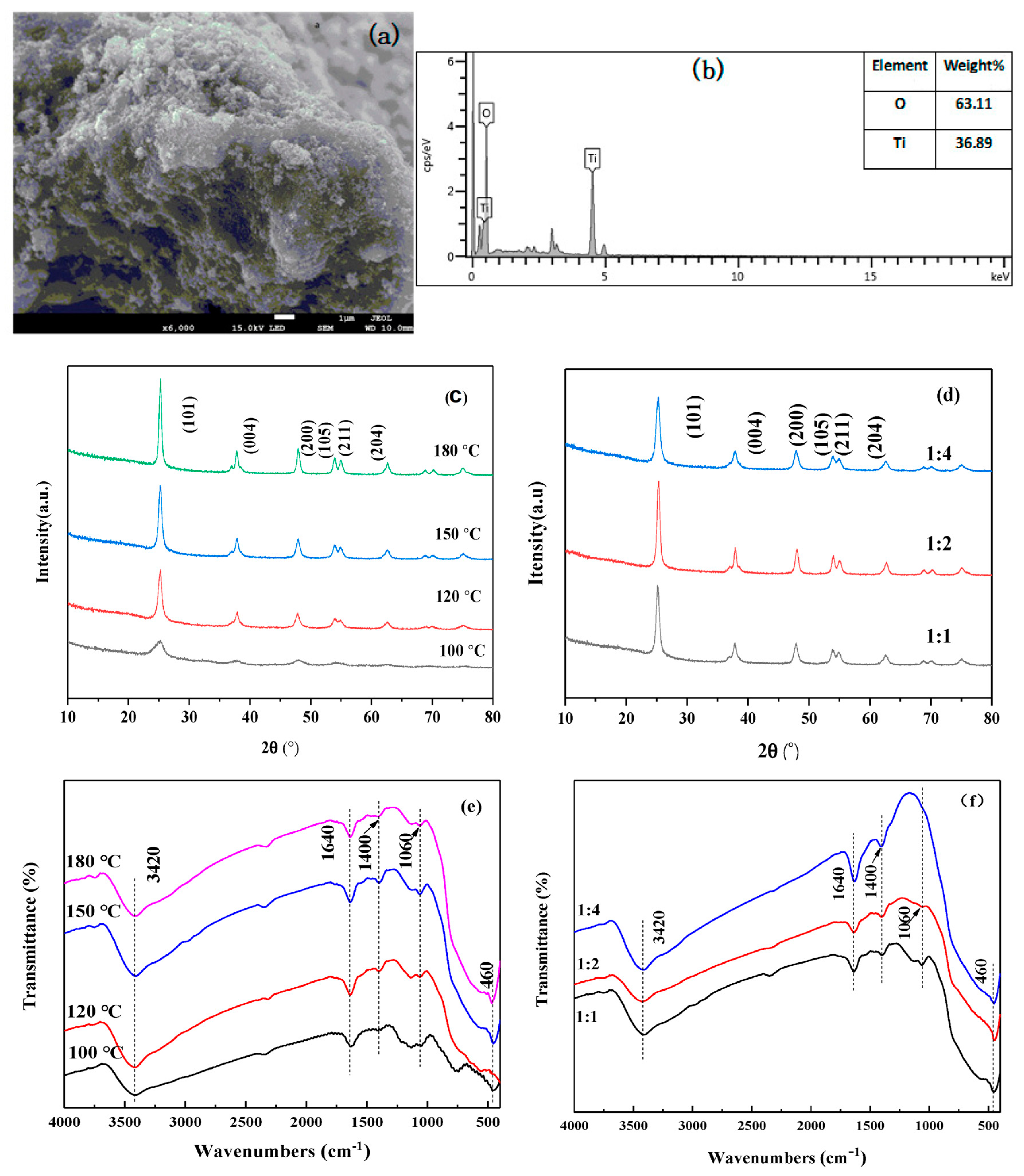
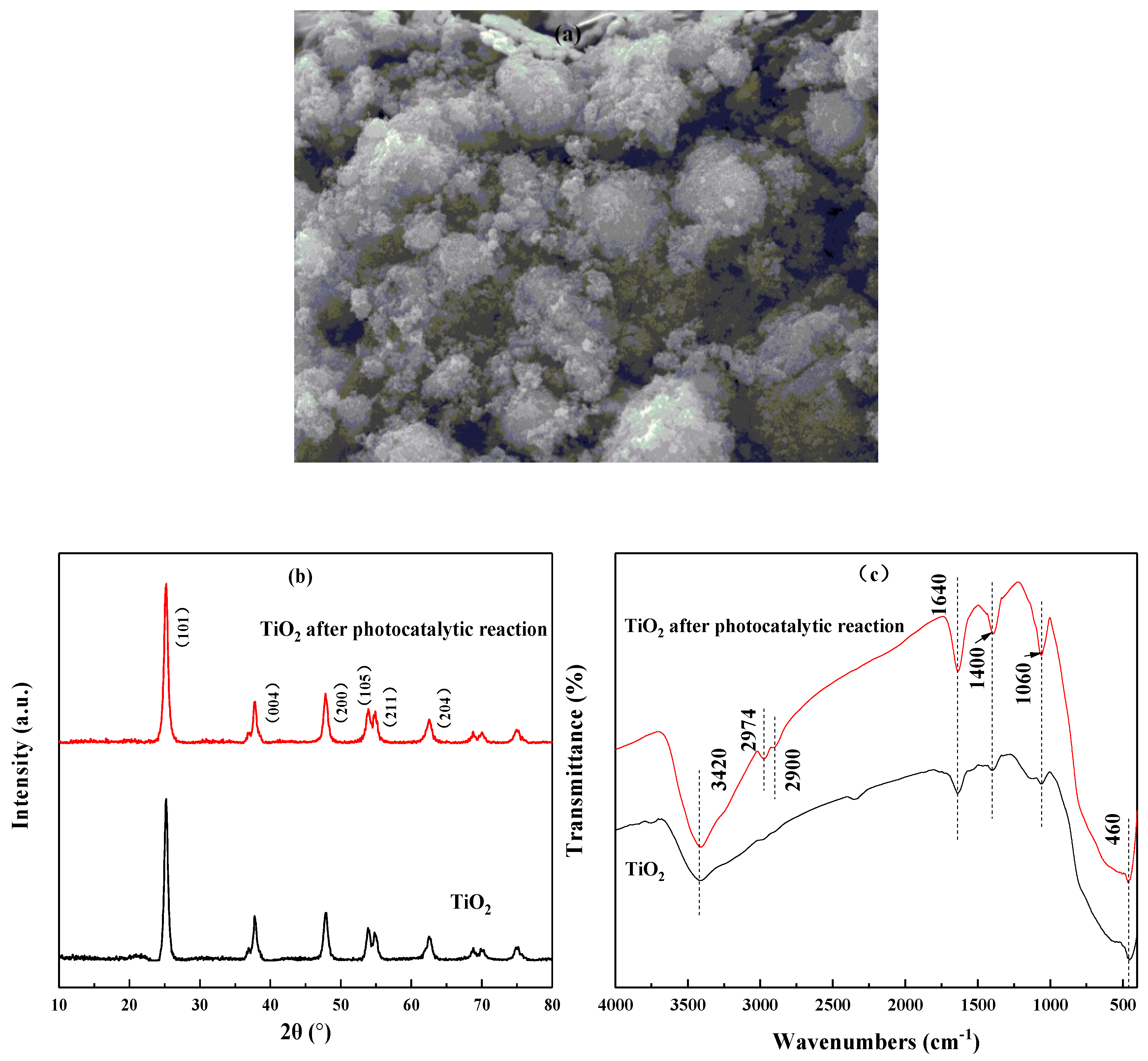
3.1. Characterization of Photocatalysts
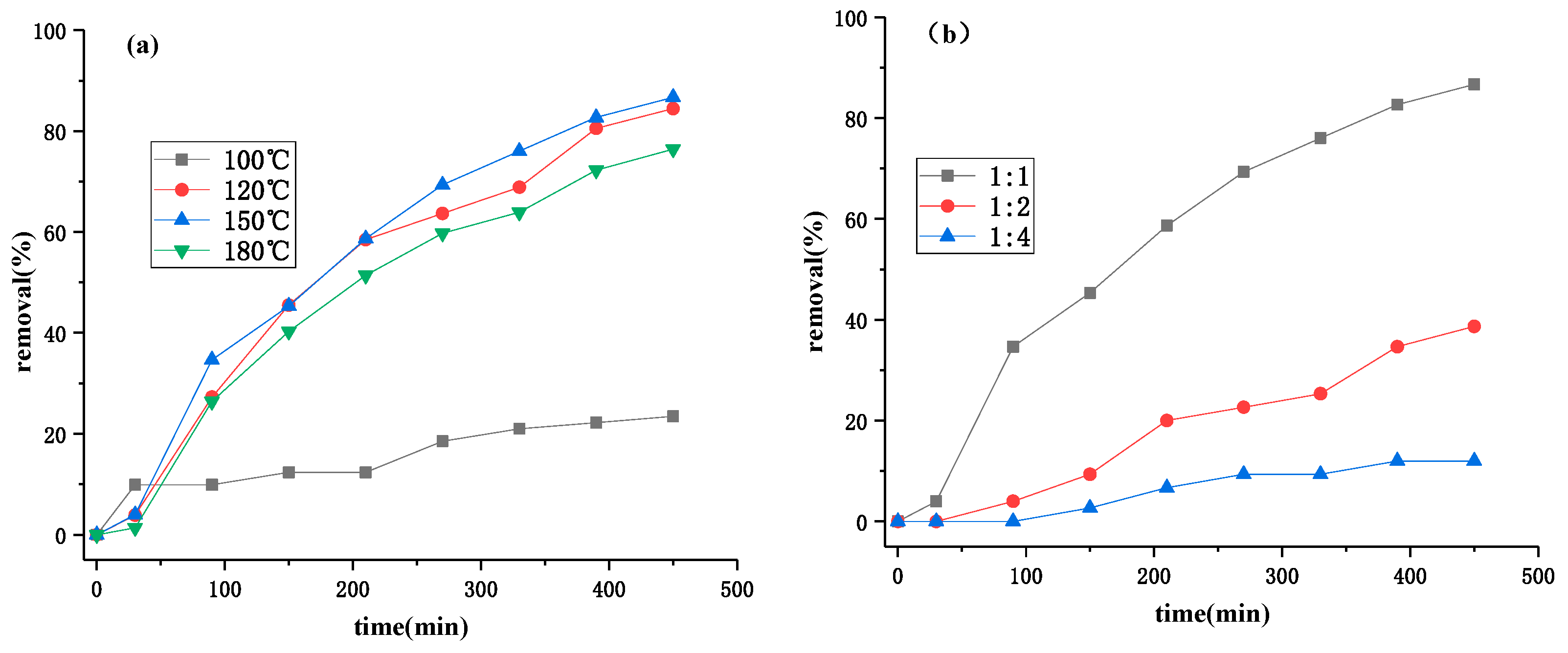
3.2. Optimization of Photocatalytic Processes
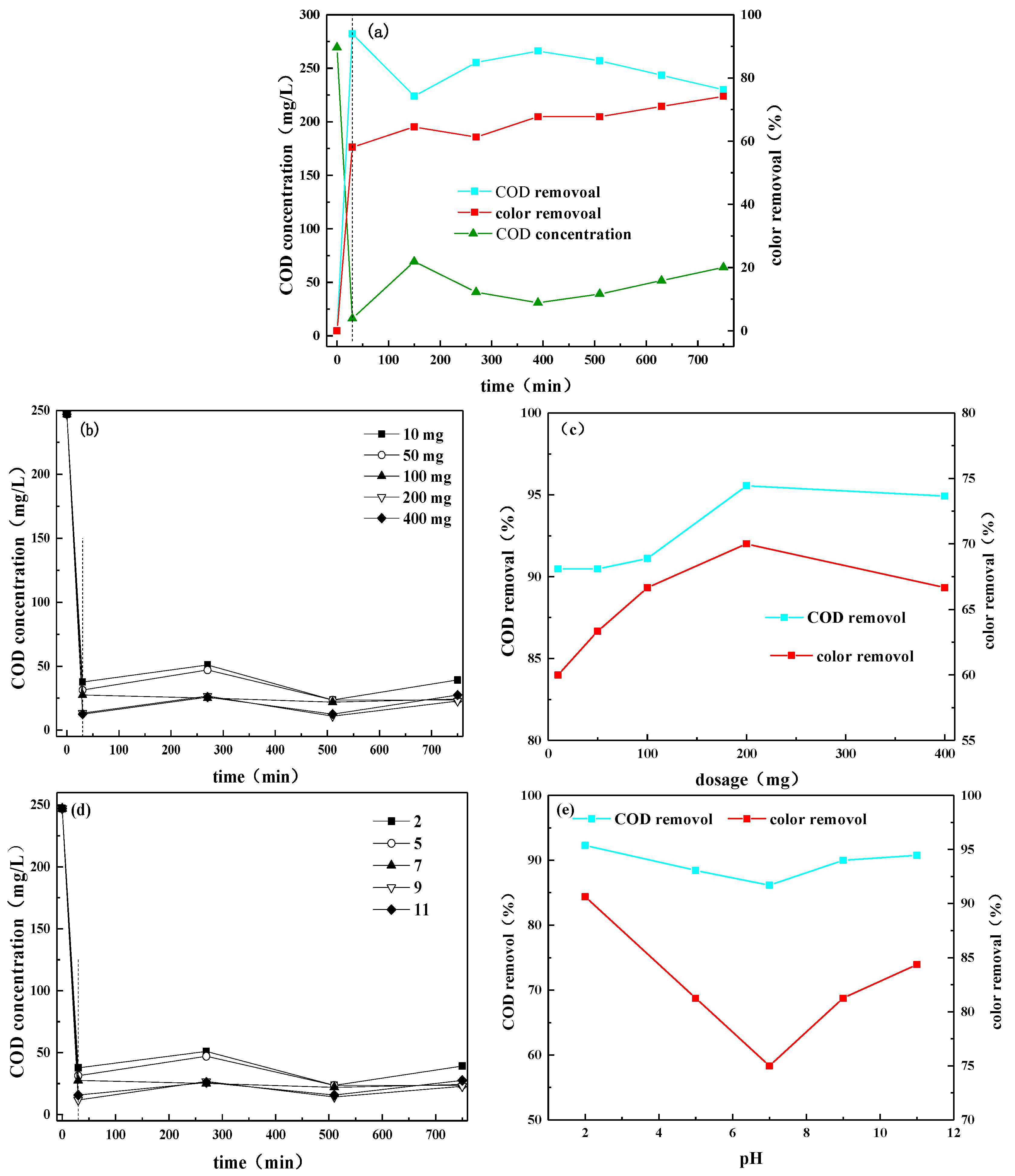
3.3. Treatment of Leachate by Aging Reactor Combined with Photocatalysis
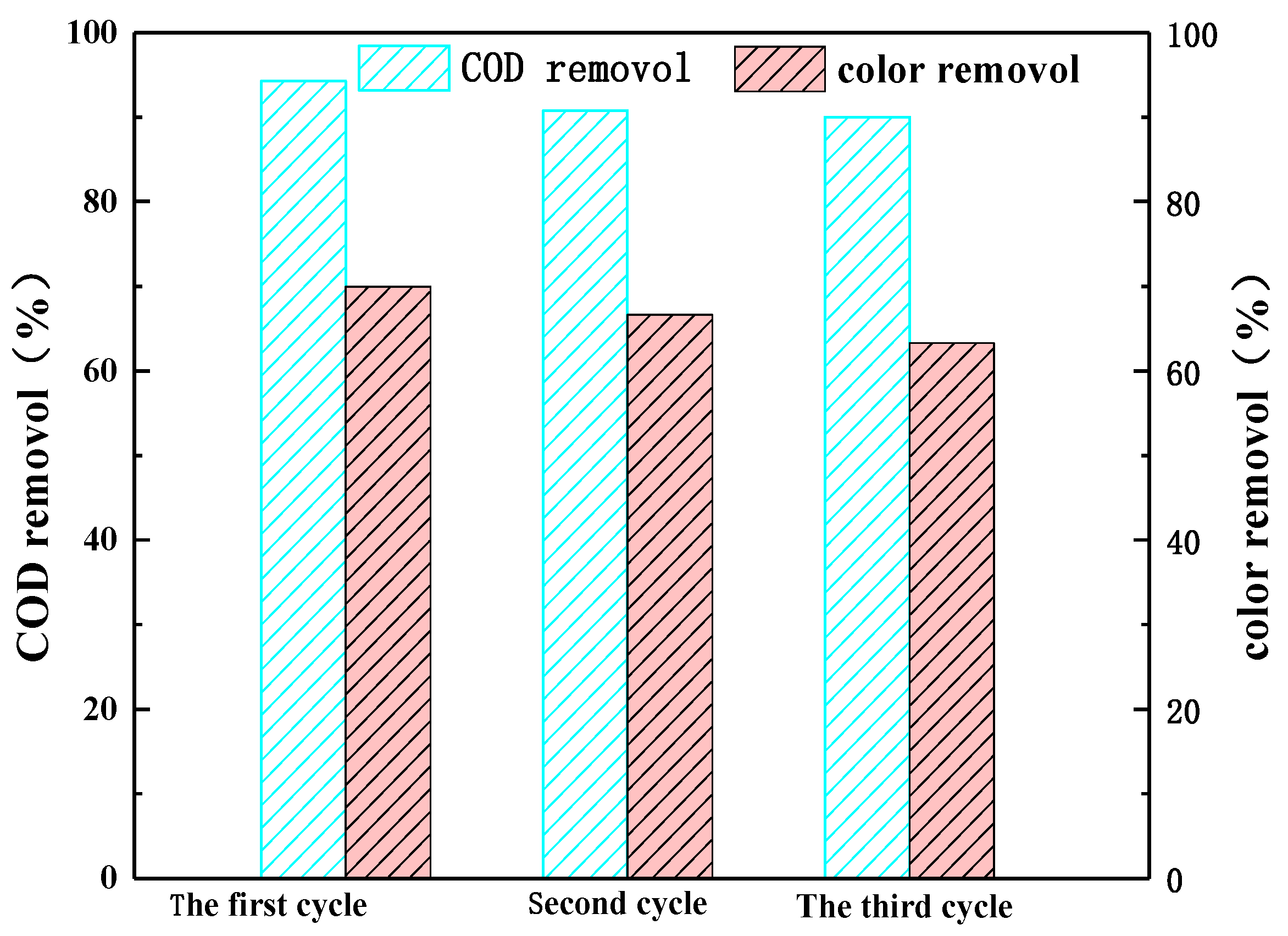
3.4. Photocatalyst Stability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, Y.; Sheehan, J.D.; Chen, Z.; Wang], S. Oxidative degradation of landfill leachate by catalysis of CeMnOx/TiO2 in super-critical water: Mechanism and kinetic study. Chem. Eng. J. 2018, 578–586. [Google Scholar]
- Yang, Y.; Liu, Z.; Demeestere, K.; Van Hulle, S. Ozonation in view of micropollutant removal from biologically treated landfill leachate: Removal efficiency, OH exposure, and surrogate-based monitoring. Chem. Eng. J. 2021, 410, 128413. [Google Scholar] [CrossRef]
- Clarke, B.O.; Anumol, T.; Barlaz, M.; Snyder, S.A. Investigating landfill leachate as a source of trace organic pollutants. Chemosphere 2015, 127, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Oloibiri, V.; Chys, M.; Audenaert, W.T.M.; Decostere, B.; He, Y.; Van Langenhove, H.; Demeestere, K.; Van Hulle, S.W.H. The present status of landfill leachate treatment and its development trend from a technological point of view. Rev. Environ. Sci. Biol. Technol. 2015, 14, 93–122. [Google Scholar] [CrossRef]
- Duyar, A.; Ciftcioglu, V.; Cirik, K.; Civelekoglu, G.; Uruş, S. Treatment of landfill leachate using single-stage anoxic moving bed biofilm reactor and aerobic membrane reactor. Sci. Total. Environ. 2021, 776, 145919. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Zeng, Y.; Cheng, Y.; He, D.; Pan, X. Recent advances in municipal landfill leachate: A review focusing on its characteristics, treatment, and toxicity assessment. Sci. Total. Environ. 2020, 703, 135468. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.; Xie, B. Use of aged refuse-based bioreactor/biofilter for landfill leachate treatment. Appl. Microbiol. Biotechnol. 2014, 98, 6543–6553. [Google Scholar] [CrossRef]
- Chys, M.; Oloibiri, V.A.; Audenaert, W.T.; Demeestere, K.; Van Hulle, S.W. Ozonation of biologically treated landfill leachate: Efficiency and insights in organic conversions. Chem. Eng. J. 2015, 277, 104–111. [Google Scholar] [CrossRef]
- Oloibiri, V.; Chys, M.; De Wandel, S.; Demeestere, K.; Van Hulle, S.W. Removal of organic matter and ammonium from landfill leachate through different scenarios: Operational cost evaluation in a full-scale case study of a Flemish landfill. J. Environ. Manag. 2017, 203, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Gu, Z.; Wen, P.; Li, Q. Degradation of refractory organic contaminants in membrane concentrates from landfill leachate by a combined coagulation-ozonation process. Chemosphere 2019, 217, 411–422. [Google Scholar] [CrossRef]
- Chys, M.; Declerck, W.; Audenaert, W.T.; Van Hulle, S. UV/H2O2, O3 and (photo-) Fenton as treatment prior to granular activated carbon filtration of biologically stabilized landfill leachate. J. Chem. Technol. Biotechnol. 2014, 90, 525–533. [Google Scholar] [CrossRef]
- Xu, Q.; Tian, Y.; Wang, S.; Ko, J.H. A comparative study of leachate quality and biogas generation in simulated anaerobic and hybrid bioreactors. Waste Manag. 2015, 41, 94–100. [Google Scholar] [CrossRef]
- Li, J.; Wu, B.; Li, Q.; Zou, Y.; Cheng, Z.; Sun, X.; Xi, B. Ex situ simultaneous nitrification-denitrification and in situ denitri-fication process for the treatment of landfill leachates. Waste Manag. 2019, 88, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Siracusa, G.; Di Gregorio, S.; Yuan, Q. COD removal from biologically stabilized landfill leachate using Advanced Oxidation Processes (AOPs). Process Saf. Environ. Protect. 2018, 120, 278–285. [Google Scholar] [CrossRef]
- Fresno, F.; Portela, R.; Suárez, S.; Coronado, J.M. Photocatalytic materials: Recent achievements and near future trends. J. Mater. Chem. A 2014, 2, 2863–2884. [Google Scholar] [CrossRef]
- Yan, M.; Wu, Y.; Liu, X. Photocatalytic nanocomposite membranes for high-efficiency degradation of tetracycline under visible light: An imitated core-shell Au-TiO2-based design. J. Alloys Compd. 2021, 855, 157548. [Google Scholar] [CrossRef]
- Chen, J.; Xiong, Y.; Duan, M.; Li, X.; Li, J.; Fang, S.W.; Qin, S.; Zhang, R. Insight into the Synergistic Effect of Adsorption-Photocatalysis for the Removal of Organic Dye Pollutants by Cr-Doped ZnO. Langmuir 2020, 36, 520–533. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, M.; Dabi, B.; Dadvar, M.; Jafarikojour, M. Photocatalysis of Phenol Using a Spinning Disc Photoreactor Immobilized with TiO2 Nanoparticles: Hydrodynamic Modeling and Reactor Optimization. Ind. Eng. Chem. Res. 2017, 56, 1739–1749. [Google Scholar] [CrossRef]
- Yu, X.; Qiu, H.; Wang, B.; Meng, Q.; Zhao, K. A ternary photocatalyst of all-solid-state Z-scheme TiO2–Au–BiOBr for efficiently degrading various dyes. J. Alloys Compd. 2020, 839, 155597. [Google Scholar] [CrossRef]
- Altina, I.; Mab, X.; Boffab, V.; Bacaksızc, E.; Magnaccad, G. Hydrothermal preparation of B–TiO2-graphene oxide ternary nanocomposite, characterization and photocatalytic degradation of bisphenol A under simulated solar irradiation. Mater. Sci. Semicond. Process. 2021, 123, 105591. [Google Scholar] [CrossRef]
- Shao, S.; Yu, J.; Love, J.B.; Fan, X. An economic approach to produce iron doped TiO2 nanorods from ilmenite for photocatalytic applications. J. Alloys Compd. 2021, 858, 158388. [Google Scholar] [CrossRef]
- Priyanka, K.; Remya, N.; Behera, M. Comparison of titanium dioxide based catalysts preparation methods in the minerali-zation and nutrients removal from greywater by solar photocatalysis. J. Clean Prod. 2019, 235, 1–10. [Google Scholar] [CrossRef]
- Seibert, D.; Borba, F.H.; Bueno, F.; Inticher, J.J.; Modenes, A.N.; Espinoza-Quinones, F.R.; Bergamasco, R. Two-stage integrated system photo-electro-Fenton and biological oxidation process assessment of sanitary landfill leachate treatment: An intermediate products study. Chem. Eng. J. 2019, 372, 471–482. [Google Scholar] [CrossRef]
- Cai, F.F.; Yang, Z.H.; Huang, J.; Zeng, G.M.; Wang, L.K.; Yang, J. Application of cetyltrimethylammonium bromide bentonite–titanium dioxide photocatalysis technology for pretreatment of aging leachate. J. Hazard. Mater. 2014, 275, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Pellenz, L.; Borba, F.H.; Daroit, D.J.; Lassen, M.F.M.; Baroni, S.; Zorzo, C.F.; Guimarães, R.E.; Espinoza-Quiñones, F.R.; Seibert, D. Landfill leachate treatment by a boron-doped diamond-based photo-electro-Fenton system integrated with biological oxidation: A toxicity, genotoxicity and by products assessment. J. Environ. Manag. 2020, 264, 110473. [Google Scholar] [CrossRef]
- Gu, Z.; Chen, W.; Wang, F.; Li, Q. A pilot-scale comparative study of bioreactor landfills for leachate decontamination and municipal solid waste stabilization. Waste Manag. 2020, 103, 113–121. [Google Scholar] [CrossRef]
- Agdag, O.N.; Aktas, D.; Simsek, O. Effects of Different Seed Substances on Anaerobic Degradation of Municipal Solid Waste in Recirculated Bioreactor. Waste Biomass-Valoriz. 2021, 12, 383–392. [Google Scholar] [CrossRef]
- Ministry of Environmental Protection of the People’s Republic of China. Standard for pollution control on the landfill site of the MSW; Ministry of Environmental Protection of the People’s Republic of China: Beijing, China, 2008.
- Wu, C.; Shen, L.; Zhang, Y.-C.; Huang, Q. Solvothermal synthesis of Cr-doped ZnO nanowires with visible light-driven photocatalytic activity. Mater. Lett. 2011, 65, 1794–1796. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, H.; Cheng, Z. Use of bioreactor landfill for nitrogen removal to enhance methane production through ex situ simultaneous nitrification-denitrification and in situ denitrification. Waste Manag. 2017, 66, 97–102. [Google Scholar] [CrossRef]
- He, J.; Yang, J.; Jiang, F.; Liu, P.; Zhu, M. Photo-assisted peroxymonosulfate activation via 2D/2D heterostructure of Ti3C2/g-C3N4 for degradation of diclofenac. Chemosphere 2020, 258, 127339. [Google Scholar] [CrossRef]
- Elleuch, L.; Messaoud, M.; Djebali, K.; Attafi, M.; Cherni, Y.; Kasmi, M.; Elaoud, A.; Trabelsi, I.; Chatti, A. A new insight into highly contaminated landfill leachate treatment using Kefir grains pre-treatment combined with Ag-doped TiO2 photocatalytic process. J. Hazard. Mater. 2020, 382, 121119. [Google Scholar] [CrossRef]
- Ebrahimian, A. Silver Doped TiO2 Nanoparticles: Preparation, Characterization and Efficient Degradation of 2,4-dichlorophenol Under Visible Light. J. Water Environ. Nanotechnol. 2016, 1, 135–144. [Google Scholar]
- Xu, C.; Yang, F.; Deng, B.; Zhuang, Y.; Li, D.; Liu, B.; Yang, W.; Li, Y. Ti3C2/TiO2 nanowires with excellent photocatalytic performance for selective oxidation of aromatic alcohols to aldehydes. J. Catal. 2020, 383, 1–12. [Google Scholar] [CrossRef]
- Peng, C.; Wang, H.; Yu, H.; Peng, F. (111) TiO2-x/Ti3C2: Synergy of active facets, interfacial charge transfer and Ti3+ doping for enhance photocatalytic activity. Mater. Res. Bull. 2017, 89, 16–25. [Google Scholar] [CrossRef]
- Lv, K.; Zuo, H.; Sun, J.; Deng, K.; Liu, S.; Li, X.; Wang, D. (Bi, C and N) codoped TiO2 nanoparticles. J. Hazard. Mater. 2009, 161, 396–401. [Google Scholar] [CrossRef]
- Hassan, M.; Zhao, Y.; Xie, B. Employing TiO2 photocatalysis to deal with landfill leachate: Current status and development. Chem. Eng. J. 2016, 285, 264–275. [Google Scholar] [CrossRef]
- Azadi, S.; Karimi-Jashni, A.; Javadpour, S.; Amiri, H. Photocatalytic treatment of landfill leachate: A comparison between N-, P-, and N-P-type TiO2 nanoparticles. Environ. Technol. Innov. 2020, 19, 100985. [Google Scholar] [CrossRef]
- Qazaq, A.; Hudaya, T.; Lee, I.; Sulidis, A.; Adesina, A. Photoremediation of natural leachate from a municipal solid waste site in a pilot-scale bubble column reactor. Catal. Commun. 2007, 8, 1917–1922. [Google Scholar] [CrossRef]
- Rojviroon, O.; Rojviroon, T.; Sirivithayapakorn, S. Removal of Color and Chemical Oxygen Demand from Landfill Leachate by Photocatalytic Process with AC/TiO2. Energy Procedia 2015, 79, 536–541. [Google Scholar] [CrossRef]
- Jyothi, K.; Yesodharan, S.; Yesodharan, E. Ultrasound (US), Ultraviolet light (UV) and combination (US + UV) assisted semiconductor catalysed degradation of organic pollutants in water: Oscillation in the concentration of hydrogen peroxide formed in situ. Ultrason. Sonochem. 2014, 21, 1787–1796. [Google Scholar] [CrossRef]
- Youngman, F. Optimization of TiO2 Photocatalyst in an Advanced Oxidation Process for the Treatment of Landfill Leachate. Ph.D. Thesis, Florida Atlantic University, Boca Raton, FL, USA, 2013. [Google Scholar]
- Miao, Z.; Wang, G.; Li, L.; Wang, C.; Zhang, X. Fabrication of black TiO2/TiO2 homojunction for enhanced photocatalytic degradation. J. Mater. Sci. 2019, 54, 14320–14329. [Google Scholar] [CrossRef]
- Jia, C.; Wang, Y.; Zhang, C.; Qin, Q. UV-TiO2 Photocatalytic Degradation of Landfill Leachate. Water Air Soil Pollut. 2011, 217, 375–385. [Google Scholar] [CrossRef]
- Wiszniowski, J.; Robert, D.; Surmacz-Górska, J.; Miksch, K.; Malato, S.; Weber, J.-V. Solar photocatalytic degradation of humic acids as a model of organic compounds of landfill leachate in pilot-plant experiments: Influence of inorganic salts. Appl. Catal. B Environ. 2004, 53, 127–137. [Google Scholar] [CrossRef]
- He, X.; Mitrano, D.M.; Nowack, B.; Bahk, Y.K.; Figi, R.; Schreiner, C.; Bürki, M.; Wang, J. Agglomeration potential of TiO2 in synthetic leachates made from the fly ash of different incinerated wastes. Environ. Pollut. 2017, 223, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Anpo, M.; Kamat, P.V. Environmentally Benign Photocatalysts; Springer: New York, NY, USA, 2010. [Google Scholar]
- Chen, C.-Y. Photocatalytic Degradation of Azo Dye Reactive Orange 16 by TiO2. Water Air Soil Pollut. 2009, 202, 335–342. [Google Scholar] [CrossRef]
- Hassan, M.; Wang, X.; Wang, F.; Wu, D.; Hussain, A.; Xie, B. Coupling ARB-based biological and photochemical (UV/TiO2 and UV/S2O82−) techniques to deal with sanitary landfill leachate. Waste Manag. 2017, 63, 292–298. [Google Scholar] [CrossRef]
- Boonnorat, J.; Honda, R.; Panichnumsin, P.; Boonapatcharoen, N.; Yenjam, N.; Krasaesueb, C.; Wachirawat, M.; Seemuang-On, S.; Jutakanoke, R.; Teeka, J.; et al. Treatment efficiency and greenhouse gas emissions of non-floating and floating bed activated sludge system with acclimatized sludge treating landfill leachate. Bioresour. Technol. 2021, 330, 124952. [Google Scholar] [CrossRef]
- Guo, X.; Li, B.; Zhao, R.; Zhang, J.; Lin, L.; Zhang, G.; Li, R.-H.; Liu, J.; Li, P.; Li, Y.; et al. Performance and bacterial community of moving bed biofilm reactors with various biocarriers treating primary wastewater effluent with a low organic strength and low C/N ratio. Bioresour. Technol. 2019, 287, 121424. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.I.; Foco, M.L.; Vieira, E.; Cassidy, J.; Silva, T.F.; Fonseca, A.; Saraiva, I.; Boaventura, R.A.; Vilar, V.J. Multistage treatment technology for leachate from mature urban landfill: Full scale operation performance and challenges. Chem. Eng. J. 2019, 376, 120573. [Google Scholar] [CrossRef]
- Gogina, E.; Gulshin, I. Simultaneous Nitrification and Denitrification with Low Dissolved Oxygen Level and C/N ratio. Procedia Eng. 2016, 153, 189–194. [Google Scholar] [CrossRef]
- Liu, T.; He, X.; Jia, G.; Xu, J.; Quan, X.; You, S. Simultaneous nitrification and denitrification process using novel sur-face-modified suspended carriers for the treatment of real domestic wastewater. Chemosphere 2020, 247, 125831. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.I.; Soares, T.F.; Silva, T.F.; Boaventura, R.A.; Vilar, V.J. Ozone-driven processes for mature urban landfill leachate treatment: Organic matter degradation, biodegradability enhancement and treatment costs for different reactors configuration. Sci. Total. Environ. 2020, 724, 138083. [Google Scholar] [CrossRef] [PubMed]
- Abu Amr, S.S.; Aziz, H.A.; Adlan, M.N.; Aziz, S.Q. Effect of Ozone and Ozone/Fenton in the Advanced Oxidation Process on Biodegradable Characteristics of Semi-aerobic Stabilized Leachate. Clean-Soil Air Water 2012, 41, 148–152. [Google Scholar] [CrossRef]
- Colombo, A.; Módenes, A.N.; Trigueros, D.E.G.; da Costa, S.I.G.; Borba, F.; Espinoza-Quiñones, F.R. Treatment of sanitary landfill leachate by the combination of photo-Fenton and biological processes. J. Clean. Prod. 2019, 214, 145–153. [Google Scholar] [CrossRef]


| NH4+–N (mg/L) | NO3–N (mg/L) | NO2–N (mg/L) | COD (mg/L) | |
|---|---|---|---|---|
| C | 5.457 | 18.209 | 0.079 | 245.302 |
| D | 1.049 | 18.226 | 0.017 | 185.955 |
| E | 18.404 | 8.5228 | 0.281 | 146.390 |
| F | 1.049 | 18.276 | 0.281 | 126.607 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Sun, X.; Shan, H.; Zhang, H.; Xi, B. Degradation of Landfill Leachate Using UV-TiO2 Photocatalysis Combination with Aged Waste Reactors. Processes 2021, 9, 946. https://doi.org/10.3390/pr9060946
Wang C, Sun X, Shan H, Zhang H, Xi B. Degradation of Landfill Leachate Using UV-TiO2 Photocatalysis Combination with Aged Waste Reactors. Processes. 2021; 9(6):946. https://doi.org/10.3390/pr9060946
Chicago/Turabian StyleWang, Chunlian, Xiaojie Sun, Huijun Shan, Hongxia Zhang, and Beidou Xi. 2021. "Degradation of Landfill Leachate Using UV-TiO2 Photocatalysis Combination with Aged Waste Reactors" Processes 9, no. 6: 946. https://doi.org/10.3390/pr9060946
APA StyleWang, C., Sun, X., Shan, H., Zhang, H., & Xi, B. (2021). Degradation of Landfill Leachate Using UV-TiO2 Photocatalysis Combination with Aged Waste Reactors. Processes, 9(6), 946. https://doi.org/10.3390/pr9060946





