Abstract
Hepatocellular carcinoma (HCC), the most common form of primary liver tumors, is the fifth cancer in the world in terms of incidence, and third in terms of mortality. Despite significant advances in the treatment of HCC, its prognosis remains bleak. Transarterial radioembolization with radiolabeled microspheres and Lipiodol has demonstrated significant effectiveness. Here we present a new, simple radiolabeling of Lipiodol with Yttrium-90, for the potential treatment of HCC.
1. Introduction
Liver tumors, either primary or metastatic, are a leading cause of death throughout the world. Primary liver cancers rank third in cancer-related deaths, behind lung and colorectal cancers [1,2], the latter being the major cause for metastatic liver cancers [3]. In 2020, primary liver cancers accounted for circa 906,000 new cases and 830,000 deaths worldwide. Primary liver cancers include essentially hepatocellular carcinoma (HCC) (representing approximately 75% of the cases) and intrahepatic cholangiocarcinoma [4,5]. Most HCC cases arise in Asia and sub-Saharan Africa, with China alone accounting for half the number of cases and deaths [6,7]. Incidence and mortality rates of liver cancer seem to be decreasing in high-risk countries, but incidence is increasing in India, Oceania, Europe and the Americas [8,9]. HCC is an aggressive disease with a grim prognosis. Median survival from the time of diagnosis is often only 6 to 20 months. Patients frequently present with advanced disease or suffer from chronic liver disease, which restricts their treatment options [10,11]. Notably, few patients are eligible for a curative treatment. In this context, loco-regional therapies are commonly used and have demonstrated some effectiveness in the management of HCC [12]. In particular, based on the fact that HCC has a vascular supply mostly dependent on the hepatic artery, while healthy liver parenchyma is essentially irrigated through the portal vein, intra-arterially delivered treatments represent a treatment method of choice for intermediate stage HCCs [13]. Transarterial radioembolization (TARE) is an emerging strategy to treat liver malignancies. It consists of the delivery of a radioactive material to the tumor through its feeding artery. Different agents and materials have been investigated and labeled with different therapeutic radioisotopes [14,15]. Currently, 90Y- and 166Ho-labeled microspheres are commercially available for the treatment of primary and secondary liver cancers.
Another potentially interesting TARE agent is Lipiodol, an oily mixture of iodinated esters of fatty acids derived from poppy seeds. Due to its adequate physico-chemical properties and prolonged retention time in the tumor, while quickly washed out from the liver, it is the vehicle of choice for chemotherapeutic agents in transarterial chemoembolization (TACE) and has been used for the delivery of radionuclides in TARE [15,16,17]. Notably, our team has successfully developed a 188Re-labeling of Lipiodol (188Re-SSS/Lipiodol), currently being investigated in clinical trials [18,19]. Yttrium-90 is another beta-emitting nuclide of interest for radionuclide therapy. It is a pure β- emitter, with a high-energy emission of 2.28 MeV, enabling a maximum tissue penetration of 12 mm. It has a half-life of 64 h, longer than the 17 h of 188Re. The absence of gamma emission is beneficial in terms of radioprotection constraints [20]. Besides its already mentioned use in microspheres for radioembolization, it has been used in peptide receptor radionuclide therapy (PRRT) and radioimmunotherapy (RIT) of various tumor types [21]. 90Y-labeling of Lipiodol has also been described [22,23,24,25]. With the exception of 131I-radiolabeling, Lipiodol labeling is reached through solubilization of a lipophilic chelate into the oily medium. One such chelate that attracted attention was 8-hydroxyquinoline, or oxine. It is known to form lipid-soluble complexes, suitable for imaging or therapy, depending on the radiometal chosen [26,27,28]. Though the poor solubility of the oxine-based complexes because of their high lipophilicity may be a drawback [29], this property is of interest for the labeling of Lipiodol. It has thus been investigated with therapeutic radionuclides, such as Yttrium-90 [23] and radiolanthanides [30,31]. However, the procedure to label Lipiodol is not straightforward, since direct extraction into Lipiodol led to poor yields. An intermediate extraction of the oxine complex into dichloromethane was thus needed. Moreover, in vivo studies showed instability of the radiotracer with increasing bone uptake over time. To date, oxine radiocomplexes have been mainly used to label blood cells for imaging of inflammation and infection [32,33]. (111In)-oxine-labeled white blood cells remain the gold standard for infection imaging.
Other lipid-soluble complexes have nonetheless been investigated to label blood cells, such as tropolone derivatives, with good results [34,35]. Tropolone, or 2-Hydroxy-2,4,6-cycloheptatrien-1-one, readily forms lipid-soluble complexes with di- or trivalent metals [36]. Besides 111In, tropolone has been used to complex 57Co [37], 59Fe [36], 64Cu [38], 67/68Ga [39,40], 89Zr [41] and 99mTc [42]. Using an analogy between oxine and tropolone, we have decided to investigate the potential usefulness of tropolone to label Lipiodol. To have a high enough lipophilicity, we have chosen to work with a more lipophilic derivative of tropolone, hinokitiol or 4-isopropyl tropolone (Figure 1). Hinokitiol, also known as β-thujaplicin, is a natural product found in the heartwood of several cupressaceous plants, used as a food additive and in hygiene products, thanks to its lack of toxicity [43]. It is known, however, to have anti-infectious and anti-proliferative properties [44,45,46]. It is also an efficient iron-chelating agent, used to restore iron transport in iron-related diseases [47], and, globally, an ionophore leading to highly lipophilic complexes [48]. It thus should be well suited for the radiolabeling of Lipiodol.
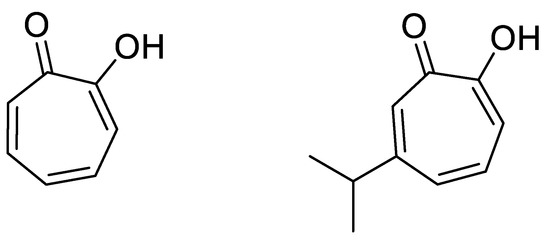
Figure 1.
Structures of Tropolone (left) and Hinokitiol (right).
2. Materials and Methods
Yttrium-90 chloride was provided by PerkinElmer Life Sciences (Waltham, MA, USA), in 0.05 M HCl solution. Lipiodol was obtained from Guerbet (Villepinte, France). Labrafac WL 1349 was a gift from Gattefossé SA (Saint-Priest, France). The other chemicals (Ligands, HPLC-grade solvents, buffer solutions) were used as received from suppliers. Experiments were performed in sealed, borosilicate glass flasks. Sealed flasks were heated on a Bioblock heating block (ThermoFisher, Waltham, MA, USA), able to heat up to 6 flasks. Activities were measured with a CRC-127R (Capintec Inc., Ramsey, NJ, USA) dose calibrator. Quality controls were carried out by TLC on Whatman 1 paper (GE Healthcare, Maidstone, UK) using MeOH with 0.1% NEt3 as mobile phase. Radiochemical Purities (RCP) were determined with a Cyclone Storage Phosphorimager (PerkinElmer, Waltham, MA, USA), using the Optiquant software.
2.1. 90Y Radiolabeling
Several parameters, such as concentration of ligand, volume and pH of the reaction mixture, incubation time and temperature, were varied extensively to obtain an optimized protocol. A 0.5 mL amount of an yttrium-90 chloride solution (3–1446 MBq), diluted in 0.1 M HCl solution (pH = 1.5), in various 1 M acetate buffers (pH = 3, 3.5, 4.65, 7 and 9), or in 1 M Tris buffer (pH = 8.3) were added to 0.5 mL of a ligand solution (c = 0.01–10 mM) in ethanol. The resulting solution was heated at 20–100 °C for 5–30 min. All experiments were completed at least in triplicate.
2.2. Extraction in Organic Phase and Log P Determination
90Y-hinokitiol complex (8–1446 MBq) prepared under optimized reaction conditions was extracted into an organic phase. Amounts of 1 mL of saline and 2 mL of organic solvent (dichloromethane, chloroform) or oily medium (Lipiodol, Labrafac WL 1349) were added. The mixture was vigorously shaken for 2 min to ensure homogeneous dispersion of the complex, and then centrifuged (3500× g, 15 min) to separate the phases, using an Awel MF 20-R centrifuge (Blain, France). The two phases were carefully collected with a 5 mL syringe equipped with an 18 gauge spinal tap needle and counted in a well-counter. The extraction yield was determined as the organic layer activity on the total (aqueous + organic) activity.
For log P determination, 10 µL of 90Y-hinokitiol (27–80 MBq) were mixed with 500 µL of n-Octanol and 500 µL of water for injection (wfi) previously equilibrated and the solution was vigorously shaken for 10 min. After separation of the phases, a 100 µL aliquot of each phase was taken and counted with a Cobra II Auto-gamma counter (Packard Bioscience, Meriden, CT, USA). Log P was calculated as follows: Log P = Log [AOct]/[Awfi], where AOct and Awfi are the measured activities of the n-Octanol and aqueous aliquots respectively.
2.3. Automated Preparation of 90Y-Hinokitiol/Lipiodol
Automation of the radiolabeling of Lipiodol was completed on a remote-controlled TADDEO module (COMECER, Castel Bolognese, Italy). C8 Sep-Pak cartridges were obtained from Waters (Milford, MA, USA) and sterile membrane filters (Minisart 0.22 µm pore size) were obtained from Sartorius (Göttingen, Germany). The content of vial A, 0.5 mL of [90Y]YCl3 (37–359 MBq) in 1 M acetate buffer pH = 4.65, was transferred into reaction vessel R. An amount of 0.5 mL of 1 mM Hinokitiol in EtOH (Vial B) was subsequently transferred to reaction vessel R. After 2 min at room temperature, the reactor’s content was then purified on a C8 Sep-Pak column, the solution being transferred to a waste vial, while the 90Y-Hinokitiol complex remained on the column. The complex was then washed with 10 mL of water, and finally eluted with 2.5 mL EtOH into vial C, through a sterile 0.22 µm filter. After EtOH evaporation, the residue was resuspended with 2 mL Lipiodol.
2.4. Stability
For the stability studies, 90Y-hinokitiol solution and 90Y-hinokitiol/Lipiodol (52–1446 MBq) were prepared under optimized reaction conditions and stored at RT. Aliquots of these solutions were analyzed on TLC at different time points over a period of seven days.
To assess possible transchelation with iron, 200 µL of freshly prepared 90Y-hinokitiol solution was incubated with 200 µL of 100 mM Fe(citrate)3 and RCP was checked at different time points.
2.5. Over Time Release in the Aqueous Phase
An amount of 1 mL of freshly prepared 90Y-hinokitiol (45–64 MBq) in Lipiodol phase was taken with a syringe and put into a 12-mL flat bottom glass vial, containing 10 mL of physiological serum (previously weighed), and the vial was closed. Activity in the vial was measured with the dose calibrator. The vial was then put inside an incubator (Fisherbrand 15 L, Fisher Scientific, Waltham, MA, USA) at 37 °C, with a smooth shaking (30 rpm). Shaking was let for 15 days. Aqueous phase aliquots (~100 µL) were taken up from the supernatant with a 1-mL syringe at different time points (e.g., 1, 24, 48, 72, 120, 168, 240, 312, 360 h) to measure out yttrium-90 released from Lipiodol. The aliquots were put in a previously weighed and carefully annotated tube (name of the sample, time point). The tubes were weighed to determine the amount of aqueous phase collected. The tubes were counted with a Cobra II Auto-gamma counter (Packard Bioscience, Meriden, CT, USA). Each sample has been realised in triplicate. Measures were corrected from counting yield and decay to determine the percentage of released activity over time. A sample of supernatant was analysed with TLC at different time points.
For comparison, other lipophilic radiotracers described in the literature to label Lipiodol were prepared according to previously published methods [18,23,25] and their release studied. An amount of 1 mL of freshly prepared 90Y-oxine (37–74 MBq), 90Y(DEDC)3(Phen) (37–74 MBq) and 188Re-SSS (82–87 MBq) in Lipiodol phase were collected with a syringe, and the above procedure was applied.
3. Results
3.1. Preparation of 90Y-Hinokitiol
Important parameters to reach an optimal radiolabeling are temperature, incubation time and the ligand concentration. If the reaction proceeds in an aqueous medium, pH is critical, while choice of the solvent is of utter importance if the reaction proceeds in an organic medium. Hinokitiol is sparingly soluble in aqueous media and soluble in organic solvents. It was thus solubilized in Ethanol to have an homogenous mixture with [90Y]-yttrium chloride and diluted in different buffers. Moreover, EtOH is suitable for the preparation of compounds for human use, and it is poorly miscible with Lipiodol, which is an advantage for Lipiodol extraction of the radiotracer, avoiding the need to evaporate residual solvent.
Temperatures between 20 and 100 °C were investigated (Figure 2A). The reaction proceeds straightforwardly at room temperature (RT), with no need to heat. Incubation time was also investigated (Figure 2B). It was shown that the best results were obtained with 5 min of incubation, radiochemical purity, as determined by TLC, tending to decrease with increasing incubation times, from 95.99 ± 5.32% to 89.65 ± 2.19% after 30 min at RT. Ligand concentrations between 10 µM and 0.1 M were investigated (Figure 2C). Results were satisfactory for concentrations above 0.5 mM. Below this, RCP decreased quickly, with only 88.45 ± 9.97% using 0.1 mM Hinokitiol in EtOH.
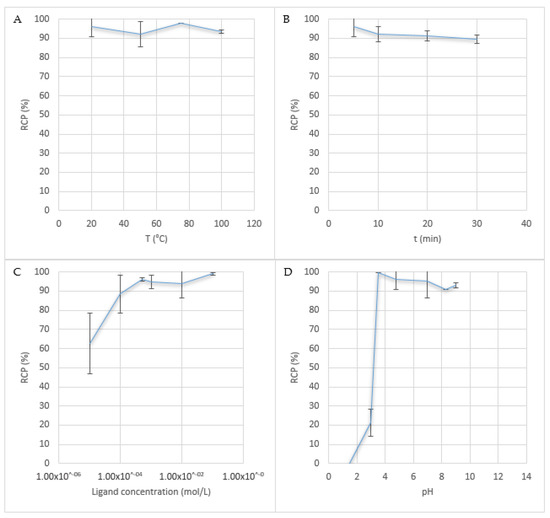
Figure 2.
Influence of the reaction conditions. (A) Temperature, (B) Time, (C) Ligand concentration and (D) pH.
[90Y]-yttrium chloride was provided as a 0.05 M HCl solution (0.1 mL), to prevent precipitation of insoluble hydroxides. It was diluted in various 1 M acetate buffer solutions (pH = 3 to 9) or diluted with HCl 0.1 M to reach a pH of 1.5 (Figure 2D). A Tris buffer solution (pH = 8.3) was also investigated. 90Y-Hinokitiol complex was obtained with an RCP > 90% for pH between 3.5 and 9. Below 3.5, RCP decreased rapidly. At pH = 1.5, no complexation occurred at all. pH > 9 was not investigated, because, at those pH, Y3+ tends to form insoluble yttrium hydroxide Y(OH)3 [49]. Results with the Tris buffer seem to be slightly poorer than those with the acetate buffer (90.8 ± 0.14%). Moreover, the Tris buffer prevents extraction of 90Y-Hinokitiol in Lipiodol, with formation of an emulsion. Best results were obtained with a pH around 4, with an RCP > 95%.
3.2. Extraction of 90Y-Hinokitiol in Organic Phase and Log P Determination
Once synthesized in optimized conditions, 90Y-Hinokitiol was extracted in organic phases (Figure 3). Its log P was determined to be 2.23. It was extracted with good yields in chlorinated solvents, with an almost quantitative extraction in chloroform (96.14 ± 4.77%). Extraction in oily mixtures was also performed with good yields (85.8 ± 0% in Labrafac WL 1349 and 88.36 ± 20.92% in Lipiodol), demonstrating the high lipophilicity of 90Y-Hinokitiol.
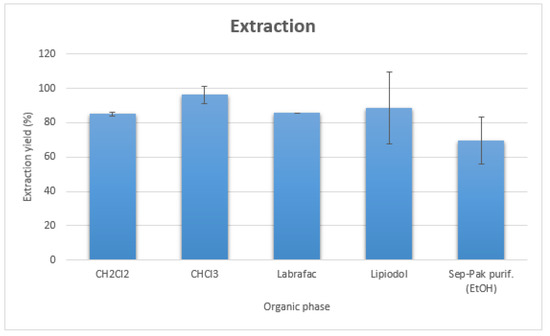
Figure 3.
Extraction yields of 90Y-Hinokitiol in various organic phases.
In view of the automation of the process, we also investigated the preparation of 90Y-Hinokitiol with solid-phase extraction instead of phase separation by centrifugation. 90Y-Hinokitiol solution was passed through a C8 Sep-Pak cartridge (Waters, Milford, MA, USA), washed with 10 mL water to remove hydrophilic impurities and eluted from the cartridge with 2 mL EtOH, with a 69.46 ± 13.59% radiochemical yield (Figure 3).
3.3. Automated Preparation of 90Y-Hinokitiol/Lipiodol
Synthesis took about 40 min, with 15 min for the evaporation of EtOH before the addition of Lipiodol (Figure 4). 90Y-Hinokitiol/Lipiodol was obtained with a radiochemical purity of 99.13 ± 1.24%. The final radiochemical yield was 62.3 ± 9.67% based on initial 90Y activity. Due to the ‘sticky’ character of the lipophilic complex, there was a significant residual activity in different parts of the module, with, for instance, circa 20% in the reaction vessel.
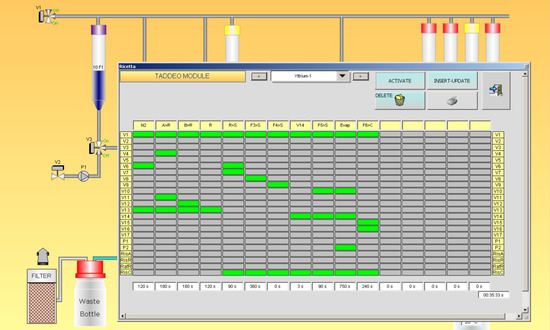
Figure 4.
Remote-controlled process for the preparation of 90Y-Hinokitiol/Lipiodol.
3.4. In Vitro Stability
In the absence or presence of Lipiodol, 90Y-Hinokitiol remained stable for 3 days. RCP of 90Y-Hinokitiol/Lipiodol was 95.17 ± 1.81% at day 3. Then a slight degradation was noticed, but RCP was still over 80% 7 days post-labeling (respectively, 80.9 ± 12.87 and 86.37 ± 20.77% for 90Y-Hinokitiol and 90Y-Hinokitiol/Lipiodol) (Figure 5).
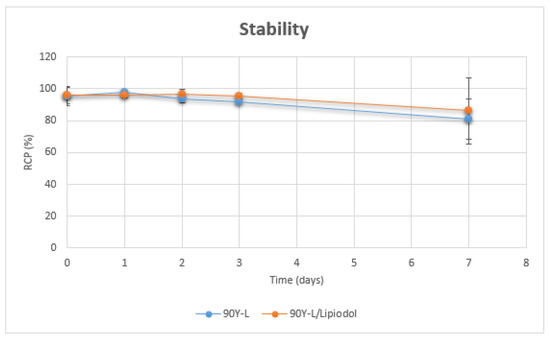
Figure 5.
Stability of 90Y-Hinokitiol and 90Y-Hinokitiol/Lipiodol.
In the presence of excess iron citrate, no transchelation between yttrium and iron was observed. RCP of 90Y-Hinokitiol remained over 90% for 6 days (Figure 6).
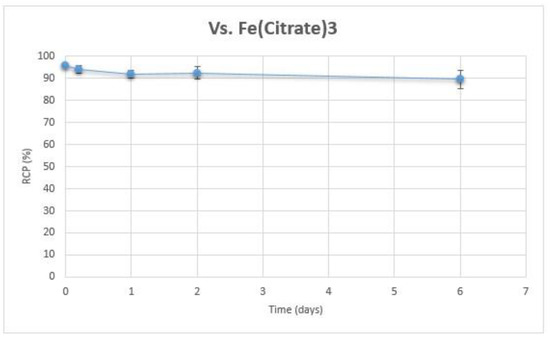
Figure 6.
Stability of 90Y-Hinokitiol vs. 100 mM Fe(citrate)3.
3.5. Over Time Release in the Aqueous Phase
When 90Y-Hinokitiol/Lipiodol was incubated with saline at 37 °C, there was a quick release of Yttrium-90 into the supernatant aqueous phase (38.56 ± 21.21% at 1 h) which then stabilized to reach 50–60% of release over 15 days (Figure 7).
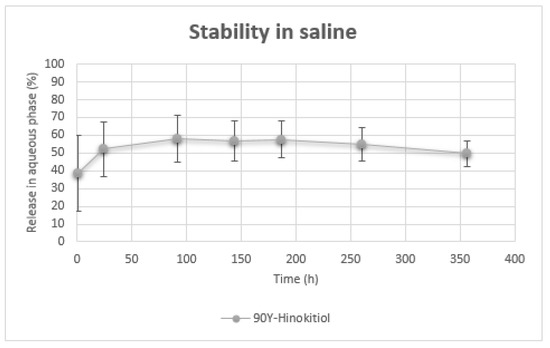
Figure 7.
Release of 90Y in aqueous phase over time.
4. Discussion
The pure beta emitter Yttrium-90 (Eβmax = 2.28 MeV, t1/2 = 64 h) is a radionuclide of choice, that has found wide use for internal radiotherapy [50]. Thus, numerous studies have demonstrated its therapeutic efficacy and safety [21,51]. Among its prominent applications is transarterial radioembolization for the treatment of primary and secondary liver cancers [52,53]. It takes advantage of the dual blood supply of the liver, which permits it to suppress the tumor feeding, while preserving the liver. Lipiodol is an embolizing agent that is particularly suited for chemo/radioembolization. When injected through the hepatic artery, it has been demonstrated to be selectively trapped into the tumor cells, where it displays a prolonged retention time, while being quickly eliminated from healthy liver cells [54,55]. Thus, the combination of Lipiodol embolization with Yttrium-90 irradiation seems attractive. To date, attempts to label Lipiodol with Yttrium-90 have been disappointing, with complicated syntheses and/or low stability [22,23,24,25]. It is worth mentioning that Lipiodol labeling is not done with the formation of a covalent bond between the Lipiodol and the chelate, except for EDTB [22], but instead is reached through solubilization of a lipophilic chelate into the oily medium. Design of new efficient lipophilic complexes is thus of prime importance to conveniently label Lipiodol with Yttrium-90. Use of biocompatible ligands or with known toxicity is also a plus for human use.
Tropolone derivatives are known to form lipophilic complexes with a wide range of metals [56]. Metal complexes based on tropolones demonstrate higher stability than with β-diketones [57,58]. Hirai et al. have shown that yttrium tropolone chelate was one of the most stable tropolone chelates among the di- and trivalent metals they investigated [59]. Hinokitiol is a natural compound already used for human applications and is the most studied tropolone derivative. It also presents a greater lipophilicity than tropolone, and a direct comparison between tropolone and hinokitiol with transuranium elements showed that, with all the metals studied, hinokitiol-based complexes were systematically more stable than their tropolone counterparts [60]. We thus postulated that labeling hinokitiol with yttrium-90 should lead to a stable lipophilic radiotracer.
90Y-Hinokitiol was obtained in 5 min at room temperature. Incubating for longer times led to decreased yields (Figure 2). The radiotracer was satisfactorily obtained with a ligand concentration up to 0.5 mM. Below this concentration, RCP of the final compound decreased rapidly. At 0.01 mM, it was only 62.55 ± 15.91%. 90Y-Hinokitiol could be obtained over a wide range of pH, between 3.5 and 9. For comparison, in the literature, 90Y-oxine was obtained in 15 min at 50 °C using a 0.1 M concentration of oxine [23]. Using a lower concentration of 6.9 mM did not permit the radiotracer to be obtained with a convenient yield.
To assess the lipophilicity of the prepared radiotracer, its log P was determined. Its value of 2.23 indicated its highly lipophilic character. This was further confirmed with the extraction results in organic solvents. 90Y-Hinokitiol was extracted with over 80% yield with all phases investigated. It was possible to directly and efficiently extract it into Lipiodol with a yield of 88.36 ± 20.92%. With the oxine chelate [23,30,31], it was necessary to first extract the radiotracer into dichloromethane, which was subsequently evaporated, before resuspension of the resulting residue into Lipiodol. This resuspension step required 50 °C heating for between 30 and 60 min. The mixed ligand (90Y)Y(dtc)3(Phen) complexes, previously developed by our team [25], were also directly extracted in Lipiodol with yields ranging from 64 to 88% depending on the length of the alkyl chain of the dithiocarbamate (dtc), while 188Re-SSS (log P = 3.33) is almost quantitatively extracted with 98.56 ± 1.2% in the Lipiodol phase [61].
We have also investigated the affinity of 90Y-Hinokitiol for Labrafac WL 1349, another potentially interesting oily medium. Labrafac WL 1349 consists of medium-chain triglycerides, mainly caprylic (C8) and capric (C10) acids. It is used as an excipient to formulate emulsions and nanoemulsions. Labrafac WL 1349 is especially employed as the lipidic core in lipid nanocapsules (LNCs), and, as such, has been used to encapsulate lipophilic radiotracers for radionuclide therapy and imaging, and in particular for HCC treatment [62,63]. 90Y-Hinokitiol was efficiently extracted into this medium with a good yield. It could thus be of interest for encapsulation into LNCs. Radiolabeled oxine has likewise been encapsulated into liposomes, and this strategy could be of interest also for radiolabeled hinokitiol [64,65].
For the preparation of high therapeutic doses, for instance in a centralized radiopharmacy, automation of the procedure appears mandatory. Automated processes have been successfully described for 188Re-labeled Lipiodol [61,66]. This automation led to a decrease in the final yield, due to a loss of activity in the various components of the module (vials, tubings, Sep-Pak cartridge), but, most importantly, allowed for a reduced dose at the fingertips up to 80% [66,67]. The feasibility of automating the preparation of 90Y-Hinokitiol/Lipiodol was therefore investigated. For this, purification of 90Y-Hinokitiol on a Sep-Pak cartridge was first studied. Using a C8 cartridge, 69.46 ± 13.59% of the activity was recovered with 2 mL of Ethanol. Synthesis was then fully automated on a Taddeo module, using a 3-vial system. Vial A contained the commercial (90Y)YCl3 solution, diluted with acetate buffer. Vial B contained the ligand solution, and Vial C was the final sterile vial to collect the desired radiotracer. The content of Vials A and B was transferred to Reactor R, then the resulting solution was purified on a C8 cartridge. After elution of 90Y-Hinokitiol from the cartridge and evaporation of EtOH, the complex was retaken in Lipiodol, with a final yield of 62.3 ± 9.67% and an RCP of 99.13 ± 1.24%. Based on our preliminary experiments with the SPE cartridge, EtOH volume to recover 90Y-Hinokitiol was slightly increased to 2.5 mL. Obtained yields are consistent—and slightly better—with those obtained in the literature with 188Re-Lipiodol (respectively, 52.68 ± 9.6% and 58 ± 3.6% for 188Re-SSS/Lipiodol and 188ReN-DEDC/Lipiodol, another successful 188Re-labeled Lipiodol developed by Duatti’s team, in Italy) [61,66].
Radiochemical purities of 90Y-Hinokitiol and 90Y-Hinokitiol/Lipiodol were regularly checked over a period of one week. The complex demonstrated a good stability, with or without the presence of Lipiodol, since its RCP remained over 90% for 3 days, before slightly decreasing to 80% at 7 days. Moreover, no transchelation with iron occurred in the presence of excess Fe(citrate)3, thus confirming the strong bonding between the ligand and the metal. Unfortunately, when 90Y-Hinokitiol/Lipiodol was incubated at 37 °C in saline, there was a quick release of activity in the aqueous supernatant (Lipiodol has a density of 1.28 at 15 °C). Half of the activity was found in the supernatant at 24 h. For comparison, we prepared 90Y-oxine/Lipiodol, 90Y(dtc)3(Phen)/Lipiodol and 188Re-SSS/Lipiodol, and repeated the same release experiment with them (Figure 8).
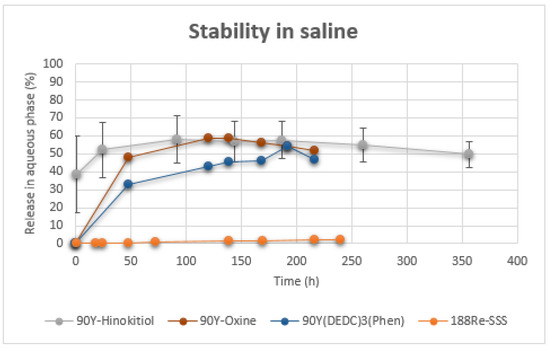
Figure 8.
Release of radioactivity (90Y, 188Re) in aqueous phase over time.
These displayed slightly better stability, at least for initial time points. However, experimental conditions used here might have been harsher than those found in the literature. For instance, Yu et al. reported 87.8% of the total radioactivity was still bound to the Lipiodol after 7 days of incubation with PBS [23], while Lopez et al. reported 82.30 ± 9.48% in the Lipiodol at 8 days for 90Y(DEDC)3(Phen) after incubation with saline [25]. However, this is expected to be more representative of physiological conditions, since radiolabeled Lipiodol will be strongly diluted when injected. In contrast, using the same conditions, 188Re-SSS/Lipiodol showed almost no release in the aqueous phase for the same period of time, with only 2.33 ± 0.28% of release at 10 days, demonstrating its still undisputed stability. Release into the aqueous supernatant could be because 90Y-Hinokitiol is not lipophilic enough to be retained in Lipiodol, or because the complex dissociates, which leads to a release of free Yttrium-90. This latter behavior would be unacceptable for in vivo use, since Yttrium-90, and lanthanides, have a tendency to fix to the bone, thus irradiating the bone marrow. This has been observed with 177Lu-oxine/Lipiodol, for which a significant leakage from the liver, with accumulation of 177Lu activity in the skeleton, was reported [31]. Subsequent analysis of the supernatant, with TLC, showed the presence of 90Y-Hinokitiol, thus indicating an insufficient affinity of 90Y-Hinokitiol with Lipiodol. Using more lipophilic tropolone derivatives might solve this problem. On the other hand, this release may potentially be due to the form of the complex. Yttrium is a trivalent metal, but favors a high coordination number (7 or 8). Depending on reaction conditions, chelating yttrium with tropolonate ligand (L) may lead to the formation of either YL3 or YL4+ [56,68,69]. To complete the coordination sphere in the case of YL3, the metal can bind a solvent molecule or the complexes tend to form small oligomers (YL3)n (n = 2–3), with increased lipophilicity and poor solubility in the aqueous phase [48,68]. The same might also be true for oxine [70]. Addition of a 1,10-phenanthroline ligand can turn this dimeric or trimeric complex into a monomeric one [71]. However, it has been suggested that, at radiotracer level, metal concentration might not be sufficient to form polynuclear species [48]. In these conditions, it is probable the complex is under the tetrakis form and therefore charged, limiting its affinity for Lipiodol. For instance, in a study by Dyrssen, 234Th-tropolone—with Th(IV)—was considerably more extracted than 90Y-tropolone in chloroform [72]. However, in our study, 90Y-Hinokitiol was almost quantitatively extracted in chloroform. Consequently, investigating more lipophilic tropolone derivatives could be of interest to increase retention into Lipiodol. Addition of a 1,10-phenanthroline or trioctylphosphine oxide (TOPO) to complete the coordination sphere, might also be worth considering [73]. Indeed, formation of a TOPO adduct on a scandium–tropolone complex increased its affinity for the organic phase [74]. In parallel, we are currently exploring the suitability of 90Y-Hinokitiol for encapsulation into HCC-targeting polymer nanoparticles [75].
5. Conclusions
90Y-Hinokitiol/Lipiodol was prepared in good yield by the solubilization of 90Y-Hinokitiol complex in Lipiodol. Preparation is fast and simple, which is an advantage for radiopharmaceutical preparation. Full automation of the labeling process is feasible. 90Y-Hinokitiol appears to be stable, even to transchelation with iron. However, poor retention in Lipiodol has been noticed. More studies are needed to increase the retention into Lipiodol. Thus, in these conditions, 90Y-Hinokitiol/Lipiodol may not be suitable for the therapy of HCC, but 90Y-Hinokitiol may be more suited for encapsulation in lipid-core nanocapsules or polymer nanoparticles.
Author Contributions
Conceptualization, N.N., E.G. and N.L.; methodology, N.N. and N.L.; validation, N.N., E.G. and N.L.; formal analysis, N.N., E.G. and N.L.; investigation, C.B., V.A., N.N., E.G. and N.L.; writing—original draft preparation, C.B. and N.L.; writing—review and editing, C.B., V.A., N.N., E.G. and N.L.; visualization, N.N., E.G. and N.L.; supervision, N.N., E.G. and N.L.; project administration, N.L.; funding acquisition, N.N., E.G. and N.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work has been supported by grants from the French National Agency for Research called “Investissements d’Avenir” IRON Labex n◦ANR-11-LABX-0018-01, SATT Ouest Valorisation, Ecole Nationale Supérieure de Chimie de Rennes (ENSCR) and Centre Eugène Marquis.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest related to this work.
References
- Akinyemiju, T.; Abera, S.; Ahmed, M.; Alam, N.; Alemayohu, M.A.; Allen, C.; Al-Raddadi, R.; Alvis-Guzman, N.; Amoako, Y.; Artaman, A.; et al. The Burden of Primary Liver Cancer and Underlying Etiologies from 1990 to 2015 at the Global, Regional, and National Level: Results from the Global Burden of Disease Study 2015. JAMA Oncol. 2017, 3, 1683–1691. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2020, 68, 394–424. [Google Scholar] [CrossRef]
- de Ridder, J.; de Wilt, J.H.; Simmer, F.; Overbeek, L.; Lemmens, V.; Nagtegaal, I. Incidence and origin of histologically confirmed liver metastases: An explorative case-study of 23,154 patients. Oncotarget 2016, 7, 55368–55376. [Google Scholar] [CrossRef]
- Ahmed, I.; Lobo, D.N. Malignant tumours of the liver. Surgery 2009, 27, 30–37. [Google Scholar] [CrossRef]
- Altekruse, S.F.; Devesa, S.S.; Dickie, L.A.; McGlynn, K.A.; Kleiner, D.E. Histological classification of liver and intrahepatic bile duct cancers in SEER registries. J. Regist. Manag. 2011, 38, 201–205. [Google Scholar]
- Frager, S.Z.; Schwartz, J.M. Hepatocellular Carcinoma: Epidemiology, Screening, and Assessment of Hepatic Reserve. Curr. Oncol. 2020, 27, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Johnston, C.J.C.; Healey, A.J. Malignant tumours of the liver. Surgery 2020, 38, 480–486. [Google Scholar] [CrossRef]
- Zhu, R.X.; Seto, W.K.; Lai, C.L.; Yuen, M.F. Epidemiology of Hepatocellular Carcinoma in the Asia-Pacific Region. Gut Liver 2016, 10, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Petrick, J.L.; Florio, A.A.; Znaor, A.; Ruggieri, D.; Laversanne, M.; Alvarez, C.S.; Ferlay, J.; Valery, P.C.; Bray, F.; McGlynn, K.A. International trends in hepatocellular carcinoma incidence, 1978–2012. Int. J. Cancer 2020, 147, 317–330. [Google Scholar] [CrossRef]
- Kulik, L.; El-Serag, H.B. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology 2019, 156, 477.e1–491.e1. [Google Scholar] [CrossRef]
- Yang, J.D.; Hainaut, P.; Gores, G.J.; Amadou, A.; Plymoth, A.; Roberts, L.R. A global view of hepatocellular carcinoma: Trends, risk, prevention and management. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 589–604. [Google Scholar] [CrossRef]
- Lencioni, R. Loco-Regional Treatment of Hepatocellular Carcinoma. Hepatology 2010, 52, 762–773. [Google Scholar] [CrossRef]
- Liapi, E.; Geschwind, J.F. Intra-arterial therapies for hepatocellular carcinoma: Where do we stand? Ann. Surg. Oncol. 2010, 17, 1234–1246. [Google Scholar] [CrossRef]
- Edeline, J.; Gilabert, M.; Garin, E.; Boucher, E.; Raoul, J.L. Yttrium-90 microsphere radioembolization for hepatocellular carcinoma. Liver Cancer 2015, 4, 16–25. [Google Scholar] [CrossRef]
- Bouvry, C.; Palard, X.; Edeline, J.; Ardisson, V.; Loyer, P.; Garin, E.; Lepareur, N. Transarterial Radioembolization (TARE) Agents beyond 90Y-Microspheres. Biomed Res. Int. 2018, 2018, 1435302. [Google Scholar] [CrossRef]
- Ahmadzadehfar, H.; Sabet, A.; Wilhelm, K.; Biersack, H.J.; Risse, J. Iodine-131-lipiodol therapy in hepatic tumours. Methods 2011, 55, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Idée, J.M.; Guiu, B. Use of Lipiodol as a drug-delivery system for transcatheter arterial chemoembolization of hepatocellular carcinoma: A review. Crit. Rev. Oncol. Hematol. 2013, 88, 530–549. [Google Scholar] [CrossRef]
- Lepareur, N.; Ardisson, V.; Noiret, N.; Garin, E. 188Re-SSS/Lipiodol: Development of a Potential Treatment for HCC from Bench to Bedside. Int. J. Mol. Imaging 2012, 2012, 278306. [Google Scholar] [CrossRef]
- Delaunay, K.; Edeline, J.; Rolland, Y.; Lepareur, N.; Laffont, S.; Palard, X.; Bouvry, C.; Le Sourd, S.; Pracht, M.; Ardisson, V.; et al. Preliminary results of the Phase 1 Lip-Re I clinical trial: Biodistribution and dosimetry assessments in hepatocellular carcinoma patients treated with 188Re-SSS Lipiodol radioembolization. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1506–1517. [Google Scholar] [CrossRef]
- Laffont, S.; Rolland, Y.; Ardisson, V.; Edeline, J.; Pracht, M.; Le Sourd, S.; Rohou, T.; Lenoir, L.; Lepareur, N.; Garin, E. Occupational radiation exposure of medical staff performing ⁹⁰Y-loaded microsphere radioembolization. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 824–831. [Google Scholar] [CrossRef]
- Tickner, B.J.; Stasiuk, G.J.; Duckett, S.B.; Angelovski, G. The use of yttrium in medical imaging and therapy: Historical background and future perspectives. Chem. Soc. Rev. 2020, 49, 6169–6185. [Google Scholar] [CrossRef]
- Wang, S.J.; Lin, W.Y.; Lui, W.Y.; Chen, M.N.; Tsai, Z.T.; Ting, G. Hepatic artery injection of yttrium-90-lipiodol: Biodistribution in rats with hepatoma. J. Nucl. Med. 1996, 37, 332–335. [Google Scholar]
- Yu, J.; Häfeli, U.O.; Sands, M.; Dong, Y. 90Y-oxine ethiodol, a potential radiopharmaceutical for the treatment of liver cancer. Appl. Radiat. Isot. 2003, 58, 567–573. [Google Scholar] [CrossRef]
- Mu, P.Y.; Jiang, X.L.; Chen, J.; Wang, J.C.; He, Q.; Zhu, Y.J.; Jin, M.J.; Li, F. Research on extracted 90Y with P204 in lipiodol for liver cancer. J. Radioanal. Nucl. Chem. 2007, 272, 669–671. [Google Scholar] [CrossRef]
- Lopez, A.; Noiret, N.; Garin, E.; Lepareur, N. Mixed-ligand complexes of yttrium-90 dialkyldithiocarbamates with 1,10-phenanthroline as a possible agent for therapy of hepatocellular carcinoma. Appl. Radiat. Isot. 2014, 94, 241–246. [Google Scholar] [CrossRef]
- Thakur, M.L.; Coss, R.; Howell, R.; Vassileva-Belnikolovska, D.; Liu, J.; Rao, S.P.; Spana, G.; Wachsberger, P.; Leeper, D.L. Role of lipid-soluble complexes in targeted tumor therapy. J. Nucl. Med. 2003, 44, 1293–1300. [Google Scholar] [PubMed]
- Savić-Gajić, I.M.; Savić, I.M. Drug design strategies with metal-hydroxyquinoline complexes. Expert Opin. Drug Discov. 2020, 15, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Giraudo, C.; Evangelista, L.; Fraia, A.S.; Lupi, A.; Quaia, E.; Cecchin, D.; Casali, M. Molecular Imaging of Pulmonary Inflammation and Infection. Int. J. Mol. Sci. 2020, 21, 894. [Google Scholar] [CrossRef]
- Firsching, F.H.; Cuca, R.C. Solubility products of the rare-earth 8-Quinolinates. J. Chem. Eng. Data 1981, 26, 116–118. [Google Scholar] [CrossRef]
- Das, T.; Chakraborty, S.; Sarma, H.D.; Venkatesh, M.; Banerjee, S. Preparation of 166Ho-oxine-lipiodol and its preliminary bioevaluation for the potential application in therapy of liver cancer. Nucl. Med. Commun. 2009, 30, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.; Das, T.; Chakraborty, S.; Sarma, H.D.; Banerjee, S.; Samuel, G.; Venkatesh, M. Preparation of 177Lu-labeled oxine in lipiodol as a possible agent for therapy of hepatocellular carcinoma: A Preliminary Animal Study. Cancer Biother. Radiopharm. 2010, 25, 539–543. [Google Scholar] [CrossRef]
- Welch, M.J.; Thakur, M.L.; Coleman, R.E.; Patel, M.; Siegel, B.A.; Ter-Pogossian, M. Gallium-68 labeled red cells and platelets: New agents for positron tomography. J. Nucl. Med. 1977, 18, 558–562. [Google Scholar] [PubMed]
- Loken, M.K.; Clay, M.E.; Carpenter, R.T.; Boudreau, R.J.; McCullough, J.J. Clinical use of indium-111 labeled blood products. Clin. Nucl. Med. 1985, 10, 902–911. [Google Scholar] [CrossRef] [PubMed]
- Datz, F.L.; Bedont, R.A.; Baker, W.J.; Alazraki, N.P.; Taylor, A., Jr. No difference in sensitivity for occult infection between tropolone- and oxine-labeled indium-111 leukocytes. J. Nucl. Med. 1985, 26, 469–473. [Google Scholar] [PubMed]
- Vallabhajosula, S.; Machac, J.; Goldsmith, S.J.; Lipszyc, H.; Badimon, L.; Rand, J.; Fuster, V. Indium-111 platelet kinetics in normal human subjects: Tropolone versus oxine methods. J. Nucl. Med. 1986, 27, 1669–1674. [Google Scholar]
- Hendershott, L.; Gentilcore, R.; Ordway, F.; Fletcher, J.; Donati, R. Tropolone: A lipid solubilizing agent for cationic metals. Eur. J. Nucl. Med. 1982, 7, 234–236. [Google Scholar] [CrossRef] [PubMed]
- Schmaljohann, J.; Karanikas, G.; Sinzinger, H. Synthesis of cobalt-55/57-complexes for radiolabelling of platelets as a potential PET imaging agent. J. Label. Compd. Radiopharm. 2001, 44, 395–403. [Google Scholar] [CrossRef]
- Socan, A.; Petrik, M.; Kolenc Peitl, P.; Krošelj, M.; Rangger, C.; Novy, Z.; Svajger, U.; Gmeiner, T.; Decristoforo, C. On-cartridge preparation and evaluation of 68Ga-, 89Zr- and 64Cu-precursors for cell radiolabelling. Nucl. Med. Biol. 2019, 71, 23–31. [Google Scholar] [CrossRef]
- Ballinger, J.R.; Boxen, I. Gallium-67-labelled red blood cells as a blood-pool marker for dual-isotope imaging. Int. J. Radiat. Appl. Instrum. Part B. Nucl. Med. Biol. 1992, 19, 79–81. [Google Scholar] [CrossRef]
- Yano, Y.; Budinger, T.F.; Ebbe, S.N.; Mathis, C.A.; Singh, M.; Brennan, K.M.; Moyer, B.R. Gallium-68 lipophilic complexes for labeling platelets. J. Nucl. Med. 1985, 26, 1429–1437. [Google Scholar]
- Ferris, T.J.; Charoenphun, P.; Meszaros, L.K.; Mullen, G.E.; Blower, P.J.; Went, M.J. Synthesis and characterisation of zirconium complexes for cell tracking with Zr-89 by positron emission tomography. Dalton Trans. 2014, 43, 14851–14857. [Google Scholar] [CrossRef] [PubMed]
- Spitznagle, L.A.; Marino, C.A.; Kasina, S. Tropolone, a ligand for a lipophilic chelate with technetium-99m. J. Radioanal. Nucl. Chem. 1982, 74, 307–313. [Google Scholar] [CrossRef]
- Imai, N.; Doi, Y.; Nabae, K.; Tamano, S.; Hagiwara, A.; Kawabe, M.; Ichihara, T.; Ogawa, K.; Shirai, T. Lack of hinokitiol (beta-thujaplicin) carcinogenicity in F344/DuCrj rats. J. Toxicol. Sci. 2006, 31, 357–370. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Saniewski, M.; Horbowicz, M.; Kanlayanarat, S. The Biological Activities of Troponoids and Their Use in Agriculture A Review. J. Hortic. Res. 2014, 22, 5–19. [Google Scholar] [CrossRef]
- Shen, Y.F.; Ho, C.C.; Shie, M.Y.; Wang, K.; Fang, H.Y. Hinokitiol-Loaded Mesoporous Calcium Silicate Nanoparticles Induce Apoptotic Cell Death through Regulation of the Function of MDR1 in Lung Adenocarcinoma Cells. Materials 2016, 9, 306. [Google Scholar] [CrossRef]
- Jayakumar, T.; Liu, C.H.; Wu, G.Y.; Lee, T.Y.; Manubolu, M.; Hsieh, C.Y.; Yang, C.H.; Sheu, J.R. Hinokitiol Inhibits Migration of A549 Lung Cancer Cells via Suppression of MMPs and Induction of Antioxidant Enzymes and Apoptosis. Int. J. Mol. Sci. 2018, 19, 939. [Google Scholar] [CrossRef] [PubMed]
- Grillo, A.S.; SantaMaria, A.M.; Kafina, M.D.; Cioffi, A.G.; Huston, N.C.; Han, M.; Seo, Y.A.; Yien, Y.Y.; Nardone, C.; Menon, A.V.; et al. Restored iron transport by a small molecule promotes absorption and hemoglobinization in animals. Science 2017, 356, 608–616. [Google Scholar] [CrossRef]
- Noro, J. Solvent extraction of several trivalent metal ions with 4-isopropyltropolone into chloroform. Anal. Sci. 1998, 14, 1099–1105. [Google Scholar] [CrossRef]
- Pourbaix, M. Atlas of Electrochemical Equilibria in Aqueous Solutions, 2nd ed.; National Association of Corrosion Engineers: Houston, TX, USA, 1974; pp. 177–182. [Google Scholar]
- Uccelli, L.; Martini, P.; Cittanti, C.; Carnevale, A.; Missiroli, L.; Giganti, M.; Bartolomei, M.; Boschi, A. Therapeutic Radiometals: Worldwide Scientific Literature Trend Analysis (2008–2018). Molecules 2019, 24, 640. [Google Scholar] [CrossRef]
- Goffredo, V.; Paradiso, A.; Ranieri, G.; Gadaleta, C.D. Yttrium-90 (90Y) in the principal radionuclide therapies: An efficacy correlation between peptide receptor radionuclide therapy, radioimmunotherapy and transarterial radioembolization therapy. Ten years of experience (1999–2009). Crit. Rev. Oncol. Hematol. 2011, 80, 393–410. [Google Scholar] [CrossRef]
- Saini, A.; Wallace, A.; Alzubaidi, S.; Knuttinen, M.G.; Naidu, S.; Sheth, R.; Albadawi, H.; Oklu, R. History and Evolution of Yttrium-90 Radioembolization for Hepatocellular Carcinoma. J. Clin. Med. 2019, 8, 55. [Google Scholar] [CrossRef]
- Pellegrinelli, J.; Chevallier, O.; Manfredi, S.; Dygai-Cochet, I.; Tabouret-Viaud, C.; Nodari, G.; Ghiringhelli, F.; Riedinger, J.-M.; Popoff, R.; Vrigneaud, J.-M.; et al. Transarterial Radioembolization of Hepatocellular Carcinoma, Liver-Dominant Hepatic Colorectal Cancer Metastases, and Cholangiocarcinoma Using Yttrium90 Microspheres: Eight-Year Single-Center Real-Life Experience. Diagnostics 2021, 11, 122. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Choi, S.I.; Kim, H.; Yoo, H.S.; Lee, Y.B. Distribution of Lipiodol in hepatocellular carcinoma. Liver Int. 1990, 10, 72–78. [Google Scholar] [CrossRef]
- Gaba, R.C.; Schwind, R.M.; Ballet, S. Mechanism of Action, Pharmacokinetics, Efficacy, and Safety of Transarterial Therapies Using Ethiodized Oil: Preclinical Review in Liver Cancer Models. J. Vasc. Interv. Radiol. 2018, 29, 413–424. [Google Scholar] [CrossRef]
- Dutt, Y.; Singh, R.P.; Katyal, M. Metal complexes with tropolones. Talanta 1969, 16, 1369–1382. [Google Scholar] [CrossRef]
- Bryant, B.E.; Fernelius, W.C.; Douglas, B.E. Formation Constants of Metal Complexes of Tropolone and Its Derivatives. I. Tropolone. J. Am. Chem. Soc. 1953, 75, 3784–3786. [Google Scholar] [CrossRef]
- Bryant, B.E.; Fernelius, W.C. Formation Constants of Metal Complexes of Tropolone and its Derivatives. II. Some Alkyltropolones. J. Am. Chem. Soc. 1954, 76, 1696–1697. [Google Scholar] [CrossRef]
- Hirai, M.; Oka, Y. Stability of Tropolone Chelates of the Bi- and Tervalent Metal Ions. Bull. Chem. Soc. Jpn. 1970, 43, 778–782. [Google Scholar] [CrossRef]
- Cilindro, L.G.; Keller, C. Complexation of some Transuranium Elements with Tropolone and β-Isopropyltropolone. Radiochim. Acta 1974, 21, 29–32. [Google Scholar] [CrossRef]
- Lepareur, N.; Ardisson, V.; Noiret, N.; Boucher, E.; Raoul, J.L.; Clément, B.; Garin, E. Automation of labelling of Lipiodol with high-activity generator-produced 188Re. Appl. Radiat. Isot. 2011, 69, 426–430. [Google Scholar] [CrossRef]
- Cahouet, A.; Denizot, B.; Hindré, F.; Passirani, C.; Heurtault, B.; Moreau, M.; Le Jeune, J.; Benoît, J. Biodistribution of dual radiolabeled lipidic nanocapsules in the rat using scintigraphy and gamma counting. Int. J. Pharm. 2002, 242, 367–371. [Google Scholar] [CrossRef]
- Vanpouille-Box, C.; Lacoeuille, F.; Roux, J.; Aubé, C.; Garcion, E.; Lepareur, N.; Oberti, F.; Bouchet, F.; Noiret, N.; Garin, E.; et al. Lipid nanocapsules loaded with rhenium-188 reduce tumor progression in a rat hepatocellular carcinoma model. PLoS ONE 2011, 6, e16926. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.E.; Yu, H.M.; Lu, Y.C.; Heish, N.N.; Tseng, Y.L.; Huang, K.L.; Chuang, K.T.; Chen, C.H.; Hwang, J.J.; Lin, W.J.; et al. Internal radiotherapy and dosimetric study for 111In/177Lu-pegylated liposomes conjugates in tumor-bearing mice. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrom. Detect. Assoc. Equip. 2006, 569, 533–537. [Google Scholar] [CrossRef]
- Gawne, P.J.; Clarke, F.; Turjeman, K.; Cope, A.P.; Long, N.J.; Barenholz, Y.; Terry, S.Y.A.; de Rosales, R.T.M. PET Imaging of Liposomal Glucocorticoids using 89Zr-oxine: Theranostic Applications in Inflammatory Arthritis. Theranostics 2020, 10, 3867–3879. [Google Scholar] [CrossRef] [PubMed]
- Uccelli, L.; Pasquali, M.; Boschi, A.; Giganti, M.; Duatti, A. Automated preparation of Re-188 lipiodol for the treatment of hepatocellular carcinoma. Nucl. Med. Biol. 2011, 38, 207–213. [Google Scholar] [CrossRef]
- Lepareur, N.; Laffont, S.; Ardisson, V.; Noiret, N.; Garin, E. Reduction of β-radiation exposure during preparation of 188Re-labelled Lipiodol for hepatocellular carcinoma treatment. Nucl. Med. Commun. 2012, 33, 205–208. [Google Scholar] [CrossRef]
- Muetterties, E.L.; Wright, C.M. Chelate Chemistry. III. Chelates of High Coordination Number. J. Am. Chem. Soc. 1965, 87, 4706–4717. [Google Scholar] [CrossRef]
- Campbell, D.L.; Moeller, T. Observations on the rare earths MLXXXI. Formation constants of tropolone chelates of the tripositive ions at 25. J. Inorg. Nucl. Chem. 1969, 31, 1077–1082. [Google Scholar] [CrossRef]
- van Deun, R.; Fias, P.; Nockemann, P.; Schepers, A.; Parac-Vogt, T.N.; van Hecke, K.; van Meervelt, L.; Binnemans, K. Rare-Earth Quinolinates: Infrared-Emitting Molecular Materials with a Rich Structural Chemistry. Inorg. Chem. 2004, 43, 8461–8469. [Google Scholar] [CrossRef]
- Bertolo, L.; Tamburini, S.; Vigato, P.A.; Porzio, W.; Macchi, G.; Meinardi, F. Tris(tropolonato)phenanthroline Lanthanide(III) Complexes as Photochemical Devices. Eur. J. Inorg. Chem. 2006, 2006, 2370–2376. [Google Scholar] [CrossRef]
- Dyrssen, D.; Uusitalo, E.; Larsen, I.; Prydz, H. Studies on the extraction of metal complexes. Acta Chem. Scand. 1955, 9, 1567–1574. [Google Scholar] [CrossRef]
- Narbutt, J.; Czerwiński, M.; Krejzler, J. Seven-Coordinate d0 and d10 Ions—Computational and Experimental Studies on Tris(tropolonato)metal(III)−TOPO Adducts. Eur. J. Inorg. Chem. 2001, 2001, 3187–3197. [Google Scholar] [CrossRef]
- Narbutt, J.; Krejzler, J. High coordination numbers of metal ions in chelate complexes: Molecular adducts of scandium tris-α-diketonates in solution. Inorg. Chim. Acta 1999, 286, 175–180. [Google Scholar] [CrossRef]
- Brossard, C.; Vlach, M.; Vène, E.; Ribault, C.; Dorcet, V.; Noiret, N.; Loyer, P.; Lepareur, N.; Cammas-Marion, S. Synthesis of poly(malic acid) derivatives end-functionalized with peptides and preparation of biocompatible nanoparticles to target hepatoma cells. Nanomaterials 2021, 11, 958. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).