Abstract
The widescale distribution of hydrogen through gas networks is promoted as a viable and cost-efficient option for optimising its application in heat, industry, and transport. It is a key step towards achieving decarbonisation targets in the UK. A key consideration before the injection of hydrogen into the UK gas networks is an assessment of the difference in hydrogen contaminants presence from different production methods. This information is essential for gas regulation and for further purification requirements. This study investigates the level of ISO 14687 Grade D contaminants in hydrogen from steam methane reforming, proton exchange membrane water electrolysis, and alkaline electrolysis. Sampling and analysis of hydrogen were carried out by the National Physical Laboratory following ISO 21087 guidance. The results of analysis indicated the presence of nitrogen in hydrogen from electrolysis, and water, carbon dioxide, and particles in all samples analysed. The contaminants were at levels below or at the threshold limits set by ISO 14687 Grade D. This indicates that the investigated production methods are not a source of contaminants for the eventual utilisation of hydrogen in different applications including fuel cell electric vehicles (FCEV’s). The gas network infrastructure will require a similar analysis to determine the likelihood of contamination to hydrogen gas.
1. Introduction
There is a global push to promote the utilisation of low carbon gases with the view to reduce greenhouse gas (GHG) emissions. This is predominantly driven by the need to mitigate the effects of climate change. Initiatives in the UK and several European countries indicate the need to replace natural gas in the conventional gas networks with hydrogen [1,2,3]. This could be key to lowering carbon emissions from hard to decarbonise sectors like transport, heat, and industry, which combined contribute to over 50% of the share of GHG emission in the UK [4,5,6,7,8]. The European Commission’s hydrogen strategy and the European Union’s hydrogen roadmap also affirms that hydrogen is a key building block for meeting Europe’s climate neutrality targets for 2050; its utilisation could lead to increased energy security with the view of reducing reliance on imported fuels [6,9]. Hydrogen can be conventionally produced from several feedstocks and processes. This includes reformation or thermochemical conversion of fossil fuels, electrolytic water splitting processes, and biological conversion of biomass residues [10,11,12,13]. Studies in the UK indicate that short term to mid-term large scale production of hydrogen for grid injection and energy applications will predominantly be carried out by electrolytic routes and thermochemical routes notably steam methane reforming; this is because of the maturity of these processes [13]. Steam methane reforming is likely to be favoured for areas with higher demand and electrolysis utilised for regional demand and grid balancing [13,14]. Therefore, the preponderant hydrogen production methods selected in this study are steam methane reforming, proton exchange membrane water electrolyser and alkaline electrolyser.
Hydrogen fuel can be distributed through various means such as the use of pipelines or the gas network, high pressure tube trailers and liquefied hydrogen tanks. The conventional gas network infrastructure is promoted due to its cost effectiveness, particularly for long distances. There are several technological challenges associated with hydrogen distribution by gas networks to the end-users (i.e., for heat application, transport and industry): Some of these include possible network pipeline material degradation, leakages, odorisation, gas metering, and gas quality [15]. The quality of gas, in particular, might be influenced by several sources in the distribution network and also from the hydrogen production process; some of the factors that might affect gas quality from the production process include the source and composition of feedstock utilised, scale of production, the level of pre-treatment carried out, the process or equipment utilised, materials utilised, and the gas clean up employed.
There are different specifications for hydrogen gas quality depending on the application of the gas; ISO 14687: 2019 stipulates the threshold or maximum concentration of specific individual contaminants that can be present in hydrogen fuel for utilisation in vehicular and stationary applications [16]. A new hydrogen purity specification has also been developed as part of the Hy4Heat programme for recommended quality requirements for hydrogen being distributed through the gas grid for domestic/commercial heating applications.
The hydrogen gas quality is influenced by the production method. Thermochemical production processes for the production of hydrogen may lead to the presence of contaminants such as methane (CH4), nitrogen (N2), carbon monoxide (CO) and carbon dioxide (CO2); other trace constituents, argon (Ar), ammonia (NH3), formic acid (HCOOH), formaldehyde (HCHO), total hydrocarbons (THC) excluding CH4, sulphur-containing compounds, and halogens) dependent on the feedstock utilised, level of pre-treatment and purification [17,18,19,20,21]. Possible contaminants from electrolytic routes to produce hydrogen are oxygen (O2) N2, CO2 and water (H2O). It is reported that the presence of other ISO 14687 grade D contaminants is unlikely in hydrogen produced from thermochemical and electrolytic routes [17,18,19,20].
A comparison of hydrogen gas quality from the different production routes is essential to understand the potential variability between hydrogen injected into the future gas network. Understanding the levels of contaminants will also support regulations and standard of hydrogen quality for gas grid injection.
The objective of this study is to ascertain the variety and level of contamination that occurs in real samples of hydrogen from conventional production methods. It presents the comparison of hydrogen gas quality from three production sources: steam methane reforming (SMR), proton exchange membrane (PEM) water electrolysis and alkaline electrolysis. A review of the literature indicates that these three hydrogen production methods are the most suitable and realistic on a large scale for gas grid injection in the short term [5,13]. SMR production method was selected as it is believed to be the most realistic way to produce hydrogen that will be injected into the gas grid on a large or centralised scale. The alkaline electrolyser was selected because of the growing assertions that it might be sustainable for distributed and regional injection into the gas grid as demonstrated in several projects occurring regionally in the UK [2]. The PEM water electrolyser was selected because of its prominent use for onsite hydrogen production in several refueling stations. This work was carried out as part of the Hydrogen Grid to Vehicle (HG2V) project [22].
2. Materials and Methods
2.1. Hydrogen Gas Sampling
Hydrogen gas from an SMR, alkaline electrolyser and PEM water electrolyser was sampled by NPL at the operating sites using a novel sampling system as detailed in Figure 1. Hydrogen from SMR was sampled at a 15 bar sampling point after the Pressure Swing Adsorption (PSA) stage. Hydrogen from the alkaline electrolyser was sampled at a 9.8 bar sampling point after a de-oxygenation unit/dryer and hydrogen from the PEM water electrolyser was sampled at a 20 bar sampling point after Temperature Swing Adsorption (TSA).
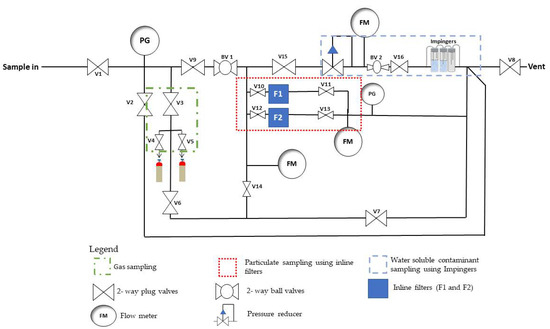
Figure 1.
Schematics of sampling system.
The same sampling methodology was applied for all the samplings. The gas sampling from all hydrogen production plants was performed by the National Physical Laboratory (NPL). Approximately 150 L of gas was collected from each source into 10 L pre-evacuated (<10−7 mbar) aluminium cylinders with spectraseal treatment (BOC, Guildford, UK). The gas samples were taken through the sampling points after plug valve 4 and 5 in the sampling system (Figure 1) after a sufficient purge of the sampling line with the sampled gas for each source. Particulates in the hydrogen gas were trapped using pre-weighed 47 mm diameter filters (0.2 µm Pore Size PTFE Filter with PFA support ring from MTL, UK) held in-line with high-pressure stainless-steel filter holders from Millipore (XX4504700, Millipore, Hertfordshire, UK). Two in-line filter holders are arranged between plug valve V10 and V11 and between plug valve V12 and V13 in the sampling system (Figure 1). The hydrogen gas volume that passed through the filter was equivalent to 400–500 L (40 min at ~10–12 L/min). Water-soluble contaminants were investigated using three in-line PFA (fluoropolymer) impingers arranged in series after plug valve V16 in the sampling system (Figure 1); 25 mL of de-ionised water was utilised as a collection liquid in two of the impinger bottles whilst the third bottle was left empty. Approximately 1000 L of hydrogen gas passed through the impinger bottles for each sampling.
2.2. Hydrogen Gas Analysis
The amount fraction of ISO 14687 Grade D contaminants in the sampled gas were quantified and evaluated at NPL’s hydrogen purity laboratory. NPL is the UKs national metrology institute and developed analytical methods to measure the hydrogen fuel contaminant listed in ISO 14687. The instruments utilised in the analysis of the sampled gas is summarized in Table 1. N2, O2 and Ar were analysed by gas chromatography (GC) (Agilent Technologies, Didcot, UK) with pulsed discharge helium ionisation detector (PDHID, VICI, CH) using helium as a carrier gas (Pre filtered helium was utilised as carrier gas (Purity > 99.9999%, (He BIP®, Air Products, London, UK))). The GC/PDHID sampling loop was 1 mL. The sample was transferred onto capillary column molsieve 5A plot (30 m × 0.53 mm × 50 µm) and a second capillary column molsieve 5A plot (50 m × 0.53 mm × 50 µm). The GC oven was set at 30 degrees Celsius. Water was measured using quartz crystal microbalance, QMA401 (Michell, Cambridgeshire, UK). Gases are sampled directly from the gas cylinder to the analyser, a valve was used to restrict the flow to 0.333 L/min for the QMA. Formic acid, formaldehyde and ammonia were measured using selected ion flow tube mass spectrometry (SIFT-MS, Syft, Christchurch, New Zealand). The measurements were performed using H3O+ reagent ions for formic acid and formaldehyde and product ions HCOOH2+ (m/z = 47 a.m.u.) and CH3O+ (m/z = 31 a.m.u). The measurements were performed using O2+ reagent ions for ammonia and the product ion NH3+ (m/z = 17 a.m.u.). The SIFT-MS vaccum pressure was set at 104 mTorr with an overflow of 140–160 mL/min. Helium was measured using gas chromatography with thermal conductivity detector (GC-TCD) (Agilent Technologies, Didcot, UK). The method used one Hayesep Q 80/100 mesh 2 m × 1/8” outer diameter × 2.0 mm inner diameter column and one Molesieve 5A 80/100 mesh 9 ft × 1/8” outer diameter × 2 mm inner diameter column with hydrogen carrier (Pre filtered hydrogen was utilised as carrier gas (Purity > 99.9999%, (H2 BIP®, Air Products, London, UK))). The loop size used for sample injection was 2 mL. Methane, carbon monoxide, carbon dioxide and total hydrocarbons excluding methane were measured GC (Peak Laboratories, California, USA) coupled with a methaniser and flame ionisation detector (FID). The method used a Haysep D column (186” × 1.5”) with nitrogen carrier with the column held at a temperature of 65 degrees Celsius. The loop size used for sample injection was 5 mL. Total sulphur compounds were measured by gas chromatography with sulphur chemiluminescence detector (GC-SCD). The analysis of the sample is performed on an Agilent 7890A gas chromatograph (Agilent, California, USA) equipped with two detectors, a flame ionisation detector and sulfur chemiluminescence detector (SCD 355, Agilent Technologies, California, USA). The GC-SCD sampling loop volume was 1 mL and the sample was then transferred onto capillary column used which is a HP-5, 30 m × 0.320 mm ID × 0.251 µm film thickness (Agilent, California, USA). The column program temperature is isothermal at 110 °C. Helium is used as a carrier gas at a flow rate of 20 mL/min. Organo-halogenated compounds were analysed using a TD-GC (Markes International, Bridgend, UK) coupled with mass spectroscopy (MS) and an FID (Agilent Technologies, Didcot, UK). The compounds were adsorbed onto a Chromosorb tube. This system desorbs the analytes from the sorbent and releases the analytes onto a U-T6SUL cold trap. A DB-VRX column 60 m × 0.25 mm with a helium carrier was used for separation. All analyses were calibrated using NPL gravimetric gas standards in hydrogen matrix gas (The gas standards utilised were in house standards prepared in accordance with ISO 6142-1 [23]). Gravimetric standards and/or dynamic standards (prepared by dilution using mass flow controller system (Bronkhorst, Ruurlo, NL)) were used to generate calibration curve ranging covering the ISO 14687 grade D threshold and the measured values (as long as it is above the limit of detection). The data was scrutinised however no result was discarded without a technical reason. The calibration curve, results of analysis and uncertainties associated were determined using NPL software XLGENline [24]. An expanded uncertainty (of k = 2) was used in this study but in some cases, a more conservative uncertainty was derived from scientific experience.

Table 1.
Analytical Methods for ISO 14687 contaminants.
The amount fraction of the particulates (mass of particulate per mass of gas passing through the filters) was calculated by taking an average of the mass of particulates from the two inline filters used during sampling. Weighing of the filters before sampling and after the passage of gas through the filters was performed using XP2U (Mettler Toledo, Royston, UK).
The de-ionised water samples from the impinger tubes were analysed by Ion Exchange Chromatography systems for a suite of Anions (Chloride, Nitrate, Sulphate) and Cations (Lithium, Sodium, Ammonium, Potassium, Magnesium, Calcium), in accordance with NPL’s in-house procedure. The Ion Chromatography systems used were ICS-1500 (carbonate/bicarbonate eluent) (Dionex, CA, USA) and ICS-2100 (MSA eluent) (Dionex, CA, USA) for anions and cations respectively, both with conductivity suppressors. The samples were analysed as supplied, no dilution or matrix matching were required. An inductively coupled plasma mass spectrometry (ICP-MS) by Agilent (Agilent 8800 Triple Quadrupole ICP-MS) was also utilised to carry out indicative analysis on the de-ionised water samples to identify the presence of additional ionic contaminants that might be present in the samples.
3. Results and Discussion
3.1. Result of Analysis
The three production routes did not show contaminants significantly above the threshold set by ISO 14687 Grade D (see Table 2).

Table 2.
Analysis of ISO 14687 contaminants found in hydrogen production sources.
The results of analysis are presented with measurement uncertainty at 95% confidence level. It should be noted that the amount fraction levels for all the compounds (except water, nitrogen, carbon dioxide, argon and particulate were below the limit of detection of NPL analytical methods. All the results of analysis from all the hydrogen production method were below the ISO 14687 threshold (except for water for PEMWE). The water amount fraction for the PEMWE is slightly higher when compared to other production methods; whilst water is a possible contaminant from this production method, water at low amount fractions as those specified by the ISO 14687 grade D specification can easily be introduced during sampling. For this reason, further work might be required to validate the accuracy of sampling and analysis of water amount fractions. Following the complexity of sampling water amount in hydrogen fraction at hydrogen production method, online measurement needs to be investigated to avoid inaccuracy during the sampling for water amount fraction in hydrogen.
Particulate analysis at low amount fraction (1 mg/kg) in low pressure hydrogen stream is a challenge for several reasons. The particulate amount fraction trapped in the inline filters was between 1.4 and 1.8 mg/kg with an uncertainty of 0.36–0.6 mg/kg. It is important to realise that the volume of gas passed through the filter was only 400–500 L and with a state-of-the-art ultra-trace balance the measurement uncertainty is few micrograms. Therefore, the amount of hydrogen gas becomes a limiting factor for this experiment. In an ideal scenario, it would have been important to sample a much larger volume 5000–10,000 L in order to improve the measurement uncertainty. The current results of analysis reflect the complexity of particulate analysis and the requirement of a large volume of gas to be passed through the filters.
3.2. Hydrogen Gas Contamination from the Three Sources
Literature indicates the possible presence of CH4, N2, CO and CO2 in the product stream of thermochemical routes to hydrogen such as SMR [17,18,19,20]. The results of analysis from this study indicates low levels of CO2 (4-8 times lower than ISO 14687 threshold) whilst the amount fractions of CH4, N2 and CO were significantly lower than the ISO 14687. This could indicate a high efficiency of the purification technique utilised in this conventional process. Asides from Ar and H2O which were identified at low fractions from the results of analysis of hydrogen from the SMR, all other gaseous ISO 14687 contaminants were lower than the limit of detection of NPLs analytical methods.
Previous studies identify O2, N2, CO2 and H2O as possible ISO 14687 contaminants from electrolytic routes to produce hydrogen with the most probable contaminant being O2 [17]. The result of analysis of hydrogen from PEM electrolyser and alkaline electrolyser indicates the presence of N2, CO2 and H2O. There is a similar profile for these contaminants in hydrogen samples from both electrolyser samples except the H2O content in hydrogen from PEM water electrolyser which is at the threshold stipulated by ISO 14687 Grade D. The results of analysis from this study confirm that the amount fraction of every other gaseous ISO 14687 contaminant were lower than ISO 14687 threshold.
This study demonstrates that the current hydrogen production methods provide hydrogen with a quality which is compatible with the most stringent standard (ISO 14687 Grade D for transport application) therefore it is suitable for injection in the gas grid as it will meet other current specification for hydrogen utilisation such as ISO 14687 Grade A. It will be important to study the actual gas network to understand if additional contamination can occur within the network and be detrimental to the hydrogen gas quality. This next activity is critical for the future transportation of hydrogen gas unto the gas network and to ensure that the end-users will benefit from the hydrogen fuel quality injected into the gas network.
3.3. Extensive Analysis for Additional Impurities
The results of analysis from this study raised several points regarding the presence of contaminants that may not have been identified in the current literature especially around particulates, hydrocarbons and water-soluble compounds.
The particulates amount fraction trapped in the inline filters was around the ISO 14687 Grade D threshold. As highlighted in Section 3.1, there are improvements to be made to the sampling method, specifically with regards to increasing the volume of hydrogen passing through the inline filters. The presence of particulates in the gas could have been derived from product gas, leachate of distribution lines/pipes or low-level degradation of equipment utilised in the plants (i.e., compressor oil, cleaning agent). As no additional analysis was carried out on the filters, it would be difficult to interpret the actual chemical composition of the contaminants which is crucial to identify the source of contamination.
Additional analysis was carried out on the sampled hydrogen gas to identify if the presence of hydrocarbon contaminants in the gas phase (i.e oil leachates) may be related to the presence of particulates. A large amount of hydrogen sample was passed through dedicated sorbent material in order to trap extremely small quantities of hydrocarbon constituents (C2–C13). Analysis by TD-GC-MS/FID indicated that the concentration of individual hydrocarbon constituents (C2–C13) in the gas for all routes of hydrogen were also below 0.08 µg/g. This indicates that the particulates trapped in the inline filters were possibly not related to oil or grease.
Trace element analysis of the hydrogen gas (Table 3) sampled using impingers did not identify any water-soluble contaminants (anions and cations) above the limit of detection of NPL analytical methods (0.20 µg/g). The trace element analysis also indicates the level of Hg in hydrogen in all three samples analysed is well below the threshold set for other quality specification for hydrogen utilisation like ISO 14687 including Type 1 Grade B (Quality specification for gaseous hydrogen; industrial fuel for power generation and heat generation except PEM fuel cell applications) which is set at 0.004 μmol/mol.

Table 3.
Trace water-soluble contaminants from all hydrogen production sources.
4. Conclusions
There is a strong case for the injection of hydrogen in the UK gas grid to promote a low carbon hydrogen economy. This could be done either as a blend of hydrogen and natural gas or as 100% hydrogen in the gas grid. If the hydrogen was sufficiently pure to meet the requirements of Hy4Heat WP2 [25] and ISO 14687 grade D [16], it could be used for heating applications, there would also be the potential to use the grid to easily transport and supply fuel to hydrogen refuelling stations across the UK.
Hydrogen gas was sampled from three different production sources (SMR, alkaline and PEM water electrolyser) for this study with the view of understanding the level and variety of contaminants in real samples of hydrogen. Comparison of the hydrogen contaminant amount fraction against the threshold set by ISO 14687 Grade D (for hydrogen vehicles) affirms that the quality of hydrogen produced from the three routes investigated is below the threshold or at the threshold for all constituents. The study highlighted that water amount fraction online measurement may be investigated due to sampling difficulty.
This study also investigated the presence of new potential contaminants in hydrogen and provides the first results for anions, cations, trace elements and C2 to C12 hydrocarbons in hydrogen from SMR, alkaline and PEM water electrolysers. The results show that anions and cations like sodium, potassium, sulphate, and calcium were not detected above the limit of detection of NPL analytical methods (0.20 µg/g).
The three sources of hydrogen sampled for this study (SMR, alkaline, and PEM water electrolyser) demonstrate that hydrogen quality from production can be compliant with ISO 14687 Grade D. It is essential to stress that contamination from the hydrogen production routes will differ from plant to plant, production routes utilised, the level of purification utilised, and operating conditions utilised. Consequently, the results from this work cannot be used as firm evidence for the expected hydrogen purity levels produced by all hydrogen production processes. Nevertheless, it gives a good indication of the level of purity that can be attained from these production routes with the purification techniques employed. This infers that H2 production methods with purification in its product line should be able to provide sufficiently pure hydrogen for its different applications (heating, transport and industry). Nevertheless, further work is required to understand possible contamination from other parts of the supply chain like the distribution network itself if the gas is transported through the conventional gas grid.
Author Contributions
Conceptualization, O.O. and T.B.; methodology, T.B.; formal analysis, O.O., N.M., S.B., K.W., S.G., B.L.; data curation, O.O.; writing—original draft preparation, O.O.; writing—review and editing, O.O., T.B. and A.M.; supervision, T.B. and A.M.; project administration, D.J.; funding acquisition, D.J. All authors have read and agreed to the published version of the manuscript.
Funding
This study was carried out as part of the first phase of the Cadent HG2V project; this was a Network Innovation Allowance (NIA) by Cadent and was led by the National Physical Laboratory (NPL). The objective of the HG2V project was to determine the feasibility and practicality in distributing hydrogen (either as pure hydrogen or enriched with natural gas) to be used in fuel cell vehicles through the UK gas grid.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to agreements with third party companies who provided the samples for analysis.
Acknowledgments
We acknowledge Cadent gas and the support of the other HG2V Project partners: Imperial Consultants (ICON), DNV-GL, and KIWA Gastec.
Conflicts of Interest
The authors declare no conflict of interest.
References
- European Commission. Powering a Climate-Neutral Economy: An EU Strategy for Energy System Integration, European Commission: Brussels, Belgium; European Commission: Brussels, Belgium, 2020. [Google Scholar]
- European Commission. Communication from the Commission to the European Parliament, the European Council, the Council, the European Economic and Social Committee and the Committee of the Regions; Panke, D., Ed.; The European Green Deal; European Commission: Brussels, Belgium, 2019. [Google Scholar]
- The Chancellor of the Exchequer. Spring Statement 2019: Written Ministerial Statement; HM Treasury: London, UK, 2019.
- Stern, J. Narratives for Natural Gas in Decarbonising European Energy Markets; Oxford Institute for Energy Studies: Oxford, UK, 2019. [Google Scholar]
- Speirs, J.; Balcombe, P.; Johnson, E.; Martin, J.; Brandon, N.; Hawkes, A. A Greener Gas Grid: What Are the Options; Sustainable Gas Institute, Imperial College London: London, UK, 2017. [Google Scholar]
- Fuel Cells and Hydrogen Joint Undertaking. Hydrogen Roadmap Europe: A Sustainable Pathway for the European Energy Transition; Publications Office of the European Union: Luxembourg, 2019. [Google Scholar]
- BEIS. 2018 UK Greenhouse Gas Emissions, Final Figure; Department for Business Energy & Industrial Strategy, Ed., Crown Copyright: London, UK, 2020. [Google Scholar]
- National Statistics. 2019 UK Greenhouse Gas Emissions, Provisional Figures; Department for Business Energy & Industrial Strategy: London, UK, 2020.
- European Commission. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions—A Hydrogen Strategy for a Climate-Neutral Europe; European Commission: Brussels, Belgium, 2020. [Google Scholar]
- Holladay, J.D.; Hu, J.; King, D.L.; Wang, Y. An overview of hydrogen production technologies. Catal. Today 2009, 139, 244–260. [Google Scholar] [CrossRef]
- Nikolaidis, P.; Poullikkas, A. A comparative overview of hydrogen production processes. Renew. Sustain. Energy Rev. 2017, 67, 597–611. [Google Scholar] [CrossRef]
- Steinberg, M.; Cheng, H.C. Modern and prospective technologies for hydrogen production from fossil fuels. Int. J. Hydrogen Energy 1989, 14, 797–820. [Google Scholar] [CrossRef]
- The Royal Society. Options for Producing Low-Carbon Hydrogen at Scale; The Royal Society: London, UK, 2018. [Google Scholar]
- Chaubey, R.; Sahu, S.; James, O.O.; Maity, S. A review on development of industrial processes and emerging techniques for production of hydrogen from renewable and sustainable sources. Renew. Sustain. Energy Rev. 2013, 23, 443–462. [Google Scholar] [CrossRef]
- National Physical Laboratory. Measurement Needs within the Hydrogen Industry; National Physical Laboratory: Teddington, UK, 2017. [Google Scholar]
- International Organization for Standardization. ISO 14687:2019(en) Hydrogen Fuel Quality—Product Specification; ISO: Geneva, Switzerland, 2019. [Google Scholar]
- Bacquart, T.; Murugan, A.; Carré, M.; Gozlan, B.; Auprêtre, F.; Haloua, F.; Aarhaug, T.A. Probability of occurrence of ISO 14687-2 contaminants in hydrogen: Principles and examples from steam methane reforming and electrolysis (water and chlor-alkali) production processes model. Int. J. Hydrog. Energy 2018, 43, 11872–11883. [Google Scholar] [CrossRef]
- Consonni, S.; Vigano, F. Decarbonized hydrogen and electricity from natural gas. Int. J. Hydrog. Energy 2005, 30, 701–718. [Google Scholar] [CrossRef]
- Kortsdottir, K.; Lindström, R.W.; Lindbergh, G. The influence of ethene impurities in the gas feed of a PEM fuel cell. Int. J. Hydrogen Energy 2013, 38, 497–509. [Google Scholar] [CrossRef]
- Papadias, D.D.; Ahmed, S.; Kumar, R.; Joseck, F. Hydrogen quality for fuel cell vehicles—A modeling study of the sensitivity of impurity content in hydrogen to the process variables in the SMR–PSA pathway. Int. J. Hydrog. Energy 2009, 34, 6021–6035. [Google Scholar] [CrossRef]
- Tuominen, R.; Nissilä, M.; Sarsama, J.; Ihonen, J. Risk Assessment: Guidance for the First Part of WP1 and WP2 Work; Fuel Cells and Hydrogen Joint Underatking; HyCoRA: Brusesels, Belgium, 2018. [Google Scholar]
- Cadent Gas Ltd. HyMotion: HG2V. In Innovation to Reduce CO2 Emissions; Cadent Gas Ltd.: Leicester, UK, 2019. [Google Scholar]
- International Organization for Standardization. Part 1: Gravimetric method for Class I mixtures. In ISO 6142-1 Gas Analysis—Preparation of Calibration Gas Mixtures; ISO: Geneva, Switzerland, 2015. [Google Scholar]
- Smith, I.; Onakunle, F. XLGENLINE Version 1.0; Document CMSC/M/06/657, National Physical Laboratory: Teddington, UK, 2007. [Google Scholar]
- Hy4Heat. Hydrogen Purity—Final Report. In Hy4heat (WP2) Hydrogen Purity & Colourant; DNV GL: Loughborough, UK, 2019. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).