Investigation on the La Replacement and Little Additive Modification of High-Performance Permanent Magnetic Strontium-Ferrite
Abstract
1. Introduction
2. Experimental Procedure
2.1. Sample Preparation
2.2. Measurements
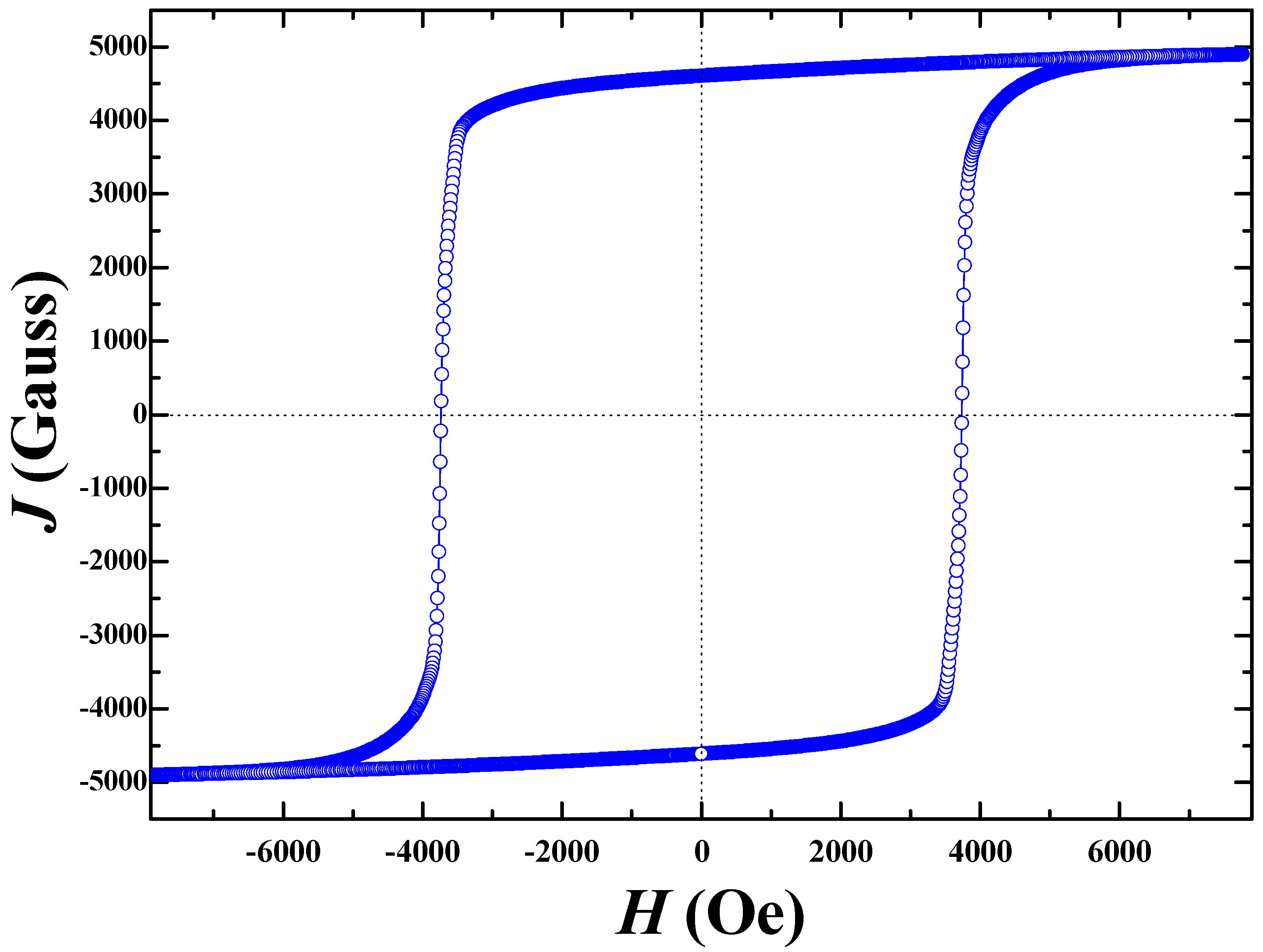
3. Result and Discussion
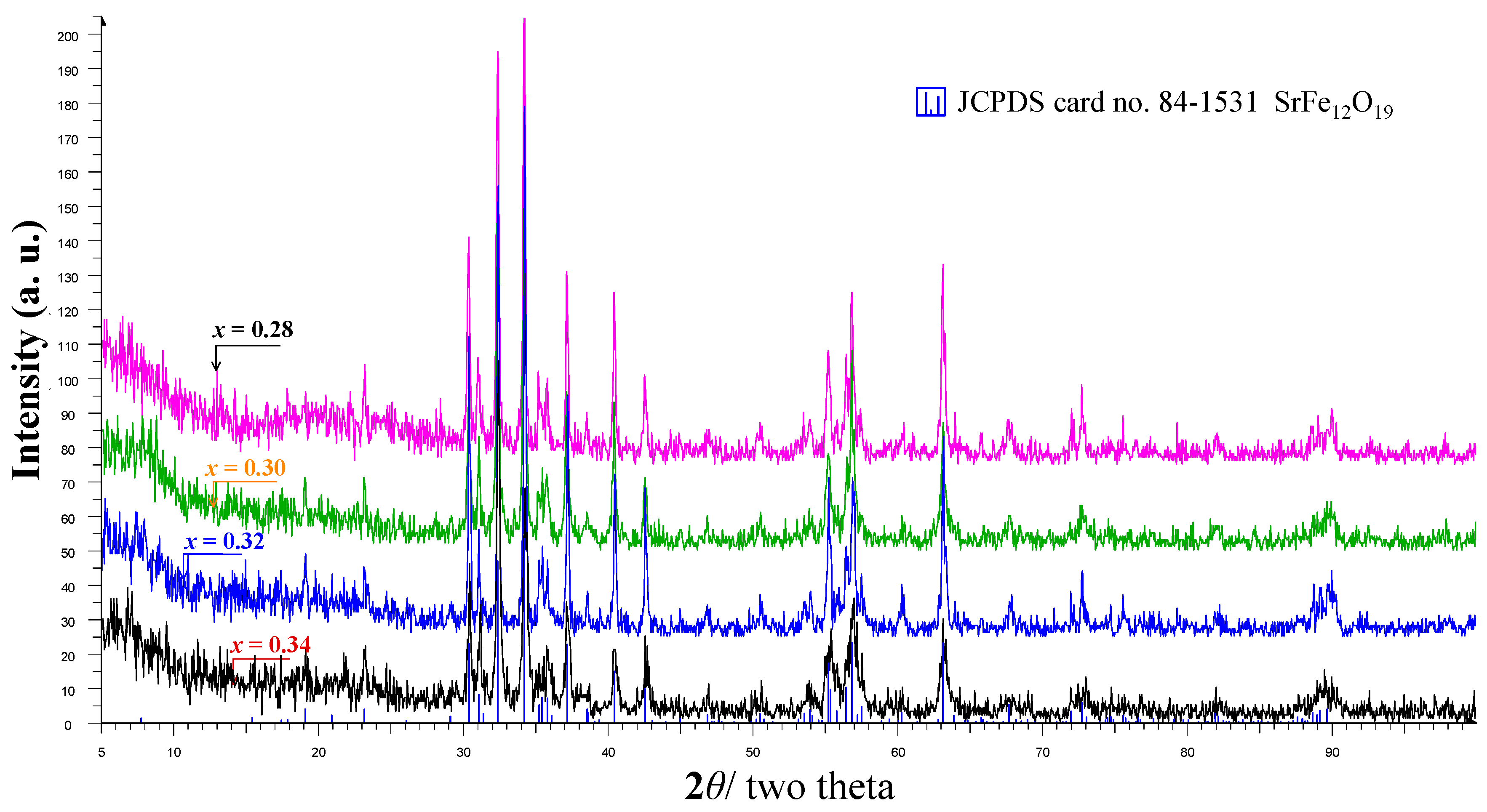
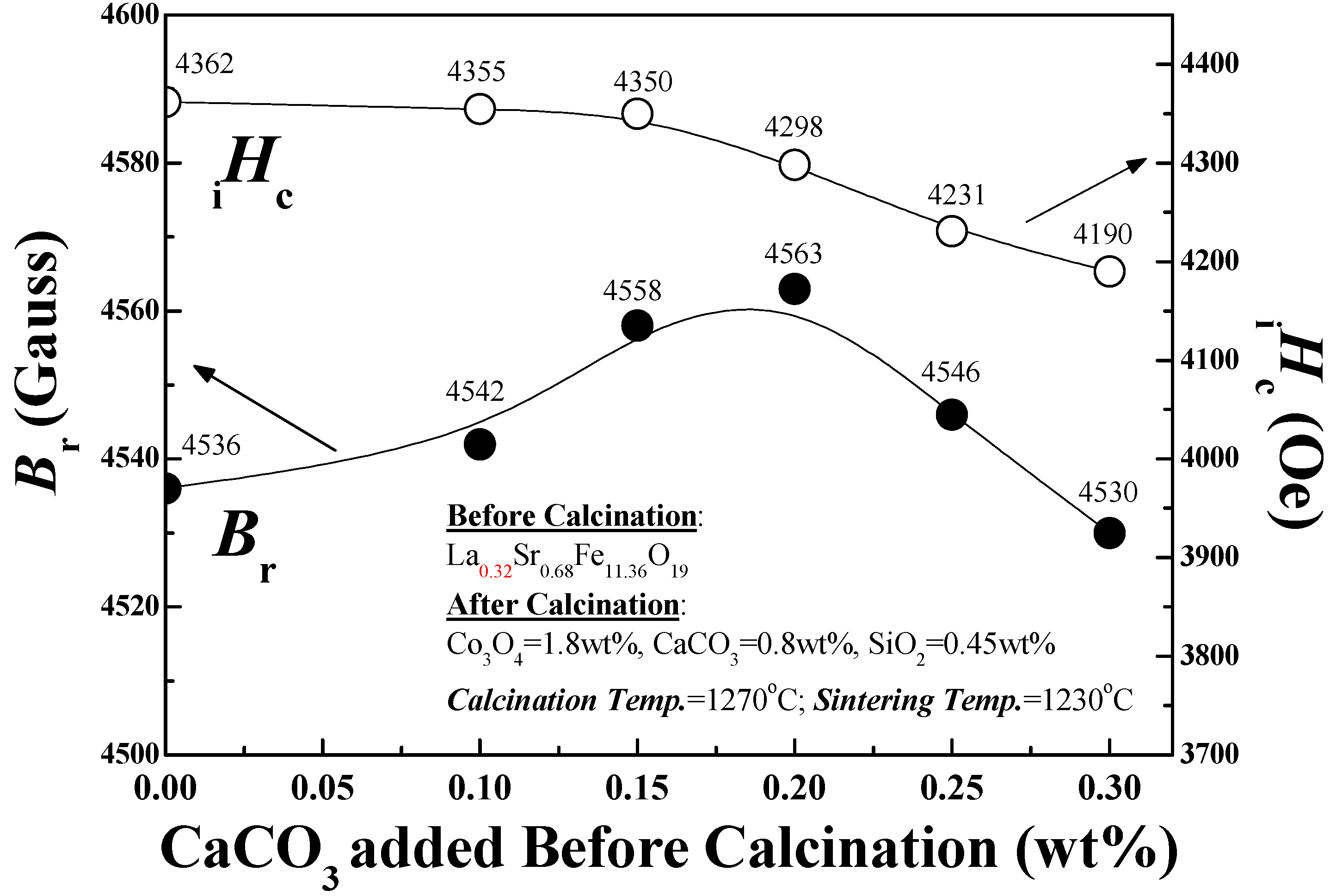
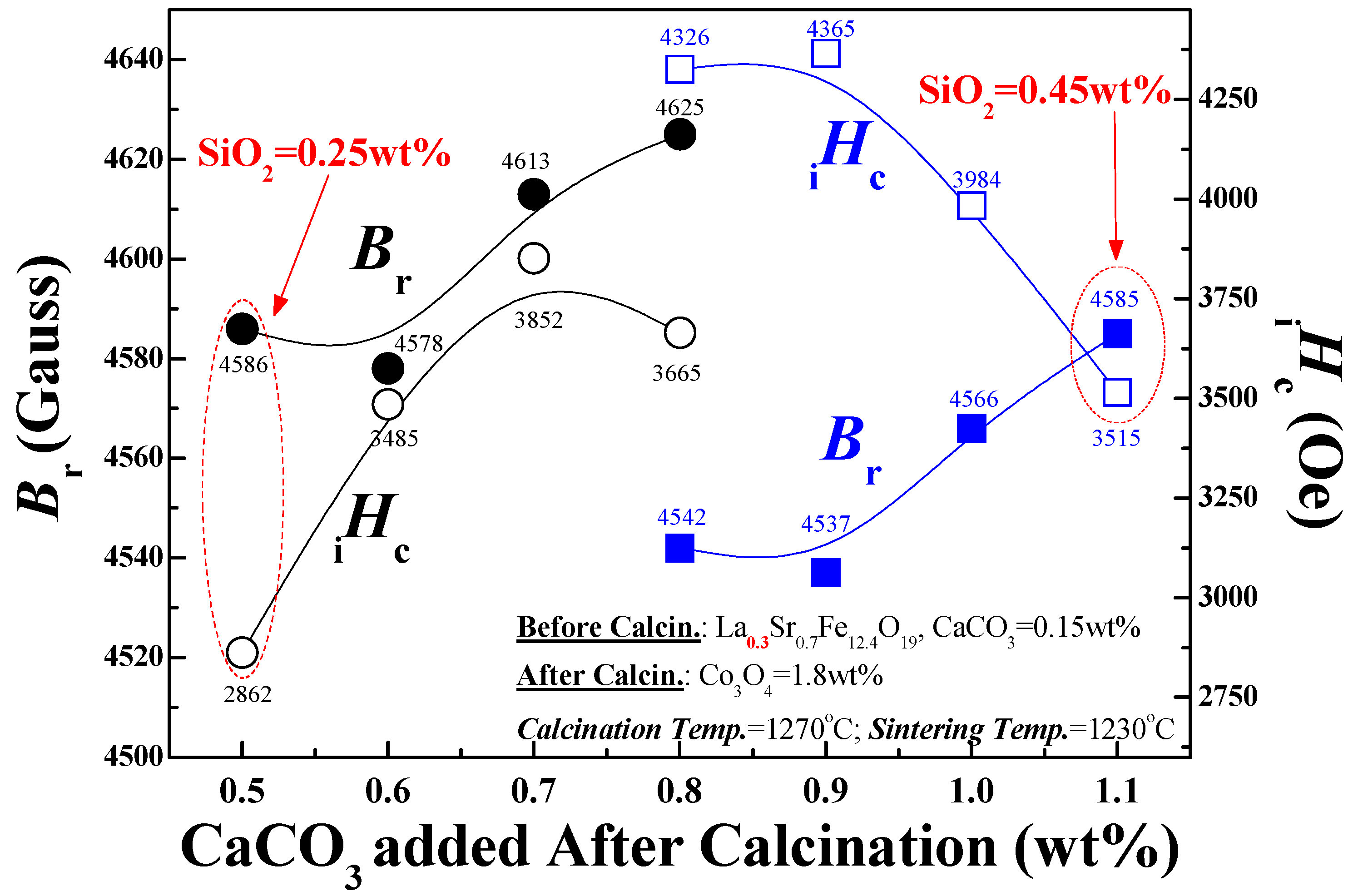
Effect of Calcined Ferrite Stoichiometry
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharrock, M.P. Particulate magnetic recording media: A review. IEEE Trans. Magn. 1989, 25, 4374–4389. [Google Scholar] [CrossRef]
- Koledintseva, M.Y.; Khanamirov, A.E.; Kitaitsev, A.A. Advances in ceramics electric and magnetic ceramics, bioceramics, Ceramics and environment. In Advances in Engineering and Applications of Hexagonal Ferrites in Russia; Sikalidis, C., Ed.; InTech: Rijeka, Croatia, 2011; pp. 61–86. [Google Scholar]
- Pullar, R.C. Hexagonal ferrites: A review of the synthesis, properties and applications of hexaferrite ceramics. Prog. Mater. Sci. 2012, 57, 1191–1334. [Google Scholar] [CrossRef]
- Lisjak, D.; Drofenik, M. The mechanism of the low-temperature formation of barium hexaferrite. J. Eur. Ceram. Soc. 2007, 27, 4515–4520. [Google Scholar] [CrossRef]
- Kim, K.; Jeon, K.W.; Moon, K.W.; Kang, M.K.; Kim, J. Effects of calcination conditions on magnetic properties in strontium ferrite synthesized by the molten salt method. IEEE Trans. Magn. 2016, 52, 2101604. [Google Scholar] [CrossRef]
- Givord, D.; Tenaud, P.; Viadieu, T. Coercivity mechanisms in ferrites and rare earth transition metal sintered magnets. IEEE Trans. Magn. 1988, 24, 1921–1923. [Google Scholar] [CrossRef]
- Huang, C.-C.; Hsiung, Y.; Kuo, M.-F. Study of the effect of specific surface area and purity of Fe2O3 on the magnetic properties of Srhexaferrites. IEEE Trans. Magn. 2016, 52, 2101909. [Google Scholar] [CrossRef]
- Liu, S. Magnetic alignment in powder magnet processing. J. Appl. Phys. 1994, 76, 6575. [Google Scholar] [CrossRef]
- Tomsia, A.P.; Glaeser, A.M. Ceramic Microstructures; Stuijts, A.L., Fulrath, R.M., Pask, J.A., Eds.; Wiley: New York, NY, USA, 1968. [Google Scholar]
- Kools, F.; Morel, A.; Tenaud, P. La–Co Substituted Sr and Ba M-Type Ferrites Magnet Properties Versus Intrinsic and Microstructural Factors; ICF-8: Kyoto, Japan, 2020; pp. 437–439. [Google Scholar]
- Morel, А.; Kools, F.; Tenaud, P. Modeling of La-Co Substituted M-type Ferrite Coercivity of Sr1-xLaxFe12-xCoxO19; ICF-8: Kyoto, Japan, 2000; pp. 434–436. [Google Scholar]
- Morel, А.; Le Breton, J.M.; Kreisel, J.; Wiesinger, G.; Kools, F.; Tenaud, P. Sublattice occupation in Sr1-xLaxFe12-xCoxO19 hexagonal ferrite analyzed by Mossbauer spectrometry and Raman spectroscopy. J. Magn. Magn. Mater. 2002, 242–245, 1405–1407. [Google Scholar] [CrossRef]
- Kools, F.; Morel, A.; Grossinger, R. LaCo-substituted ferrite magnets, a new class of high grade ceramic magnets; intrinsic and microstructural aspects. J. Magn. Magn. Mater. 2002, 242–245, 1270–1276. [Google Scholar]
- Tenaud, P.; Morel, A.; Kools, F.; le Breton, J.M.; Lechevallier, L. Recent improvement of hard ferrite permanent magnets based on La-Co substitution. J. Alloys Compd. 2004, 370, 331–334. [Google Scholar] [CrossRef]
- Grössinger, R.; Blanco, C.T.; Küpferling, M. Magnetic properties of a new family of rare-earth substituted ferrites. Phys. B Condens. Matter 2003, 327, 202–207. [Google Scholar] [CrossRef]
- Kikuchi, T.; Nakamura, T.; Yamasaki, T.; Nakanishi, M.; Fujii, T.; Takada, J.; Ikeda, Y. Magnetic properties of La-Co substituted M-type strontium hexaferrites prepared by polymerizable complex method. J. Magn. Magn. Mater. 2010, 322, 2381–2385. [Google Scholar] [CrossRef]
- Iida, K.; Minachi, Y.; Masuzawa, K.; Kawakami, M.; Nishio, H.; Taguchi, H. Hgh-performance ferrite magnets: M-type Sr-ferrite containing lanthanum and cobalt. J. Magn. Soc. Jpn. 1999, 23, 1093–1096. [Google Scholar] [CrossRef]
- Nishio, H.; Iida, K.; Minachi, Y.; Masuzawa, K.; Kawakami, M.; Taguchi, H. Magnetocrystalline anisotropy of M-type Sr-ferrite containing lanthanum and cobalt. J. Magn. Soc. Jpn. 1999, 23, 1097–1100. [Google Scholar] [CrossRef]
- Ogata, Y.; Takami, T.; Kubota, Y. Development of La-Co substituted ferrite magnets. J. Jpn. Soc. Powder Powder Metall. 2003, 50, 636–641. [Google Scholar] [CrossRef]
- Huang, C.H.; Jiang, A.I.; Liou, C.H.; Wang, Y.I.; Lee, C.H.; Hung, T.O.; Shaw, C.H.; Hung, Y.U.; Kuo, M.I.; Cheng, C.H. Magnetic property enhancement of cobalt-free M-type strontium hexagonal ferrites by CaCO3 and SiO2 addition. Intermetallics 2017, 89, 111–117. [Google Scholar] [CrossRef]
- Ding, J.; Street, R.; Nishio, H. Magnetic properties of Ba- and Srhexaferrite prepared by mechanical alloying. J. Magn. Magn. Mater. 1996, 164, 385–389. [Google Scholar] [CrossRef]
- Lechevallier, L.; le Breton, J.M.; Wang, J.F.; Harris, I.R. Structural and Mössbauer analyses of ultrafine Sr1−xLaxFe12−xZnxO19 and Sr1−xLaxFe12−xCoxO19 hexagonal ferrites synthesized by chemical co-precipitation. J. Phys. Condens. Matter. 2004, 16, 5359–5376. [Google Scholar] [CrossRef]
- Bai, J.; Liu, X.; Xie, T.; Wei, F.; Yang, Z. The effects of La–Zn substitution on the magnetic properties of Sr-magnetoplumbite ferrite nano-particles. Mater. Sci. Eng. B 2000, 68, 182–185. [Google Scholar] [CrossRef]
- Huang, C.H.; Jiang, A.I.; Hung, Y.U.; Liou, C.H.; Wang, Y.I.; Lee, C.H.; Hung, T.O.; Shaw, C.H.; Kuo, M.I.; Chengd, C.H. Influence of CaCO3 and SiO2 additives on magnetic properties of M-type Sr ferrites. J. Magn. Magn. Mater. 2018, 451, 288–294. [Google Scholar] [CrossRef]
- Huang, C.H.; Mo, C.H.; Hsiao, T.S.; Chen, G.U.; Lu, S.H.; Tai, Y.E.; Hsu, H.S.; Cheng, C.H. Preparation and magnetic properties of high performance Ca-Sr based M-type hexagonal ferrites. Results Mater. 2020, 8, 10050. [Google Scholar]
- Kaneko, Y.; Kitajima, K.; Takusagawa, N. Effects of CaO and SiO2 addition on the microstructure and intrinsic coercivity of sintered Sr ferrite. J. Eur. Ceram. Soc. 1992, 100, 1413–1417. [Google Scholar] [CrossRef]
- Arendt, R.H. Liquid-phase sintering of magnetically isotropic and anisotropic compacts of BaFe12O19 and SrFe12O19. J. Appl. Phys. 1973, 44, 3300–3305. [Google Scholar] [CrossRef]
- Haberey, F. Glasbildung durch SiO2-Zusatz bei der Herstellung von Ba- und Sr-Hexaferriten. Ber. Dtsch. Keram. Ges. 1978, 55, 297–301. [Google Scholar]
- TDK Co. Available online: https://product.tdk.com/en/search/magnet/magnet/ferrite/info?part_no=FB9N (accessed on 1 May 2021).

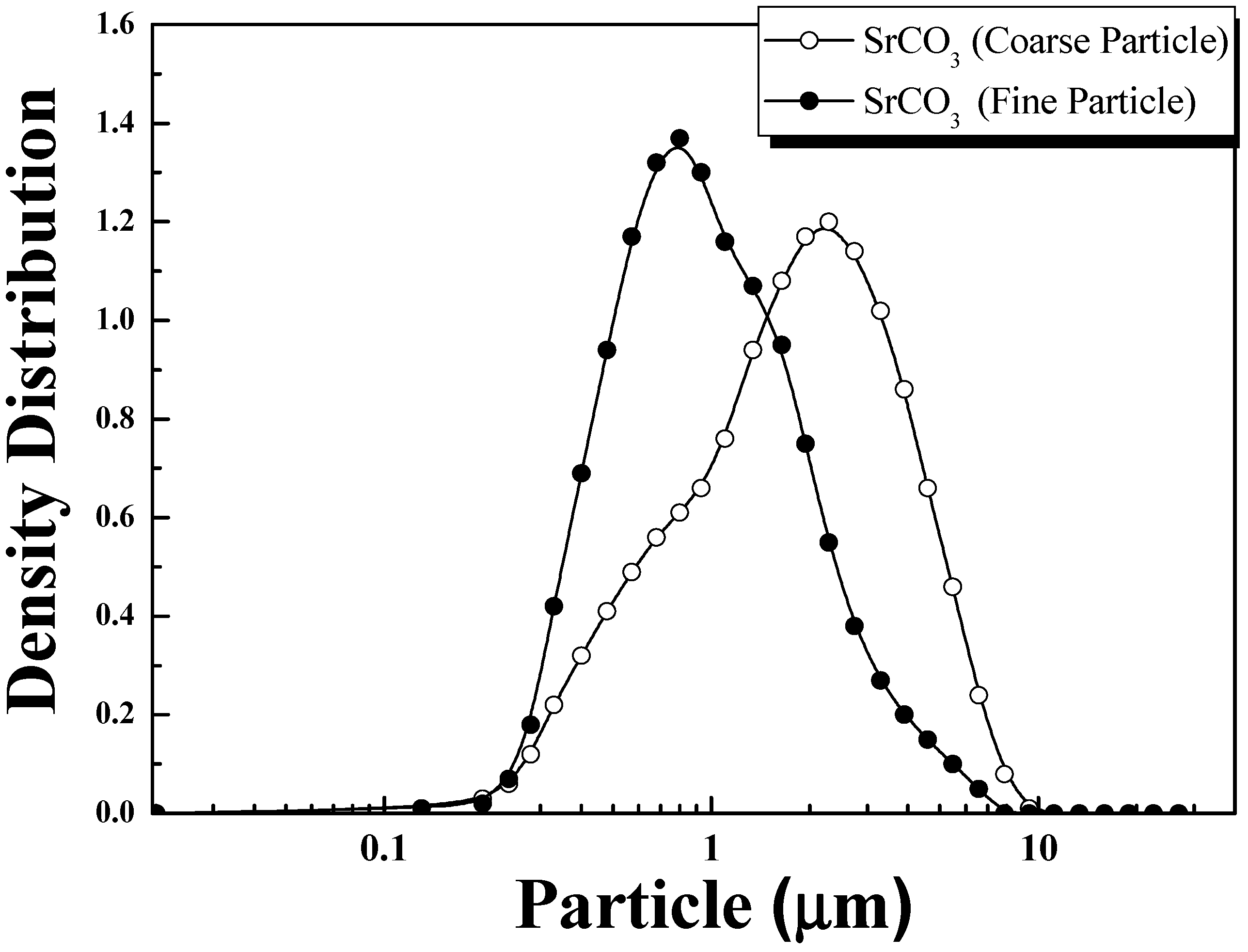
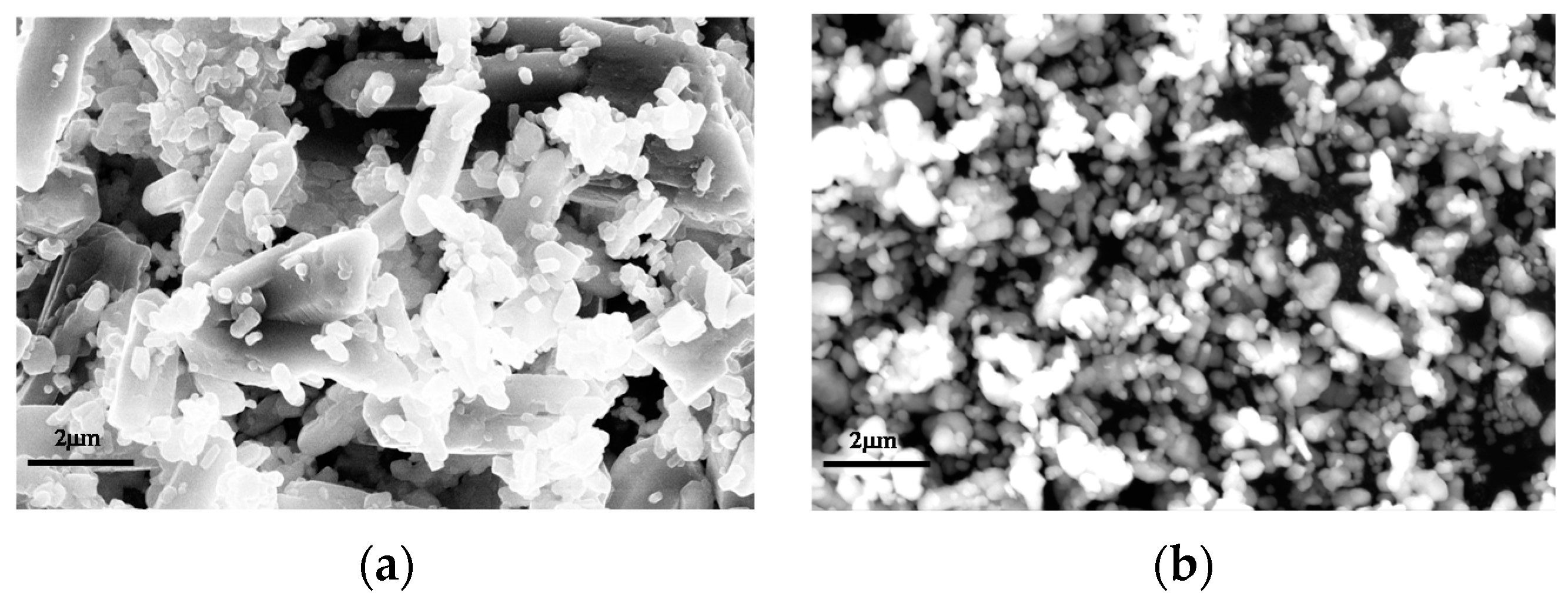
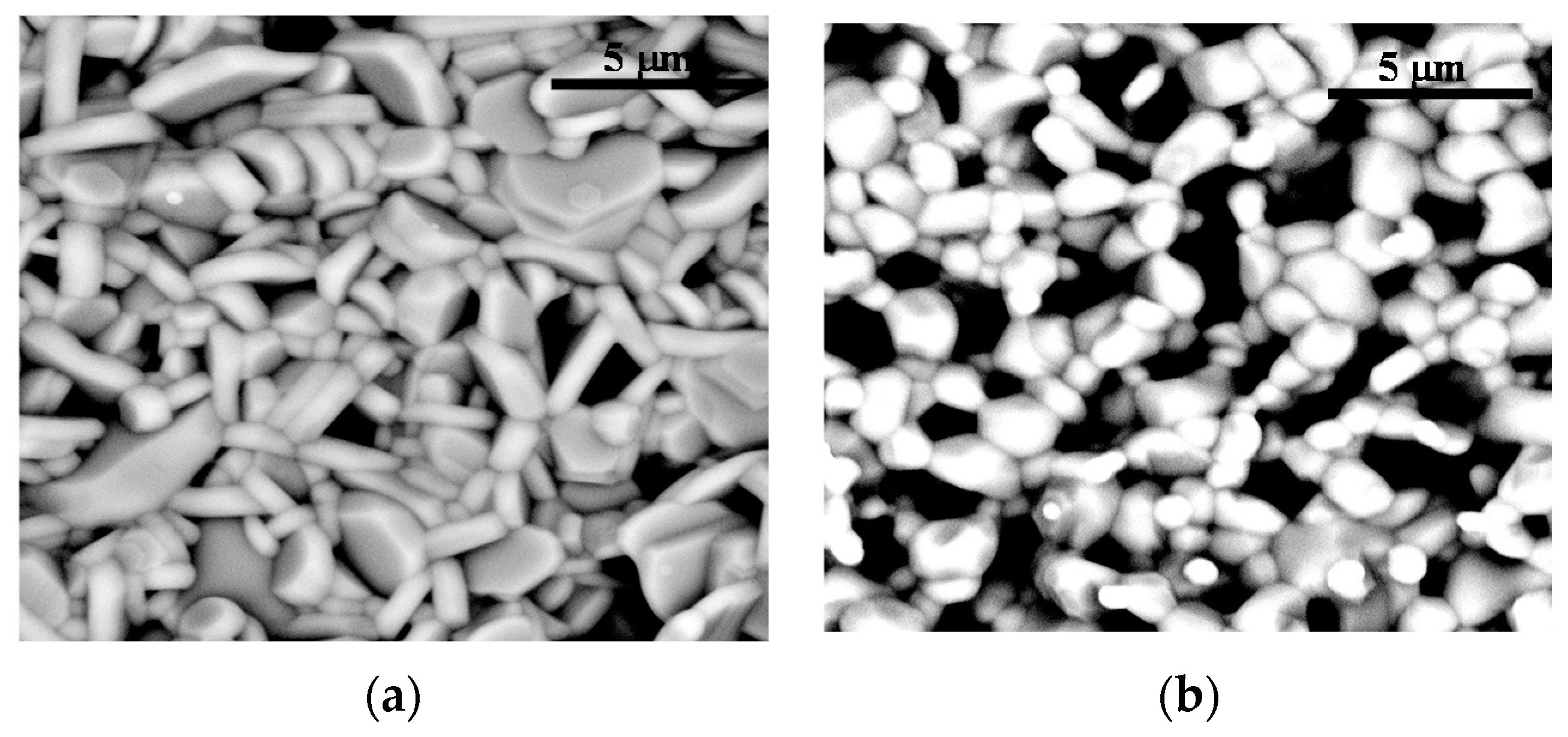



| D10 (μm) | D50 (μm) | D90 (μm) | |
|---|---|---|---|
| SrSO3 (Coarse Particle) | 0.70 ± 0.02 | 2.50 ± 0.02 | 6.10 ± 0.02 |
| SrSO3 (Coarse Particle) | 0.50 ± 0.02 | 1.00 ± 0.02 | 2.30 ± 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.-C.; Mo, C.-C.; Chen, G.-M.; Hsu, H.-H.; Shu, G.-J. Investigation on the La Replacement and Little Additive Modification of High-Performance Permanent Magnetic Strontium-Ferrite. Processes 2021, 9, 1034. https://doi.org/10.3390/pr9061034
Huang C-C, Mo C-C, Chen G-M, Hsu H-H, Shu G-J. Investigation on the La Replacement and Little Additive Modification of High-Performance Permanent Magnetic Strontium-Ferrite. Processes. 2021; 9(6):1034. https://doi.org/10.3390/pr9061034
Chicago/Turabian StyleHuang, Ching-Chien, Chin-Chieh Mo, Guan-Ming Chen, Hsiao-Hsuan Hsu, and Guo-Jiun Shu. 2021. "Investigation on the La Replacement and Little Additive Modification of High-Performance Permanent Magnetic Strontium-Ferrite" Processes 9, no. 6: 1034. https://doi.org/10.3390/pr9061034
APA StyleHuang, C.-C., Mo, C.-C., Chen, G.-M., Hsu, H.-H., & Shu, G.-J. (2021). Investigation on the La Replacement and Little Additive Modification of High-Performance Permanent Magnetic Strontium-Ferrite. Processes, 9(6), 1034. https://doi.org/10.3390/pr9061034







