UVA-LED Technology’s Treatment Efficiency and Cost in a Competitive Trial Applied to the Photo-Fenton Treatment of Landfill Leachate
Abstract
1. Introduction
2. Material and Methods
2.1. Mature Landfill Leachate Characteristics
2.2. Chemicals
2.3. Coagulation
2.4. Photo-Fenton Process
2.5. Analytical Determinations
3. Results and Discussion
3.1. Coagulation Pre-Treatment
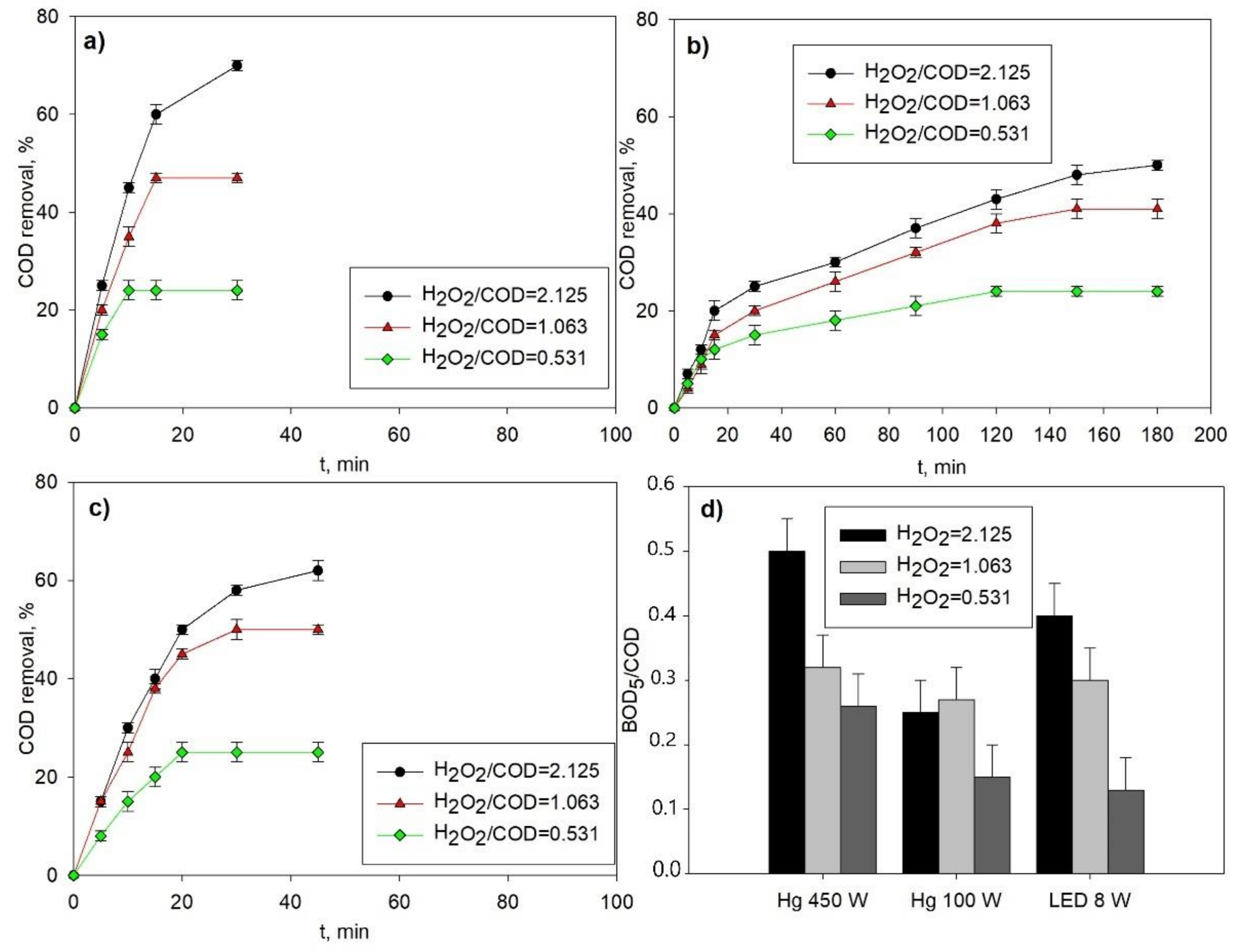
3.2. Photo-Fenton Oxidation: Comparative Application of Different UV Sources
3.3. Energy Consumption and Its Preliminary Economic Assessment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pelkonen, M.; Wang, Y. Leachate direct-discharge limits and incentives related to landfill aftercare costs. J. Mater. Cycles Waste Manag. 2017, 19, 413–422. [Google Scholar] [CrossRef]
- Tripathy, B.K.; Kumar, M. Sequential coagulation/flocculation and microwave-persulfate processes for landfill leachate treatment: Assessment of bio-toxicity, effect of pretreatment and cost-analysis. Waste Manag. 2019, 85, 18–29. [Google Scholar] [CrossRef]
- Iskander, S.M.; Zhao, R.; Pathak, A.; Gupta, A.; Pruden, A.; Novak, J.T.; He, Z. A review of landfill leachate induced ultraviolet quenching substances: Sources, characteristics, and treatment. Water Res. 2018, 145, 297–311. [Google Scholar] [CrossRef]
- Wang, K.; Li, L.; Tan, F.; Wu, D. Treatment of landfill leachate using activated sludge technology: A review. Archaea 2018, 2018, 1039453. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Zeng, Y.; Cheng, Y.; He, D.; Pan, X. Recent advances in municipal landfill leachate: A review focusing on its characteristics, treatment, and toxicity assessment. Sci. Total Environ. 2020, 703, 135468. [Google Scholar] [CrossRef]
- Bove, D.; Merello, S.; Frumento, D.; Al Arni, S.; Aliakbarian, B.; Converti, A. A Critical Review of Biological Processes and Technologies for Landfill Leachate Treatment. Chem. Eng. Technol. 2015, 38, 2115–2126. [Google Scholar] [CrossRef]
- Colombo, A.; Módenes, A.N.; Trigueros, D.E.G.; da Costa, S.I.G.; Borba, F.H.; Espinoza-Quiñones, F.R. Treatment of sanitary landfill leachate by the combination of photo-Fenton and biological processes. J. Clean. Prod. 2019, 214, 145–153. [Google Scholar] [CrossRef]
- Hilles, A.H.; Amr, S.S.A.; Hussein, R.A.; El-Sebaie, O.D.; Arafa, A.I. Performance of combined sodium persulfate/H2O2 based advanced oxidation process in stabilized landfill leachate treatment. J. Environ. Manag. 2016, 166, 493–498. [Google Scholar] [CrossRef]
- Iskander, S.M.; Novak, J.T.; Brazil, B.; He, Z. Percarbonate oxidation of landfill leachates towards removal of ultraviolet quenchers. Environ. Sci. Water Res. Technol. 2017, 3, 1162–1170. [Google Scholar] [CrossRef]
- Jia, C.; Wang, Y.; Zhang, C.; Qin, Q. UV-TiO2 photocatalytic degradation of landfill leachate. Water Air Soil Pollut. 2011, 217, 375–385. [Google Scholar] [CrossRef]
- Litter, M.I.; Slodowicz, M. An overview on heterogeneous Fenton and photoFenton reactions using zerovalent iron materials. J. Adv. Oxid. Technol. 2017, 20, 19. [Google Scholar] [CrossRef]
- Turro, E.; Giannis, A.; Cossu, R.; Gidarakos, E.; Mantzavinos, D.; Katsaounis, A. Electrochemical oxidation of stabilized landfill leachate on DSA electrodes. J. Hazard. Mater. 2011, 190, 460–465. [Google Scholar] [CrossRef]
- Umar, M.; Aziz, H.A.; Yusoff, M.S. Trends in the use of Fenton, electro-Fenton and photo-Fenton for the treatment of landfill leachate. Waste Manag. 2010, 30, 2113–2121. [Google Scholar] [CrossRef]
- Wu, C.; Chen, W.; Gu, Z.; Li, Q. A review of the characteristics of Fenton and ozonation systems in landfill leachate treatment. Sci. Total Environ. 2020, 143131. [Google Scholar] [CrossRef]
- Bautista, P.; Mohedano, A.F.; Casas, J.A.; Zazo, J.A.; Rodriguez, J.J. An overview of the application of Fenton oxidation to industrial wastewaters treatment. J. Chem. Technol. Biotechnol. 2008, 83, 1323–1338. [Google Scholar] [CrossRef]
- Deng, Y.; Englehardt, J.D. Treatment of landfill leachate by the Fenton process. Water Res. 2006, 40, 3683–3694. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.-H.; Dong, H.; Zhao, L.; Wang, D.-X.; Meng, D. A review on Fenton process for organic wastewater treatment based on optimization perspective. Sci. Total Environ. 2019, 670, 110–121. [Google Scholar] [CrossRef]
- Cassano, D.; Zapata, A.; Brunetti, G.; Del Moro, G.; Di Iaconi, C.; Oller, I. Comparison of several combined/integrated biological-AOPs setups for the treatment of municipal landfill leachate: Minimization of operating costs and effluent toxicity. Chem. Eng. J. 2011, 172, 250–257. [Google Scholar] [CrossRef]
- Durán, A.; Monteagudo, J.; San Martín, I. Photocatalytic treatment of an industrial effluent using artificial and solar UV radiation: An operational cost study on a pilot plant scale. J. Environ. Manag. 2012, 98, 1–4. [Google Scholar] [CrossRef]
- Silva, T.F.; Ferreira, R.; Soares, P.A.; Manenti, D.R.; Fonseca, A.; Saraiva, I. Insights into solar photo-Fenton reaction parameters in the oxidation of a sanitary landfill leachate at lab-scale. J. Environ. Manag. 2015, 164, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Singa, P.K.; Isa, M.H.; Ho, Y.-C.; Lim, J.-W. Mineralization of hazardous waste landfill leachate using photo-fenton process. In E3S Web of Conferences; EDP Sciences: Evry, France, 2018. [Google Scholar]
- Amor, C.; De Torres-Socías, E.; Peres, J.A.; Maldonado, M.I.; Oller, I.; Malato, S. Mature landfill leachate treatment by coagulation/flocculation combined with Fenton and solar photo-Fenton processes. J. Hazard. Mater. 2015, 286, 261–268. [Google Scholar] [CrossRef]
- Gomes, A.I.; Silva, T.F.; Duarte, M.A.; Boaventura, R.A.; Vilar, V.J. Cost-effective solar collector to promote photo-Fenton reactions: A case study on the treatment of urban mature leachate. J. Clean. Prod. 2018, 199, 369–382. [Google Scholar] [CrossRef]
- Silva, T.F.; Fonseca, A.; Saraiva, I.; Boaventura, R.A.; Vilar, V.J. Scale-up and cost analysis of a photo-Fenton system for sanitary landfill leachate treatment. Chem. Eng. J. 2016, 283, 76–88. [Google Scholar] [CrossRef]
- Will, I.; Moraes, J.; Teixeira, A.; Guardani, R.; Nascimento, C. Photo-Fenton degradation of wastewater containing organic compounds in solar reactors. Sep. Purif. Technol. 2004, 34, 51–57. [Google Scholar] [CrossRef]
- Smaoui, Y.; Mseddi, S.; Ayadi, N.; Sayadi, S.; Bouzid, J. Evaluation of influence of coagulation/flocculation and Fenton oxidation with iron on landfill leachate treatment. Environ. Prot. Eng. 2019, 45. [Google Scholar] [CrossRef]
- Zhao, J.; Ouyang, F.; Yang, Y.; Tang, W. Degradation of recalcitrant organics in nanofiltration concentrate from biologically pretreated landfill leachate by ultraviolet-Fenton method. Sep. Purif. Technol. 2020, 235, 116076. [Google Scholar] [CrossRef]
- Tejera, J.; Miranda, R.; Hermosilla, D.; Urra, I.; Negro, C.; Blanco, Á. Treatment of a Mature Landfill Leachate: Comparison between Homogeneous and Heterogeneous Photo-Fenton with Different Pretreatments. Water 2019, 11, 1849. [Google Scholar] [CrossRef]
- Borba, F.H.; Leichtweis, J.; Bueno, F.; Pellenz, L.; Inticher, J.J.; Seibert, D. Pollutant removal and acute toxicity assessment (Artemia salina) of landfill leachate treated by photo-Fenton process mediated by oxalic acid. J. Water Process Eng. 2019, 28, 159–168. [Google Scholar] [CrossRef]
- Seibert, D.; Diel, T.; Welter, J.B.; de Souza, A.L.; Módenes, A.N.; Espinoza-Quiñones, F.R. Performance of photo-Fenton process mediated by Fe (III)-carboxylate complexes applied to degradation of landfill leachate. J. Environ. Chem. Eng. 2017, 5, 4462–4470. [Google Scholar] [CrossRef]
- Rocha, E.M.; Vilar, V.J.; Fonseca, A.; Saraiva, I.; Boaventura, R.A. Landfill leachate treatment by solar-driven AOPs. Sol. Energy 2011, 85, 46–56. [Google Scholar] [CrossRef]
- Tejera, J.; Hermosilla, D.; Miranda, R.; Gascó, A.; Alonso, V.; Negro, C.; Blanco, A. Assessing an Integral Treatment for Landfill Leachate Reverse Osmosis Concentrate. Catalysts 2020, 10, 1389. [Google Scholar] [CrossRef]
- Liang, X.; Zhu, X.; Butler, E.C. Comparison of four advanced oxidation processes for the removal of naphthenic acids from model oil sands process water. J. Hazard. Mater. 2011, 190, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Hatchard, C.; Parker, C.A. A new sensitive chemical actinometer—II. Potassium ferrioxalate as a standard chemical actinometer. Proc. R. Soc. Lond. Ser. Math. Phys. Sci. 1956, 235, 518–536. [Google Scholar]
- Montalti, M.; Credi, A.; Prodi, L.; Gandolfi, M.T. Handbook of Photochemistry; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Kim, S.-M.; Geissen, S.-U.; Vogelpohl, A. Landfill leachate treatment by a photo-assisted Fenton reaction. Water Sci. Technol. 1997, 35, 239–248. [Google Scholar] [CrossRef]
- Federation, Water Environmental; APH Association. Standard Methods for the Examination of Water and Wastewater; American Public Health Association (APHA): Washington, DC, USA, 2005. [Google Scholar]
- Pobiner, H. Determination of hydroperoxides in hydrocarbon by conversion to hydrogen peroxide and measurement by titanium complexing. Anal. Chem. 1961, 33, 1423–1426. [Google Scholar] [CrossRef]
- Hermosilla, D.; Cortijo, M.; Huang, C.P. Optimizing the treatment of landfill leachate by conventional Fenton and photo-Fenton processes. Sci. Total Environ. 2009, 407, 3473–3481. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Value | Parameter | Value |
|---|---|---|---|
| pH | 8.2 ± 0.1 | Chloride, mg L−1 | 3000 ± 137 |
| Conductivity, mS cm−1 | 17.3 ± 0.1 | Sulfate, mg L−1 | 125 ± 23 |
| UV-254, cm−1 | 27.5 ± 0.1 | Aluminum, mg L−1 | 5.50 ± 0.03 |
| Color, mg Pt L−1 | 17,300 ± 200 | Iron, mg L−1 | 8.50 ± 0.01 |
| COD, mg O2 L−1 | 4961 ± 495 | Chromium, mg L−1 | 1.9 ± 0.05 |
| BOD5, mg O2 L−1 | 149 ± 26 | Sodium, mg L−1 | 2152 ± 215 |
| BOD5/COD | 0.03 ± 0.01 | Potassium, mg L−1 | 1219 ± 122 |
| TOC, mg C L−1 | 1980 ± 10 | Magnesium, mg L−1 | 98 ± 6 |
| TS, mg L−1 | 21,290 ± 1030 | Calcium, mg L−1 | 134 ± 4 |
| TSS, mg L−1 | 1370 ± 20 | Silicon, mg L−1 | 15 ± 1 |
| TDS, mg L−1 | 18,970 ± 230 | Zinc, mg L−1 | 0.61 ± 0.06 |
| Alkalinity, mg CaCO3 L−1 | 13,244 ± 100 | Nickel, mg L−1 | 0.26 ± 0.03 |
| TNb, mg N L−1 | 1600 ± 10 | Copper, mg L−1 | 0.03 ± 0.01 |
| Parameter | Raw Leachate | Coagulated Leachate * |
|---|---|---|
| pH | 8.2 ± 0.1 | 2.8 ± 0.1 |
| Conductivity, mS cm−1 | 17.3 ± 0.1 | 22.3 ± 0.1 |
| COD, mg O2 L−1 | 4961 ± 495 | 1538 ± 150 (69%) |
| BOD5, mg O2 L−1 | 149 ± 26 | 46 ± 15 (69%) |
| BOD5/COD | 0.03 ± 0.01 | 0.03 ± 0.01 |
| TOC, mg C L−1 | 1980 ± 10 | 614 ± 6 (69%) |
| Dissolved iron, mg L−1 | 8.50 ± 0.01 | 300 ± 20 |
| UV-254, cm−1 | 27.5 ± 0.1 | 3.85 ± 0.05 (86%) |
| Color, mg Pt L−1 | 17,300 ± 200 | 1750 ± 100 (90%) |
| TNb, mg N L−1 | 1600 ± 10 | 1600 ± 10 |
| Parameter | Hg 450 W | Hg 100 W | LED 8 W | ||||||
|---|---|---|---|---|---|---|---|---|---|
| [H2O2]0/COD0 | 0.531 | 1.063 | 2.125 | 0.531 | 1.063 | 2.125 | 0.531 | 1.063 | 2.125 |
| pH | 3.2 ± 0.1 | 3.6 ± 0.1 | 4.0 ± 0.1 | 3.0 ± 0.1 | 3.3 ± 0.1 | 3.8 ± 0.1 | 3.3 ± 0.1 | 3.6 ± 0.1 | 3.8 ± 0.1 |
| Conductivity | 22.5 ± 0.1 | 22.8 ± 0.1 | 23.4 ± 0.1 | 22.4 ± 0.1 | 22.5 ± 0.1 | 22.9 ± 0.1 | 22.2 ± 0.1 | 22.6 ± 0.1 | 23.0 ± 0.1 |
| mS cm−1 | |||||||||
| COD | 1169 ± 100 | 815 ± 80 | 461 ± 40 | 1169 ± 150 | 907 ± 90 | 769 ± 80 | 1153 ± 110 | 769 ± 70 | 564 ± 50 |
| mg O2 L−1 | (76%) | (84%) | (91%) | (76%) | (82%) | (84%) | (77%) | (84%) | (89%) |
| BOD5 | 304 ± 30 | 261 ± 30 | 230 ± 20 | 175 ± 20 | 245 ± 20 | 192 ± 20 | 150 ± 30 | 231 ± 20 | 234 ± 20 |
| mg O2 L−1 | |||||||||
| BOD5/COD | 0.26 ± 0.05 | 0.32 ± 0.05 | 0.50 ± 0.05 | 0.15 ± 0.05 | 0.27 ± 0.05 | 0.25 ± 0.05 | 0.13 ± 0.05 | 0.30 ± 0.05 | 0.41 ± 0.05 |
| TOC | 556 ± 40 | 495 ± 50 | 297 ± 30 | 594 ± 60 | 554 ± 50 | 495 ± 50 | 535 ± 50 | 436 ± 40 | 356 ± 30 |
| mg C L−1 | (72%) | (75%) | (85%) | (70%) | (72%) | (75%) | (73%) | (78%) | (82%) |
| Dissolved Fe mg L−1 | 290 ± 20 | 285 ± 30 | 270 ± 20 | 295 ± 30 | 280 ± 30 | 275 ± 20 | 285 ± 20 | 280 ± 30 | 275 ± 20 |
| UV-254 | 2.31 ± 0.05 | 1.54 ± 0.05 | 0.77 ± 0.05 | 2.28 ± 0.05 | 1.70 ± 0.05 | 1.0 ± 0.05 | 2.20 ± 0.05 | 1.35 ± 0.05 | 0.85 ± 0.05 |
| cm−1 | (92%) | (94%) | (97%) | (92%) | (94%) | (96%) | (92%) | (95%) | (97%) |
| Color | 450 ± 20 | 360 ± 20 | 200 ± 30 | 500 ± 40 | 380 ± 20 | 230 ± 40 | 400 ± 20 | 340 ± 30 | 210 ± 20 |
| mg Pt L−1 | (97%) | (98%) | (99%) | (97%) | (98%) | (99%) | (98%) | (98%) | (99%) |
| TNb | 1600 ± 100 | 1600 ± 100 | 1600 ± 100 | 1600 ± 100 | 1600 ± 100 | 1600 ± 100 | 1600 ± 100 | 1600 ± 100 | 1600 ± 100 |
| mg N L−1 | |||||||||
| UV Source | [H2O2]0/COD0 | COD Removal % | BOD5/COD | t min | E kWh m−3 | Cost EUR m−3 | Cost EUR kg COD−1 |
|---|---|---|---|---|---|---|---|
| Hg (450 W) | 0.531 | 24 ± 2 | 0.26 ± 0.05 | 10 | 42 | 4.62 | 12.5 |
| 1.063 | 47 ± 3 | 0.32 ± 0.05 | 15 | 64 | 7.04 | 9.7 | |
| 2.125 | 70 ± 4 | 0.50 ± 0.05 | 30 | 129 | 14.20 | 13.2 | |
| Hg (100 W) | 0.531 | 24 ± 2 | 0.15 ± 0.05 | 120 | 114 | 12.50 | 33.9 |
| 1.063 | 41 ± 4 | 0.27 ± 0.05 | 150 | 143 | 15.71 | 24.9 | |
| 2.125 | 50 ± 3 | 0.25 ± 0.05 | 180 | 171 | 18.80 | 24.4 | |
| LED (8 W) | 0.531 | 25 ± 1 | 0.13 ± 0.05 | 20 | 27 | 2.93 | 7.6 |
| 1.063 | 50 ± 2 | 0.30 ± 0.05 | 30 | 40 | 4.40 | 5.7 | |
| 2.125 | 62 ± 4 | 0.40 ± 0.05 | 45 | 60 | 6.60 | 6.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tejera, J.; Gascó, A.; Hermosilla, D.; Alonso-Gomez, V.; Negro, C.; Blanco, Á. UVA-LED Technology’s Treatment Efficiency and Cost in a Competitive Trial Applied to the Photo-Fenton Treatment of Landfill Leachate. Processes 2021, 9, 1026. https://doi.org/10.3390/pr9061026
Tejera J, Gascó A, Hermosilla D, Alonso-Gomez V, Negro C, Blanco Á. UVA-LED Technology’s Treatment Efficiency and Cost in a Competitive Trial Applied to the Photo-Fenton Treatment of Landfill Leachate. Processes. 2021; 9(6):1026. https://doi.org/10.3390/pr9061026
Chicago/Turabian StyleTejera, Javier, Antonio Gascó, Daphne Hermosilla, Víctor Alonso-Gomez, Carlos Negro, and Ángeles Blanco. 2021. "UVA-LED Technology’s Treatment Efficiency and Cost in a Competitive Trial Applied to the Photo-Fenton Treatment of Landfill Leachate" Processes 9, no. 6: 1026. https://doi.org/10.3390/pr9061026
APA StyleTejera, J., Gascó, A., Hermosilla, D., Alonso-Gomez, V., Negro, C., & Blanco, Á. (2021). UVA-LED Technology’s Treatment Efficiency and Cost in a Competitive Trial Applied to the Photo-Fenton Treatment of Landfill Leachate. Processes, 9(6), 1026. https://doi.org/10.3390/pr9061026












