Effect of Steam Quality on Extra-Heavy Crude Oil Upgrading and Oil Recovery Assisted with PdO and NiO-Functionalized Al2O3 Nanoparticles
Abstract
1. Introduction
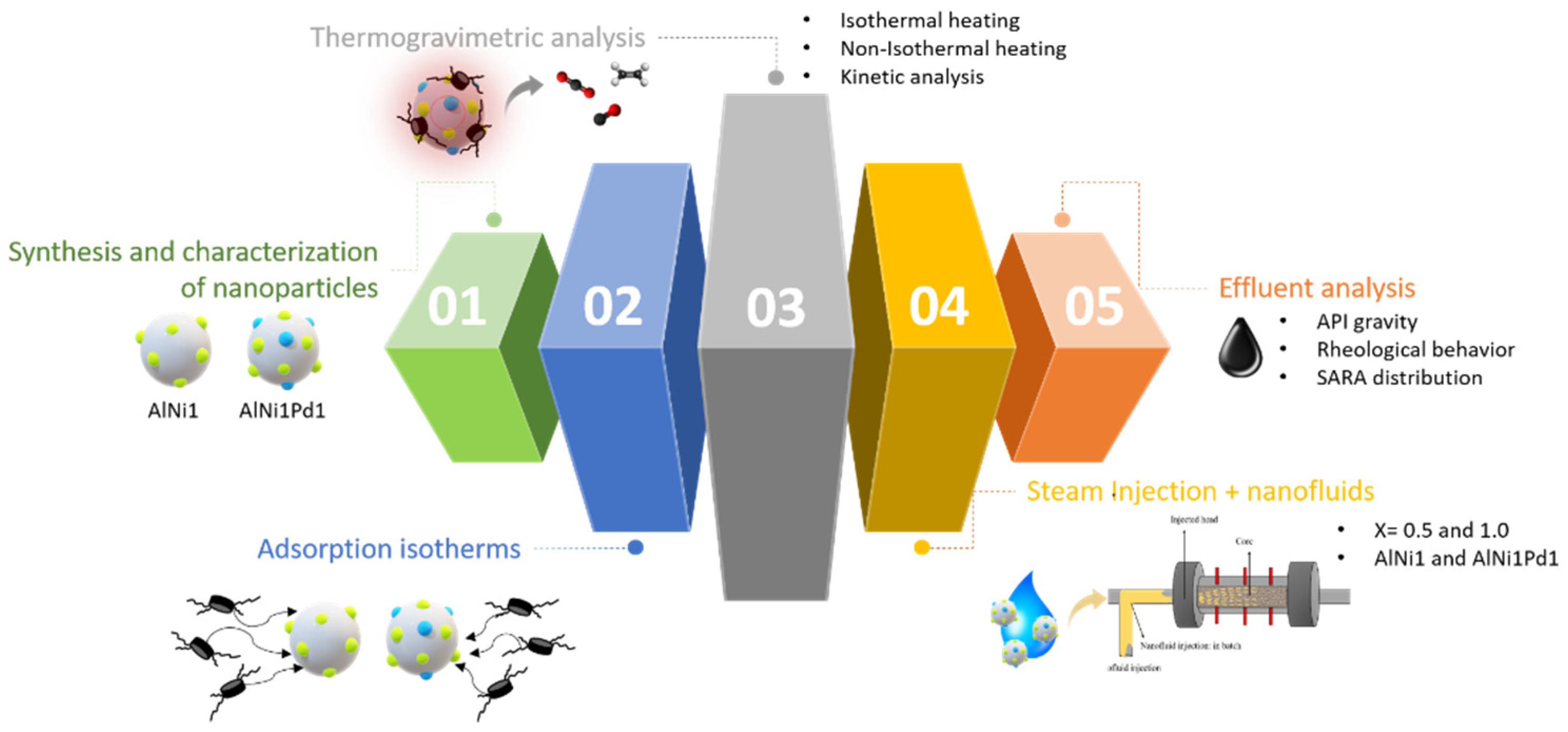
2. Materials and Methods
2.1. Materials
Porous Media
2.2. Methods
2.2.1. Nanoparticles Characterization
2.2.2. Adsorption Batch Experiments
2.2.3. Catalytic Decomposition of Asphaltenes
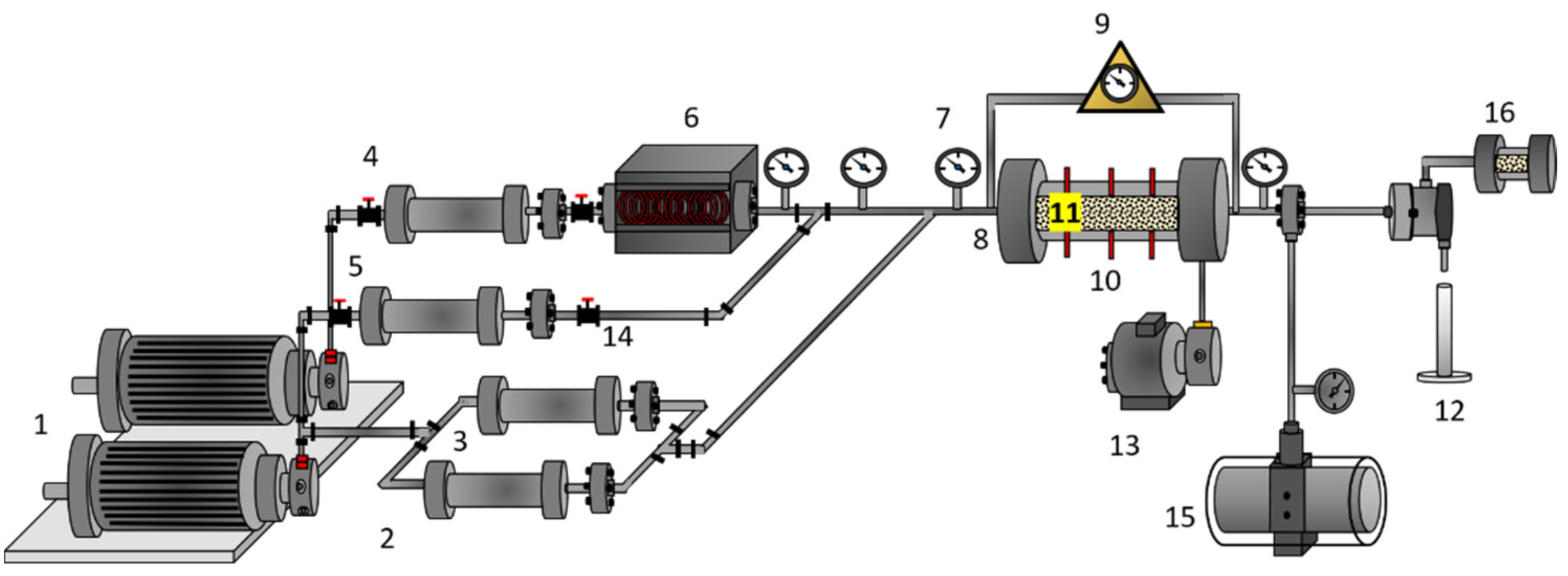
2.2.4. Displacement Tests
2.2.5. Effluent Analysis
3. Results
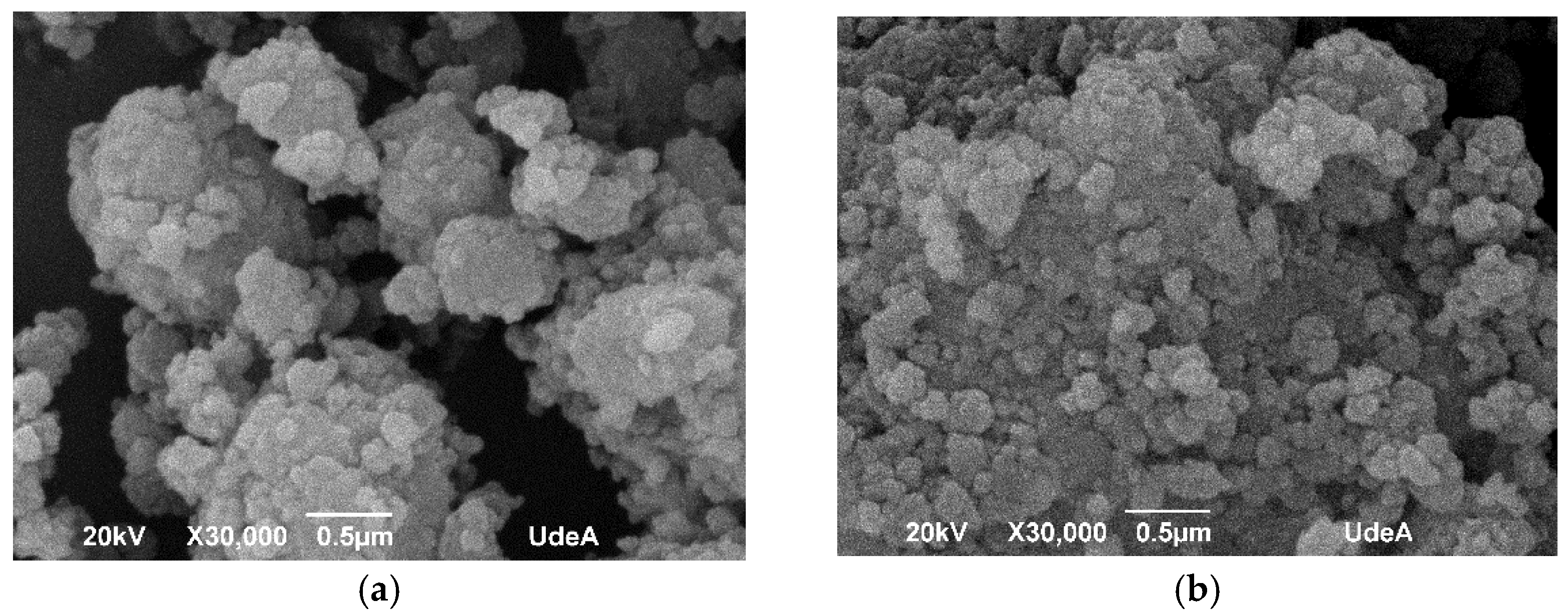
3.1. Nanoparticles Characterization
3.2. Adsorption Experiments
3.3. Thermogravimetric Analysis
3.3.1. Non-Isothermal Thermogravimetric Experiments
3.3.2. Isothermal Thermogravimetric Experiments and Kinetic Analysis
3.4. Displacement Test
3.4.1. Waterflooding and Steam Injection without Nanoparticles at Different Qualities
3.4.2. Steam Injection with Nanoparticles at Different Qualities
3.5. Effluent Characterization
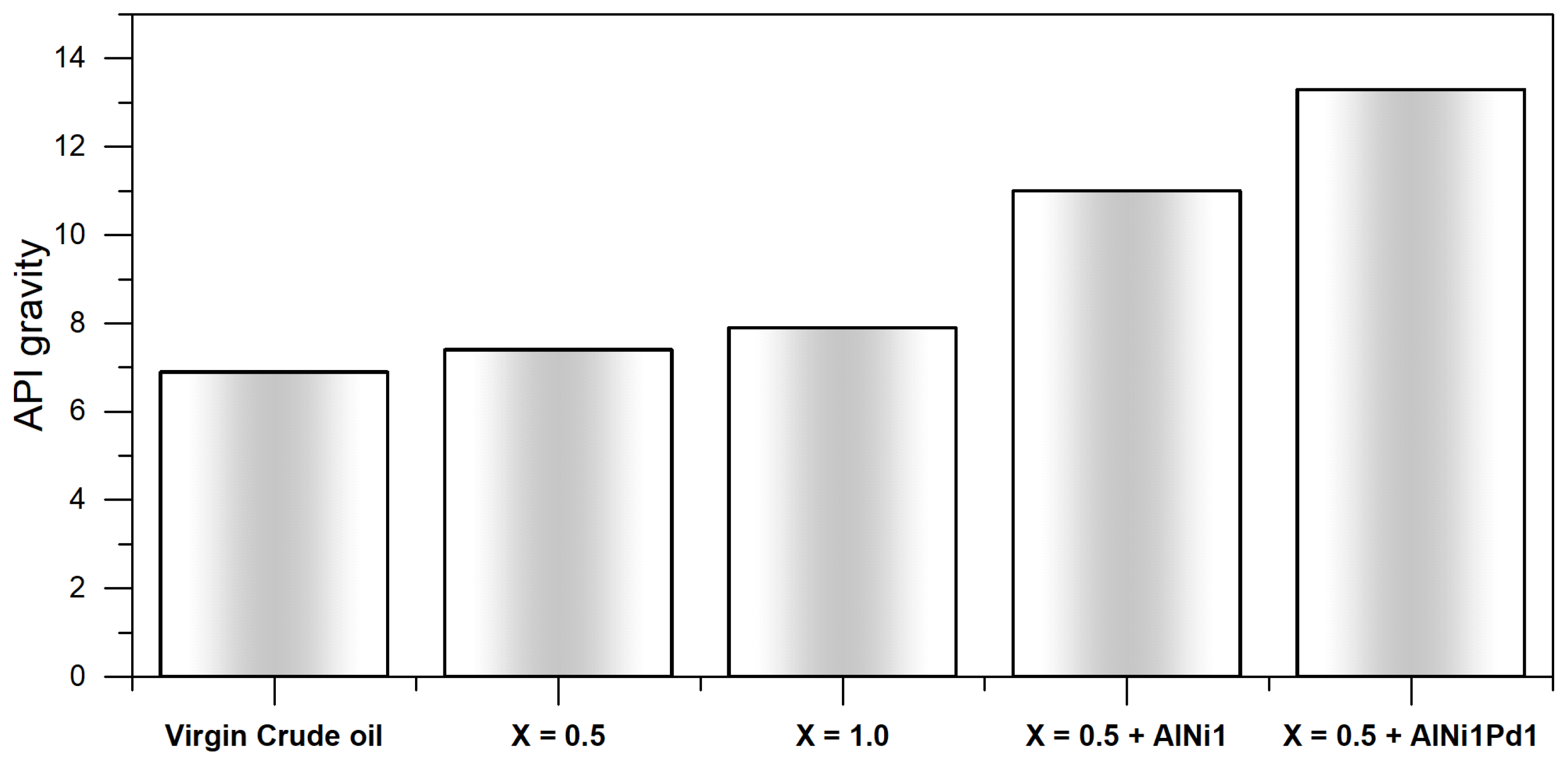
3.5.1. API Gravity Changes
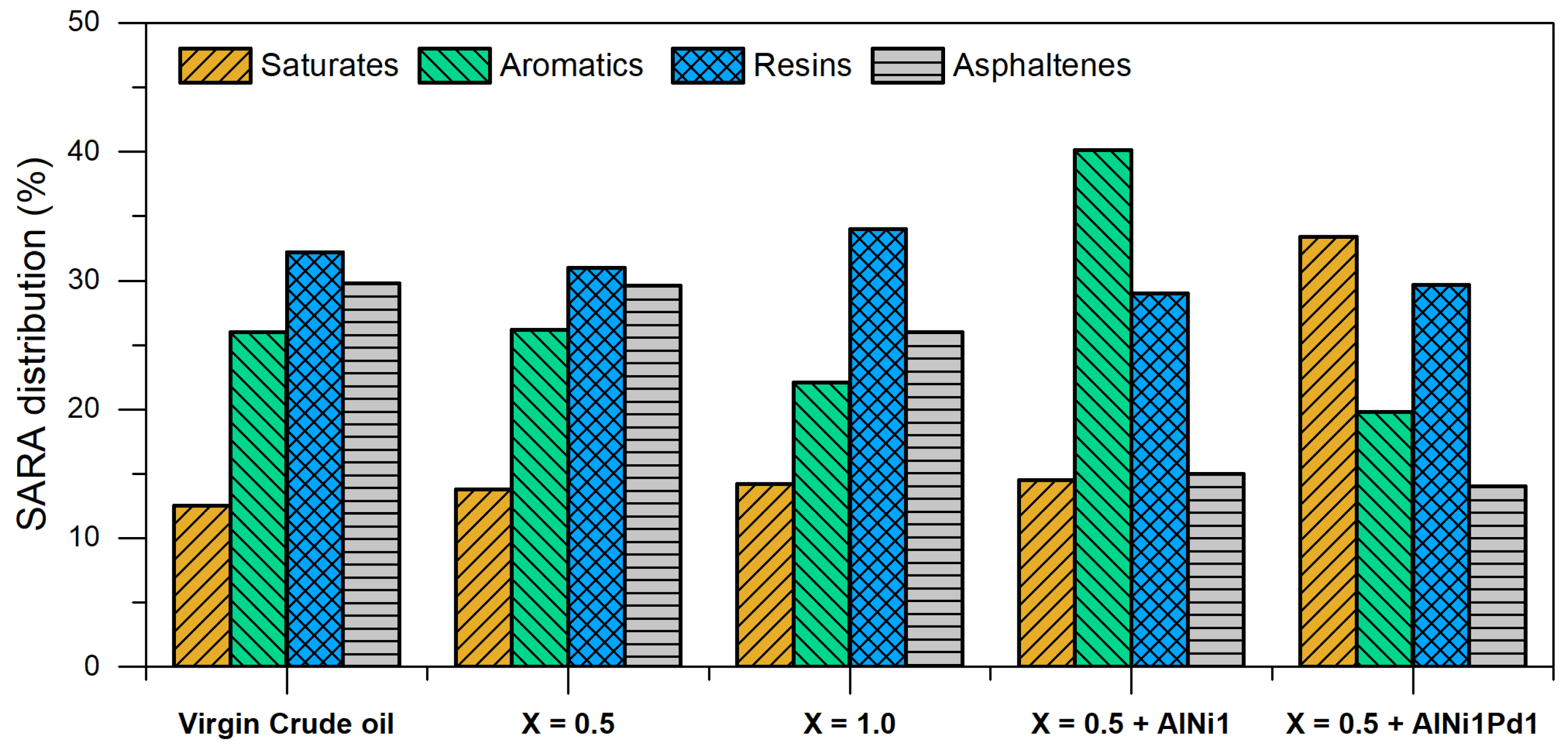
3.5.2. SARA Composition Changes
3.5.3. Simulated Distillation
3.5.4. Rheological Behavior Analysis
4. Conclusions
- The steam injection at 0.5 and 1.0 quality increased the oil recovery by 3.0% and 7.0%, respectively, regarding the base curve (waterflooding). After nanofluid injection, the steam at 0.5 quality achieved an increase in oil recovery of 20.0% and 13.0% for AlNi1 and AlNi1Pd1 nanoparticles, respectively. When steam was injected at 1.0 quality for both nanoparticles evaluated, no incremental oil was produced. The adsorption capacity was determined through batch adsorption experiments, obtaining higher asphaltene adsorption for the AlNi1Pd1 sample.
- The catalytic experiments show that AlNi1 and AlNi1Pd1 samples achieve 100% conversion of adsorbed asphaltenes at 220 °C during 140 and 175 min, respectively.
- All functionalized nanoparticles tested in this work exhibit low energy activation compared with the virgin asphaltenes and a high gasification rate. The reaction mechanism depends on the metal’s nature in the nanoparticles’ surface and the interaction between different metals on the same surface.
- The change in steam quality did not affect the crude oil upgrading (API and SARA content). However, AlNi1 and AlN1Pd1-based nanofluids increased de API gravity from 6.9° to 11°, and 13.3° and the asphaltene content was reduced near to 30% and 50%, respectively, and the highest viscosity reduction percentage was 99% in for AlNi1Pd1.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AlNi1 | Alumina nanoparticles doped with 1.0% in mass fraction of Ni. |
| AlNi1Pd1 | Alumina nanoparticles doped with 1.0% in mass fraction of Ni and Pd. |
| TEOR | Thermal Enhanced Oil Recovery |
| HO | Heavy oil |
| EHO | Extra-heavy oil |
| SBET (m2) | Surface area estimates by the Brunauer-Emmett-Teller surface method |
| EDX | Energy Dispersive X-ray spectroscopy |
| SEM | Scanning Electron Microscopy |
| DLS | Dynamic Light Scattering |
| Kabs | Absolute permeability |
| ko | Oil effective permeability |
| DVR | Degree of viscosity reduction |
| R% | Residue content |
| (cP) | Reference viscosity |
| (cP) | Viscosity after treatment |
| (cP) | Viscosity at the infinite shear rate |
| (cP) | Viscosity at zero shear rate |
| H (mg g−1) | Henry’s law constant |
| K (g g−1) | Adsorbate association degree |
| (g g−1) | Maximum adsorbed amount of adsorbate |
| (mg L−1) | Equilibrium concentration |
| (mg L−1) | Initial concentration of asphaltene in the solution |
| (°C) | Temperature |
| (kJ mol−1) | Activation energy |
| X | Steam quality |
| Reaction rate |
References
- Davarpanah, A.; Mirshekari, B. Experimental investigation and mathematical modeling of gas diffusivity by carbon dioxide and methane kinetic adsorption. Ind. Eng. Chem. Res. 2019, 58, 12392–12400. [Google Scholar] [CrossRef]
- Hu, X.; Xie, J.; Cai, W.; Wang, R.; Davarpanah, A. Thermodynamic effects of cycling carbon dioxide injectivity in shale reservoirs. J. Pet. Sci. Eng. 2020, 195, 107717. [Google Scholar] [CrossRef]
- Li, S.; Lu, C.; Wu, M.; Hu, Z.; Li, Z.; Wang, Z. New insight into CO2 huff-n-puff process for extraheavy oil recovery via viscosity reducer agents: An experimental study. J. CO2 Util. 2020, 42, 101312. [Google Scholar] [CrossRef]
- Medina, O.E.; Olmos, C.; Lopera, S.H.; Cortés, F.B.; Franco, C.A. Nanotechnology Applied to Thermal Enhanced Oil Recovery Processes: A Review. Energies 2019, 12, 4671. [Google Scholar] [CrossRef]
- Galeano-Caro, D.; Villegas, J.P.; Sánchez, J.H.; Cortés, F.B.; Lopera, S.H.; Franco, C.A. Injection of Nanofluids with Fluorosurfactant-Modified Nanoparticles Dispersed in a Flue Gas Stream at Very Low Concentration for Enhanced Oil Recovery (EOR) in Tight Gas–Condensate Reservoirs. Energy Fuels 2020, 34, 12517–12526. [Google Scholar] [CrossRef]
- Villegas, J.; Moncayo-Riascos, I.; Galeano-Caro, D.; Riazi, M.; Franco, C.A.; Cortés, F.B. Functionalization of γ-Alumina and Magnesia Nanoparticles with a Fluorocarbon Surfactant to Promote Ultra Gas-Wet Surfaces: Experimental and Theoretical Approach. ACS Appl. Mater. Interfaces 2020, 12, 13510–13520. [Google Scholar] [CrossRef] [PubMed]
- Green, D.W.; Willhite, G.P. Enhanced Oil Recovery; Henry L. Doherty Memorial Fund of AIME, Society of Petroleum Engineers: Richardson, TX, USA, 1998; Volume 6. [Google Scholar]
- Escobar, E.; Valko, P.; Lee, W.; Rodriguez, M. Optimization methodology for cyclic steam injection with horizontal wells. In Proceedings of the SPE/CIM International Conference on Horizontal Well Technology, Calgary, AB, Canada, 6–8 November 2000. [Google Scholar]
- Morte, M.K. Relative Permeability Determination for Steam Injection Processes: An Analytical Approach. Master’s Thesis, Texas A & M University, College Station, TX, USA, 2016. [Google Scholar]
- Hong, K. Effects of steam quality and injection rate on steamflood performance. SPE Reserv. Eng. 1994, 9, 290–296. [Google Scholar] [CrossRef]
- Kirmani, F.U.D.; Raza, A.; Gholami, R.; Haidar, M.Z.; Fareed, C.S. Analyzing the effect of steam quality and injection temperature on the performance of steam flooding. Energy Geosci. 2021, 2, 83–86. [Google Scholar] [CrossRef]
- Kusumastuti, I.; Erfando, T.; Hidayat, F. Effects of Various Steam Flooding Injection Patterns and Steam Quality to Recovery Factor. J. Earth Energy Eng. 2019, 8, 33–39. [Google Scholar] [CrossRef]
- Alvarez, J.; Han, S. Current overview of cyclic steam injection process. J. Pet. Sci. Res. 2013, 2, 116–127. [Google Scholar]
- Nasr, T.; Beaulieu, G.; Golbeck, H.; Heck, G. Novel expanding solvent-SAGD process “ES-SAGD”. In Proceedings of the Canadian International Petroleum Conference, Calgary, AB, Canada, 11–13 June 2002. [Google Scholar]
- Zhao, Y. Laboratory experiment and field application of high pressure and high quality steam flooding. J. Pet. Sci. Eng. 2020, 189, 107016. [Google Scholar] [CrossRef]
- Dong, X.; Liu, H.; Chen, Z.; Wu, K.; Lu, N.; Zhang, Q. Enhanced oil recovery techniques for heavy oil and oilsands reservoirs after steam injection. Appl. Energy 2019, 239, 1190–1211. [Google Scholar] [CrossRef]
- Wen, S.; Zhao, Y.; Liu, Y.; Hu, S. A study on catalytic aquathermolysis of heavy crude oil during steam stimulation. In Proceedings of the International Symposium on Oilfield Chemistry, Houston, TX, USA, 28 February–2 March 2007. [Google Scholar]
- Franco, C.A.; Montoya, T.; Nassar, N.N.; Pereira-Almao, P.; Cortés, F.B. Adsorption and subsequent oxidation of colombian asphaltenes onto nickel and/or palladium oxide supported on fumed silica nanoparticles. Energy Fuels 2013, 27, 7336–7347. [Google Scholar] [CrossRef]
- Arias-Madrid, D.; Medina, O.E.; Gallego, J.; Acevedo, S.; Correa-Espinal, A.A.; Cortés, F.B.; Franco, C.A. NiO, Fe2O3, and MoO3 Supported over SiO2 Nanocatalysts for Asphaltene Adsorption and Catalytic Decomposition: Optimization through a Simplex–Centroid Mixture Design of Experiments. Catalysts 2020, 10, 569. [Google Scholar] [CrossRef]
- Lozano, M.M.; Franco, C.A.; Acevedo, S.A.; Nassar, N.N.; Cortés, F.B. Effects of resin I on the catalytic oxidation of n-C7 asphaltenes in the presence of silica-based nanoparticles. RSC Adv. 2016, 6, 74630–74642. [Google Scholar] [CrossRef]
- Cardona, L.; Arias-Madrid, D.; Cortés, F.B.; Lopera, S.H.; Franco, C.A. Heavy oil upgrading and enhanced recovery in a steam injection process assisted by NiO-and PdO-Functionalized SiO2 nanoparticulated catalysts. Catalysts 2018, 8, 132. [Google Scholar] [CrossRef]
- Medina, O.E.; Gallego, J.; Restrepo, L.G.; Cortés, F.B.; Franco, C.A. Influence of the Ce4+/Ce3+ Redox-couple on the cyclic regeneration for adsorptive and catalytic performance of NiO-PdO/CeO2±δ nanoparticles for n-C7 asphaltene steam gasification. Nanomaterials 2019, 9, 734. [Google Scholar] [CrossRef]
- Medina Erao, O.E.; Gallego, J.; Olmos, C.M.; Chen, X.; Cortés, F.B.; Franco, C.A. Effect of Multifunctional Nanocatalysts on n-C7 Asphaltene Adsorption and Subsequent Oxidation under High Pressure Conditions. Energy Fuels 2020, 34, 6261–6278. [Google Scholar] [CrossRef]
- Medina, O.E.; Hurtado, Y.; Caro-Velez, C.; Cortés, F.B.; Riazi, M.; Lopera, S.H.; Franco, C.A. Improvement of steam injection processes through nanotechnology: An approach through in situ upgrading and foam injection. Energies 2019, 12, 4633. [Google Scholar] [CrossRef]
- Medina, O.E.; Caro-Vélez, C.; Gallego, J.; Cortés, F.B.; Lopera, S.H.; Franco, C.A. Upgrading of Extra-Heavy Crude Oils by Dispersed Injection of NiO–PdO/CeO2±δ Nanocatalyst-Based Nanofluids in the Steam. Nanomaterials 2019, 9, 1755. [Google Scholar] [CrossRef]
- Medina, O.E.; Gallego, J.; Arias-Madrid, D.; Cortés, F.B.; Franco, C.A. Optimization of the load of transition metal oxides (Fe2O3, Co3O4, NiO and/or PdO) onto CeO2 nanoparticles in catalytic steam decomposition of n-C7 asphaltenes at low temperatures. Nanomaterials 2019, 9, 401. [Google Scholar] [CrossRef]
- Medina, O.E.; Gallego, J.; Acevedo, S.; Riazi, M.; Ocampo-Pérez, R.; Cortés, F.B.; Franco, C.A. Catalytic Conversion of n-C7 Asphaltenes and Resins II into Hydrogen Using CeO2-Based Nanocatalysts. Nanomaterials 2021, 11, 1301. [Google Scholar] [CrossRef]
- Nassar, N.N.; Franco, C.A.; Montoya, T.; Cortés, F.B.; Hassan, A. Effect of oxide support on Ni–Pd bimetallic nanocatalysts for steam gasification of n-C7 asphaltenes. Fuel 2015, 156, 110–120. [Google Scholar] [CrossRef]
- Nassar, N.N.; Hassan, A.; Pereira-Almao, P. Effect of surface acidity and basicity of aluminas on asphaltene adsorption and oxidation. J. Colloid Interface Sci. 2011, 360, 233–238. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Q.; Fan, Z. Enhanced in situ combustion of heavy crude oil by nickel oxide nanoparticles. Int. J. Energy Res. 2019, 43, 3399–3412. [Google Scholar] [CrossRef]
- Marei, N.N.; Nassar, N.N.; Hmoudah, M.; El-Qanni, A.; Vitale, G.; Hassan, A. Nanosize effects of NiO nanosorbcats on adsorption and catalytic thermo-oxidative decomposition of vacuum residue asphaltenes. Can. J. Chem. Eng. 2017, 95, 1864–1874. [Google Scholar] [CrossRef]
- Medina, O.E.; Galeano-Caro, D.; Castelo-Quibén, J.; Ocampo-Pérez, R.; Perez-Cadenas, A.F.; Carrasco-Marín, F.; Franco, C.A.; Corteś, F.B. Monolithic carbon xerogels-metal composites for crude oil removal from oil in-saltwater emulsions and subsequent regeneration through oxidation process: Composites synthesis, adsorption studies, and oil decomposition experiments. Microporous and Mesoporous Mater. 2021, 319, 111039. [Google Scholar] [CrossRef]
- Nassar, N.N.; Hassan, A.; Pereira-Almao, P. Comparative oxidation of adsorbed asphaltenes onto transition metal oxide nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2011, 384, 145–149. [Google Scholar] [CrossRef]
- Mateus, L.; Moreno-Castilla, C.; López-Ramón, M.V.; Cortés, F.B.; Álvarez, M.Á.; Medina, O.E.; Franco, C.A.; Yebra-Rodríguez, Á. Physicochemical characteristics of calcined MnFe2O4 solid nanospheres and their catalytic activity to oxidize para-nitrophenol with peroxymonosulfate and n-C7 asphaltenes with air. J. Environ. Manag. 2021, 281, 111871. [Google Scholar] [CrossRef]
- Afzal, S.; Nikookar, M.; Ehsani, M.R.; Roayaei, E. An experimental investigation of the catalytic effect of Fe2O3 nanoparticle on steam injection process of an Iranian reservoir. Iran. J. Oil Gas Sci. Technol. 2014, 3, 27–36. [Google Scholar]
- Hamedi Shokrlu, Y.; Babadagli, T. In-situ upgrading of heavy oil/bitumen during steam injection by use of metal nanoparticles: A study on in-situ catalysis and catalyst transportation. SPE Reserv. Eval. Eng. 2013, 16, 333–344. [Google Scholar] [CrossRef]
- Tiab, D.; Donaldson, E. Chapter 5—Capillary pressure. In Petrophysics, 2nd ed.; Gulf Professional Publishing: Burlington, MA, USA, 2004; pp. 313–359. [Google Scholar]
- Cardona Rojas, L. Efecto de Nanopartículas en Procesos con Inyección de Vapor a Diferentes Calidades. Master’s Thesis, Universidad Nacional de Colombia-Sede Medellín, Medellín, Colombia, 2017. [Google Scholar]
- Naderi, M. Surface Area: Brunauer–Emmett–Teller (BET). In Progress in Filtration and Separation; Elsevier: Alperton, London, UK, 2015; pp. 585–608. [Google Scholar]
- Acosta, L.; Galeano-Caro, D.; Medina, O.E.; Cortés, F.B.; Franco, C.A. Nano-Intermediate of Magnetite Nanoparticles Supported on Activated Carbon from Spent Coffee Grounds for Treatment of Wastewater from Oil Industry and Energy Production. Processes 2021, 9, 63. [Google Scholar] [CrossRef]
- Whitaker, S. Flow in porous media I: A theoretical derivation of Darcy’s law. Transp. Porous Media 1986, 1, 3–25. [Google Scholar] [CrossRef]
- Giraldo, J.; Benjumea, P.; Lopera, S.; Cortés, F.B.; Ruiz, M.A. Wettability alteration of sandstone cores by alumina-based nanofluids. Energy Fuels 2013, 27, 3659–3665. [Google Scholar] [CrossRef]
- Montes, D.; Henao, J.; Taborda, E.A.; Gallego, J.; Cortés, F.B.; Franco, C.A. Effect of Textural Properties and Surface Chemical Nature of Silica Nanoparticles from Different Silicon Sources on the Viscosity Reduction of Heavy Crude Oil. ACS Omega 2020, 5, 5085–5097. [Google Scholar] [CrossRef] [PubMed]
- Montes, D.; Orozco, W.; Taborda, E.A.; Franco, C.A.; Cortés, F.B. Development of nanofluids for perdurability in viscosity reduction of extra-heavy oils. Energies 2019, 12, 1068. [Google Scholar] [CrossRef]
- Bansal, V.; Krishna, G.; Chopra, A.; Sarpal, A. Detailed hydrocarbon characterization of RFCC feed stocks by NMR spectroscopic techniques. Energy Fuels 2007, 21, 1024–1029. [Google Scholar] [CrossRef]
- Shi, Q.; Hou, D.; Chung, K.H.; Xu, C.; Zhao, S.; Zhang, Y. Characterization of heteroatom compounds in a crude oil and its saturates, aromatics, resins, and asphaltenes (SARA) and non-basic nitrogen fractions analyzed by negative-ion electrospray ionization Fourier transform ion cyclotron resonance mass spectrometry. Energy Fuels 2010, 24, 2545–2553. [Google Scholar]
- Hashemi, R.; Nassar, N.N.; Pereira-Almao, P. Transport behavior of multimetallic ultradispersed nanoparticles in an oil-sands-packed bed column at a high temperature and pressure. Energy Fuels 2012, 26, 1645–1655. [Google Scholar] [CrossRef]
- Cross, W.E., Jr. The psychology of Nigrescence: Revising the Cross model. In Handbook of Multicultural Counseling; APA PsycNet: Washington, DC, USA, 1995. [Google Scholar]
- Sharma, A.; Saito, I.; Nakagawa, H.; Miura, K. Effect of carbonization temperature on the nickel crystallite size of a Ni/C catalyst for catalytic hydrothermal gasification of organic compounds. Fuel 2007, 86, 915–920. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, D.; Zhao, P.; Niu, Z.; Peng, Q.; Li, Y. Monodispersed Pd−Ni nanoparticles: Composition control synthesis and catalytic properties in the Miyaura−Suzuki reaction. Inorg. Chem. 2011, 50, 2046–2048. [Google Scholar] [CrossRef]
- Nassar, N.N.; Hassan, A.; Pereira-Almao, P. Application of nanotechnology for heavy oil upgrading: Catalytic steam gasification/cracking of asphaltenes. Energy Fuels 2011, 25, 1566–1570. [Google Scholar] [CrossRef]
- Medina, O.E.; Gallego, J.; Nassar, N.N.; Acevedo, S.A.; Cortés, F.B.; Franco, C.A. Thermo-Oxidative Decomposition Behaviors of Different Sources of n-C7 Asphaltenes at High-Pressure Conditions. Energy Fuels 2020, 34, 8740–8758. [Google Scholar] [CrossRef]
- Siddiqui, M.N. Catalytic pyrolysis of Arab Heavy residue and effects on the chemistry of asphaltene. J. Anal. Appl. Pyrolysis 2010, 89, 278–285. [Google Scholar] [CrossRef]
- Franco, C.A.; Nassar, N.N.; Montoya, T.; Ruíz, M.A.; Cortés, F.B. Influence of asphaltene aggregation on the adsorption and catalytic behavior of nanoparticles. Energy Fuels 2015, 29, 1610–1621. [Google Scholar] [CrossRef]
- Hou, J. The Feasibility Study of High Pressure Steam Flooding after Water Flooding for Common Heavy Oil Reservoir. J. Eng. Res. 2017, 5, 200–219. [Google Scholar]
- Ruiz, S.M.; Campo, L.T.C.; Gómez, L.R.O.; Navarro, S.F.M. Cálculo de la eficiencia térmica de un proceso de inyección continua vapor en yacimientos estratificados. Fuentes El Reventón Energético 2013, 11, 4. [Google Scholar]
- Alemán-Vázquez, L.; Cano-Domínguez, J.; García-Gutiérrez, J. Effect of tetralin, decalin and naphthalene as hydrogen donors in the upgrading of heavy oils. Procedia Eng. 2012, 42, 532–539. [Google Scholar] [CrossRef]
- Iwamoto, M.; Yoda, Y.; Yamazoe, N.; Seiyama, T. Study of metal oxide catalysts by temperature programmed desorption. 4. Oxygen adsorption on various metal oxides. J. Phys. Chem. 1978, 82, 2564–2570. [Google Scholar] [CrossRef]
- Jana, N.R.; Wang, Z.; Pal, T. Redox catalytic properties of palladium nanoparticles: Surfactant and electron donor—Acceptor effects. Langmuir 2000, 16, 2457–2463. [Google Scholar] [CrossRef]
- Hashemi, R.; Nassar, N.N.; Pereira Almao, P. In situ upgrading of Athabasca bitumen using multimetallic ultradispersed nanocatalysts in an oil sands packed-bed column: Part 1. Produced liquid quality enhancement. Energy Fuels 2013, 28, 1338–1350. [Google Scholar] [CrossRef]
- Zhang, Z.; Barrufet, M.A.; Lane, R.H.; Mamora, D.D. Experimental study of in-situ upgrading for heavy oil using hydrogen donors and catalyst under steam injection condition. In Proceedings of the SPE Heavy Oil Conference Canada, Calgary, AB, Canada, 12–14 June 2012. [Google Scholar]
- Palencia, J.; Delgado, J.P.; Luis, M.Á.; Labrador Sánchez, H. Evaluación del crudo extra pesado Carabobo mediante Hidrotratamiento con variación de la temperatura utilizando un catalizador mesoporoso MCM-41. Rev. Ing. UC 2012, 19, 69–75. [Google Scholar]
- Dong, W.; Ledentu, V.; Sautet, P.; Eichler, A.; Hafner, J. Hydrogen adsorption on palladium: A comparative theoretical study of different surfaces. Surf. Sci. 1998, 411, 123–136. [Google Scholar] [CrossRef]
- Ospina Gómez, N.A. Evaluación de la aplicación de nanofluidos para mejoramiento in-situ del crudo pesado. Master’s Thesis, Universidad Nacional de Colombia-Sede Medellín, Medellín, Colombia, 2015. [Google Scholar]
- Hashemi, R.; Nassar, N.N.; Pereira Almao, P. Enhanced heavy oil recovery by in situ prepared ultradispersed multimetallic nanoparticles: A study of hot fluid flooding for Athabasca bitumen recovery. Energy Fuels 2013, 27, 2194–2201. [Google Scholar] [CrossRef]
- Santos, R.; Loh, W.; Bannwart, A.; Trevisan, O. An overview of heavy oil properties and its recovery and transportation methods. Braz. J. Chem. Eng. 2014, 31, 571–590. [Google Scholar] [CrossRef]
- Aristizábal-Fontal, J.E.; Cortés, F.B.; Franco, C.A. Viscosity reduction of extra heavy crude oil by magnetite nanoparticle-based ferrofluids. Adsorpt. Sci. Technol. 2018, 36, 23–45. [Google Scholar] [CrossRef]







| System | Porous Medium 1 | Porous Medium 2 |
|---|---|---|
| Mineralogy | Silica 99% | Silica 99% |
| Length (cm) | 60 | 60 |
| Diameter (cm) | 2.5 | 2.5 |
| Porous volume (mL) | 131.5 | 132 |
| Porosity (%) | 38 | 38 |
| Absolute permeability | 9080 | 9100 |
| Nanoparticle injected (500 mg∙L−1) | AlNi1Pd1 | AlNi1 |
| Type of Nanoparticle | Pd (%) EDX | Ni (%) EDX | Dispersion (%) | Average Crystal Size (nm) | ||
|---|---|---|---|---|---|---|
| AlNi1 | - | 1.9 | 4.48 | - | 4.50 | - |
| AlNi1Pd1 | 0.98 | 1.0 | 5.40 | 9.90 | 2.20 | 4.10 |
| Material | Temperature (°C) | ||
|---|---|---|---|
| Virgin Asphaltenes | 360 | 211.41 | 0.012 |
| 370 | 0.018 | ||
| 380 | 0.032 | ||
| AlNi1 | 200 | 30.39 | 0.039 |
| 210 | 0.050 | ||
| 220 | 0.078 | ||
| AlNi1Pd1 | 200 | 64.65 | 0.083 |
| 210 | 0.090 | ||
| 220 | 0.127 |
| Parameters Cross Model | Virgin Crude Oil | Oil + X = 50% | Oil + X = 100% | Oil + X = 50% + AlNi1 | Oil + X = 50% + AlNi1Pd1 |
|---|---|---|---|---|---|
| DRV % | - | 8.9 | 32.5 | 92.4 | 99.0 |
| × 103 (cp) | 184 | 162 | 153 | 3.5 | 2.7 |
| × 103 (cp) | 2811 | 2390 | 1302 | 93 | 3 |
| %RMS | 0.143 | 0.062 | 0.071 | 0.051 | 0.041 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardona, L.; Medina, O.E.; Céspedes, S.; Lopera, S.H.; Cortés, F.B.; Franco, C.A. Effect of Steam Quality on Extra-Heavy Crude Oil Upgrading and Oil Recovery Assisted with PdO and NiO-Functionalized Al2O3 Nanoparticles. Processes 2021, 9, 1009. https://doi.org/10.3390/pr9061009
Cardona L, Medina OE, Céspedes S, Lopera SH, Cortés FB, Franco CA. Effect of Steam Quality on Extra-Heavy Crude Oil Upgrading and Oil Recovery Assisted with PdO and NiO-Functionalized Al2O3 Nanoparticles. Processes. 2021; 9(6):1009. https://doi.org/10.3390/pr9061009
Chicago/Turabian StyleCardona, Luisana, Oscar E. Medina, Santiago Céspedes, Sergio H. Lopera, Farid B. Cortés, and Camilo A. Franco. 2021. "Effect of Steam Quality on Extra-Heavy Crude Oil Upgrading and Oil Recovery Assisted with PdO and NiO-Functionalized Al2O3 Nanoparticles" Processes 9, no. 6: 1009. https://doi.org/10.3390/pr9061009
APA StyleCardona, L., Medina, O. E., Céspedes, S., Lopera, S. H., Cortés, F. B., & Franco, C. A. (2021). Effect of Steam Quality on Extra-Heavy Crude Oil Upgrading and Oil Recovery Assisted with PdO and NiO-Functionalized Al2O3 Nanoparticles. Processes, 9(6), 1009. https://doi.org/10.3390/pr9061009










