Polyphenolic Profiling of Green Waste Determined by UPLC-HDMSE
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Sample Preparation
2.3. Analytical Instrumentation
2.4. UPLC Conditions
2.5. Synapt G2-Si Conditions
2.6. Data Processing
3. Results and Discussion
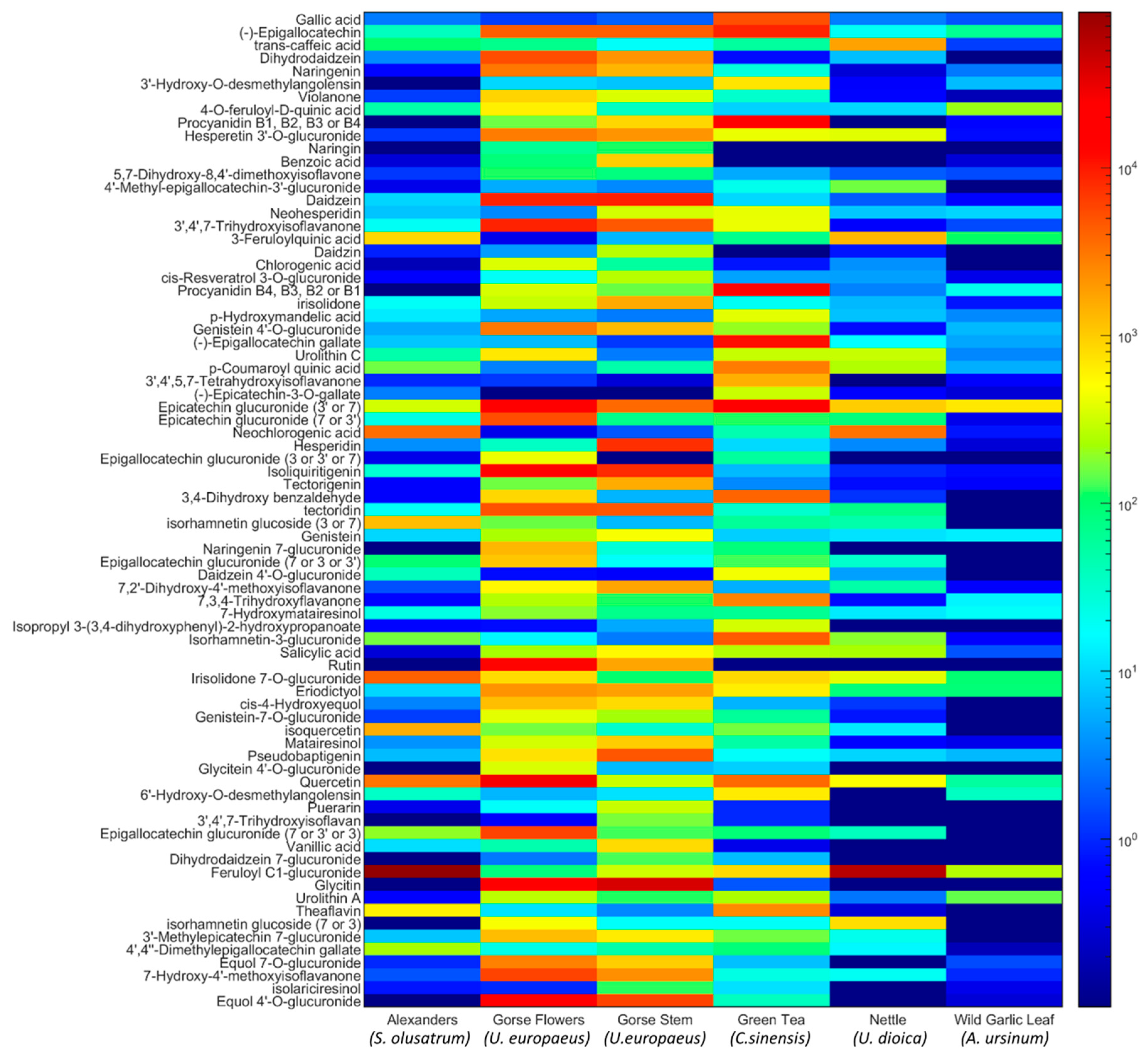
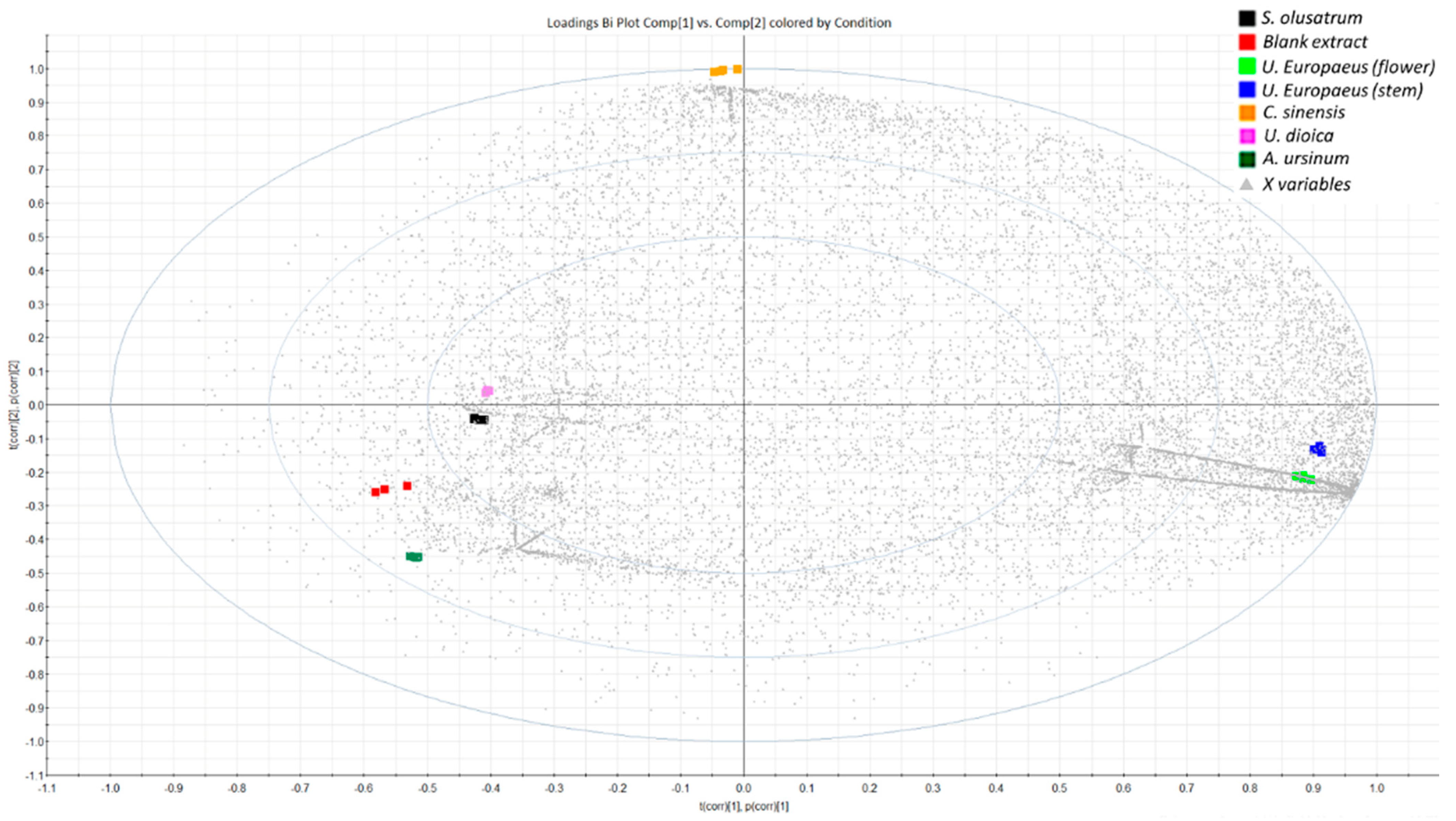
3.1. Bioactive Phenolic Compounds in the Green Waste Extracts
3.2. Tentative Identification of Polyphenols
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sheldon, R.A. Green and sustainable manufacture of chemicals from biomass: State of the art. Green Chem. 2014, 16, 950–963. [Google Scholar] [CrossRef]
- Langsdorf, A.; Volkmar, M.; Holtmann, D.; Ulber, R. Material utilization of green waste: A review on potential valorization methods. Bioresourc Bioprocess. 2021, 8, 19. [Google Scholar] [CrossRef]
- Barba, F.J.; Zhu, Z.Z.; Koubaa, M.; Sant’Ana, A.S.; Orlien, V. Green alternative methods for the extraction of antioxidant bioactive compounds from winery wastes and by-products: A review. Trends Food Sci. Technol. 2016, 49, 96–109. [Google Scholar] [CrossRef]
- De La Iglesia, R.; Milagro, F.I.; Campión, J.; Boqué, N.; Martínez, J.A. Healthy properties of proanthocyanidins. BioFactors 2010, 36, 159–168. [Google Scholar] [CrossRef]
- Krikorian, R.; Kalt, W.; Mcdonald, J.E.; Shidler, M.D.; Summer, S.S.; Stein, A.L. Cognitive performance in relation to urinary anthocyanins and their flavonoid-based products following blueberry supplementation in older adults at risk for dementia. J. Funct. Foods 2019, 103667. [Google Scholar] [CrossRef]
- Preedy, V.; Zibadi, S.; Watson, R. Polyphenols in Human Health and Disease; Academic Press: New York, NY, USA, 2014; Volume 1, ISBN 9780123984562. [Google Scholar]
- Uyama, H.; Kobayashi, S. Enzymatic synthesis of polyphenols. Curr. Org. Chem. 2003, 7, 1387–1397. [Google Scholar] [CrossRef]
- Potter, C.M.; Jones, D.L. Polyphenolic Profiling of Forestry Waste by UPLC-HDMSe. Processes 2020, 8, 1411. [Google Scholar] [CrossRef]
- Wu, F.F.; Sun, H.; Wei, W.F.; Han, Y.; Wang, P.; Dong, T.W.; Yan, G.L.; Wang, X.J. Rapid and global detection and characterization of the constituents in ShengMai San by ultra-performance liquid chromatography-high-definition mass spectrometry. J. Sep. Sci. 2011, 34, 3194–3199. [Google Scholar] [CrossRef]
- Wang, P.; Sun, H.; Lv, H.; Sun, W.; Yuan, Y.; Han, Y.; Wang, D.W.; Zhang, A.H.; Wang, X.J. Thyroxine and reserpine-induced changes in metabolic profiles of rat urine and the therapeutic effect of Liu Wei Di Huang Wan detected by UPLC-HDMS. J. Pharm. Biomed. Anal. 2010, 53, 631–645. [Google Scholar] [CrossRef]
- Da Silva Pinto, M. Tea: A new perspective on health benefits. Food Res. Int. 2013, 53, 558–567. [Google Scholar] [CrossRef]
- Sapozhnikova, Y. Development of liquid chromatography-tandem mass spectrometry method for analysis of polyphenolic compounds in liquid samples of grape juice, green tea and coffee. Food Chem. 2014, 150, 87–93. [Google Scholar] [CrossRef]
- Vos, F.; Crespy, V.; Chaffaut, L.; Mennen, L.; Knox, C.; Neveu, V. Phenol-Explorer: An online comprehensive database on polyphenol contents in foods. Database 2010, 2010, 1–9. [Google Scholar] [CrossRef]
- Pandey, R.; Chandra, P.; Arya, K.R.; Kumar, B. Development and validation of an ultra high performance liquid chromatography electrospray ionization tandem mass spectrometry method for the simultaneous determination of selected flavonoids in Ginkgo biloba. J. Sep. Sci. 2014, 37, 3610–3618. [Google Scholar] [CrossRef]
- Lopes, N.P.; Demarque, D.P.; Crotti, A.E.M.; Vessecchi, R.; Lopes, J. Fragmentation reactions using electrospray ionization mass spectrometry: An important tool for the structural elucidation and characterization of synthetic and natural products. RSC-Nat. Prod. Rep. 2016, 33, 432. [Google Scholar] [CrossRef]
- Ahn, H.J.; You, J.; Park, S.; Li, Z.; Choe, D. RSC Advances and their contribution to improved anti-inflammatory activity. RSC Adv. 2020, 10, 5339–5350. [Google Scholar] [CrossRef]
- Chaitanya, M.V.N.L.; Suresh, P.; Dhanabal, P.; Jubie, S. Human Topopoisons From Weeds: A Review. Curr. Tradit. Med. 2018, 4, 4–15. [Google Scholar] [CrossRef]
- Andersen Oyvind, M.; Dersen Markham, K.R. Flavonoids: Chemistry, Biochemistry and Applications; CRC Press: Boca Raton, FL, USA, 2006; Volume 45, ISBN 9780849320217. [Google Scholar]
- Gülçin, I. Antioxidant activity of caffeic acid (3,4-dihydroxycinnamic acid). Toxicology 2006, 217, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Barsky, L.; Cook-Wiens, G.; Doyle, M.; Shufelt, C.; Rogers, W.; Reis, S.; Pepine, C.J.; Noel Bairey Merz, C. Phytoestrogen blood levels and adverse outcomes in women with suspected ischemic heart disease. Eur. J. Clin. Nutr. 2020. [Google Scholar] [CrossRef] [PubMed]
- Zaw, J.J.T.; Howe, P.R.C.; Wong, R.H.X. Does phytoestrogen supplementation improve cognition in humans? A systematic review. Ann. N. Y. Acad. Sci. 2017, 1403, 150–163. [Google Scholar] [CrossRef]
- Costa, E.M.D.M.B.; Pimenta, F.C.; Luz, W.C.; De Oliveira, V. Selection of filamentous fungi of the Beauveria genus able to metabolize quercetin like mammalian cells. Braz. J. Microbiol. 2008, 39, 405–408. [Google Scholar] [CrossRef]
- Marvalin, C.; Azerad, R. Microbial glucuronidation of polyphenols. J. Mol. Catal. B Enzym. 2011, 73, 43–52. [Google Scholar] [CrossRef]
- Salehi, B.; Fokou, P.V.T.; Sharifi-Rad, M.; Zucca, P.; Pezzani, R.; Martins, N.; Sharifi-Rad, J. The therapeutic potential of naringenin: A review of clinical trials. Pharmaceuticals 2019, 12, 11. [Google Scholar] [CrossRef] [PubMed]
- Tutunchi, H.; Naeini, F.; Ostadrahimi, A.; Hosseinzadeh-Attar, M.J. Naringenin, a flavanone with antiviral and anti-inflammatory effects: A promising treatment strategy against COVID-19. Phyther. Res. 2020, 1, 1–11. [Google Scholar] [CrossRef]
- Alberca, R.W.; Teixeira, F.M.E.; Beserra, D.R.; de Oliveira, E.A.; de Andrade, M.M.S.; Pietrobon, A.J.; Sato, M.N. Perspective: The Potential Effects of Naringenin in COVID-19. Front. Immunol. 2020, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Fernández de Simón, B.; Esteruelas, E.; Muñoz, Á.M.; Cadahía, E.; Hernández, M.T.; Estrella, I.; Martinez, J. Polyphenols in red wine aged in acacia (Robinia pseudoacacia) and oak (Quercus petraea) wood barrels. Anal. Chim. Acta 2012, 732, 83–90. [Google Scholar] [CrossRef]
- Duan, J.; Guan, Y.; Mu, F.; Guo, C.; Zhang, E.; Yin, Y.; Wei, G.; Zhu, Y.; Cui, J.; Cao, J.; et al. Protective effect of butin against ischemia/reperfusion-induced myocardial injury in diabetic mice: Involvement of the AMPK/GSK-3β/Nrf2 signaling pathway. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Hollman, P.C.H.; Bijsman, M.N.C.P.; Van Gameren, Y.; Cnossen, E.P.J.; De Vries, J.H.M.; Katan, M.B. The sugar moiety is a major determinant of the absorption of dietary flavonoid glycosides in man. Free Radic. Res. 1999, 31, 569–573. [Google Scholar] [CrossRef]
- Rabago Smith, M.; Kindl, E.D.; Williams, I.R.; Moorman, V.R. 5,7,3′,4′-Hydroxy substituted flavonoids reduce the heme of cytochrome c with a range of rate constants. Biochimie 2019, 162, 167–175. [Google Scholar] [CrossRef]
- Liu, Y.C.; Yan, X.N. Eriodictyol inhibits survival and inflammatory responses and promotes apoptosis in rheumatoid arthritis fibroblast-like synoviocytes through AKT/FOXO1 signaling. J. Cell. Biochem. 2019, 120, 14628–14635. [Google Scholar] [CrossRef]
- Lv, P.; Yu, J.; Xu, X.; Lu, T.; Xu, F. Eriodictyol inhibits high glucose-induced oxidative stress and inflammation in retinal ganglial cells. J. Cell. Biochem. 2019, 120, 5644–5651. [Google Scholar] [CrossRef]
- HighChem LLC mzCloud. Available online: https://www.mzcloud.org/ (accessed on 1 December 2020).
- The, S.N. A Review on the Medicinal Plant Dalbergia odorifera Species: Phytochemistry and Biological Activity. Evid.-Based Complement. Altern. Med. 2017, 1. [Google Scholar] [CrossRef]
- Deesamer, S.; Kokpol, U.; Chavasiri, W.; Douillard, S.; Peyrot, V.; Vidal, N.; Combes, S.; Finet, J.P. Synthesis and biological evaluation of isoflavone analogues from Dalbergia oliveri. Tetrahedron 2007, 63, 12986–12993. [Google Scholar] [CrossRef]
- Wen, R.; Lv, H.; Jiang, Y.; Tu, P. Anti-inflammatory isoflavones and isoflavanones from the roots of Pongamia pinnata (L.) Pierre. Bioorganic Med. Chem. Lett. 2018, 28, 1050–1055. [Google Scholar] [CrossRef]
- Peng, F.; Du, Q.; Peng, C.; Wang, N.; Tang, H.; Xie, X.; Shen, J.; Chen, J. A Review: The Pharmacology of Isoliquiritigenin. Phyther. Res. 2015, 29, 969–977. [Google Scholar] [CrossRef]
- Park, E.-K.; Shin, Y.-W.; Lee, H.-U.; Lee, C.S.; Kim, D. Passive Cutaneous Anaphylaxis-Inhibitory Action of Tectorigenin, a Metabolite of Tectridin by Intestinal Microflora. Biol. Pharm. Bull. 2004, 27, 1099–1102. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jing, W.; Qu, W.; Liu, Z.; Zhang, D.; Qi, X.; Liu, L. Tectorigenin inhibits inflammation and pulmonary fibrosis in allergic asthma model of ovalbumin-sensitized guinea pigs. J. Pharm. Pharmacol. 2020, 72, 956–968. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Nomura, T.; Jokura, H.; Kitamura, N.; Saiki, A.; Fujii, A. Chlorogenic acid-enriched green coffee bean extract affects arterial stiffness assessed by the cardio-ankle vascular index in healthy men: A pilot study. Int. J. Food Sci. Nutr. 2019, 70, 901–908. [Google Scholar] [CrossRef]
- Drynan, J.W.; Clifford, M.N.; Obuchowicz, J.; Kuhnert, N. The chemistry of low molecular weight black tea polyphenols. Nat. Prod. Rep. 2010, 27, 417–462. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y. Cancer chemopreventive potential of procyanidin. Toxicol. Res. 2017, 33, 273–282. [Google Scholar] [CrossRef]
- Wiciski, M.; Leis, K.; Szyperski, P.; W˛ Eclewicz, M.M.; Mazur, E.; Pawlak-Osiska, K. ARTICLE IN PRESS Impact of resveratrol on exercise performance: A review Effet du resveratrol sur la performance physique: Revue générale. Sci. Sport. 2018, 1. [Google Scholar] [CrossRef]
- Qualley, A.V.; Widhalm, J.R.; Adebesin, F.; Kish, C.M.; Dudareva, N. Completion of the core β-oxidative pathway of benzoic acid biosynthesis in plants. Proc. Natl. Acad. Sci. USA 2012, 109, 16383–16388. [Google Scholar] [CrossRef]
- Raskin, I. Role of salicylic acid in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1992, 43, 439–463. [Google Scholar] [CrossRef]
- Senaratna, T.; Merritt, D.; Dixon, K.; Bunn, E.; Touchell, D.; Sivasithamparam, K. Benzoic acid may act as the functional group in salicylic acid and derivatives in the induction of multiple stress tolerance in plants. Plant Growth Regul. 2003, 39, 77–81. [Google Scholar] [CrossRef]
- Ganesan, K.; Xu, B. A critical review on phytochemical profile and health promoting effects of mung bean (Vigna radiata). Food Sci. Hum. Wellness 2018, 7, 11–33. [Google Scholar] [CrossRef]
- Kang, K.A.; Zhang, R.; Piao, M.J.; Ko, D.O.; Wang, Z.H.; Kim, B.J.; Park, J.W.; Kim, H.S.; Kim, D.H.; Hyun, J.W. Protective effect of irisolidone, a metabolite of kakkalide, against hydrogen peroxide induced cell damage via antioxidant effect. Bioorganic Med. Chem. 2008, 16, 1133–1141. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wu, X.; Yang, X.; Zhang, Y.; Wang, L.; Li, X.; Qiu, Y. Comprehensive quality evaluation of Lignum Caraganae and rapid discrimination of Caragana jubata and Caragana changduensis based on characteristic compound fingerprints by HPLC-UV and HPLC-MS/MS coupled with chemometrics analysis. Phytochem. Anal. 2020, 31, 846–860. [Google Scholar] [CrossRef]
- Al-Maharik, N. Isolation of naturally occurring novel isoflavonoids: An update. Nat. Prod. Rep. 2019, 36, 1156–1195. [Google Scholar] [CrossRef]
- Iranshahi, M.; Rezaee, R.; Parhiz, H.; Roohbakhsh, A.; Soltani, F. Protective effects of flavonoids against microbes and toxins: The cases of hesperidin and hesperetin. Life Sci. 2015, 137, 125–132. [Google Scholar] [CrossRef]
- Li, D.; Mitsuhashi, S.; Ubukata, M. Protective effects of hesperidin derivatives and their stereoisomers against advanced glycation end-products formation. Pharm. Biol. 2012, 50, 1531–1535. [Google Scholar] [CrossRef] [PubMed]
- Stanisławska, I.J.; Piwowarski, J.P.; Granica, S.; Kiss, A.K. The effects of urolithins on the response of prostate cancer cells to non-steroidal antiandrogen bicalutamide. Phytomedicine 2018, 1. [Google Scholar] [CrossRef]
- García-Villalba, R.; Beltrán, D.; Espín, J.C.; Selma, M.V.; Tomás-Barberán, F.A. Time course production of urolithins from ellagic acid by human gut microbiota. J. Agric. Food Chem. 2013, 61, 8797–8806. [Google Scholar] [CrossRef]
- Kundu, A. Vanillin biosynthetic pathways in plants. Planta 2017, 245, 1069–1078. [Google Scholar] [CrossRef]
- Bylund, A.; Saarinen, N.; Zhang, J.X.; Bergh, A.; Widmark, A.; Johansson, A.; Lundin, E.; Adlercreutz, H.; Hallmans, G.; Stattin, P.; et al. Anticancer effects of a plant lignan 7-hydroxymatairesinol on a prostate cancer model in vivo. Urol. Oncol. Semin. Orig. Investig. 2005, 23, 380–381. [Google Scholar] [CrossRef]
- Milder, I.E.J.; Feskens, E.J.M.; Arts, I.C.W.; De Mesquita, H.B.B.; Hollman, P.C.H.; Kromhout, D. Intake of the Plant Lignans Secoisolariciresinol, Matairesinol, Lariciresinol, and Pinoresinol in Dutch Men and Women 1. Nutr. Epidemiol. 2005, 135, 1202–1207. [Google Scholar] [CrossRef] [PubMed]
- Jeevanandam, J.; Madhumitha, R.; Saraswathi, N.T. Identification of potential phytochemical lead against diabetic cataract: An in silico approach. J. Mol. Struct. 2020, 1226, 129428. [Google Scholar] [CrossRef]
- Chiu, F.L.; Lin, J.K. HPLC analysis of naturally occurring methylated catechins, 3″- and 4″-methyl-epigallocatechin gallate, in various fresh tea leaves and commercial teas and their potent inhibitory effects on inducible nitric oxide synthase in macrophages. J. Agric. Food Chem. 2005, 53, 7035–7042. [Google Scholar] [CrossRef]
- Yano, S.; Fujimura, Y.; Umeda, D.; Miyase, T.; Yamada, K.; Tachibana, H. Relationship between the biological activities of methylated derivatives of (-)-epigallocatechin-3-O-gallate (EGCG) and their cell surface binding activities. J. Agric. Food Chem. 2007, 55, 7144–7148. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Khan, I.A.; ur-Rehman, M.; Gilani, S.A.; Mehmood, Z.; Mubarak, M.S. Anticancer potential of quercetin: A comprehensive review. Phyther. Res. 2018, 32, 2109–2130. [Google Scholar] [CrossRef] [PubMed]
- Emami, S.; Ghanbarimasir, Z. Recent advances of chroman-4-one derivatives: Synthetic approaches and bioactivities. Eur. J. Med. Chem. 2015, 93, 539–563. [Google Scholar] [CrossRef]
- Shyamal, K.; Jash, G.B. Recent progress in the research of naturally occurring flavonoids: A look through. Signpost Open Access J. Org. Biomol. Chem. 2013, 1, 65–168. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Potter, C.M.; Jones, D.L. Polyphenolic Profiling of Green Waste Determined by UPLC-HDMSE. Processes 2021, 9, 824. https://doi.org/10.3390/pr9050824
Potter CM, Jones DL. Polyphenolic Profiling of Green Waste Determined by UPLC-HDMSE. Processes. 2021; 9(5):824. https://doi.org/10.3390/pr9050824
Chicago/Turabian StylePotter, Colin M., and David L. Jones. 2021. "Polyphenolic Profiling of Green Waste Determined by UPLC-HDMSE" Processes 9, no. 5: 824. https://doi.org/10.3390/pr9050824
APA StylePotter, C. M., & Jones, D. L. (2021). Polyphenolic Profiling of Green Waste Determined by UPLC-HDMSE. Processes, 9(5), 824. https://doi.org/10.3390/pr9050824





