Time-Dependent Viscous Flow Behavior of a Hydrophobic Fumed Silica Suspension
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Sample Preparation
2.2. Rheological Experiments
2.3. Resting Time
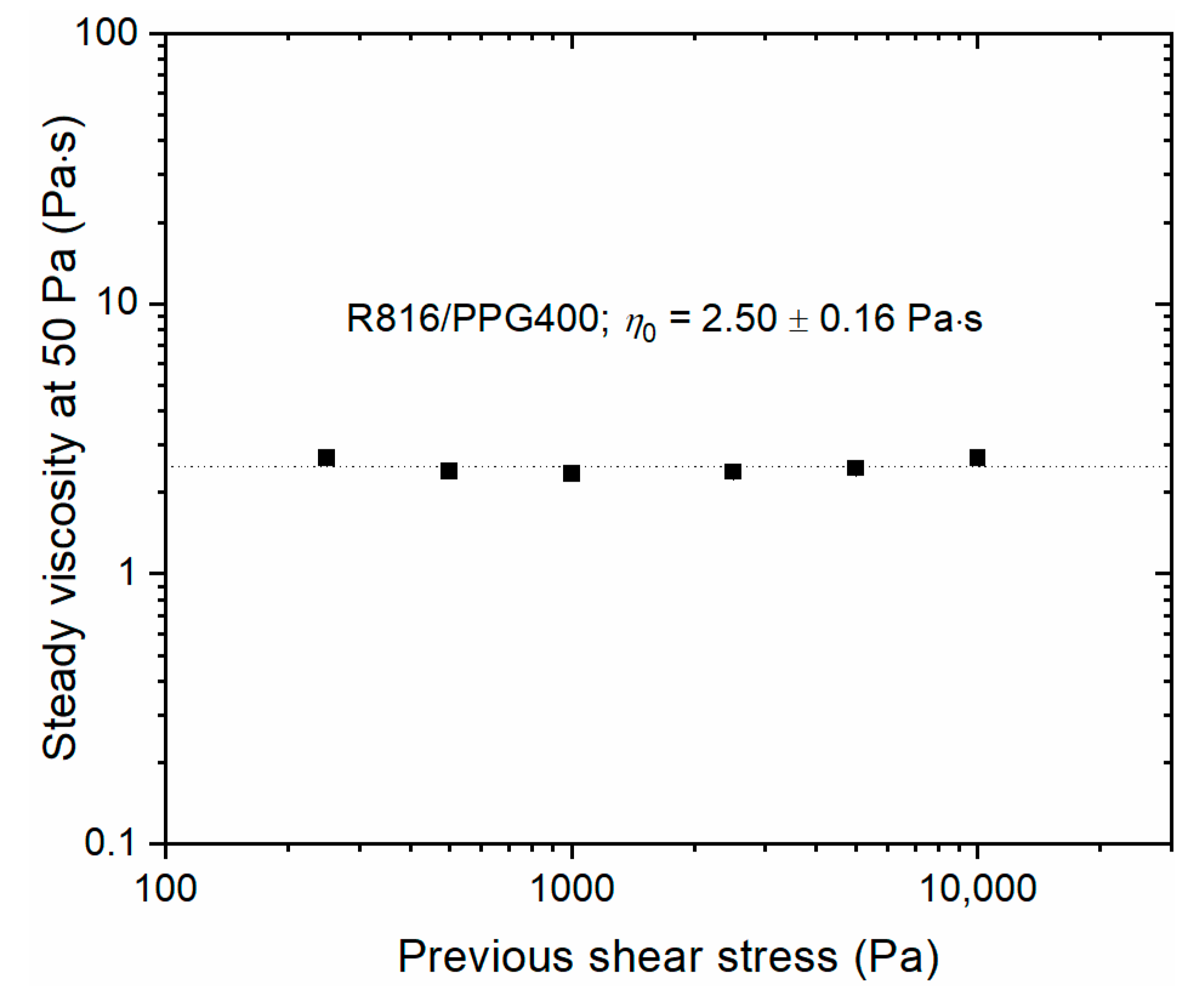
2.4. Limit of Reversibility
3. Results and Discussion
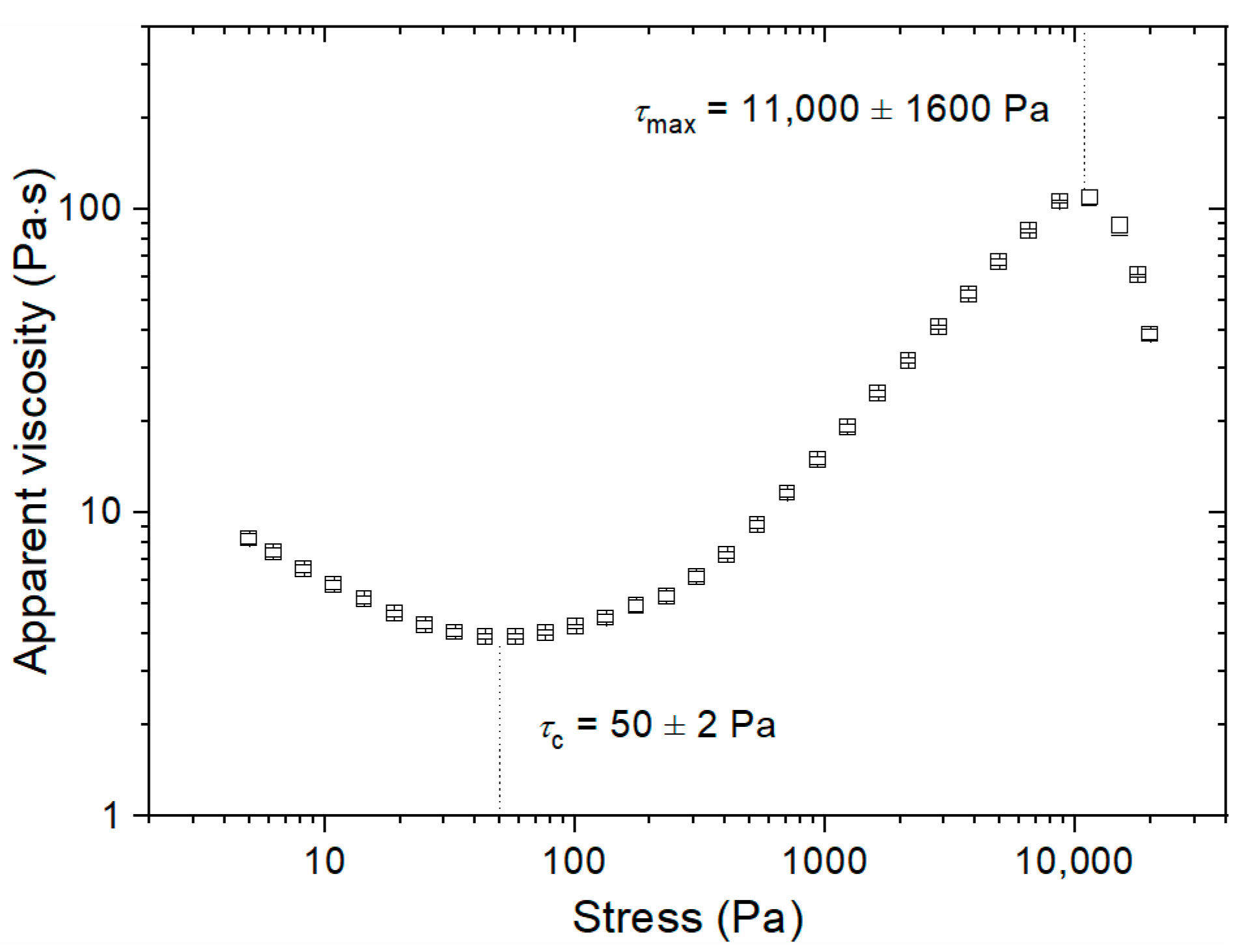
3.1. Steady Shear Experiments
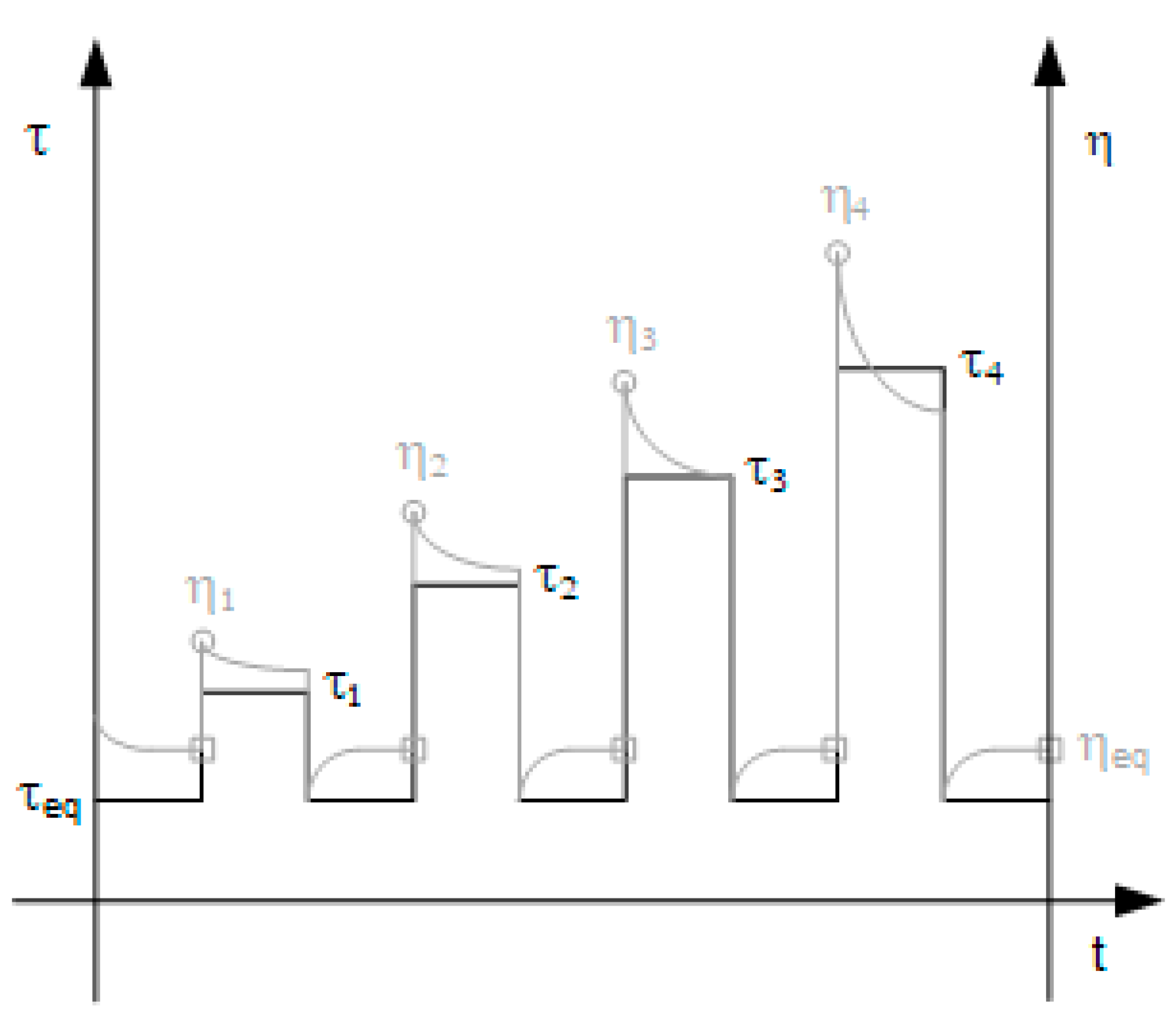
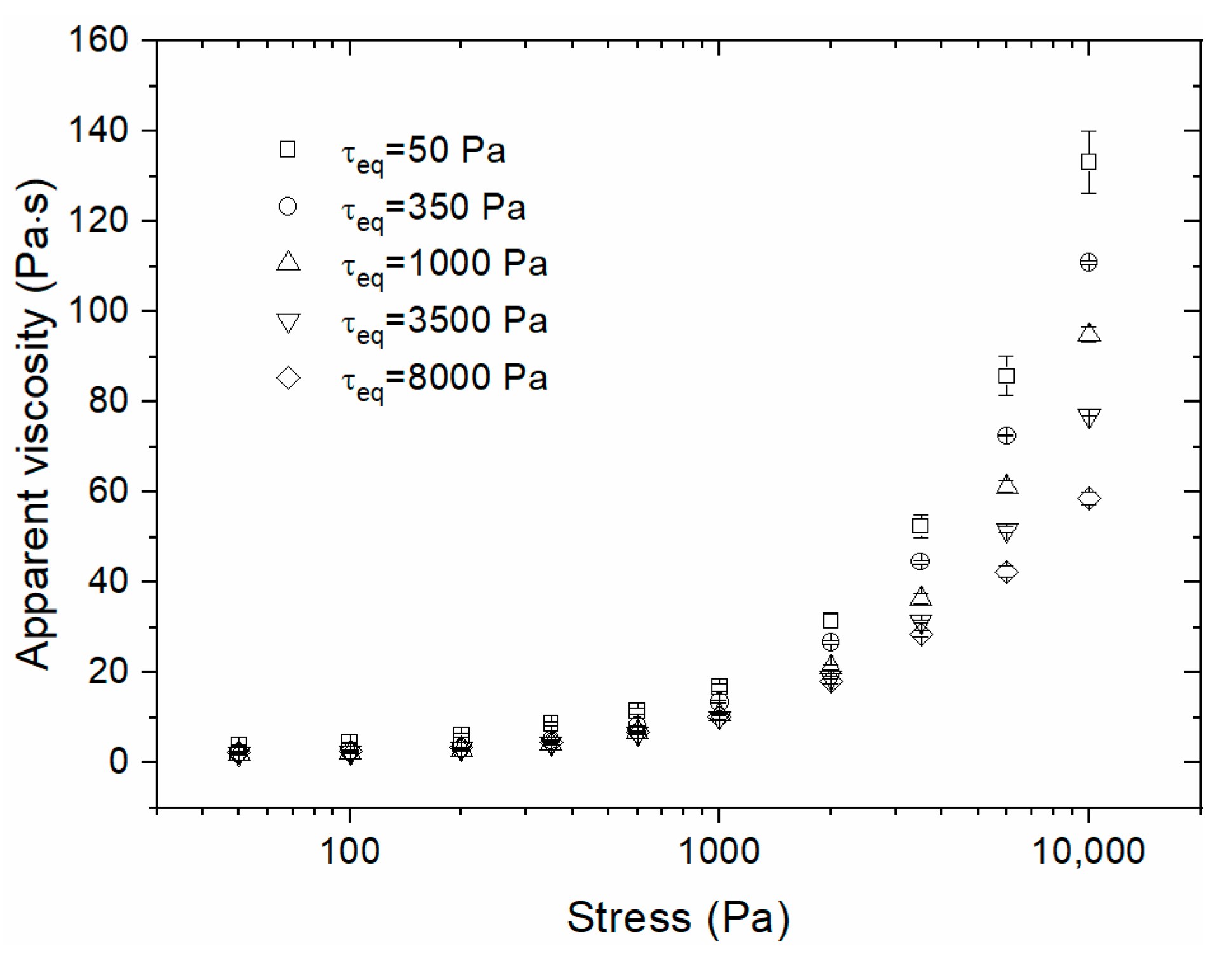
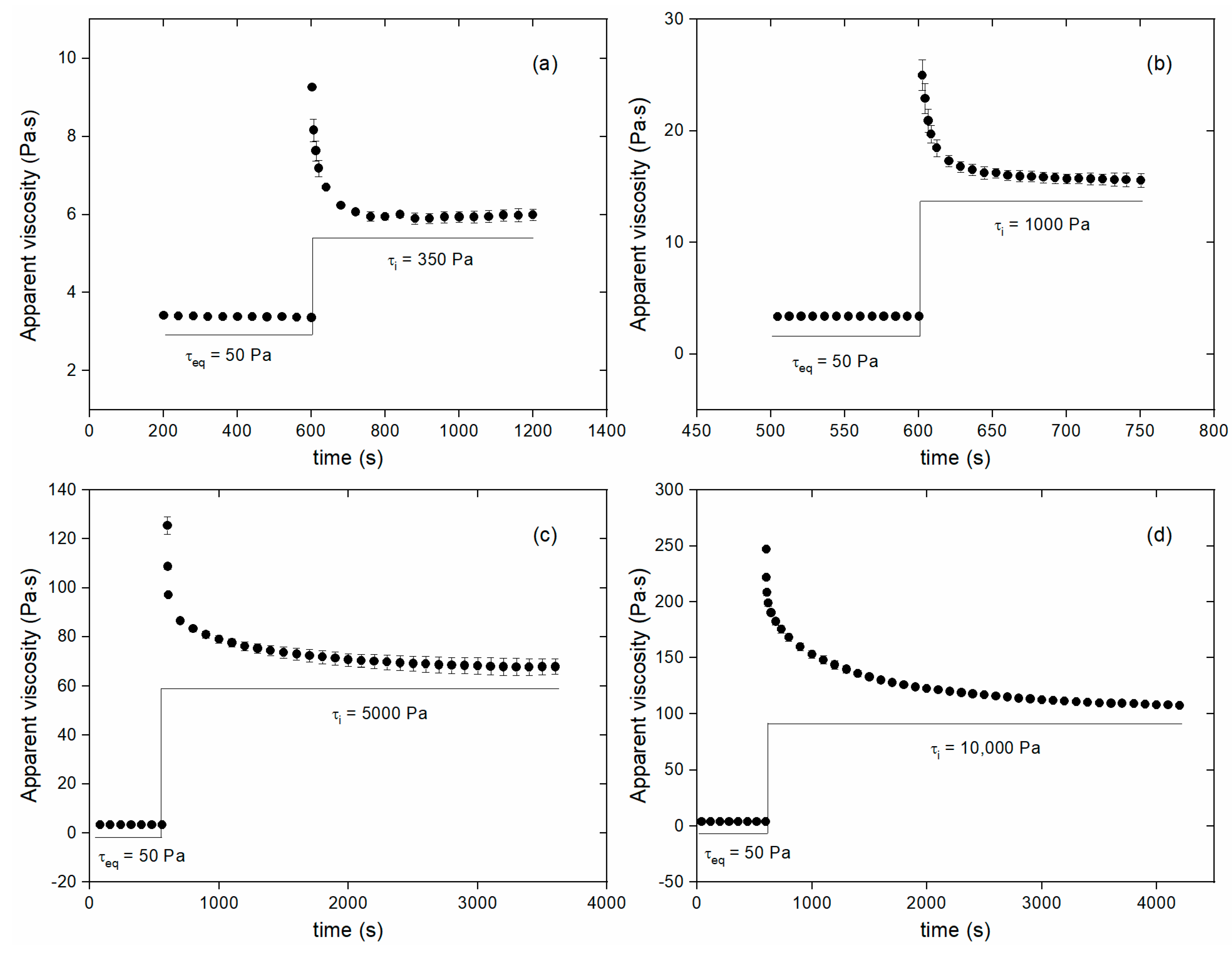
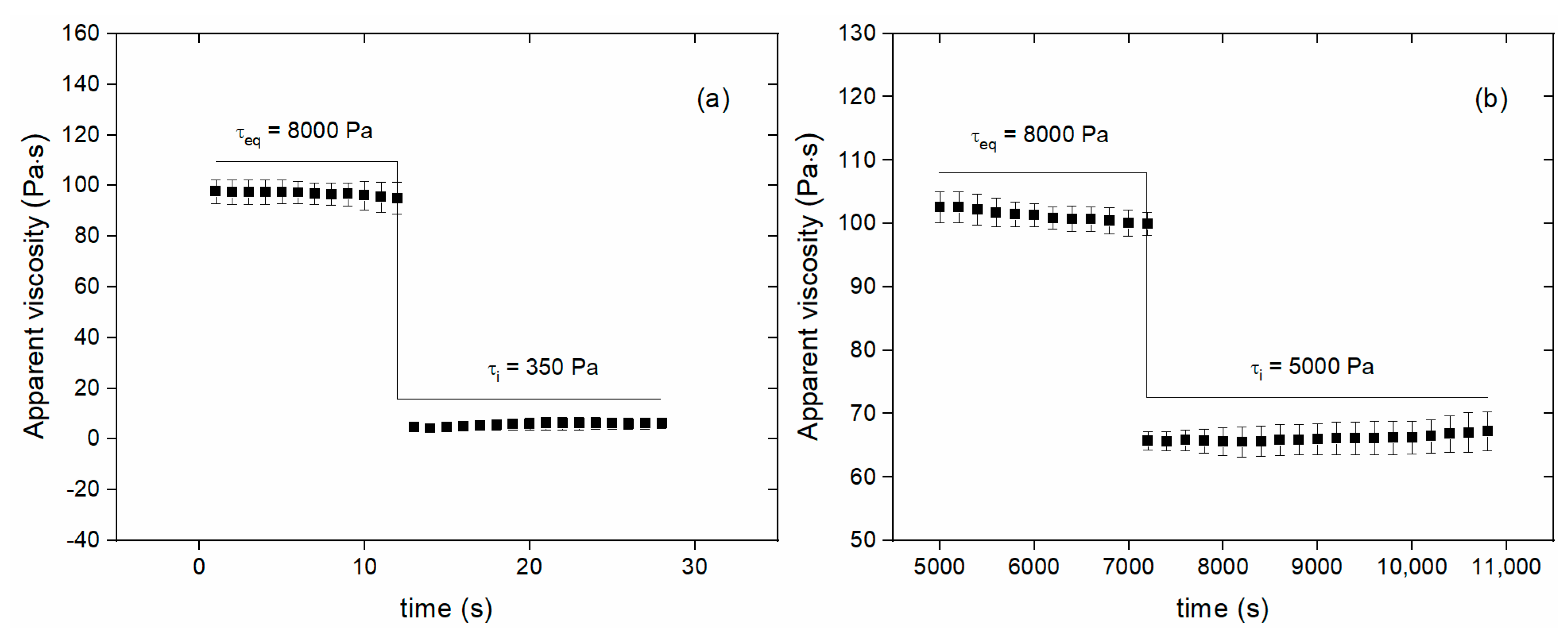
3.2. Step experiments
4. Conclusions
- The steady viscosity response depends on the previous state of aggregation, indicating the existence of aggregates (hydroclusters) in the suspension at rest;
- The suspension showed apparent thixotropic behavior in the shear-thickening region, contrary to what has been usually assumed. This result can be justified by the fast hydroclusters formation due to step-up in shear stress followed by the erosion of these aggregates caused by Brownian thermal diffusion of silica particles.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barnes, H.A. Thixotropy-a review. J. Non-Newton Fluid 1997, 70, 1–33. [Google Scholar] [CrossRef]
- Mewis, J.; Wagner, N.J. Thixotropy. Adv. Colloid Interface 2009, 147–148, 214–227. [Google Scholar] [CrossRef] [PubMed]
- Buitenhuis, J.; Pönitsch, M. Negative thixotropy of polymer solutions. 1. A model explaining time-dependent viscosity. Colloid Polym. Sci. 2003, 281, 253–259. [Google Scholar] [CrossRef]
- Larson, R.G. Constitutive equations for thixotropic fluids. J. Rheol. 2015, 59, 595–611. [Google Scholar] [CrossRef]
- Ostwald, W. Ueber die Geschwindigkeitsfunktion der Viskositat Disperser Systeme. I. Kolloid Z. 1925, 36, 99–117. [Google Scholar] [CrossRef]
- De Kee, D.; Chan Man Fong, C.F. Rheological properties of structured fluids. Polym. Eng. Sci. 1994, 34, 438–445. [Google Scholar] [CrossRef]
- Fredrickson, A.G. A model for the thixotropy of suspensions. AIChE J. 1970, 16, 436–441. [Google Scholar] [CrossRef]
- Alvarez, M.; Canet, W. Time-independent and time-dependent rheological characterization of vegetable-based infant purees. J. Food Eng. 2013, 114, 449–464. [Google Scholar] [CrossRef]
- Jarny, S.; Roussel, N.; Rodts, S.; Bertrand, F.; Le Roy, R.; Coussot, P. Rheological behavior of cement pastes from MRI velocimetry. Cement. Concr. Res. 2005, 35, 1873–1881. [Google Scholar] [CrossRef]
- Klein, B.; Hallbom, D.J. Modifying the rheology of nickel laterite suspensions. Miner Eng. 2002, 15, 745–749. [Google Scholar] [CrossRef]
- Roseley, A.S.M.; Rojo, E.; Ansell, M.P.; Smedley, D. Creep response of thixotropic ambient temperature cure adhesives measured by DMTA in static tension and shear. Int. J. Adhes. Adhes. 2011, 31, 575–582. [Google Scholar] [CrossRef]
- Keshtkar, M.; Heuzey, M.C.; Carreau, P.J. Rheological behavior of fiber-filled model suspensions: Effect of fiber flexibility. J. Rheol. 2009, 53, 631–650. [Google Scholar] [CrossRef]
- Quemada, D. Rheological modelling of complex fluids. I. The concept of effective volume fraction revisited. Eur. Phys. J. Appl. Phys. 1998, 1, 119–127. [Google Scholar] [CrossRef]
- Murshed, S.M.S.; Leong, K.C.; Yang, C. Investigations of thermal conductivity and viscosity of nanofluids. Int. J. Therm. Sci. 2008, 47, 560–568. [Google Scholar] [CrossRef]
- Mueller, S.; Llewellin, E.W.; Mader, H.M. The rheology of suspensions of solid particles. Proc. R. Soc. A 2010, 466, 1201–1228. [Google Scholar] [CrossRef]
- Mewis, J.; Wagner, N.J. Colloidal Suspension Rheology; Cambridge University Press: New York, NY, USA, 2012. [Google Scholar]
- Barnes, H.A.; Hutton, J.F.; Walters, K. An Introduction to Rheology; Elsevier: Amsterdam, The Netherlands, 1989. [Google Scholar]
- Abu-Jdayil, B. Modelling the time-dependent rheological behavior of semisolid foodstuffs. J. Food Eng. 2003, 57, 97–102. [Google Scholar] [CrossRef]
- Bekkour, K.; Scrivener, O. Time-Dependent and Flow Properties of Foams. Mech. Time-Depend Mat. 1998, 2, 171–193. [Google Scholar] [CrossRef]
- Sha, J.; Zhang, F.; Zhang, H. Thixotropic flow behavior in chemical pulp fibre suspensions. Bioresources 2016, 11, 3481–3493. [Google Scholar] [CrossRef][Green Version]
- Calero, N.; Santos, J.; Muñoz, J. Methodology to estimate the yield stress applied to ultraconcentrated detergents as model systems. Chem. Eng. Sci. 2017, 166, 115–121. [Google Scholar] [CrossRef]
- Chen, Q.; Xuan, S.; Jiang, W.; Cao, S.; Gong, X. Shear time dependent viscosity of polystyrene-ethylacrylate based shear thickening fluid. Smart Mater. Struct. 2016, 25, 045005. [Google Scholar] [CrossRef]
- Rubio-Hernández, F.J.; Sánchez-Toro, J.H.; Páez-Flor, N.M. Testing shear thinning/thixotropy and shear thickening/antithixotropy relationships in a fumed silica suspension. J. Rheol. 2020, 64, 785–797. [Google Scholar] [CrossRef]
- Galindo-Rosales, F.J.; Rubio-Hernández, F.J.; Velázquez-Navarro, J.F. Shear-thickening behavior of Aerosil® R816 nanoparticles suspensions in polar organic liquids. Rheol. Acta 2009, 48, 699–708. [Google Scholar] [CrossRef]
- Rubio-Hernández, F.J.; Gómez-Merino, A.I.; Páez-Flor, N.M.; Velázquez-Navarro, J.F. On the steady shear behavior of hydrophobic fumed silica suspensions in PPG and PEG of low molecular weight. Soft Mater. 2017, 15, 55–63. [Google Scholar] [CrossRef]
- Rubio-Hernández, F.J.; Páez-Flor, N.M.; Velázquez-Navarro, J.F. Why monotonous and non-monotonous steady-flow curves can be obtained with the same non-Newtonian fluid? A single explanation. Rheol. Acta 2018, 57, 389–396. [Google Scholar] [CrossRef]
- Chryss, A.G.; Bhattacharya, S.N.; Pullum, L. Rheology of shear thickening suspensions and the effects of wall slip in torsional flow. Rheol. Acta 2005, 45, 124–131. [Google Scholar] [CrossRef]
- Yoshimura, A.; Prud’homme, R.K. Wall Slip Corrections for Couette and Parallel Disk Viscometers. J. Rheol. 1988, 32, 53–67. [Google Scholar] [CrossRef]
- Raghavan, S.R.; Khan, S.A. Shear-induced microstructural changes in flocculated suspensions of fumed silica. J. Rheol. 1995, 39, 1311–1325. [Google Scholar] [CrossRef]
- Barnes, H.A. Shear-thickening (“dilatancy”) in suspensions of nonaggregating solid particles dispersed in Newtonian liquids. J. Rheol. 1989, 33, 329–366. [Google Scholar] [CrossRef]
- Eisenlauer, J.; Killmann, E. Stability of colloidal silica (aerosil) hydrosols. I. Preparation and characterization of silica (aerosil) hydrosol. J Colloid Interface Sci. 1980, 74, 108–119. [Google Scholar] [CrossRef]
- Raghavan, S.R.; Khan, S.A. Shear-thickening response of fumed silica suspensions under steady and oscillatory shear. J. Colloid Interface Sci. 1997, 185, 57–67. [Google Scholar] [CrossRef]
- Amiri, A.; Øye, G.; Sjöblom, J. Stability and flow-induced flocculation of fumed silica suspensions in mixture of water-glycerol. J. Disper. Sci. Technol. 2012, 33, 1247–1256. [Google Scholar] [CrossRef]
- Asija, N.; Chouhan, H.; Gebremeskel, S.A.; Bhatnagar, N. Influence of particle size on the low and high strain rate behavior of dense colloidal dispersions of nanosilica. J. Nanopart. Res. 2017, 19, 21. [Google Scholar] [CrossRef]
- Brown, E.; Jaeger, H.M. Shear thickening in concentrated suspensions: Phenomenology, mechanisms and relations to jamming. Rep. Prog. Phys. 2014, 77, 046602. [Google Scholar] [CrossRef] [PubMed]
- Wagner, N.J.; Brady, J.F. Shear thickening in colloidal dispersions. Phys. Today 2009, 62, 27–32. [Google Scholar] [CrossRef]
- Kalman, D.P.; Wagner, N.J. Microstructure of shear-thickening concentrated suspensions determined by flow-USANS. Rheol. Acta 2009, 48, 897–908. [Google Scholar] [CrossRef]
- Cheng, X.; McCoy, J.H.; Israelachvili, J.N.; Cohen, I. Imaging the microscopic structure of shear thinning and thickening colloidal suspensions. Science 2011, 333, 1276–1279. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Hernández, F.J.; Gómez-Merino, A.I.; Delgado-García, R.; Páez-Flor, N.M. An activation energy approach for viscous flow: A complementary tool for the study of microstructural evolutions in sheared suspensions. Powder Tech. 2017, 308, 318–323. [Google Scholar] [CrossRef]
- Cheng, D.C.H. Yield stress: A time-dependent property and how to measure it. Rheol. Acta 1986, 25, 542–554. [Google Scholar] [CrossRef]
- Galindo-Rosales, F.J.; Rubio-Hernández, F.J.; Velázquez-Navarro, J.F.; Gómez-Merino, A.I. Structural level of silica-fumed aqueous suspensions. J. Am. Ceram. Soc. 2007, 90, 1641–1643. [Google Scholar] [CrossRef]
- Raghavan, S.R.; Hou, J.; Baker, G.L.; Khan, S.A. Colloidal interactions between particles with tethered nonpolar chains dispersed in polar media: Direct correlation between dynamic rheology and interaction parameters. Langmuir 2000, 16, 1066–1077. [Google Scholar] [CrossRef]
- Barthel, H.; Dreyer, M.; Gottschalk-Gaudig, T.; Litvinov, V.; Nikitina, E. Fumed silica—Rheological additive for adhesives, resins, and paints. Macromol. Symp. 2002, 187, 573–584. [Google Scholar] [CrossRef]
- Dullaert, K.; Mewis, J. A structural kinetics model for thixotropy. J. Non-Newton Fluid 2006, 139, 21–30. [Google Scholar] [CrossRef]
- Galindo-Rosales, F.J.; Rubio-Hernandez, F.J. Static and dynamic yield stresses of Aerosil® 200 suspensions in polypropylene glycol. Appl. Rheol. 2010, 20, 22787. [Google Scholar]
- Ilyin, S.O.; Pupchenkov, G.S.; Krasheninnikov, A.I.; Kulichikhin, V.G.; Malkin, A.Y. Rheology of aqueous poly(ethylene oxide) solutions reinforced with bentonite clay. Colloid J. 2013, 75, 267–273. [Google Scholar] [CrossRef]
- Manion, S.J.; Johnson, L.L.; Fernando, R.H. Shear-thickening in aqueous surfactant-associative thickener mixtures. J. Coat Technol. Res. 2011, 8, 299–309. [Google Scholar] [CrossRef]
- Krishnamurthy, S.; Battacharya, P.; Phelan, P.E. Enhanced mass transport in nanofluids. Nano Lett. 2006, 6, 419–423. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez, J.H.; Rubio-Hernández, F.J.; Páez-Flor, N.M. Time-Dependent Viscous Flow Behavior of a Hydrophobic Fumed Silica Suspension. Processes 2021, 9, 807. https://doi.org/10.3390/pr9050807
Sánchez JH, Rubio-Hernández FJ, Páez-Flor NM. Time-Dependent Viscous Flow Behavior of a Hydrophobic Fumed Silica Suspension. Processes. 2021; 9(5):807. https://doi.org/10.3390/pr9050807
Chicago/Turabian StyleSánchez, Jorge H., Francisco J. Rubio-Hernández, and Nicolás M. Páez-Flor. 2021. "Time-Dependent Viscous Flow Behavior of a Hydrophobic Fumed Silica Suspension" Processes 9, no. 5: 807. https://doi.org/10.3390/pr9050807
APA StyleSánchez, J. H., Rubio-Hernández, F. J., & Páez-Flor, N. M. (2021). Time-Dependent Viscous Flow Behavior of a Hydrophobic Fumed Silica Suspension. Processes, 9(5), 807. https://doi.org/10.3390/pr9050807





