Selective Adsorption of CR (VI) onto Amine-Modified Passion Fruit Peel Biosorbent
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of DAPFP
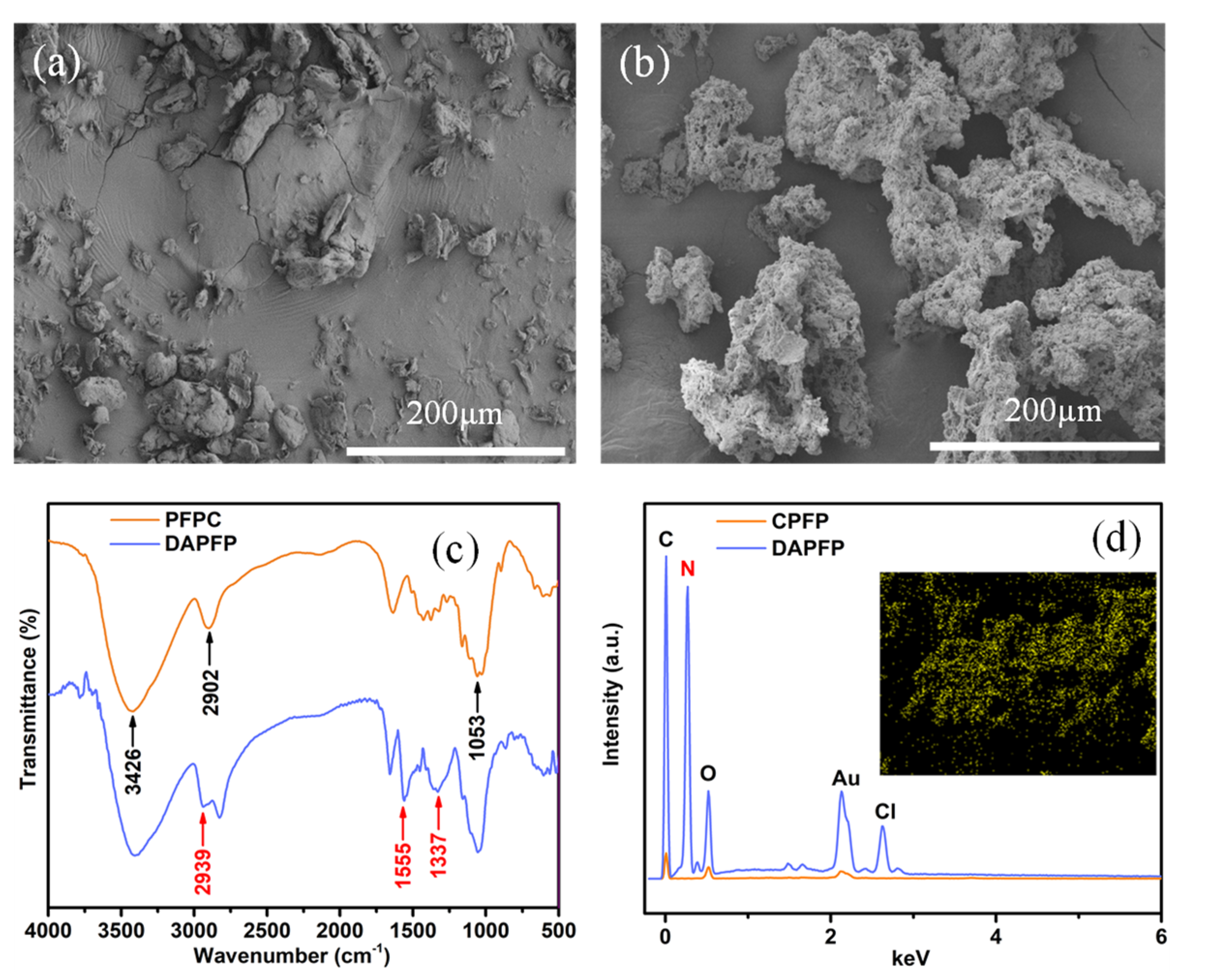
2.3. Characterizations
2.4. Adsorption Experiment
2.5. Adsorption–Desorption Recycling Experiment
3. Results
3.1. Morphological and Structural Analysis
3.2. Adsorption Study
3.2.1. Influence of pH on the Adsorption of Cr (VI)
3.2.2. Influence of Initial Concentration on the Removal of Cr (VI)
3.2.3. Study on the Adsorption Kinetics
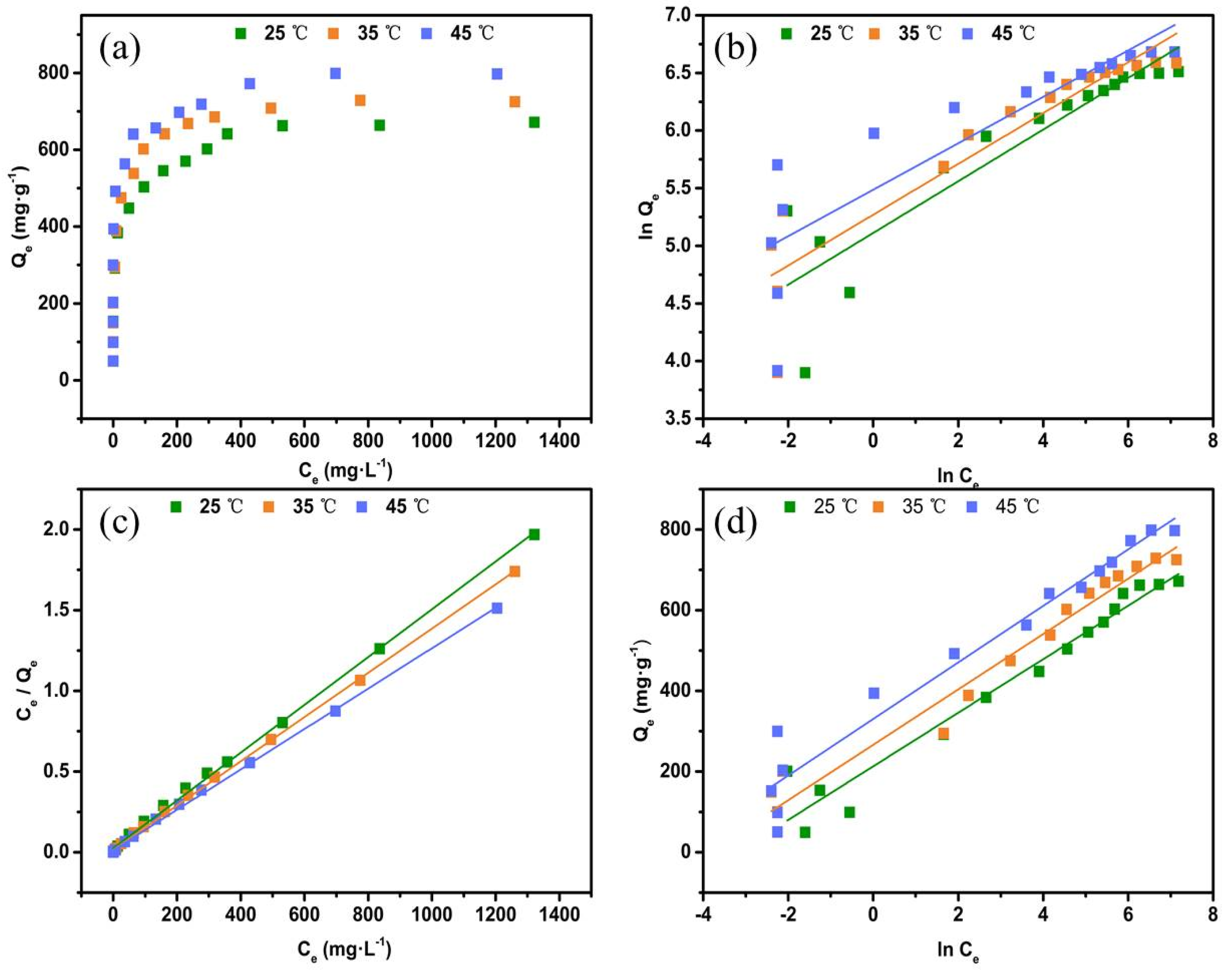
3.2.4. Adsorption Isotherm
3.2.5. Thermodynamic Study
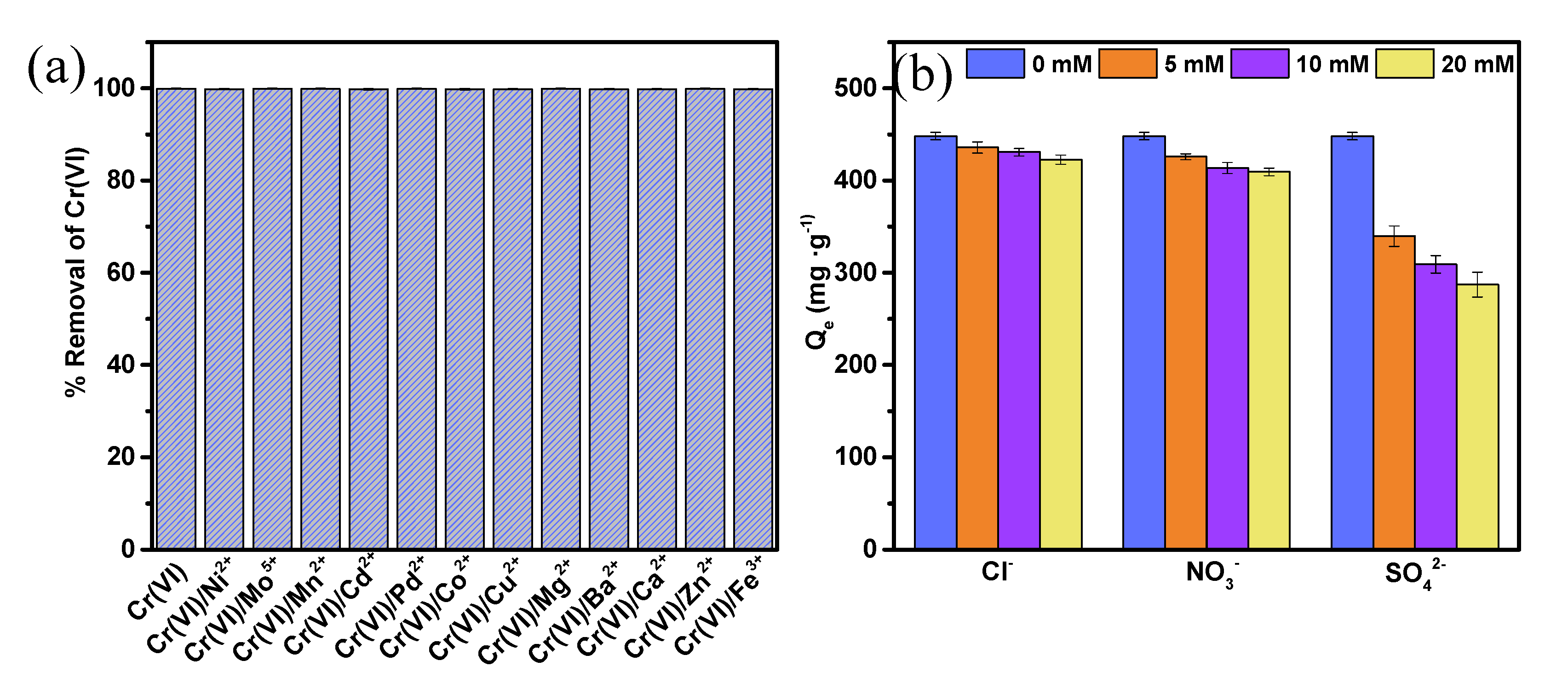
3.2.6. Effect of Coexistent Ions
3.2.7. Adsorption of Low-Level Cr(VI) (to Meet the WHO Requirement) and Regeneration
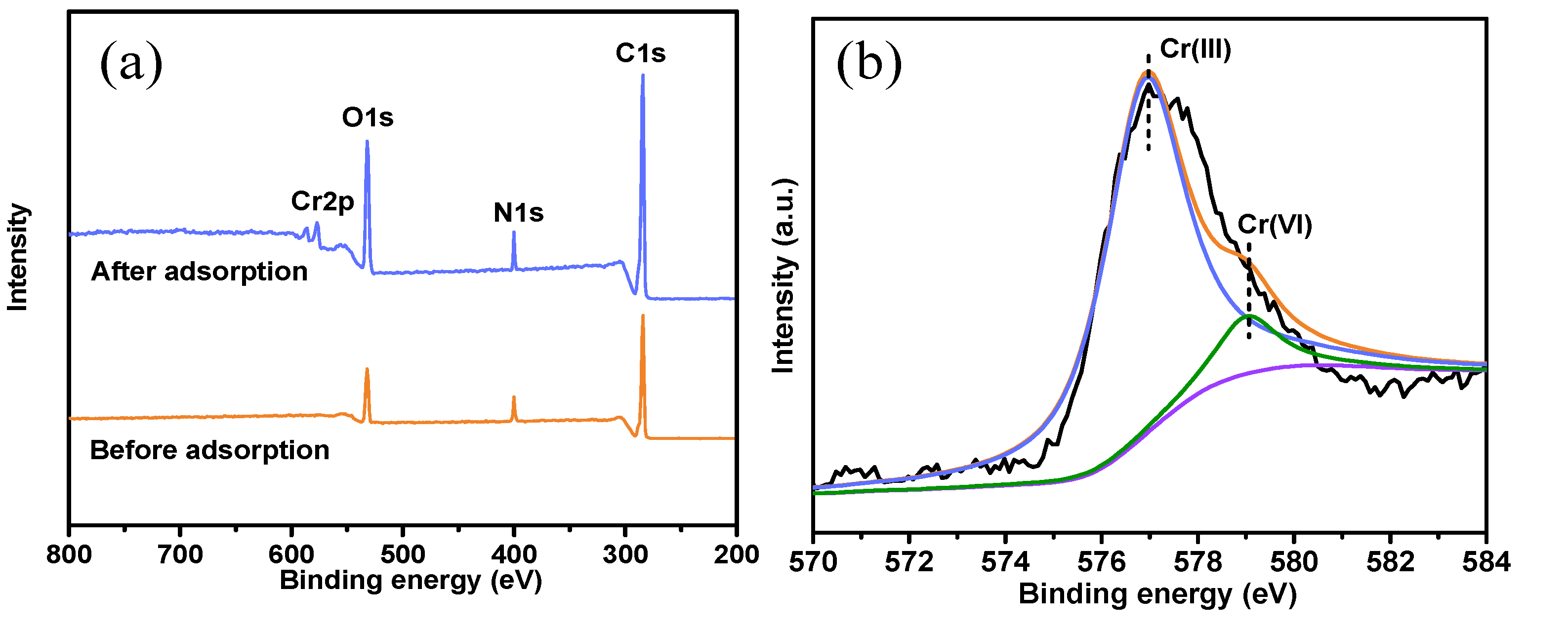
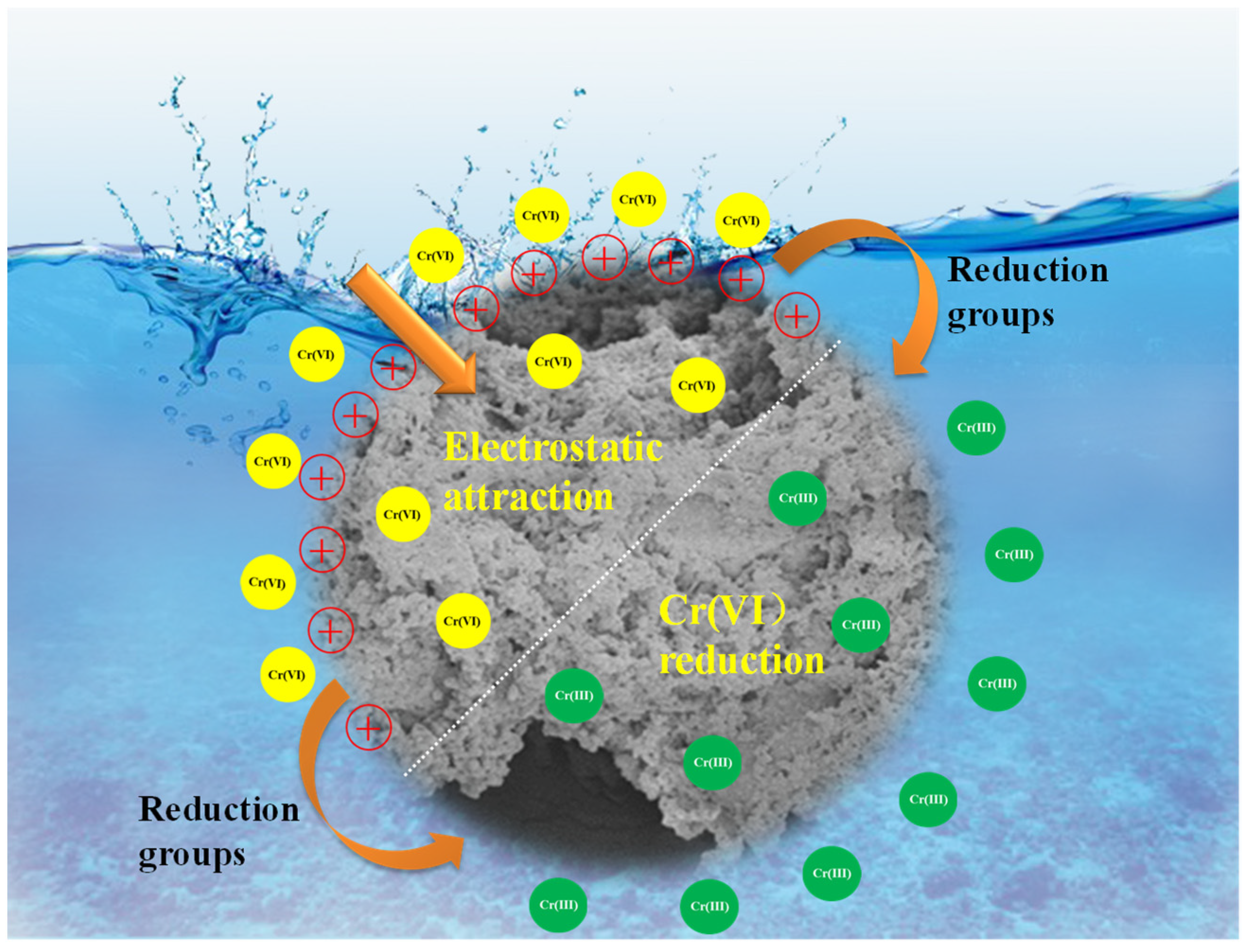
3.3. Confirmation of Adsorption Behaviors and Adsorption Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qiu, B.; Gu, H.; Yan, X.; Guo, J.; Wang, Y.; Sun, D.; Wang, Q.; Khan, M.; Zhang, X.; Weeks, B.L.; et al. Cellulose derived magnetic mesoporous carbon nanocomposites with enhanced hexavalent chromium removal. J. Mater. Chem. A 2014, 2, 17454–17462. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Z.; Wang, J.; Li, Q.; Wang, X.; Zhao, J.; Li, Z.; Wang, J.; Li, Q.; Wang, X. Capsular polypyrrole hollow nanofibers: An efficient recyclable adsorbent for hexavalent chromium removal. J. Mater. Chem. A 2015, 3, 15124–15132. [Google Scholar] [CrossRef]
- Ko, Y.J.; Choi, K.; Lee, S.; Jung, K.W.; Hong, S.; Mizuseki, H.; Choi, J.W.; Lee, W.S. Strong chromate-adsorbent based on pyrrolic nitrogen structure: An experimental and theoretical study on the adsorption mechanism. Water Res. 2018, 145, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Ying, Y.; Liu, Y.; Wang, X.; Mao, Y.; Cao, W.; Hu, P.; Peng, X. Two-dimensional titanium carbide for efficiently reductive removal of highly toxic chromium(VI) from water. ACS Appl. Mater. Inter. 2015, 7, 1795–1803. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Shang, T.; Jin, X.; Gao, J.; Zhao, Q. Study of chromium(VI) removal from aqueous solution using nitrogen-enriched activated carbon based bamboo processing residues. RSC Adv. 2015, 5, 784–790. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, L.; Li, C.; Yang, W.; Song, T.; Tang, C.; Meng, Y.; Dai, S.; Wang, H.; Chai, L.; et al. Synthesis of core-shell magnetic Fe3O4@poly(m-phenylenediamine) particles for chromium reduction and adsorption. Environ. Sci. Technol. 2015, 49, 5654–5662. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, J.; Chen, R.; Yu, P.; Guo, S.; Wang, X. Adsorption and reduction of chromium(VI) from aqueous solution using polypyrrole/calcium rectorite composite adsorbent. Water Res. 2019, 160, 148–157. [Google Scholar] [CrossRef]
- Xie, B.; Shan, C.; Xu, Z.; Li, X.; Zhang, X.; Chen, J.; Pan, B. One-step removal of Cr(VI) at alkaline pH by UV/sulfite process: Reduction to Cr(III) and in situ Cr(III) precipitation. Chem. Eng. J. 2017, 308, 791–797. [Google Scholar] [CrossRef]
- Goyal, R.K.; Jayakumar, N.S.; Hashim, M.A. A comparative study of experimental optimization and response surface optimization of Cr removal by emulsion ionic liquid membrane. J. Hazard. Mater. 2011, 195, 383–390. [Google Scholar] [CrossRef]
- Dharnaik, A.S.; Ghosh, P.K. Hexavalent chromium [Cr(VI)] removal by the electrochemical ion-exchange process. Environ. Technol. 2014, 35, 2272–2279. [Google Scholar] [CrossRef]
- Wang, L.L.; Wang, J.Q.; Zheng, Z.X.; Xiao, P. Cloud point extraction combined with high-performance liquid chromatography for speciation of chromium(III) and chromium(VI) in environmental sediment samples. J. Hazard. Mater. 2010, 177, 114–118. [Google Scholar] [CrossRef]
- Liu, W.; Ni, J.; Yin, X. Synergy of photocatalysis and adsorption for simultaneous removal of Cr(VI) and Cr(III) with TiO2 and titanate nanotubes. Water Res. 2014, 53, 12–25. [Google Scholar] [CrossRef]
- Tan, J.; Song, Y.; Huang, X.; Zhou, L. Facile functionalization of natural peach gum polysaccharide with multiple amine groups for highly efficient removal of toxic hexavalent chromium (Cr(VI)) ions from water. ACS Omega 2018, 3, 17309–17318. [Google Scholar] [CrossRef]
- Su, H.; Chong, Y.; Wang, J.; Long, D.; Qiao, W.; Ling, L. Nanocrystalline celluloses-assisted preparation of hierarchical carbon monoliths for hexavalent chromium removal. J. Colloid. Interface. Sci. 2018, 510, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Gong, K.; Hu, Q.; Cheng, X.; Zhou, J.; Dong, M.; Wang, N.; Ding, T.; Qiu, B.; Guo, Z. Optimizing nanocarbon shell in zero-valent iron nanoparticles for improved electron utilization in Cr(VI) reduction. Chemosphere 2020, 242, 125235. [Google Scholar] [CrossRef] [PubMed]
- Wan, Z.; Cho, D.W.; Tsang, D.C.W.; Li, M.; Sun, T.; Verpoort, F. Concurrent adsorption and micro-electrolysis of Cr(VI) by nanoscale zerovalent iron/biochar/Ca-alginate composite. Environ. Pollut. 2019, 247, 410–420. [Google Scholar] [CrossRef]
- Pal, A.; Uddin, K.; Thu, K.; Saha, B.B.; Kil, H.S.; Yoon, S.H.; Miyawaki, J. Synthesis of High Grade Activated Carbons From Waste Biomass. Mater. Sci. Eng. 2020, 4, 584–595. [Google Scholar]
- Pal, A.; Uddin, K.; Saha, B.B.; Thu, K.; Kil, H.S.; Yoon, S.H.; Miyawaki, J. A benchmark for CO2 uptake onto newly synthesized biomass-derived activated carbons. Appl. Energy 2020, 264, 114720. [Google Scholar] [CrossRef]
- Miretzky, P.; Cirelli, A.F. Cr(VI) and Cr(III) removal from aqueous solution by raw and modified lignocellulosic materials: A review. J. Hazard. Mater. 2010, 180, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Luo, W.; Luo, T.; Zhou, Q.; Li, H.; Jing, L. A study on adsorption of Cr (VI) by modified rice straw: Characteristics, performances and mechanism. J. Clean. Prod. 2018, 196, 626–634. [Google Scholar] [CrossRef]
- Rangabhashiyam, S.; Selvaraju, N. Efficacy of unmodified and chemically modified Swietenia mahagoni shells for the removal of hexavalent chromium from simulated wastewater. J. Mol. Liq. 2015, 209, 487–497. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Sillanpää, M.; Witek-Krowiak, A. Agricultural waste peels as versatile biomass for water purification—A review. Chem. Eng. J. 2015, 270, 244–271. [Google Scholar] [CrossRef]
- Da Silva Lima, L.K.; De Jesus, O.N.; Soares, T.L.; Dos Santos, I.S.; De Oliveira, E.J.; Filho, M.A.C. Growth, physiological, anatomical and nutritional responses of two phenotypically distinct passion fruit species (Passiflora L.) and their hybrid under saline conditions. Sci. Hortic. 2020, 263, 109037. [Google Scholar] [CrossRef]
- Guo, R.; Tian, S.; Li, X.; Wu, X.; Liu, X.; Li, D.; Liu, Y.; Ai, L.; Song, Z.; Wu, Y. Pectic polysaccharides from purple passion fruit peel: A comprehensive study in macromolecular and conformational characterizations. Carbohydr. Polym. 2020, 229, 115406. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.C.; Freitas, A.L.P.; Barros, F.C.N.; Lins, K.O.A.L.; Alves, A.P.N.N.; Alencar, N.M.N.; De Figueiredo, I.S.T.; Pessoa, C.; De Moraes, M.O.; Lotufo, L.V.C.; et al. Polysaccharide isolated from Passiflora edulis: Characterization and antitumor properties. Carbohydr. Polym. 2012, 87, 139–145. [Google Scholar] [CrossRef]
- Zhao, R.; Li, X.; Sun, B.; Li, Y.; Li, Y.; Yang, R.; Wang, C. Branched polyethylenimine grafted electrospun polyacrylonitrile fiber membrane: A novel and effective adsorbent for Cr(VI) remediation in wastewater. J. Mater. Chem. A 2017, 5, 1133–1144. [Google Scholar] [CrossRef]
- Liu, C.; Jin, R.-N.; Ouyang, X.-K.; Wang, Y.-G. Adsorption behavior of carboxylated cellulose nanocrystal-polyethyleneimine composite for removal of Cr(VI) ions. Appl. Surf. Sci. 2017, 408, 77–87. [Google Scholar] [CrossRef]
- Li, H.; Fu, Z.; Yan, C.; Huang, J.; Liu, Y.N.; Kirin, S.I. Hydrophobic-hydrophilic post-cross-linked polystyrene/poly (methyl acryloyl diethylenetriamine) interpenetrating polymer networks and its adsorption properties. J. Colloid. Interface Sci. 2016, 463, 61–68. [Google Scholar] [CrossRef]
- Igberase, E.; Osifo, P.O. Application of diethylenetriamine grafted on glyoxal cross-linked chitosan composite for the effective removal of metal ions in batch system System. Int. J. Biol. Macromol. 2019, 134, 1145–1155. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.N.; Tamboli, S.R.; Sutar, D.S.; Jadhav, S.R.; Marathe, J.V.; Shaikh, A.A.; Prajapati, A.A. Batch and continuous studies for adsorption of anionic dye onto waste tea residue: Kinetic, equilibrium, breakthrough and reusability studies. J. Clean. Prod. 2020, 252, 119778. [Google Scholar] [CrossRef]
- Song, Y.; Tan, J.; Wang, G.; Zhou, L. Superior amine-rich gel adsorbent from peach gum polysaccharide for highly efficient removal of anionic dyes. Carbohydr. Polym. 2018, 199, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Liu, C.; Anderson, D.P.; Chang, P.R. Porous cellulose spheres: Preparation, modification and adsorption properties. Chemosphere 2016, 165, 399–408. [Google Scholar] [CrossRef]
- Chen, Q.; Zheng, J.; Wen, L.; Yang, C.; Zhang, L. A multi-functional-group modified cellulose for enhanced heavy metal cadmium adsorption: Performance and quantum chemical mechanism. Chemosphere 2019, 224, 509–518. [Google Scholar] [CrossRef]
- Xing, L.; Gu, J.; Zhang, W.; Tu, D.; Hu, C. Cellulose I and II nanocrystals produced by sulfuric acid hydrolysis of Tetra pak cellulose I. Carbohydr. Polym. 2018, 192, 184–192. [Google Scholar] [CrossRef]
- Gan, C.; Liu, Y.; Tan, X.; Wang, S.; Zeng, G.; Zheng, B.; Li, T.; Jiang, Z.; Liu, W. Effect of porous zinc-biochar nanocomposites on Cr(VI) adsorption from aqueous solution. RSC Adv. 2015, 5, 35107–35115. [Google Scholar] [CrossRef]
- Yan, Y.Z.; An, Q.D.; Xiao, Z.Y.; Zhai, S.R.; Zhai, B.; Shi, Z. Interior multi-cavity/surface engineering of alginate hydrogels with polyethylenimine for highly efficient chromium removal in batch and continuous aqueous systems. J. Mater. Chem. A 2017, 5, 17073–17087. [Google Scholar] [CrossRef]
- Chen, X.; Li, P.; Kang, Y.; Zeng, X.; Xie, Y.; Zhang, Y.; Wang, Y.; Xie, T. Preparation of temperature-sensitive xanthan/NIPA hydrogel using citric acid as crosslinking agent for bisphenol A adsorption. Carbohydr. Polym. 2019, 206, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yue, X.; Xu, W.; Zhang, H.; Li, F. Amino modification of rice straw-derived biochar for enhancing its cadmium (II) ions adsorption from water. J. Hazard. Mater. 2019, 379, 120783. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zheng, P.; Ma, X. The modification of carbon materials with carbon disulfide for the removal of Pb2+. Powder Technol. 2016, 301, 1–9. [Google Scholar] [CrossRef]
- Song, W.; Gao, B.; Zhang, T.; Xu, X.; Huang, X.; Yu, H.; Yue, Q. High-capacity adsorption of dissolved hexavalent chromium using amine-functionalized magnetic corn stalk composites. Bioresour. Technol. 2015, 190, 550–557. [Google Scholar] [CrossRef]
- Zhou, G.; Luo, J.; Liu, C.; Chu, L.; Crittenden, J. Efficient heavy metal removal from industrial melting effluent using fixed-bed process based on porous hydrogel adsorbents. Water Res. 2018, 131, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Duranoğlu, D.; Trochimczuk, A.W.; Beker, U. Kinetics and thermodynamics of hexavalent chromium adsorption onto activated carbon derived from acrylonitrile-divinylbenzene copolymer. Chem. Eng. J. 2012, 187, 193–202. [Google Scholar] [CrossRef]
- Jain, M.; Garg, V.K.; Kadirvelu, K. Adsorption of hexavalent chromium from aqueous medium onto carbonaceous adsorbents prepared from waste biomass. J. Environ. Manag. 2010, 91, 949–957. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, J.; Achari, G.; Yu, J.; Cai, W. Cr(VI) removal from aqueous solutions by hydrothermal synthetic layered double hydroxides: Adsorption performance, coexisting anions and regeneration studies. Colloids Surf. A Physicochem. Eng. Asp. 2014, 457, 33–40. [Google Scholar] [CrossRef]
- Ji, M.; Su, X.; Zhao, Y.; Qi, W.; Wang, Y.; Chen, G.; Zhang, Z. Effective adsorption of Cr(VI) on mesoporous Fe-functionalized Akadama clay: Optimization, selectivity, and mechanism. Appl. Surf. Sci. 2015, 344, 128–136. [Google Scholar] [CrossRef]
- Molnár, Á.; Papp, A. Catalyst recycling-A survey of recent progress and current status. Coord. Chem. Rev. 2017, 349, 1–65. [Google Scholar] [CrossRef]
- Kloprogge, J.T.; Hickey, L.; Frost, R.L. The effect of varying synthesis conditions on zinc chromium hydrotalcite: A spectroscopic study. Mater. Chem. Phys. 2005, 89, 99–109. [Google Scholar] [CrossRef]
- Han, S.; Zang, Y.; Gao, Y.; Yue, Q.; Zhang, P.; Kong, W.; Jin, B.; Xu, X.; Gao, B. Co-monomer polymer anion exchange resin for removing Cr(VI) contaminants: Adsorption kinetics, mechanism and performance. Sci. Total Environ. 2020, 709, 136002. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; An, Q.; Xiao, Z.; Zheng, W.; Zhai, S. Flexible core-shell/bead-like alginate@PEI with exceptional adsorption capacity, recycling performance toward batch and column sorption of Cr(VI). Chem. Eng. J. 2017, 313, 475–486. [Google Scholar] [CrossRef]
- Ding, J.; Pu, L.; Wang, Y.; Wu, B.; Yu, A.; Zhang, X.; Pan, B.; Zhang, Q.; Gao, G. Adsorption and reduction of Cr(VI) together with Cr(III) sequestration by polyaniline confined in pores of polystyrene beads. Environ. Sci. Technol. 2018, 52, 12602–12611. [Google Scholar] [CrossRef] [PubMed]


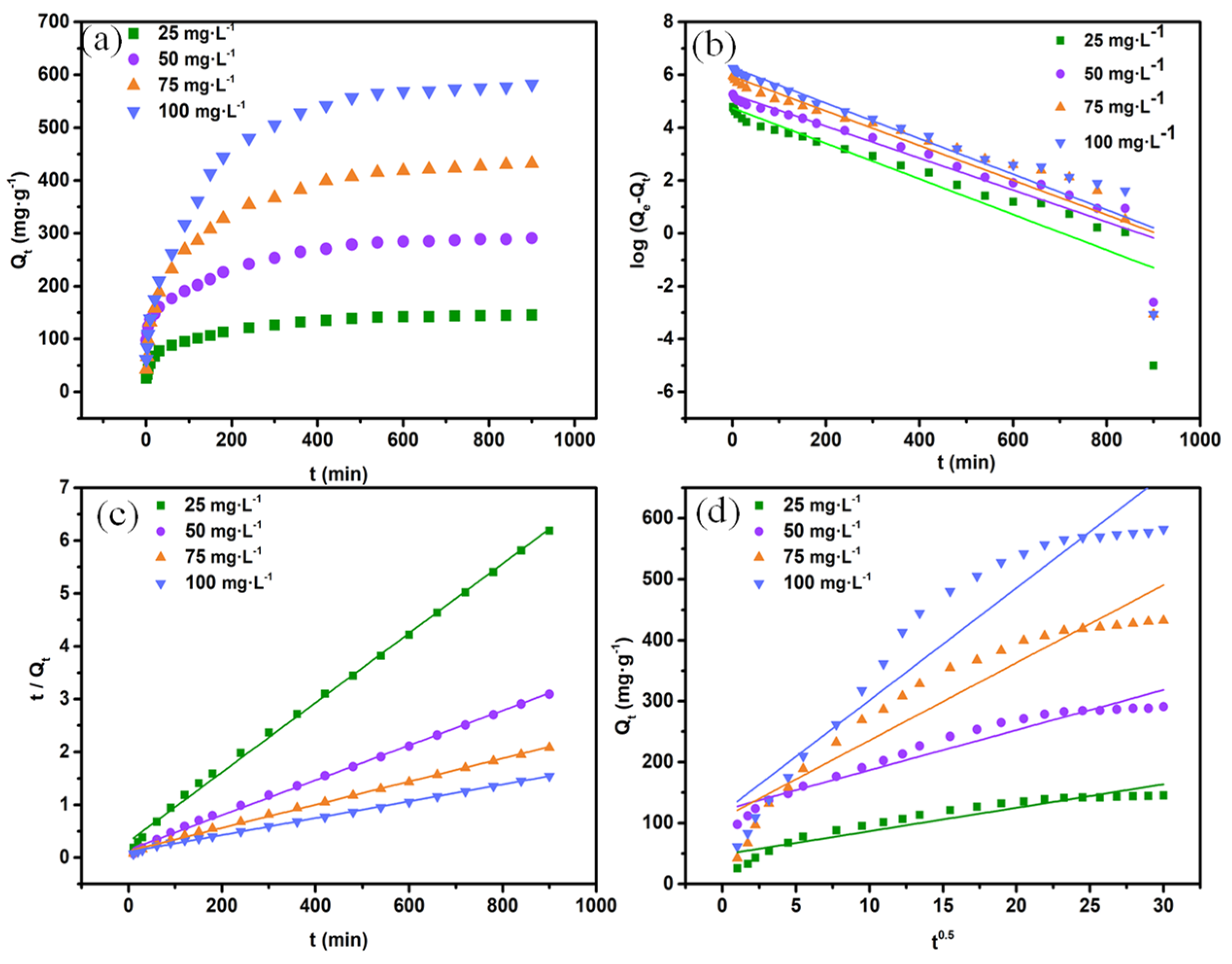


| Pseudo-First-Order Model | Pseudo-Second-Order Mode | Intraparticle Diffusion Model | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Qe-exp (mg/g−1) | Ln (Qe − Qt) = lnQe − k1t | t/Qt = 1/k2Qe2 + t/Qe | Qt = kit0.5 + C | |||||||
| k1 (min−1) | Qe (mg/g−1) | R2 | k2 (min−1) | Qe (mg/g−1) | R2 | ki | C | R2 | ||
| 25 | 145.51 | 0.00672 | 114.43 | 0.841 | 0.00014 | 151.98 | 0.998 | 3.84244 | 48.18 | 0.898 |
| 50 | 291.02 | 0.00603 | 192.48 | 0.906 | 0.00007 | 303.03 | 0.997 | 6.57357 | 121.03 | 0.943 |
| 75 | 432.05 | 0.00655 | 379.93 | 0.871 | 0.00003 | 456.62 | 0.998 | 12.73691 | 108.27 | 0.907 |
| 100 | 582.05 | 0.00673 | 531.93 | 0.869 | 0.00002 | 628.93 | 0.998 | 18.42881 | 117.03 | 0.916 |
| Isotherm Model | Parameters | 25 | 35 | 45 |
|---|---|---|---|---|
| Langmuir: Ce/qe = Ce/qm + 1/qmKL | qm (mg/g−1) | 675.67 | 729.92 | 800.11 |
| KL (L/mg−1) | 0.0601 | 0.0913 | 0.1014 | |
| R2 | 0.9985 | 0.9993 | 0.9987 | |
| Freundlich: lnqe = lnKF + bFlnCe | KF (mg/g−1) | 165.94 | 194.41 | 241.44 |
| bF | 0.2241 | 0.2202 | 0.2014 | |
| R2 | 0.8174 | 0.8561 | 0.7497 | |
| Temkin: qe = BlnKT + BlnCe | KT (L/mg−1) | 24.71 | 48.39 | 115.51 |
| B (kJ−2/mol−2) | 66.3780 | 68.6297 | 70.05829 | |
| R2 | 0.9568 | 0.9704 | 0.9560c |
| Temperature (K) | ∆G (kJ/mol−1) | ∆S (J/mol−1/K−1) | ∆H (kJ/mol−1) |
|---|---|---|---|
| 298 | −1.741 | 38.64 | 9.773 |
| 308 | −2.127 | ||
| 318 | −2.514 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Zheng, J.; You, S.; Du, L.; Liu, C.; Chen, K.; Liu, Y.; Ma, L. Selective Adsorption of CR (VI) onto Amine-Modified Passion Fruit Peel Biosorbent. Processes 2021, 9, 790. https://doi.org/10.3390/pr9050790
Zhao X, Zheng J, You S, Du L, Liu C, Chen K, Liu Y, Ma L. Selective Adsorption of CR (VI) onto Amine-Modified Passion Fruit Peel Biosorbent. Processes. 2021; 9(5):790. https://doi.org/10.3390/pr9050790
Chicago/Turabian StyleZhao, Xiaolei, Junli Zheng, Shaohong You, Linlin Du, Chongmin Liu, Kaiwei Chen, Yuanli Liu, and Lili Ma. 2021. "Selective Adsorption of CR (VI) onto Amine-Modified Passion Fruit Peel Biosorbent" Processes 9, no. 5: 790. https://doi.org/10.3390/pr9050790
APA StyleZhao, X., Zheng, J., You, S., Du, L., Liu, C., Chen, K., Liu, Y., & Ma, L. (2021). Selective Adsorption of CR (VI) onto Amine-Modified Passion Fruit Peel Biosorbent. Processes, 9(5), 790. https://doi.org/10.3390/pr9050790






