Study on a Novel Filter Media Incorporating with Core Shell Nanoencapsulated Phase Change Material: Fabrication and Evaluation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Apparatus
2.3. Synthetic Procedures of Nanoencapsuled Phase Change Material with Silica Shell (NPCMs)
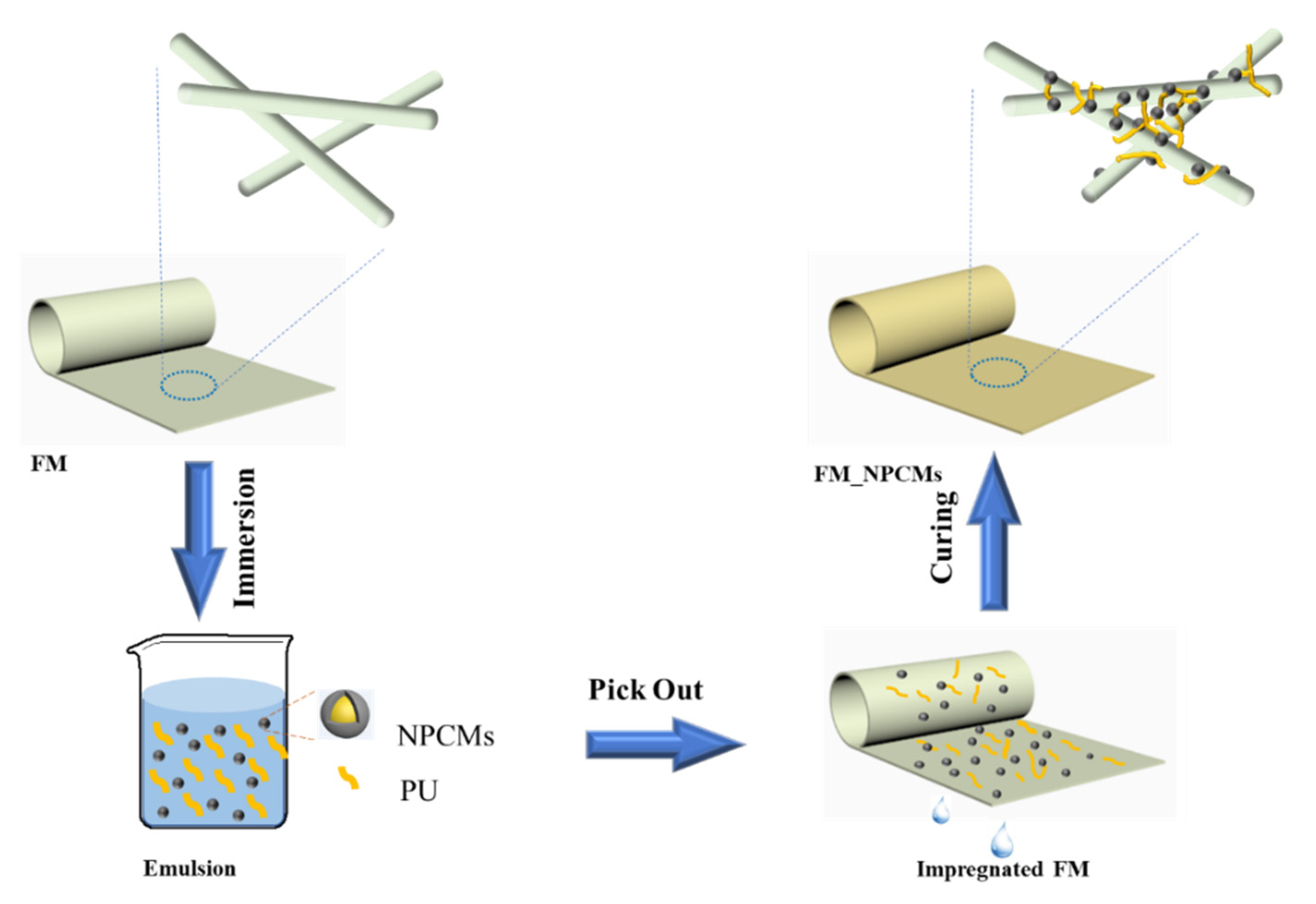
2.4. Fabrication of Filter Media with NPCMs
2.5. Characterization
3. Results and Discussion
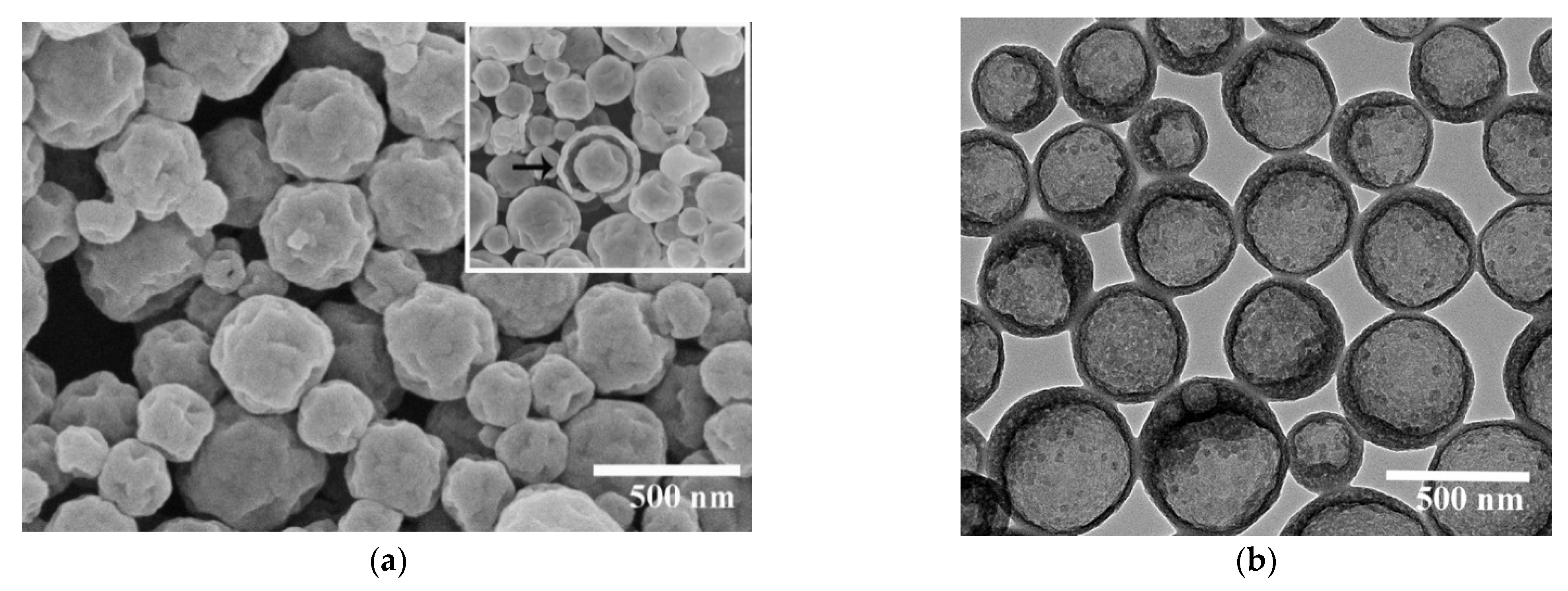
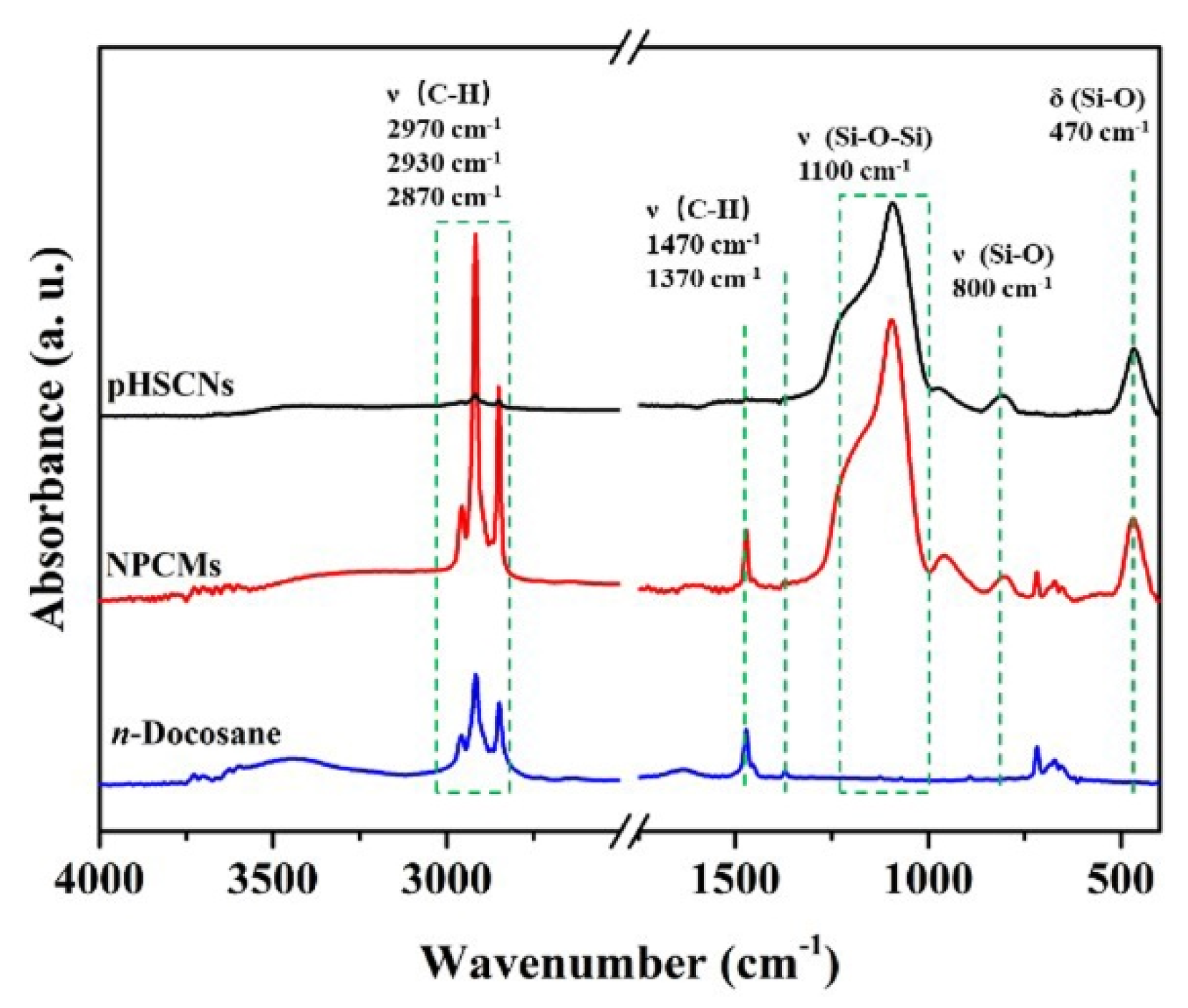
3.1. Preparation of Nano-Encapsuled Phase Change Material with Silica Shell
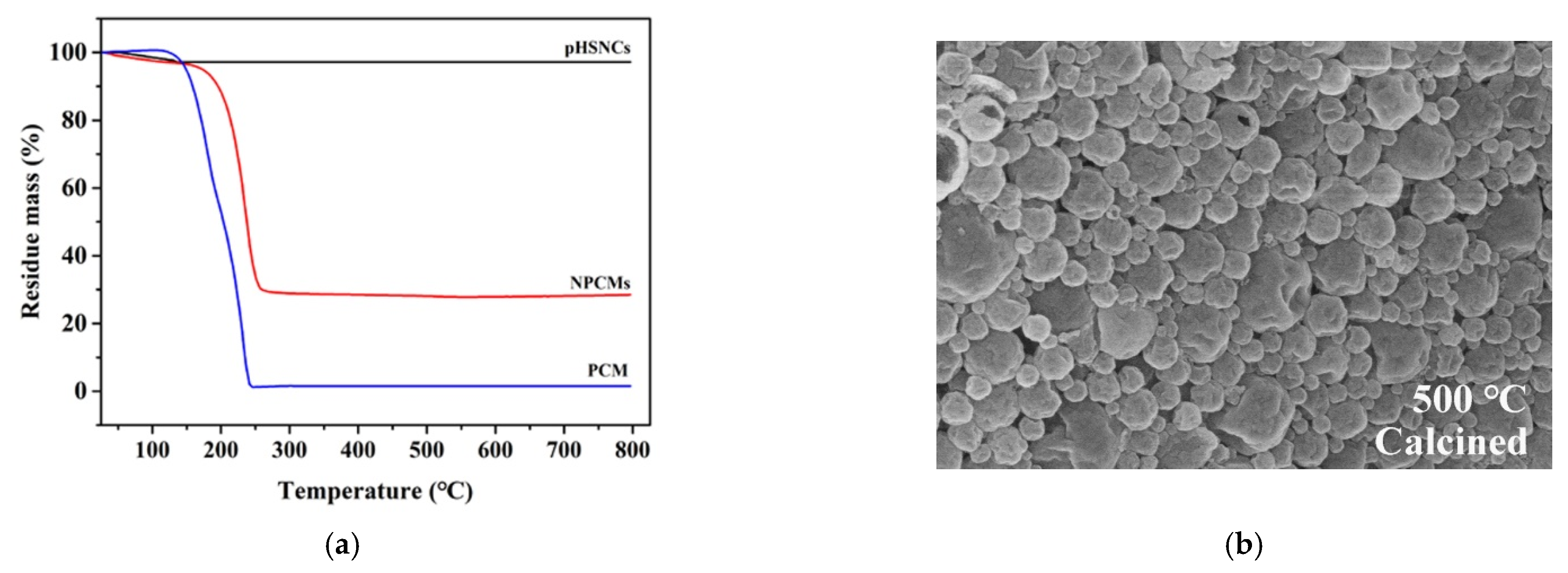
3.2. Thermal Stability of Nano-Encapsuled PCMs with Silica Shell
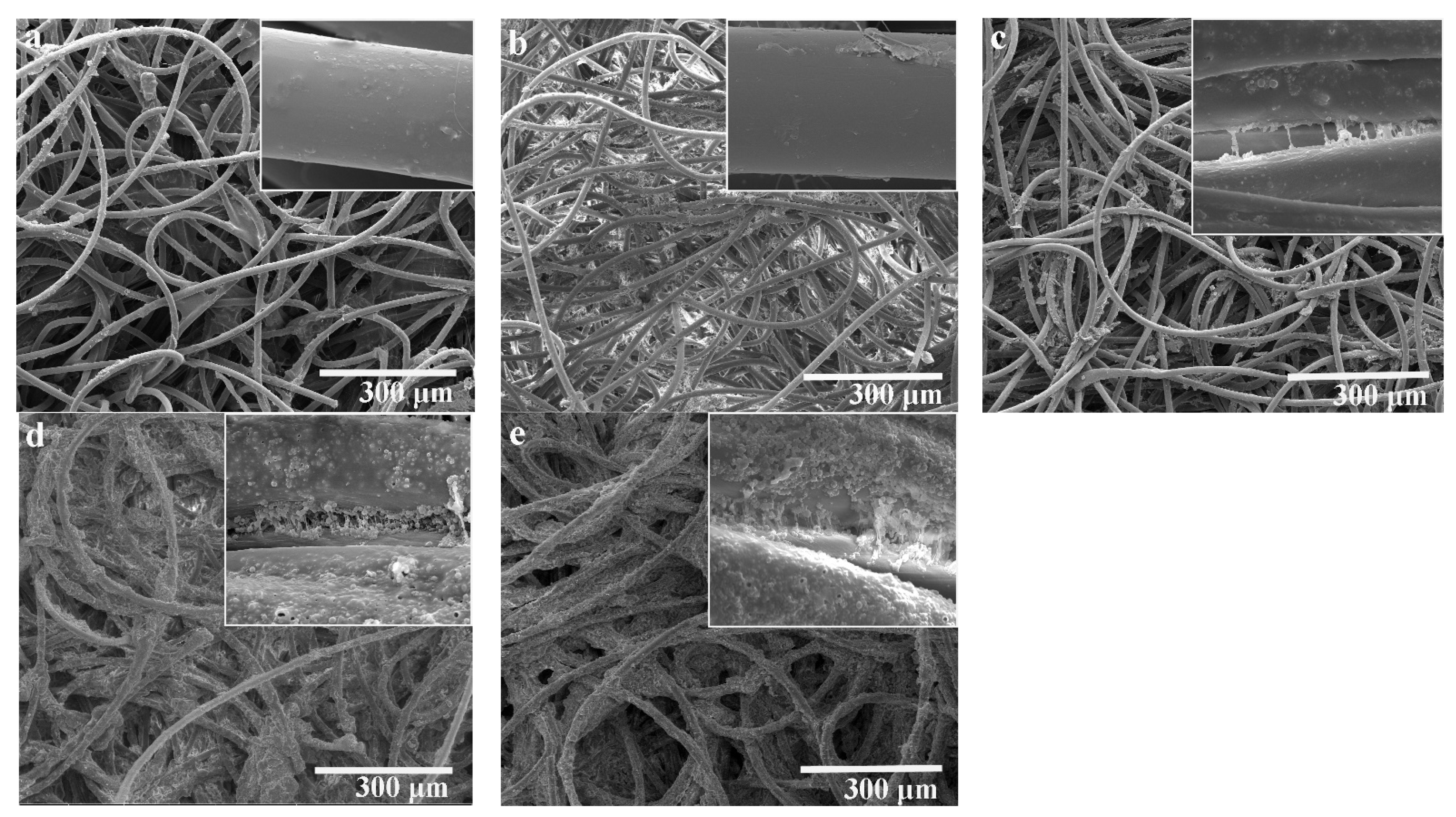
3.3. Microsture and Filtration Performance of Filter Media with NPCMs
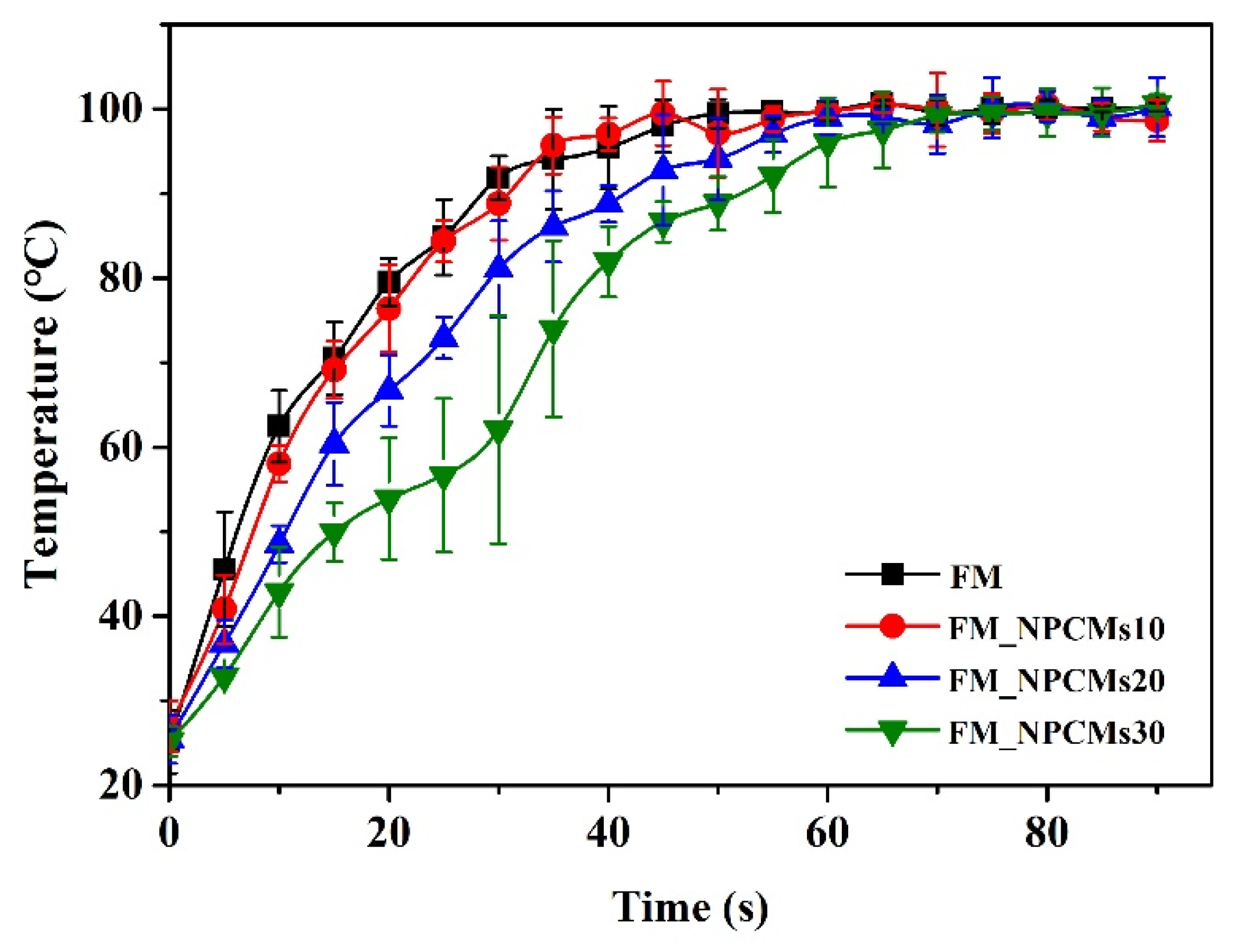
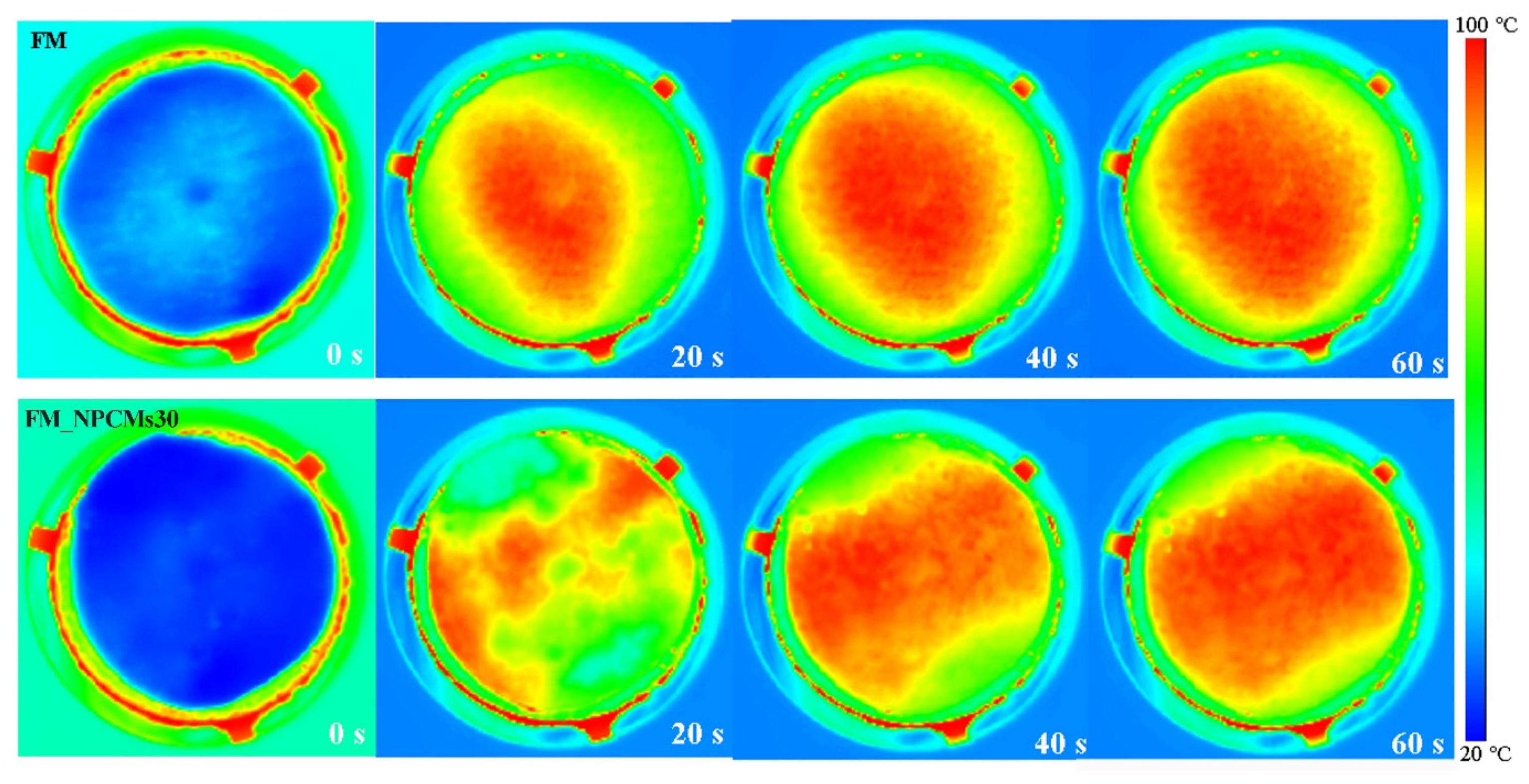
3.4. Thermal Performance of Filter Media with NPCMs
3.5. Environmental Endurance of Filter Media with NPCMs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lin, J.-H.; Huang, C.-L.; Li, T.-T.; Lee, Y.-M.; Wu, Z.-H.; Lou, C.-W. Extended PTFE fabrics used as high-temperature filter clothes: Manufacturing technique and chemical stability evaluation. J. Text. Inst. 2015, 106, 793–799. [Google Scholar] [CrossRef]
- Boudhan, R.; Joubert, A.; Durécu, S.; Gueraoui, K.; Le Coq, L. Influence of air humidity on particle filtration performance of a pulse-jet bag filter. J. Aerosol. Sci. 2019, 130, 1–9. [Google Scholar] [CrossRef]
- Kurtz, O.; Meyer, J.; Kasper, G. Influence of filter operating parameters on fine dust emissions from pulse-cleaned filter bags. Chem. Eng. Technol. 2016, 39, 435–443. [Google Scholar] [CrossRef]
- Wang, A.; Song, Q.; Tu, G.; Wang, H.; Yue, Y.; Yao, Q. Influence of flue gas cleaning system on characteristics of PM2.5 emission from coal-fired power plants. Int. J. Coal Sci. Technol. 2014, 1, 4–12. [Google Scholar] [CrossRef]
- Patnaik, A.; Anandjiwala, R.D. Reasons for filter bag failure and method development to improve its life span. Chem. Eng. Technol. 2016, 39, 529–534. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M.; Naffakh, M. Synthesis and characterization of nitrated and aminated poly(phenylene sulfide) derivatives for advanced applications. Mater. Chem. Phys. 2012, 131, 605–614. [Google Scholar] [CrossRef]
- Ye, Y.; Wang, Z. Effect of corona discharge on polyphenylene sulfide filter material of electrostatic–bag composite precipitators. Ind. Eng. Chem. Res. 2018, 57, 1319–1330. [Google Scholar] [CrossRef]
- Wang, C.-T.; Lee, S.-T.; Li, L.-Y.; Syu, J.-Y.; Kuo, C.-C.; Lin, W.-Y. A Novel Charged Fibrous Media Characterized by Higher Efficiency and Lower Pressure Drop. Aerosol. Sci. Technol. 2019, 53, 1441–1452. [Google Scholar] [CrossRef]
- Liu, Y.-S.; Qian, X.-M.; Wang, L.; Qian, Y.; Bai, H.; Wang, X.-B. Hierarchical micro/nanofibrous filter for effective fine-particle capture. Powder Technol. 2020, 360, 1192–1199. [Google Scholar] [CrossRef]
- Li, X.-Q.; Wang, N.; Fan, G.; Yu, J.-Y.; Gao, J.; Sun, G.; Ding, B. Electreted polyetherimide–silica fibrous membranes for enhanced filtration of fine particles. J. Colloid Interface Sci. 2015, 439, 12–20. [Google Scholar] [CrossRef]
- Su, J.-F.; Yang, G.-H.; Cheng, C.-L.; Huang, C.; Xu, H.; Ke, Q.-F. Hierarchically structured TiO2/PAN nanofibrous membranes for high-efficiency air filtration and toluene degradation. J. Colloid Interface Sci. 2017, 507, 386–396. [Google Scholar] [CrossRef]
- Bortolassi, A.C.C.; Nagarajan, S.; Lima, B.D.A.; Guerra, V.G.; Aguiar, M.L.; Huon, V.; Soussan, L.; Cornu, D.; Miele, P.; Bechelany, M. Efficient nanoparticles removal and bactericidal action of electrospun nanofibers membranes for air filtration. Mat. Sci. Eng. C Mater. 2019, 102, 718–729. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Sun, Y.; Chen, Z.; Gao, Y. A multi-functional textile that combines self-cleaning, water-proofing and VO2-based temperature-responsive thermoregulating. Sol. Energy Mater. Sol. Cells 2017, 159, 102–111. [Google Scholar] [CrossRef]
- Zhou, D.; Zhao, C.Y.; Tian, Y. Review on thermal energy storage with Phase change materials (PCMs) in building applications. Appl. Energy 2012, 92, 593–605. [Google Scholar] [CrossRef]
- Xie, J.; Wang, W.; Liu, J.; Pan, S. Thermal performance analysis of PCM components heat storage using mechanical ventilation: Experimental results. Energy Build 2016, 123, 169–178. [Google Scholar] [CrossRef]
- Seong, Y.-B.; Energies, J.-H.L.J. Energy saving potentials of phase change materials applied to lightweight building envelopes. Energies 2013, 6, 5219–5230. [Google Scholar] [CrossRef]
- Dincer, I.; Dost, S. A perspective on thermal energy storage systems for solar energy applications. Int. J. Energy Res. 1996, 20, 547–557. [Google Scholar] [CrossRef]
- Koca, A.; Oztop, H.F.; Koyun, T.; Varol, Y. Energy and exergy analysis of a latent heat storage system with phase change material for a solar collector. Renew. Energy 2008, 33, 567–574. [Google Scholar] [CrossRef]
- Sarier, N.; Onder, E. Organic phase change materials and their textile applications: An overview. Thermochim. Acta 2012, 540, 7–60. [Google Scholar] [CrossRef]
- Sun, D.; Iqbal, K. Synthesis of functional nanocapsules and their application to cotton fabric for thermal management. Cellulose 2017, 24, 3525–3543. [Google Scholar] [CrossRef]
- Iqbal, K.; Sun, D. Synthesis of nanoencapsulated Glauber’s salt using PMMA shell and its application on cotton for thermoregulating effect. Cellulose 2018, 25, 2103–2113. [Google Scholar] [CrossRef]
- Lu, P.; Chen, W.; Fan, J.; Ghaban, R.; Zhu, M. Thermally triggered nanocapillary encapsulation of lauric acid in polystyrene hollow fibers for efficient thermal energy storage. ACS Sustain. Chem. Eng. 2018, 6, 2656–2666. [Google Scholar] [CrossRef]
- Zhang, G.H.; Bon, S.A.F.; Zhao, C.Y. Synthesis, characterization and thermal properties of novel nanoencapsulated phase change materials for thermal energy storage. Sol. Energy 2012, 86, 1149–1154. [Google Scholar] [CrossRef]
- Su, J.-F.; Huang, Z.; Ren, L. High compact melamine-formaldehyde microPCMs containing n-octadecane fabricated by a two-step coacervation method. Colloid Polym. Sci. 2007, 285, 1581–1591. [Google Scholar] [CrossRef]
- Kahraman Döğüşcü, D.; Kızıl, Ç.; Biçer, A.; Sarı, A.; Alkan, C. Microencapsulated n-alkane eutectics in polystyrene for solar thermal applications. Sol. Energy 2018, 160, 32–42. [Google Scholar] [CrossRef]
- Jiang, Z.; Yang, W.; He, F.; Xie, C.; Fan, J.; Wu, J.; Zhang, K. Modified phase change microcapsules with calcium carbonate and graphene oxide shells for enhanced energy storage and leakage prevention. ACS Sustain. Chem. Eng. 2018, 6, 5182–5191. [Google Scholar] [CrossRef]
- Tang, F.; Liu, L.; Alva, G.; Jia, Y.; Fang, G. Synthesis and properties of microencapsulated octadecane with silica shell as shape–stabilized thermal energy storage materials. Sol. Energy Mater. Sol. Cells 2017, 160, 1–6. [Google Scholar] [CrossRef]
- Ciriminna, R.; Sciortino, M.; Alonzo, G.; Schrijver, A.D.; Pagliaro, M. From molecules to systems: Sol−gel microencapsulation in silica-based materials. Chem. Rev. 2011, 111, 765–789. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yan, K.-L.; Hu, C.-Y.; Zhao, Y.-L.; Chen, Z.; Zhu, X.-M.; Möller, M. Encapsulation of enzymes in silica nanocapsules formed by an amphiphilic precursor polymer in water. J. Mater. Chem. B 2015, 3, 1261–1267. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Li, Q.; Zhu, Y.; Chen, K.; Tian, C.; Wang, J.; Bai, R. Nanoencapsulation of n-octadecane phase change material with silica shell through interfacial hydrolysis and polycondensation in miniemulsion. Energy 2015, 93, 1684–1692. [Google Scholar] [CrossRef]
- Paneliya, S.; Khanna, S.; Singh, A.P.; Patel, Y.K.; Vanpariya, A.; Makani, N.H.; Banerjee, R.; Mukhopadhyay, I. Core shell paraffin/silica nanocomposite: A promising phase change material for thermal energy storage. Renew. Energy 2021, 167, 591–599. [Google Scholar] [CrossRef]
- Zhu, X.-M.; Jaumann, M.; Peter, K.; Möller, M.; Melian, C.; Adams-Buda, A.; Demco, D.E.; Blümich, B.J.M. One-pot synthesis of hyperbranched polyethoxysiloxanes. Macromolecules 2006, 39, 1701–1708. [Google Scholar] [CrossRef]
- Zhao, Y.-L.; Liu, J.; Chen, Z.; Zhu, X.-M.; Möller, M. Hybrid nanostructured particles via surfactant-free double miniemulsion polymerization. Nat. Commun. 2018, 9, 1918. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhao, Y.-L.; Zhao, Y.; Thomas, H.; Zhu, X.-M.; Möller, M. Inclusion of phase-change materials in submicron silica capsules using a surfactant-free emulsion approach. Langmuir 2018, 34, 10397–10406. [Google Scholar] [CrossRef] [PubMed]




| Sample | Pore Size (μm) | Penetration Rate (×10−4%) | Standard Deviation | |||
|---|---|---|---|---|---|---|
| Mean | Maximum | Minimum | Standard Deviation | |||
| FM | 15.43 | 43.79 | 4.62 | 5.64 | 457 | 31 |
| FM_PU | 12.23 | 39.22 | 3.33 | 5.28 | 386 | 44 |
| FM_NPCMs10 | 10.38 | 37.77 | 3.23 | 5.25 | 273 | 60 |
| FM_NPCMs20 | 2.50 | 28.49 | 1.40 | 4.83 | 81 | 37 |
| FM_NPCMs30 | 1.98 | 29.67 | 1.55 | 5.14 | 5 | 23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Chang, S.; Song, G.; Liu, J.; Shen, H. Study on a Novel Filter Media Incorporating with Core Shell Nanoencapsulated Phase Change Material: Fabrication and Evaluation. Processes 2021, 9, 731. https://doi.org/10.3390/pr9050731
Zhang C, Chang S, Song G, Liu J, Shen H. Study on a Novel Filter Media Incorporating with Core Shell Nanoencapsulated Phase Change Material: Fabrication and Evaluation. Processes. 2021; 9(5):731. https://doi.org/10.3390/pr9050731
Chicago/Turabian StyleZhang, Chi, Shuo Chang, Gaoju Song, Jianlin Liu, and Henggen Shen. 2021. "Study on a Novel Filter Media Incorporating with Core Shell Nanoencapsulated Phase Change Material: Fabrication and Evaluation" Processes 9, no. 5: 731. https://doi.org/10.3390/pr9050731
APA StyleZhang, C., Chang, S., Song, G., Liu, J., & Shen, H. (2021). Study on a Novel Filter Media Incorporating with Core Shell Nanoencapsulated Phase Change Material: Fabrication and Evaluation. Processes, 9(5), 731. https://doi.org/10.3390/pr9050731





