Revealing of Supercritical Water Gasification Process of Lignin by Reactive Force Field Molecular Dynamics Simulations
Abstract
1. Introduction
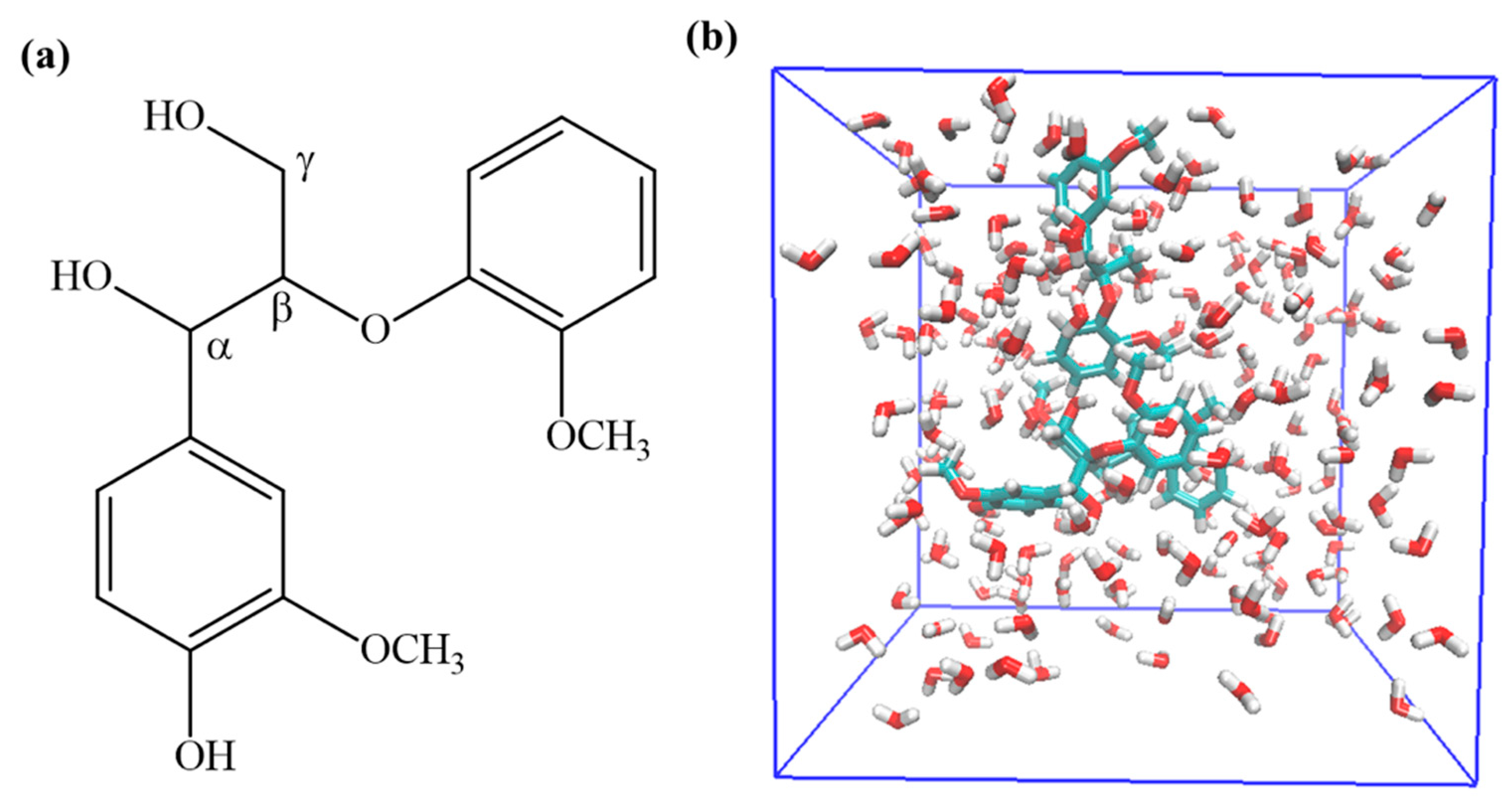
2. Computational Details
3. Results and Discussion
3.1. The Impact of Low Temperatures: 1000 K and 1500 K
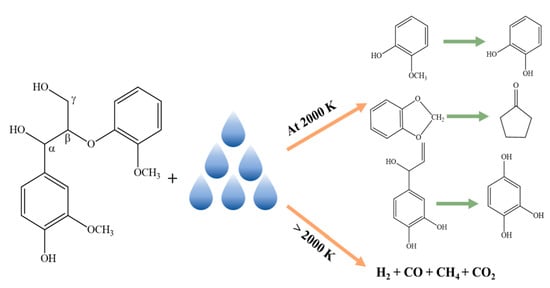
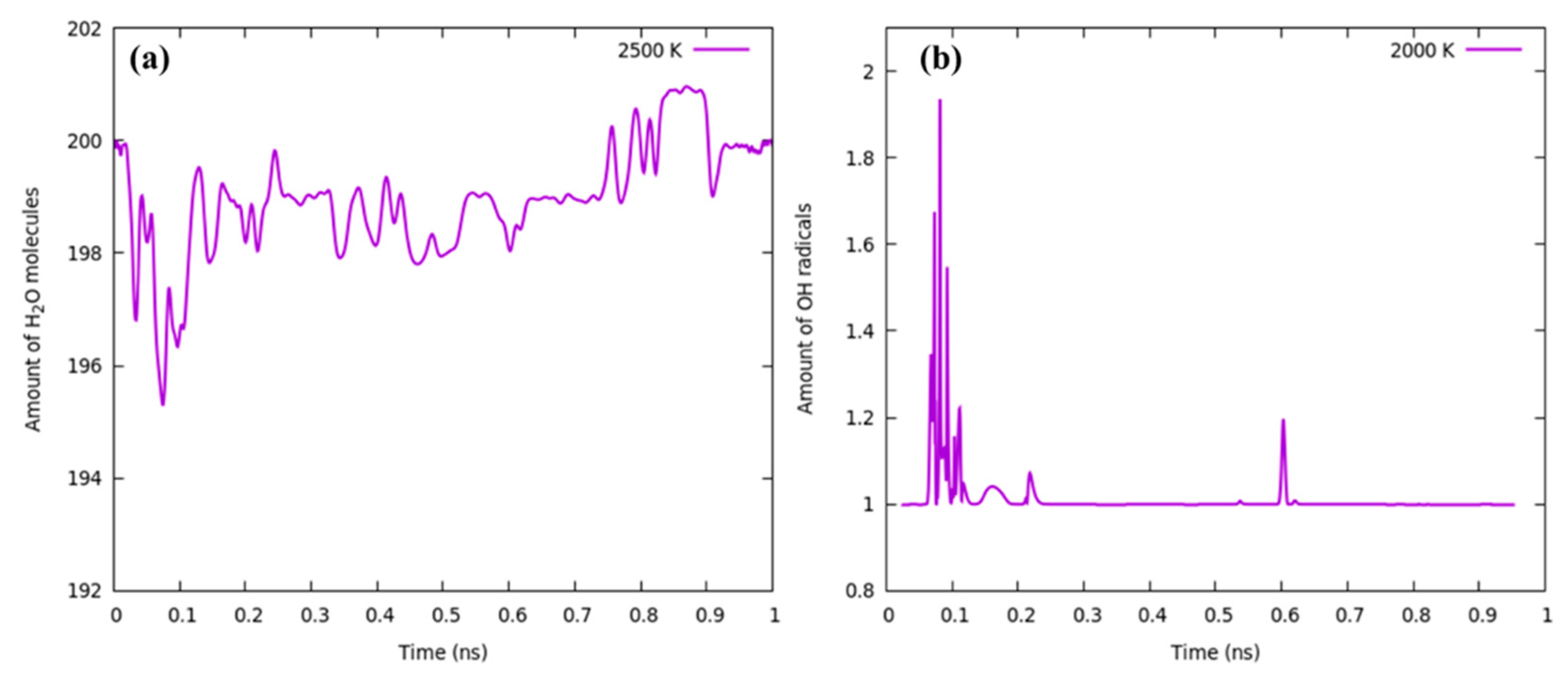
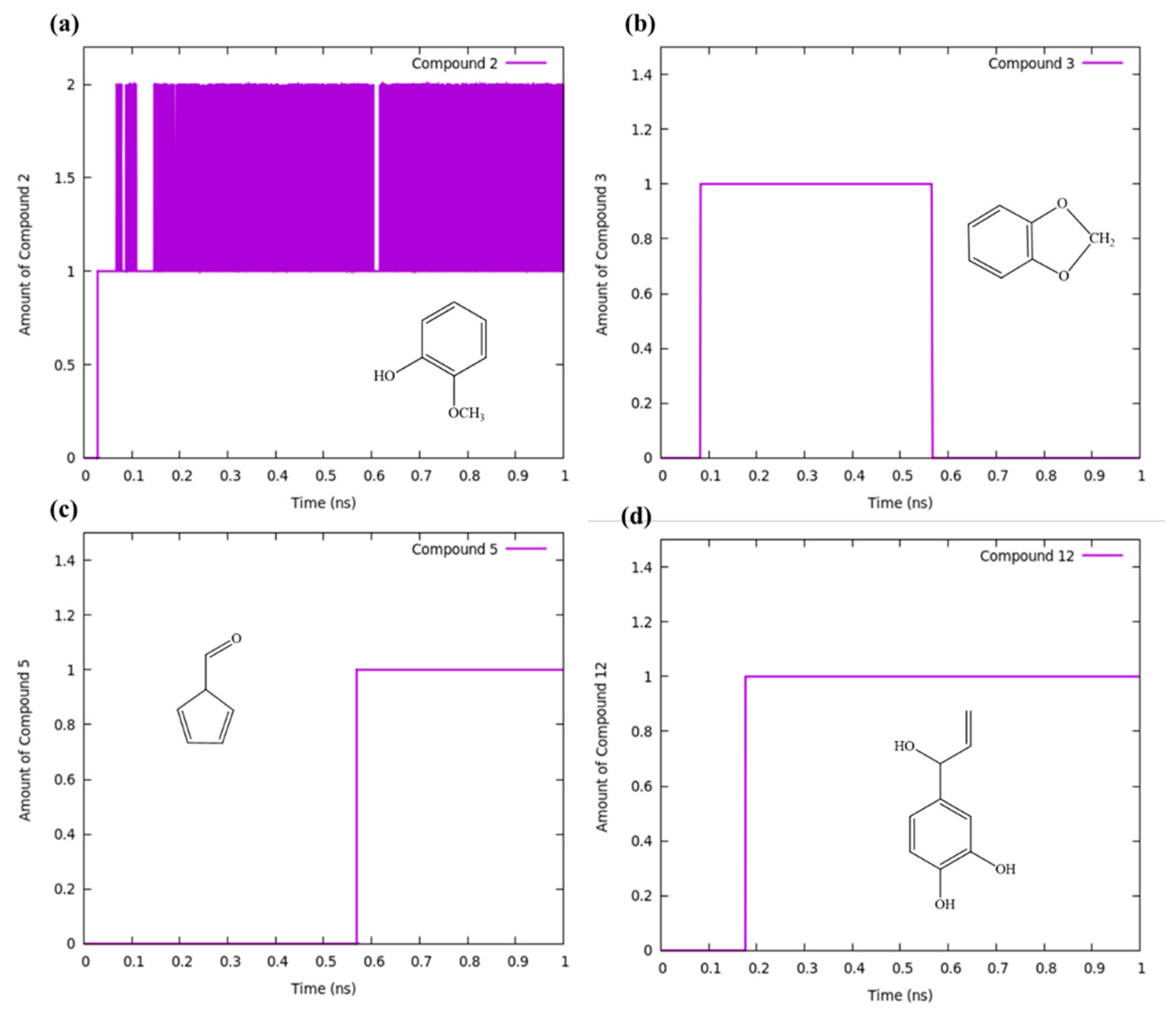
3.2. At Temperature 2000 K
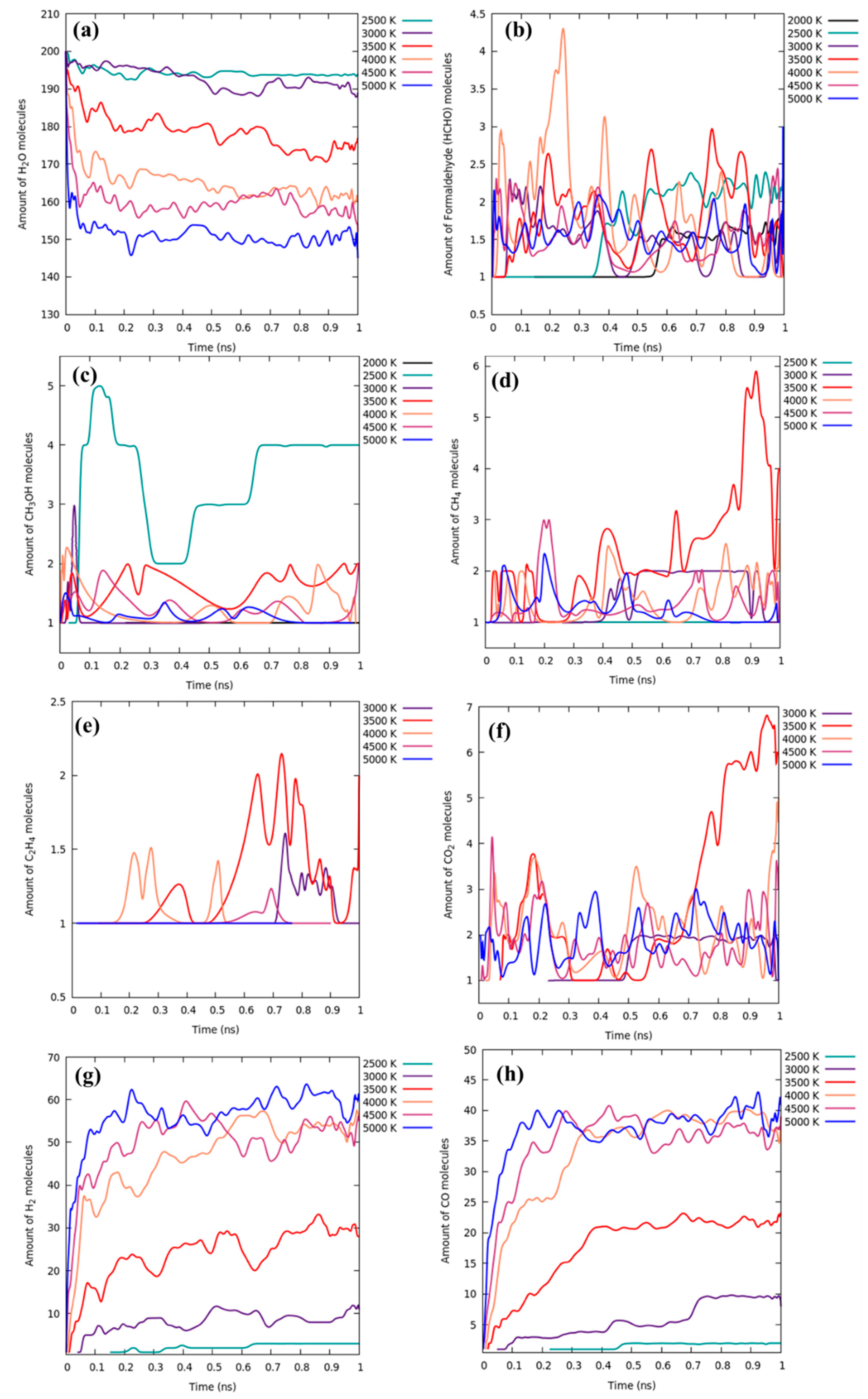
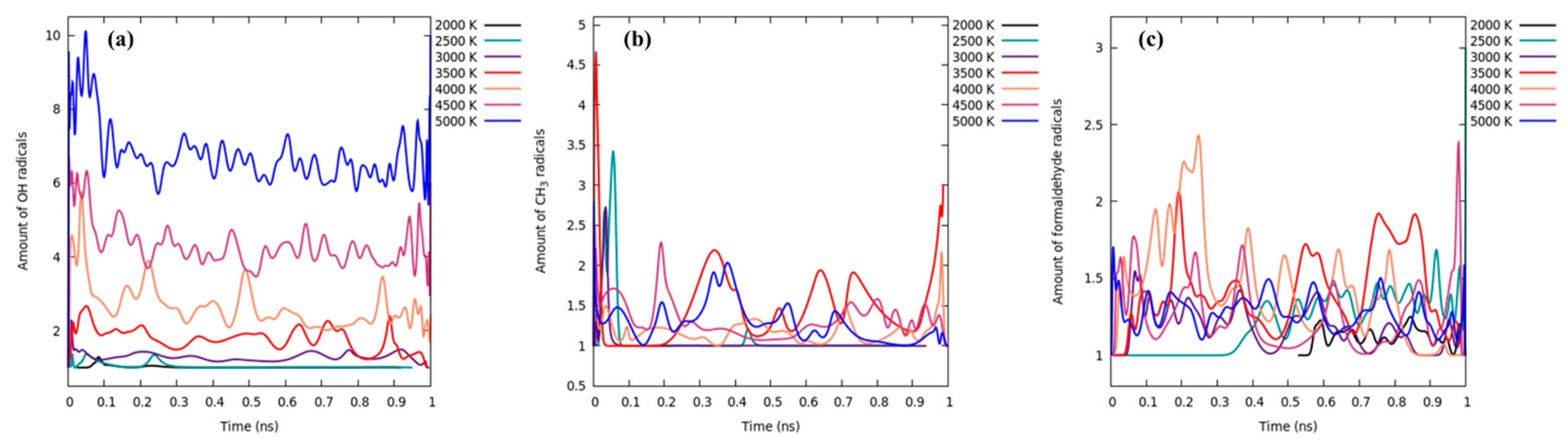
3.3. 2500 K and Higher Temperature
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahorsu, R.; Medina, F.; Constantí, M. Significance and Challenges of Biomass as a Suitable Feedstock for Bioenergy and Biochemical Production: A Review. Energies 2018, 11, 3366. [Google Scholar] [CrossRef]
- Lebaka, V.R. Potential Bioresources as Future Sources of Biofuels Production: An Overview. Biofuel Technol. 2013, 223–258. [Google Scholar] [CrossRef]
- Commodities 2021: Climate Change Policy Targets to Stimulate EU Biodiesel Consumption|S&P Global Platts. Available online: https://www.spglobal.com/platts/en/market-insights/latest-news/agriculture/122320-commodities-2021-climate-change-policy-targets-to-stimulate-eu-biodiesel-consumption (accessed on 24 March 2021).
- Sandak, A.; Sandak, J. Utilization of FT-NIR for Proper Biomass Conversion. In Proceedings of the NIR2013-A1—Agriculture, Environment, La Grande-Motte, France, 2 June 2013. [Google Scholar]
- Anukam, A.; Berghel, J. Biomass Pretreatment and Characterization: A Review. Biomass 2020. [Google Scholar] [CrossRef]
- Lee, S.; Shah, Y.T. Biofuels and Bioenergy: Processes and Technologies; CRC Press: Boca Raton, FL, USA, 2012; ISBN 978-1-4200-8955-4. [Google Scholar]
- Poletto, M. Lignin: Trends and Applications; BoD—Books on Demand: Norderstedt, Germany, 2018; ISBN 978-953-51-3901-0. [Google Scholar]
- Pandey, M.P.; Kim, C.S. Lignin Depolymerization and Conversion: A Review of Thermochemical Methods. Chem. Eng. Technol. 2011, 34, 29–41. [Google Scholar] [CrossRef]
- Holladay, J.E.; Bozell, J.J.; White, J.F.; Johnson, D. Results of Screening for Potential Candidates from Biorefinery Lignin. In Top Value Added Candidates Biomass; U.S. Department of Energy: Washington, DC, USA, 2007; pp. 53–55. [Google Scholar]
- Sinha, S.; Jhalani, A.; Ravi, M.R.; Ray, A. Modelling of Pyrolysis in Wood: A Review. SESI J. 2000, 10, 41–62. [Google Scholar]
- Guan, Q.; Mao, T.; Zhang, Q.; Miao, R.; Ning, P.; Gu, J.; Tian, S.; Chen, Q.; Chai, X.-S. Catalytic Gasification of Lignin with Ni/Al2O3–SiO2 in Sub/Supercritical Water. J. Supercrit. Fluids 2014, 95, 413–421. [Google Scholar] [CrossRef]
- Barati, M.; Babatabar, M.; Tavasoli, A.; Dalai, A.K.; Das, U. Hydrogen Production via Supercritical Water Gasification of Bagasse Using Unpromoted and Zinc Promoted Ru/γ-Al2O3 Nanocatalysts. Fuel Process. Technol. 2014, 123, 140–148. [Google Scholar] [CrossRef]
- Safari, F.; Tavasoli, A.; Ataei, A.; Choi, J.-K. Hydrogen and Syngas Production from Gasification of Lignocellulosic Biomass in Supercritical Water Media. Int. J. Recycl. Org. Waste Agric. 2015, 4, 121–125. [Google Scholar] [CrossRef]
- Reddy, S.N.; Nanda, S.; Dalai, A.K.; Kozinski, J.A. Supercritical Water Gasification of Biomass for Hydrogen Production. Int. J. Hydrog. Energy 2014, 39, 6912–6926. [Google Scholar] [CrossRef]
- Guo, L.J.; Lu, Y.J.; Zhang, X.M.; Ji, C.M.; Guan, Y.; Pei, A.X. Hydrogen Production by Biomass Gasification in Supercritical Water: A Systematic Experimental and Analytical Study. Catal. Today 2007, 129, 275–286. [Google Scholar] [CrossRef]
- Saxena, R.C.; Seal, D.; Kumar, S.; Goyal, H.B. Thermo-Chemical Routes for Hydrogen Rich Gas from Biomass: A Review. Renew. Sustain. Energy Rev. 2008, 12, 1909–1927. [Google Scholar] [CrossRef]
- Calzavara, Y.; Joussot-Dubien, C.; Boissonnet, G.; Sarrade, S. Evaluation of Biomass Gasification in Supercritical Water Process for Hydrogen Production. Energy Convers. Manag. 2005, 46, 615–631. [Google Scholar] [CrossRef]
- Resende, F.L.P.; Savage, P.E. Kinetic Model for Noncatalytic Supercritical Water Gasification of Cellulose and Lignin. AIChE J. 2010, 56, 2412–2420. [Google Scholar] [CrossRef]
- Pinkard, B.R.; Gorman, D.J.; Tiwari, K.; Rasmussen, E.G.; Kramlich, J.C.; Reinhall, P.G.; Novosselov, I.V. Supercritical Water Gasification: Practical Design Strategies and Operational Challenges for Lab-Scale, Continuous Flow Reactors. Heliyon 2019, 5, e01269. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Oshima, Y.; Matsumura, Y. Gasification of Biomass Model Compounds and Real Biomass in Supercritical Water. Biomass Bioenergy 2004, 26, 71–78. [Google Scholar] [CrossRef]
- Yoshida, T.; Matsumura, Y. Gasification of Cellulose, Xylan, and Lignin Mixtures in Supercritical Water. Ind. Eng. Chem. Res. 2001, 40, 5469–5474. [Google Scholar] [CrossRef]
- Van Duin, A.C.T.; Dasgupta, S.; Lorant, F.; Goddard, W.A. ReaxFF: A Reactive Force Field for Hydrocarbons. J. Phys. Chem. A 2001, 105, 9396–9409. [Google Scholar] [CrossRef]
- Jin, H.; Wu, Y.; Guo, L.; Su, X. Molecular Dynamic Investigation on Hydrogen Production by Polycyclic Aromatic Hydrocarbon Gasification in Supercritical Water. Int. J. Hydrog. Energy 2016, 41, 3837–3843. [Google Scholar] [CrossRef]
- Jin, H.; Wu, Y.; Zhu, C.; Guo, L.; Huang, J. Molecular Dynamic Investigation on Hydrogen Production by Furfural Gasification in Supercritical Water. Int. J. Hydrog. Energy 2016, 41, 16064–16069. [Google Scholar] [CrossRef]
- Jin, H.; Chen, B.; Zhao, X.; Cao, C. Molecular Dynamic Simulation of Hydrogen Production by Catalytic Gasification of Key Intermediates of Biomass in Supercritical Water. J. Energy Resour. Technol. 2017, 140. [Google Scholar] [CrossRef]
- Rismiller, S.C.; Groves, M.M.; Meng, M.; Dong, Y.; Lin, J. Water Assisted Liquefaction of Lignocellulose Biomass by ReaxFF Based Molecular Dynamic Simulations. Fuel 2018, 215, 835–843. [Google Scholar] [CrossRef]
- Zheng, M.; Wang, Z.; Li, X.; Qiao, X.; Song, W.; Guo, L. Initial Reaction Mechanisms of Cellulose Pyrolysis Revealed by ReaxFF Molecular Dynamics. Fuel 2016, 177, 130–141. [Google Scholar] [CrossRef]
- Zhang, T.; Li, X.; Qiao, X.; Zheng, M.; Guo, L.; Song, W.; Lin, W. Initial Mechanisms for an Overall Behavior of Lignin Pyrolysis through Large-Scale ReaxFF Molecular Dynamics Simulations. Energy Fuels 2016, 30, 3140–3150. [Google Scholar] [CrossRef]
- Zhang, T.; Li, X.; Guo, L.; Guo, X. Reaction Mechanisms in Pyrolysis of Hardwood, Softwood, and Kraft Lignin Revealed by ReaxFF MD Simulations. Energy Fuels 2019, 33, 11210–11225. [Google Scholar] [CrossRef]
- Li, H.; Xu, B.; Jin, H.; Luo, K.; Fan, J. Molecular Dynamics Investigation on the Lignin Gasification in Supercritical Water. Fuel Process. Technol. 2019, 192, 203–209. [Google Scholar] [CrossRef]
- Han, Y.; Chen, F.; Ma, T.; Gong, H.; Al-Shwafy, K.W.A.; Li, W.; Zhang, J.; Zhang, M. Size Effect of a Ni Nanocatalyst on Supercritical Water Gasification of Lignin by Reactive Molecular Dynamics Simulations. Ind. Eng. Chem. Res. 2019, 58, 23014–23024. [Google Scholar] [CrossRef]
- Liu, X.; Wang, T.; Chu, J.; He, M.; Li, Q.; Zhang, Y. Understanding Lignin Gasification in Supercritical Water Using Reactive Molecular Dynamics Simulations. Renew. Energy 2020, 161, 858–866. [Google Scholar] [CrossRef]
- Zakzeski, J.; Bruijnincx, P.C.A.; Jongerius, A.L.; Weckhuysen, B.M. The Catalytic Valorization of Lignin for the Production of Renewable Chemicals. Chem. Rev. 2010, 110, 3552–3599. [Google Scholar] [CrossRef]
- Ponnuchamy, V.; Gordobil, O.; Diaz, R.H.; Sandak, A.; Sandak, J. Fractionation of Lignin Using Organic Solvents: A Combined Experimental and Theoretical Study. Int. J. Biol. Macromol. 2020. [Google Scholar] [CrossRef]
- Schmidt, M.W.; Baldridge, K.K.; Boatz, J.A.; Elbert, S.T.; Gordon, M.S.; Jensen, J.H.; Koseki, S.; Matsunaga, N.; Nguyen, K.A.; Su, S.; et al. General Atomic and Molecular Electronic Structure System. J. Comput. Chem. 1993, 14, 1347–1363. [Google Scholar] [CrossRef]
- Zhang, Y.; He, H.; Dong, K.; Fan, M.; Zhang, S. A DFT Study on Lignin Dissolution in Imidazolium-Based Ionic Liquids. RSC Adv. 2017, 7, 12670–12681. [Google Scholar] [CrossRef]
- Ju, Z.; Xiao, W.; Yao, X.; Tan, X.; Simmons, B.A.; Sale, K.L.; Sun, N. Theoretical Study on the Microscopic Mechanism of Lignin Solubilization in Keggin-Type Polyoxometalate Ionic Liquids. Phys. Chem. Chem. Phys. 2020, 22, 2878–2886. [Google Scholar] [CrossRef]
- Melián-Rodríguez, M.; Saravanamurugan, S.; Meier, S.; Kegnæs, S.; Riisager, A. Ru-Catalyzed Oxidative Cleavage of Guaiacyl Glycerol-β-Guaiacyl Ether-a Representative β-O-4 Lignin Model Compound. Catalysts 2019, 9, 832. [Google Scholar] [CrossRef]
- Zhu, Y.; Han, Z.; Fu, L.; Liu, C.; Zhang, D. Cleavage of the β–O–4 Bond in a Lignin Model Compound Using the Acidic Ionic Liquid 1-H-3-Methylimidazolium Chloride as Catalyst: A DFT Mechanistic Study. J. Mol. Model. 2018, 24, 322. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhong, W.; Wu, K.; Wei, G.; Hu, Z.; Zheng, W.; Ruan, H.; Zhang, H.; Xiao, R. Catalytic Pyrolysis Mechanism of β-O-4 Type of Lignin Dimer: The Role of H Proton. Energy Fuels 2021, 35, 575–582. [Google Scholar] [CrossRef]
- Morales, G.; Iglesias, J.; Melero, J.A. Sustainable Catalytic Conversion of Biomass for the Production of Biofuels and Bioproducts. Catalysts 2020, 10, 581. [Google Scholar] [CrossRef]
- Drage, T.C.; Vane, C.H.; Abbott, G.D. The Closed System Pyrolysis of β-O-4 Lignin Substructure Model Compounds. Org. Geochem. 2002, 33, 1523–1531. [Google Scholar] [CrossRef]
- He, T.; Zhang, Y.; Zhu, Y.; Wen, W.; Pan, Y.; Wu, J.; Wu, J. Pyrolysis Mechanism Study of Lignin Model Compounds by Synchrotron Vacuum Ultraviolet Photoionization Mass Spectrometry. Energy Fuels 2016, 30, 2204–2208. [Google Scholar] [CrossRef]
- Hu, B.; Zhang, B.; Xie, W.; Jiang, X.; Liu, J.; Lu, Q. Recent Progress in Quantum Chemistry Modeling on the Pyrolysis Mechanisms of Lignocellulosic Biomass. Energy Fuels 2020, 34, 10384–10440. [Google Scholar] [CrossRef]
- Martínez, L.; Andrade, R.; Birgin, E.G.; Martínez, J.M. PACKMOL: A package for building initial configurations for molecular dynamics simulations. J. Comput. Chem. 2009, 30, 2157–2164. [Google Scholar] [CrossRef]
- Chenoweth, K.; van Duin, A.C.T.; Goddard, W.A. ReaxFF Reactive Force Field for Molecular Dynamics Simulations of Hydrocarbon Oxidation. J. Phys. Chem. A 2008, 112, 1040–1053. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Cao, L.; Chin, C.-H.; Ren, H.; Zhang, J.Z.H.; Zhu, T. ReacNetGenerator: An Automatic Reaction Network Generator for Reactive Molecular Dynamics Simulations. Phys. Chem. Chem. Phys. 2020, 22, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, H. Lignin Pyrolysis Reactions. J. Wood Sci. 2017, 63, 117–132. [Google Scholar] [CrossRef]
- McGinty, D.; Letizia, C.S.; Api, A.M. Fragrance Material Review on 1,3-Benzodioxole-5-Propanol, α-Methyl-, 5-Acetate. Food Chem. Toxicol. 2012, 50, S330–S332. [Google Scholar] [CrossRef]
- Pingali, S.R.K.; Jursic, B.S. Microwave-Assisted Synthesis of 1,3-Benzodioxole Derivatives from Catechol and Ketones or Aldehydes. Tetrahedron Lett. 2011, 52, 4371–4374. [Google Scholar] [CrossRef]
- Zhu, C.; Guo, L.; Jin, H.; Ou, Z.; Wei, W.; Huang, J. Gasification of Guaiacol in Supercritical Water: Detailed Reaction Pathway and Mechanisms. Int. J. Hydrog. Energy 2018, 43, 14078–14086. [Google Scholar] [CrossRef]
- Cao, C.; Xie, Y.; Li, L.; Wei, W.; Jin, H.; Wang, S.; Li, W. Supercritical Water Gasification of Lignin and Cellulose Catalyzed with Co-Precipitated CeO2–ZrO2. Energy Fuels 2021. [Google Scholar] [CrossRef]
- Miliotti, E.; Dell’Orco, S.; Lotti, G.; Rizzo, A.M.; Rosi, L.; Chiaramonti, D. Lignocellulosic Ethanol Biorefinery: Valorization of Lignin-Rich Stream through Hydrothermal Liquefaction. Energies 2019, 12, 723. [Google Scholar] [CrossRef]
- Okuda, K.; Umetsu, M.; Takami, S.; Adschiri, T. Disassembly of Lignin and Chemical Recovery—Rapid Depolymerization of Lignin without Char Formation in Water–Phenol Mixtures. Fuel Process. Technol. 2004, 85, 803–813. [Google Scholar] [CrossRef]
- Toledano, A.; Serrano, L.; Labidi, J. Improving Base Catalyzed Lignin Depolymerization by Avoiding Lignin Repolymerization. Fuel 2014, 116, 617–624. [Google Scholar] [CrossRef]
- Chakar, F.S.; Ragauskas, A.J. Review of Current and Future Softwood Kraft Lignin Process Chemistry. Ind. Crop. Prod. 2004, 20, 131–141. [Google Scholar] [CrossRef]

| H2O | → | H˙ | + | HO˙ | ||
| 2 H˙ | → | H2 | ||||
| HCOOH | → | HCOO˙ | + | H˙ | ||
| HCOO˙ | → | CO2 | + | H˙ | ||
| HCOO˙ | → | CO | + | HO˙ | ||
| HCOO˙ | + | H˙ | → | CO2 | + | H2 |
| HCOO˙ | + | OH˙ | → | CO2 | + | H2O |
| H2O2 | → | HOO˙ | + | H˙ | ||
| H2O2 | → | O2 | + | H2 | ||
| H2O2 | → | 2 HO˙ | ||||
| CH4 | + | OH˙ | → | CH3˙ | + | H2O |
| CH3˙ | + | H˙ | → | CH2˙˙ | + | H2 |
| CH2˙˙ | → | C2H4 | ||||
| CH3˙ | + | H2 | → | CH4 | + | H˙ |
| CH3˙ | + | H2O | → | CH3OH | + | H˙ |
| CH3OH | + | HO˙ | → | [CH2OH] ˙ | + | H2O |
| CH3OH | + | H˙ | → | [CH2OH] ˙ | + | H2 |
| [CH2OH] ˙ | + | H2 | → | CH4 | + | HO˙ |
| [CH2OH] ˙ | + | H2O | → | CH3O˙ | + | HO˙ |
| CH3O˙ | + | H˙ | → | CO | + | 2H2 |
| HO-C≡C-OH | + | 4 H˙ | → | ˙C≡C˙ | + | 2 H2O |
| ˙C≡C˙ | + | 2 H˙ | → | HC≡CH | ||
| HC≡CH | + | 2 H˙ | → | H2C=CH2 | ||
| H2C=CH2 | + | 4 H˙ | → | 2 CH4 | ||
| CH4 | + | H2O | → | CO | + | 6 H2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ponnuchamy, V.; Sandak, J.; Sandak, A. Revealing of Supercritical Water Gasification Process of Lignin by Reactive Force Field Molecular Dynamics Simulations. Processes 2021, 9, 714. https://doi.org/10.3390/pr9040714
Ponnuchamy V, Sandak J, Sandak A. Revealing of Supercritical Water Gasification Process of Lignin by Reactive Force Field Molecular Dynamics Simulations. Processes. 2021; 9(4):714. https://doi.org/10.3390/pr9040714
Chicago/Turabian StylePonnuchamy, Veerapandian, Jakub Sandak, and Anna Sandak. 2021. "Revealing of Supercritical Water Gasification Process of Lignin by Reactive Force Field Molecular Dynamics Simulations" Processes 9, no. 4: 714. https://doi.org/10.3390/pr9040714
APA StylePonnuchamy, V., Sandak, J., & Sandak, A. (2021). Revealing of Supercritical Water Gasification Process of Lignin by Reactive Force Field Molecular Dynamics Simulations. Processes, 9(4), 714. https://doi.org/10.3390/pr9040714