Effect of Drying on Nutritional Composition of Atlantic Sea Cucumber (Cucumaria frondosa) Viscera Derived from Newfoundland Fisheries
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Samples
2.2.1. Air Drying
2.2.2. Freeze Drying
2.3. Proximate Analysis
2.4. Total Lipid Class and Fatty Acid Composition
2.4.1. Lipid Extraction
2.4.2. Lipid Class Determination
2.4.3. Preparation of Fatty Acid Methyl Esters (FAMEs)
2.4.4. Fatty Acid Composition Analysis
2.4.5. Separation of Phospholipids
2.4.6. Preparation of FAMEs of Phospholipids and Fatty Acid Composition Analysis
2.5. Amino Acid Analysis
2.5.1. Hydrolysis of Dried SCV
2.5.2. Derivatization and Measurement of Amino Acids
2.6. Statistical Analysis
3. Results and Discussion
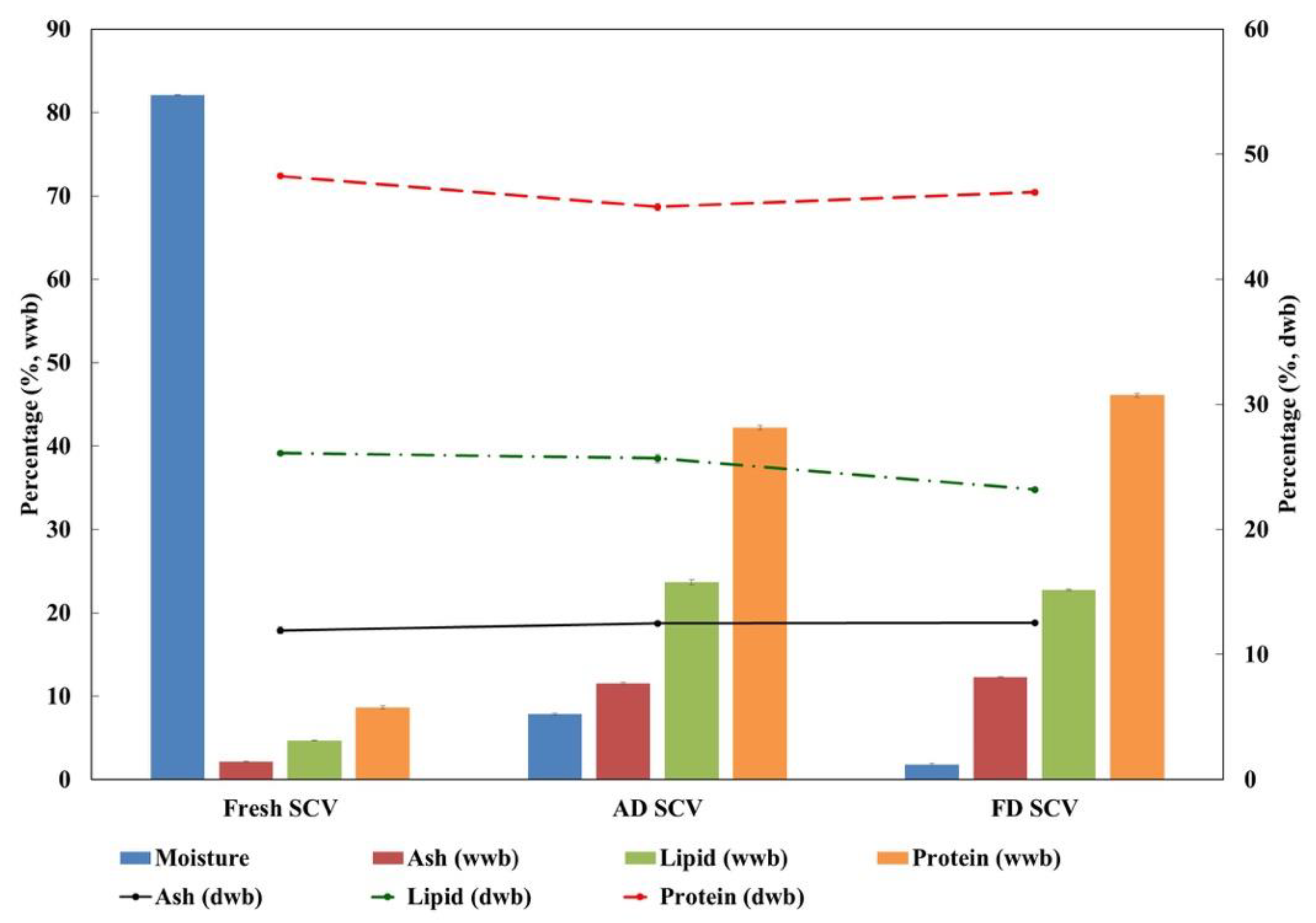
3.1. Proximate Analysis
3.2. Total Lipid Class
3.3. Fatty Acid Composition
3.3.1. Total Fatty Acid Composition
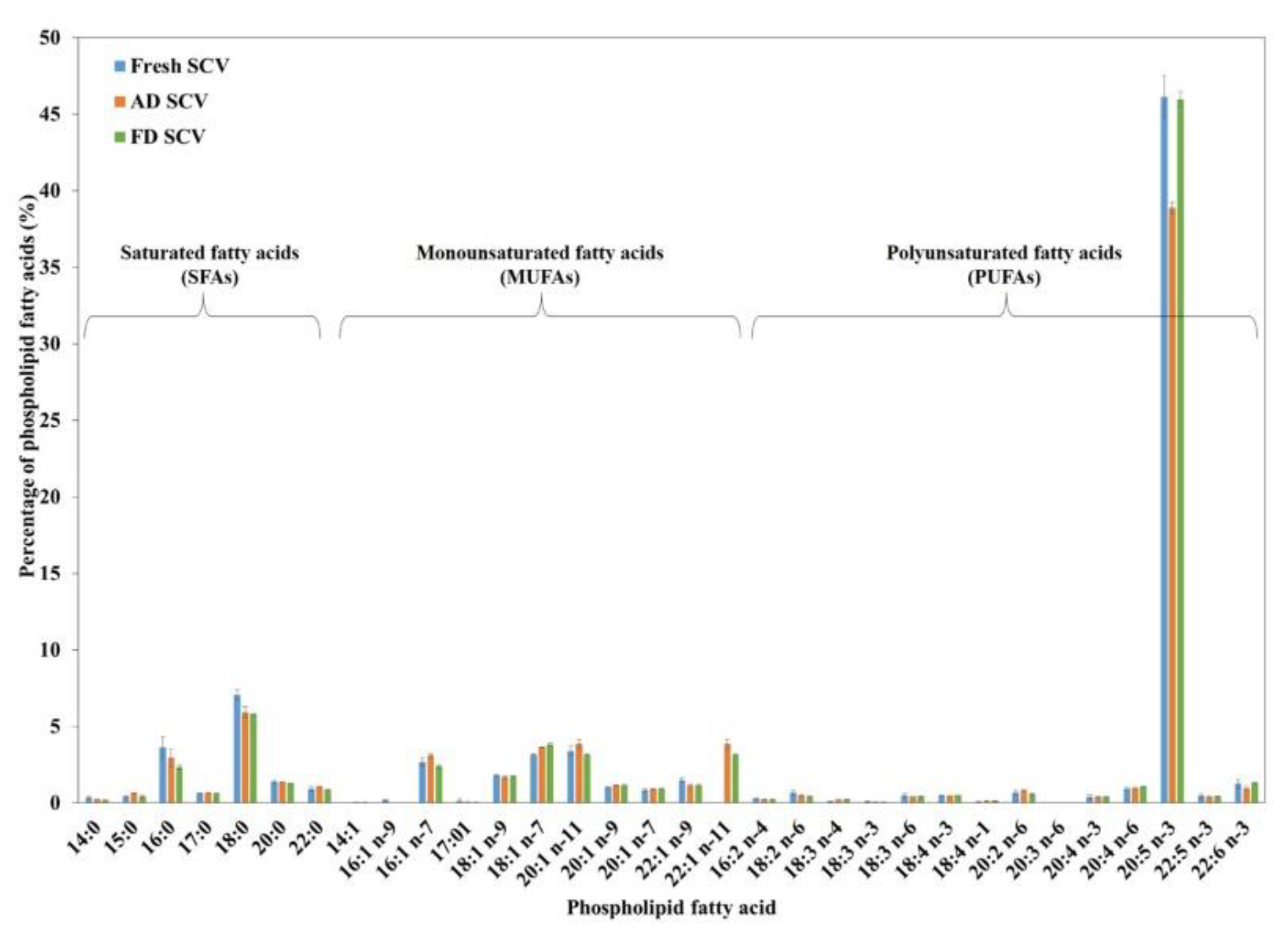
3.3.2. Phospholipid Fatty Acid Composition
3.4. Total Amino Acid Composition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Conand, C. Tropical sea cucumber fisheries: Changes during the last decade. Mar. Pollut. Bull. 2018, 133, 590–594. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Hu, C.; Fan, S. Chemical composition and nutritional quality of sea cucumbers. J. Sci. Food Agric. 2010, 90, 2469–2474. [Google Scholar] [CrossRef] [PubMed]
- Hossain, A.; Dave, D.; Shahidi, F. Northern sea cucumber (Cucumaria frondosa): A potential candidate for functional food, nutraceutical, and pharmaceutical sector. Mar. Drugs 2020, 18, 274. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Feng, W.; Hu, S.; Liang, S.; An, N.; Mao, Y. Bioactive compounds of sea cucumbers and their therapeutic effects. Chin. J. Oceanol. Limnol. 2016, 34, 549–558. [Google Scholar] [CrossRef]
- Pangestuti, R.; Arifin, Z. Medicinal and health benefit effects of functional sea cucumbers. J. Tradit. Complement. Med. 2018, 8, 341–351. [Google Scholar] [CrossRef]
- Tridge Sea Cucumber. Available online: https://www.tridge.com/intelligences/sea-cucumber/production (accessed on 18 August 2020).
- Hamel, J.-F.; Mercier, A. Population status, fisheries and trade of sea cucumbers in temperate areas of the Northern Hemisphere. Sea Cucumbers A Glob. Rev. Fish. Trade 2008, 516, 257–291. [Google Scholar]
- Fisheries and Oceans Canada. Fisheries and Oceans Canada Sea Cucumber Stock Status Update in NAFO Subdivision 3PS; Fisheries and Oceans Canada: St. John’s, NL, Canada, 2018. [Google Scholar]
- Zhang, Y.; Chang, H. Research progress on the structural components and function of sea cucumber viscera and their application. Sci. Technol. Food Ind. 2014, 13, 382–386. [Google Scholar]
- Mamelona, J.; Saint-Louis, R.; Pelletier, É. Proximate composition and nutritional profile of by-products from green urchin and Atlantic sea cucumber processing plants. Int. J. Food Sci. Technol. 2010, 45, 2119–2126. [Google Scholar] [CrossRef]
- Hou, Y.; Shavandi, A.; Carne, A.; Bekhit, A.A.; Ng, T.B.; Cheung, R.C.; Bekhit, A.E. Marine shells: Potential opportunities for extraction of functional and health-promoting materials. Crit. Rev. Environ. Sci. Technol. 2016, 46, 1047–1116. [Google Scholar] [CrossRef]
- Shavandi, A.; Hou, Y.; Carne, A.; McConnell, M.; Bekhit, A.E.D.A. Marine waste utilization as a source of functional and health compounds. In Advances in Food and Nutrition Research; Toldra, F., Ed.; Academic Press: Cambridge, MA, USA, 2019; Volume 87, pp. 187–254. ISBN 9780128160497. [Google Scholar]
- Mamelona, J.; Pelletier, É. Producing high antioxidant activity extracts from Echinoderm byproducts by using pressured liquid extraction. Biotechnology 2010, 9, 523–528. [Google Scholar] [CrossRef][Green Version]
- Zhou, X.; Zhou, D.-Y.; Yin, F.-W.; Song, L.; Liu, Y.-X.; Xie, H.-K.; Gang, K.-Q.; Zhu, B.-W.; Shahidi, F. Glycerophospholipids in sea cucumber (Stichopus japonicus) and its processing by-products serve as bioactives and functional food ingredients. J. Food Bioact. 2018, 1, 134–142. [Google Scholar] [CrossRef]
- Mamelona, J.; Pelletier, É.; Girard-Lalancette, K.; Legault, J.; Karboune, S.; Kermasha, S. Quantification of phenolic contents and antioxidant capacity of Atlantic sea cucumber, Cucumaria frondosa. Food Chem. 2007, 104, 1040–1047. [Google Scholar] [CrossRef]
- Zhong, C.; Sun, L.C.; Yan, L.J.; Lin, Y.C.; Liu, G.M.; Cao, M.J. Production, optimisation and characterisation of angiotensin converting enzyme inhibitory peptides from sea cucumber (Stichopus japonicus) gonad. Food Funct. 2018, 9, 594–603. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Wu, H.T.; Zhu, B.W.; Dong, X.P.; Zhang, M.M.; Li, Y.L. Identification of antioxidative oligopeptides derived from autolysis hydrolysates of sea cucumber (Stichopus japonicus) guts. Eur. Food Res. Technol. 2012, 234, 895–904. [Google Scholar] [CrossRef]
- Yan, M.; Tao, H.; Qin, S. Effect of enzyme type on the antioxidant activities and functional properties of enzymatic hydrolysates from sea cucumber (Cucumaria frondosa) viscera. J. Aquat. Food Prod. Technol. 2016, 25, 940–952. [Google Scholar] [CrossRef]
- Moses, J.A.; Norton, T.; Alagusundaram, K.; Tiwari, B.K. Novel drying techniques for the food industry. Food Eng. Rev. 2014, 6, 43–55. [Google Scholar] [CrossRef]
- Guiné, R.P.F. The drying of foods and its effect on the physical-chemical, sensorial and nutritional Properties. ETP Int. J. Food Eng. 2018, 4, 93–100. [Google Scholar] [CrossRef]
- Oetjen, G.-W.; Haseley, P. Freeze-Drying, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2004. [Google Scholar]
- Mejia-Meza, E.I.; Yanez, J.A.; Davies, N.M.; Rasco, B.; Younce, F.; Remsberg, C.M.; Clary, C. Improving nutritional value of dried blueberries (Vaccinium corymbosum L.) combining microwave-vacuum, hot-air drying and freeze drying technologies. Int. J. Food Eng. 2008, 4. [Google Scholar] [CrossRef]
- Asami, D.K.; Hong, Y.J.; Barrett, D.M.; Mitchell, A.E. Comparison of the total phenolic and ascorbic acid content of freeze-dried and air-dried marionberry, strawberry, and corn grown using conventional, organic, and sustainable agricultural practices. J. Agric. Food Chem. 2003, 51, 1237–1241. [Google Scholar] [CrossRef]
- Barbosa, J.; Borges, S.; Amorim, M.; Pereira, M.J.; Oliveira, A.; Pintado, M.E.; Teixeira, P. Comparison of spray drying, freeze drying and convective hot air drying for the production of a probiotic orange powder. J. Funct. Foods 2015, 17, 340–351. [Google Scholar] [CrossRef]
- Ratti, C. Hot air and freeze-drying of high-value foods: A review. J. Food Eng. 2001, 49, 311–319. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists. Official Methods of Analysis of AOAC International, 17th ed.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Parrish, C.C. Determination of total lipid, lipid classes, and fatty acids in aquatic samples. In Lipids in Freshwater Ecosystems; Arts, M.T., Wainman, B.C., Eds.; Springer: New York, NY, USA, 1999; pp. 4–20. [Google Scholar]
- Hooper, T.; Parrish, C.C. Profiling neutral lipids in individual fish larvae by using short-column gas chromatography with flame ionization detection. Limnol. Oceanogr. Methods 2009, 7, 411–428. [Google Scholar] [CrossRef]
- Parrish, C.C. Separation of aquatic lipid classes by chromarod thin-layer chromatography with measurement by latroscan flame ionization detection. Can. J. Fish. Aquat. Sci. 1987, 44, 722–731. [Google Scholar] [CrossRef]
- Fountoulakis, M.; Lahm, H.-W. Hydrolysis and amino acid composition analysis of proteins. J. Chromatogr. A 1998, 826, 109–134. [Google Scholar] [CrossRef]
- Stenerson, K.K. The derivatization and analysis of amino acids by GC-MS. Rep. US 2011, 25, 1–3. [Google Scholar]
- Gianasi, B.L.; Parrish, C.C.; Hamel, J.F.; Mercier, A. Influence of diet on growth, reproduction and lipid and fatty acid composition in the sea cucumber Cucumaria frondosa. Aquac. Res. 2017, 48, 3413–3432. [Google Scholar] [CrossRef]
- Dave, D.; Liu, Y.; Clark, L.; Dave, N.; Trenholm, S.; Westcott, J. Availability of marine collagen from Newfoundland fisheries and aquaculture waste resources. Bioresour. Technol. Rep. 2019, 100271. [Google Scholar] [CrossRef]
- Zhong, Y.; Khan, M.A.; Shahidi, F. Compositional characteristics and antioxidant properties of fresh and processed sea cucumber (Cucumaria frondosa). J. Agric. Food Chem. 2007, 55, 1188–1192. [Google Scholar] [CrossRef]
- Karlsdottir, M.; Arason, S.; Thorarinsdottir, K.; Nguyen, M.V.; Kristinsson, H. Lipid degradation of cod liver during frozen storage as influenced by temperature, packaging method, and seasonal variation. J. Aquat. Food Prod. Technol. 2016, 25, 802–810. [Google Scholar] [CrossRef]
- Vaidya, H.; Cheema, S.K. Sea cucumber and blue mussel: New sources of phospholipid enriched omega-3 fatty acids with a potential role in 3T3-L1 adipocyte metabolism. Food Funct. 2014, 5, 3287–3295. [Google Scholar] [CrossRef] [PubMed]
- Swanson, D.; Block, R.; Mousa, S.A. Omega-3 fatty acids EPA and DHA: Health benefits throughout life. J. Adv. Nutr. 2012, 3, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Dave, D.; Ramakrishnan, V.V.; Trenholm, S.; Manuel, H.; Pohling, J.; Murphy, W. Marine oils as potential feedstock for biodiesel production: Physicochemical characterization. J. Bioprocess. Biotech. 2014, 4, 10001678. [Google Scholar] [CrossRef]
- Simopoulos, A.P. An increase in the Omega-6/Omega-3 fatty acid ratio increases the risk for obesity. Nutrients 2016, 8, 128. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Evolutionary aspects of diet: The omega-6/omega-3 ratio and the brain. Mol. Neurobiol. 2011, 44, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Dinicolantonio, J.J.; O’Keefe, J.H. Importance of maintaining a low omega-6/omega-3 ratio for reducing inflammation. Open Heart 2018, 5, 1–4. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef]
- Burri, L.; Hoem, N.; Banni, S.; Berge, K. Marine omega-3 phospholipids: Metabolism and biological activities. Int. J. Mol. Sci. 2012, 13, 15401–15419. [Google Scholar] [CrossRef]
- Küllenberg, D.; Taylor, L.A.; Schneider, M.; Massing, U. Health effects of dietary phospholipids. Lipids Health Dis. 2012, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, X.; Zhang, T.; Wang, C.; Huang, Z.; Luo, X.; Deng, Y. A review on phospholipids and their main applications in drug delivery systems. Asian J. Pharm. Sci. 2015, 10, 81–98. [Google Scholar] [CrossRef]
- Ahmmed, M.K.; Ahmmed, F.; Tian, H.; Carne, A.; Bekhit, A.E.D. Marine omega-3 (n-3) phospholipids: A comprehensive review of their properties, sources, bioavailability, and relation to brain health. Compr. Rev. Food Sci. Food Saf. 2020, 19, 64–123. [Google Scholar] [CrossRef]
- Araujo, P.; Zhu, H.; Breivik, J.F.; Hjelle, J.I.; Zeng, Y. Determination and structural elucidation of triacylglycerols in krill oil by chromatographic techniques. Lipids 2014, 49, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Ramprasath, V.R.; Eyal, I.; Zchut, S.; Jones, P.J. Enhanced increase of omega-3 index in healthy individuals with response to 4-week n-3 fatty acid supplementation from krill oil versus fish oil. Lipids Health Dis. 2013, 12, 1–11. [Google Scholar] [CrossRef]
- Mamelona, J.; Saint-Louis, R.; Pelletier, É. Nutritional composition and antioxidant properties of protein hydrolysates prepared from echinoderm byproducts. Int. J. Food Sci. Technol. 2010, 45, 147–154. [Google Scholar] [CrossRef]
- Sun, L.; Xia, W. Effect of steam cooking on muscle and protein heatdenature of tuna. Food Mach. 2010, 26, 22–25. [Google Scholar]
- Deng, Y.; Luo, Y.; Wang, Y.; Zhao, Y. Effect of different drying methods on the myosin structure, amino acid composition, protein digestibility and volatile profile of squid fillets. Food Chem. 2015, 171, 168–176. [Google Scholar] [CrossRef]
- Kim, B.-S.; Oh, B.-J.; Lee, J.-H.; Yoon, Y.S.; Lee, H.-I. Effects of various drying methods on physicochemical characteristics and textural features of yellow croaker (Larimichthys polyactis). Foods 2020, 9, 196. [Google Scholar] [CrossRef]
- Zaenuri, M.; Anggoro, S.; Kusumaningrum, H.P.S. Nutritional value of sea cucumber [Paracaudina Australis (Semper, 1868)]. Aquat. Procedia 2016, 7, 271–276. [Google Scholar] [CrossRef]
- Dutta, S.; Ray, S.; Nagarajan, K. Glutamic acid as anticancer agent: An overview. Saudi Pharm. J. 2013, 21, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Bajwa, M.A.; Zahoor, T.; Butt, T.M.; Atiq, M.; Sahi, S.T. Microbial production of L-isoleucine from different substrates using locally isolated bacteria. Int. J. Agric. Biol. 2010, 12, 668–672. [Google Scholar]
- Moorthi, P.P.; Gunasekaran, S.; Ramkumaar, G.R. Vibrational spectroscopic studies of isoleucine by quantum chemical calculations. Spectrochim. Acta—Part A Mol. Biomol. Spectrosc. 2014, 124, 365–374. [Google Scholar] [CrossRef]
- Fernstrom, J.D.; Fernstrom, M.H. Tyrosine, phenylalanine, and catecholamine synthesis and function in the brain. J. Nutr. 2007, 137, 1539S–1547S. [Google Scholar] [CrossRef]
- Meyers, S. Use of neurotransmitter precursors for treatment of depression. Altern. Med. Rev. 2000, 5, 64–71. [Google Scholar] [PubMed]
- Gundersen, R.Y.; Vaagenes, P.; Breivik, T.; Fonnum, F.; Opstad, P.K. Glycine—An important neurotransmitter and cytoprotective agent. Acta Anaesthesiol. Scand. 2005, 49, 1108–1116. [Google Scholar] [CrossRef]
- Bordbar, S.; Anwar, F.; Saari, N. High-value components and bioactives from sea cucumbers for functional foods—A review. Mar. Drugs 2011, 9, 1761–1805. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
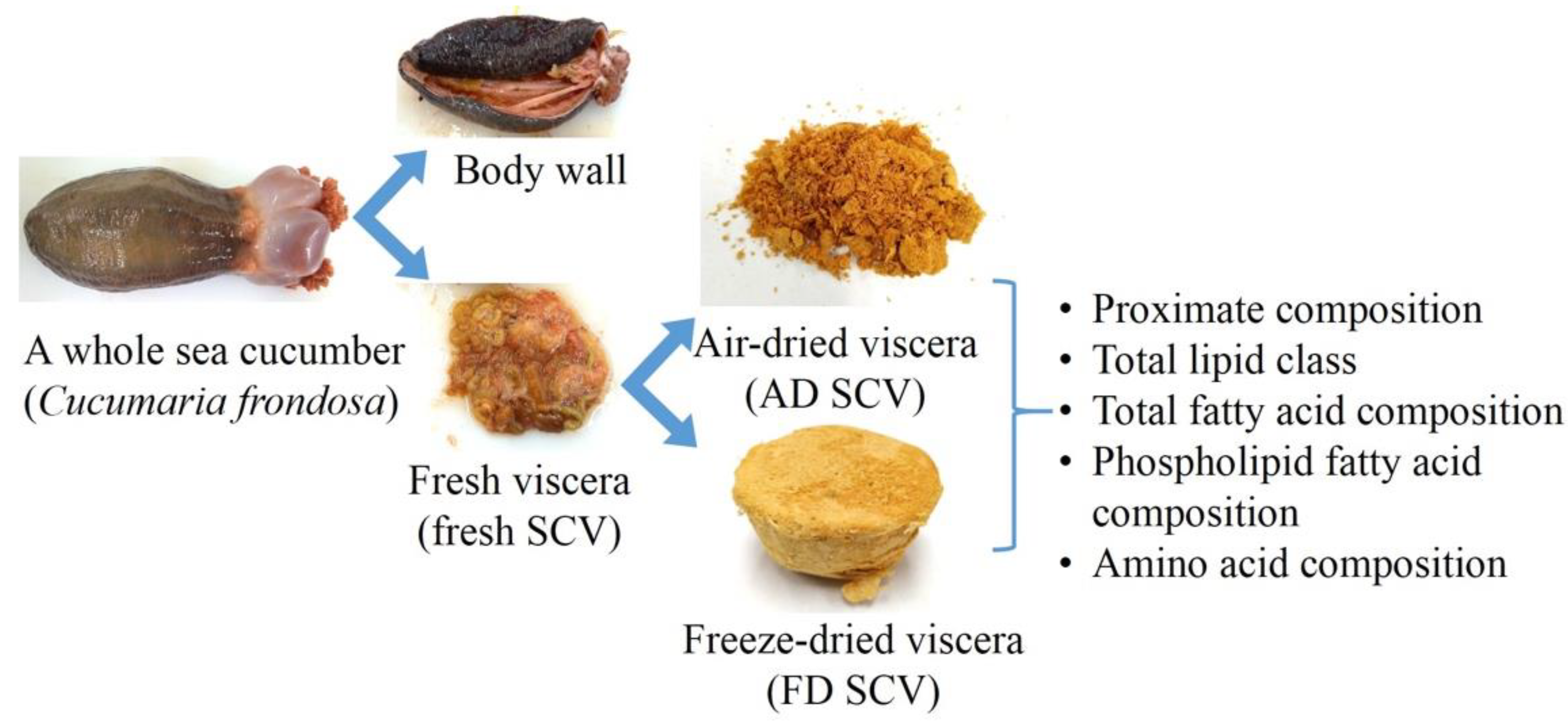



| Parameter | Fresh SCV | AD SCV | FD SCV |
|---|---|---|---|
| Percent of wet tissue (wwb, Mean ± SD) | |||
| Moisture (%) | 82.07 ± 0.03 | 7.84 ± 0.14 | 1.79 ± 0.07 |
| Ash (%) | 2.14 ± 0.05 | 11.51 ± 0.14 | 12.31 ± 0.05 |
| Lipid (%) | 4.68 ± 0.01 | 23.68 ± 0.33 | 22.77 ± 0.12 |
| Protein (%) | 8.65 ± 0.17 | 42.20 ± 0.29 | 46.12 ± 0.20 |
| Percent of dry matter (dwb, Mean ± SD) | |||
| Ash (%) | 11.95 ± 0.29 a | 12.49 ± 0.15 b | 12.53 ± 0.05 b |
| Lipid (%) | 26.12 ± 0.03 a | 25.69 ± 0.36 a | 23.19 ± 0.12 b |
| Protein (%) | 48.26 ± 0.17 a | 45.79 ± 0.31 b | 46.96 ± 0.20 a,b |
| Lipid Class | Fresh SCV (%) | AD SCV (%) | FD SCV (%) |
|---|---|---|---|
| Hydrocarbons | 0.05 ± 0.09 | 0.24 ± 0.28 | 0.30 ± 0.43 |
| Steryl esters/wax esters | 0.10 ± 0.17 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Ethyl esters | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Methyl esters | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Ethyl ketones | 0.16 ± 0.27 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Methyl ketones | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Glyceryl ethers | 26.87 ± 1.93 | 22.15 ± 0.64 | 23.14 ± 2.48 |
| Triacylglycerols | 32.93 ± 1.96 | 13.20 ± 0.90 | 26.30 ± 3.29 |
| Free fatty acids | 7.75 ± 1.21 | 28.75 ± 0.20 | 6.84 ± 0.36 |
| Alcohols | 0.00 ± 0.00 | 4.01 ± 0.35 | 0.51 ± 0.72 |
| Sterols | 1.92 ± 0.15 | 1.04 ± 0.18 | 0.86 ± 0.21 |
| Diacylglycerols | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Acetone mobile polar lipids | 3.60 ± 1.70 | 2.85 ± 2.61 | 4.91 ± 3.30 |
| Phospholipids | 26.63 ± 1.31 | 27.77 ± 3.20 | 37.13 ± 8.78 |
| Type | Isomer | Systematic Name | Fresh SCV | AD SCV | FD SCV |
|---|---|---|---|---|---|
| Saturated fatty acids (SFAs) | 14:0 | Tetradecanoic acid | 3.39 ± 0.04 | 3.66 ± 0.04 | 3.79 ± 0.09 |
| 15:0 | Pentadecanoic acid | 12.27 ± 0.10 | 13.12 ± 0.06 | 12.81 ± 0.04 | |
| 16:0 | Hexadecanoic acid | 4.90 ± 0.09 | 4.73 ± 0.17 | 4.66 ± 0.02 | |
| 17:0 | Heptadecanoic acid | 0.99 ± 0.01 | 0.55 ± 0.54 | 1.19 ± 0.02 | |
| 18:0 | Octadecanoic acid | 3.45 ± 0.03 | 3.37 ± 0.05 | 3.46 ± 0.02 | |
| 20:0 | Eicosanoic acid | 0.48± 0.01 | 0.50 ± 0.04 | 0.52 ± 0.01 | |
| 22:0 | Docosanoic acid | 0.45 ± 0.08 | 0.53 ± 0.06 | 0.54 ± 0.01 | |
| Subtotal (SFAs) | 25.93 | 26.46 | 26.97 | ||
| Monounsaturated fatty acids (MUFAs) | 14:1 | Tetradecenoic acid | 0.37 ± 0.00 | 0.40 ± 0.00 | 0.40 ± 0.00 |
| 16:1 n-9 | cis-7-Hexadecenoic acid | 0.00 ± 0.00 | 0.06 ± 0.01 | 0.00 ± 0.00 | |
| 16:1 n-7 | Hexadecenoic acid | 17.86 ± 0.05 | 18.20 ± 0.05 | 18.16 ± 0.08 | |
| 17:1 | Heptadecenoic acid | 0.33 ± 0.05 | 0.43 ± 0.14 | 0.42 ± 0.08 | |
| 18:1 n-9 | Octadecenoic acid | 2.61 ± 0.08 | 2.47 ± 0.04 | 2.59 ± 0.06 | |
| 18:1 n-7 | cis-Vaccenic acid | 2.87 ± 0.04 | 2.89 ± 0.02 | 2.94 ± 0.06 | |
| 20:1 n-11 | Gadoleic acid | 1.07 ± 0.04 | 1.14 ± 0.03 | 1.07 ± 0.03 | |
| 20:1 n-9 | Eicosenoic acid | 0.82 ± 0.03 | 0.84 ± 0.01 | 0.85 ± 0.05 | |
| 20:1 n-7 | Paullinic acid | 0.26 ± 0.00 | 0.30 ± 0.00 | 0.26 ± 0.05 | |
| 22:1 n-9 | Erucic acid | 0.81 ± 0.01 | 0.81 ± 0.14 | 0.77 ± 0.06 | |
| 22:1 n-11 | Docosenoic acid | 0.00 ± 0.00 | 1.14 ± 0.03 | 1.07 ± 0.03 | |
| Subtotal (MUFAs) | 27.01 | 28.73 | 28.60 | ||
| Polyunsaturated fatty acids (PUFAs) | 16:2 n-4 | Hexadecadienoic acid | 1.10 ± 0.00 | 1.13 ± 0.01 | 1.13 ± 0.02 |
| 18:2 n-6 | Octadecadienoic acid | 0.38 ± 0.00 | 0.34 ± 0.02 | 0.39 ± 0.05 | |
| 18:3 n-4 | Octadecatrienoic acid | 0.08 ± 0.02 | 0.13 ± 0.04 | 0.15 ± 0.00 | |
| 18:3 n-3 | 15-Octadecatrienoic acid | 0.06 ± 0.01 | 0.09 ± 0.02 | 0.10 ± 0.01 | |
| 18:3 n-6 | 12-Octadecatrienoic acid | 0.09 ± 0.02 | 0.25 ± 0.01 | 0.25 ± 0.02 | |
| 18:4 n-3 | 6,9,12,15-Octadecatetraenoic acid | 1.30 ± 0.01 | 1.31 ± 0.01 | 1.31 ± 0.03 | |
| 18:4 n-1 | Octadeca-9,11,13,15-tetraenoic acid | 0.23 ± 0.01 | 0.23 ± 0.00 | 0.23 ± 0.01 | |
| 20:2 n-6 | 11,14-Eicosadienoic acid | 0.17 ± 0.03 | 0.24± 0.03 | 0.22 ± 0.01 | |
| 20:3 n-6 | 8,11,14-Eicosatrienoic acid | 0.00 ± 0.00 | 0.00 ± 0.01 | 0.00 ± 0.01 | |
| 20:4 n-3 | 8,11,14,17-Eicosatetraenoic acid | 0.18 ± 0.07 | 0.25 ± 0.05 | 0.25 ± 0.02 | |
| 20:4 n-6 | 5,8,11,14-Eicosatetraenoic acid | 0.39 ± 0.01 | 0.42 ± 0.07 | 0.38 ± 0.01 | |
| 20:5 n-3 | Eicosapentaenoic acid (EPA) | 27.76 ± 0.07 | 28.71 ± 0.20 | 27.97 ± 0.11 | |
| 22:5 n-3 | Docosapentaenoic acid (DPA) | 0.41 ± 0.03 | 0.41 ± 0.04 | 0.38 ± 0.01 | |
| 22:6 n-3 | Docosahexaenoic acid (DHA) | 0.88 ± 0.01 | 0.87 ± 0.01 | 0.85 ± 0.00 | |
| Other PUFAs | 6.53 ± 0.27 | 5.74 ± 0.18 | 6.31 ± 0.12 | ||
| Subtotal (PUFAs) | 33.01 | 40.14 | 39.91 | ||
| Total Omega-3 PUFAs | 30.58 | 30.58 | 31.65 | ||
| Total Omega-6 PUFAs | 1.02 | 1.02 | 1.26 | ||
| Other fatty acids | 14.05 | 4.68 | 4.52 | ||
| Species | Total Omega-3 PUFAs (%) | Total Omega-6 PUFAs (%) | Omega-6 /Omega-3 | EPA (%) | Reference |
|---|---|---|---|---|---|
| Fresh SCV | 30.58 | 1.02 | 0.03 | 27.76 | The present study |
| AD SCV | 31.65 | 1.26 | 0.04 | 28.71 | |
| FD SCV | 30.85 | 1.24 | 0.04 | 27.97 | |
| Crude farmed Atlantic salmon oil | 9.93 | 15.11 | 1.52 | 4.63 | Dave et al. [38] |
| Crude seal oil | 16.27 | 2.03 | 0.12 | 7.12 | |
| Crude cod liver oil | 20.77 | 2.29 | 0.11 | 8.52 | |
| Crude wild Pacific salmon oil | 21.18 | 2.26 | 0.11 | 9.54 |
| Type | Isomer | Systematic Name | Fresh SCV | AD SCV | FD SCV |
|---|---|---|---|---|---|
| Saturated fatty acids (SFAs) | 14:0 | Tetradecanoic acid | 0.35 ± 0.11 | 0.25 ± 0.01 | 0.18 ± 0.03 |
| 15:0 | Pentadecanoic acid | 0.44 ± 0.03 | 0.65 ± 0.03 | 0.43 ± 0.07 | |
| 16:0 | Hexadecanoic acid | 3.62 ± 0.68 | 2.96 ± 0.60 | 2.34 ± 0.13 | |
| 17:0 | Heptadecanoic acid | 0.62 ± 0.03 | 0.66 ± 0.02 | 0.64 ± 0.01 | |
| 18:0 | Octadecanoic acid | 7.08 ± 0.32 | 5.93 ± 0.38 | 5.81 ± 0.02 | |
| 20:0 | Eicosanoic acid | 1.37 ± 0.12 | 1.34 ±0.02 | 1.29 ± 0.03 | |
| 22:0 | Docosanoic acid | 0.93 ± 0.18 | 1.07 ± 0.03 | 0.87 ± 0.04 | |
| Subtotal (SFAs) | 14.41 | 12.85 | 11.56 | ||
| Monounsaturated fatty acids (MUFAs) | 14:1 | Tetradecenoic acid | 0.00 ± 0.00 | 0.04 ± 0.01 | 0.03 ± 0.00 |
| 16:1 n-9 | cis-7-Hexadecenoic acid | 0.19 ± 0.06 | 0.00 ± 0.00 | 0.01 ± 0.02 | |
| 16:1 n-7 | Hexadecenoic acid | 2.68 ± 0.29 | 3.09 ± 0.13 | 2.40 ± 0.14 | |
| 17:1 | Heptadecenoic acid | 0.12 ± 0.18 | 0.07 ± 0.00 | 0.03 ± 0.00 | |
| 18:1 n-9 | Octadecenoic acid | 1.81 ± 0.10 | 1.69 ± 0.07 | 1.75 ± 0.03 | |
| 18:1 n-7 | cis-Vaccenic acid | 3.15 ± 0.07 | 3.63 ± 0.02 | 3.84 ± 0.08 | |
| 20:1 n-11 | Gadoleic acid | 3.40 ± 0.32 | 3.86 ± 0.30 | 3.15 ± 0.07 | |
| 20:1 n-9 | Eicosenoic acid | 1.03 ± 0.02 | 1.15 ± 0.02 | 1.16 ± 0.04 | |
| 20:1 n-7 | Paullinic acid | 0.84 ± 0.11 | 0.91 ± 0.03 | 0.94 ± 0.03 | |
| 22:1 n-9 | Erucic acid | 1.49 ± 0.14 | 1.82 ± 0.05 | 1.76 ± 0.06 | |
| 22:1 n-11 | Docosenoic acid | 0.00 ± 0.00 | 3.86 ± 0.30 | 3.15 ± 0.07 | |
| Subtotal (MUFAs) | 14.71 | 19.46 | 17.60 | ||
| Polyunsaturated fatty acids (PUFAs) | 16:2 n-4 | Hexadecadienoic acid | 0.28 ± 0.05 | 0.22 ± 0.01 | 0.25 ± 0.00 |
| 18:2 n-6 | Octadecadienoic acid | 0.65 ± 0.14 | 0.48 ± 0.06 | 0.44 ± 0.03 | |
| 18:3 n-4 | Octadecatrienoic acid | 0.09 ± 0.03 | 0.20 ± 0.00 | 0.24 ± 0.01 | |
| 18:3 n-3 | 15-Octadecatrienoic acid | 0.10 ± 0.03 | 0.09 ± 0.01 | 0.08 ± 0.00 | |
| 18:3 n-6 | 12-Octadecatrienoic acid | 0.50 ± 0.11 | 0.42 ± 0.00 | 0.42 ± 0.01 | |
| 18:4 n-3 | 6,9,12,15-Octadecatetraenoic acid | 0.49 ± 0.03 | 0.48 ± 0.01 | 0.52 ± 0.01 | |
| 18:4 n-1 | Octadeca-9,11,13,15-tetraenoic acid | 0.08 ± 0.03 | 0.14 ±0.01 | 0.13 ± 0.03 | |
| 20:2 n-6 | 11,14-Eicosadienoic acid | 0.67 ± 0.13 | 0.84 ± 0.02 | 0.58 ± 0.02 | |
| 20:3 n-6 | 8,11,14-Eicosatrienoic acid | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| 20:4 n-3 | 8,11,14,17-Eicosatetraenoic acid | 0.39 ± 0.15 | 0.40 ± 0.02 | 0.38 ± 0.02 | |
| 20:4 n-6 | 5,8,11,14-Eicosatetraenoic acid | 0.93 ± 0.07 | 0.97 ± 0.03 | 1.10 ± 0.02 | |
| 20:5 n-3 | Eicosapentaenoic acid (EPA) | 46.13 ± 1.39 | 38.88 ± 0.36 | 45.97 ± 0.50 | |
| 22:5 n-3 | Docosapentaenoic acid (DPA) | 0.46 ± 0.12 | 0.39 ± 0.01 | 0.42 ± 0.02 | |
| 22:6 n-3 | Docosahexaenoic acid (DHA) | 1.26 ± 0.27 | 0.97 ± 0.13 | 1.32 ± 0.01 | |
| Other PUFAs | 14.14 ± 4.74 | 10.46 ± 0.69 | 8.89 ± 0.27 | ||
| Subtotal (PUFAs) | 52.02 | 54.93 | 60.72 | ||
| Total Omega-3 PUFAs | 48.82 | 48.82 | 41.21 | ||
| Total Omega-6 PUFAs | 2.75 | 2.75 | 2.71 | ||
| Other fatty acids | 18.87 | 12.77 | 10.12 | ||
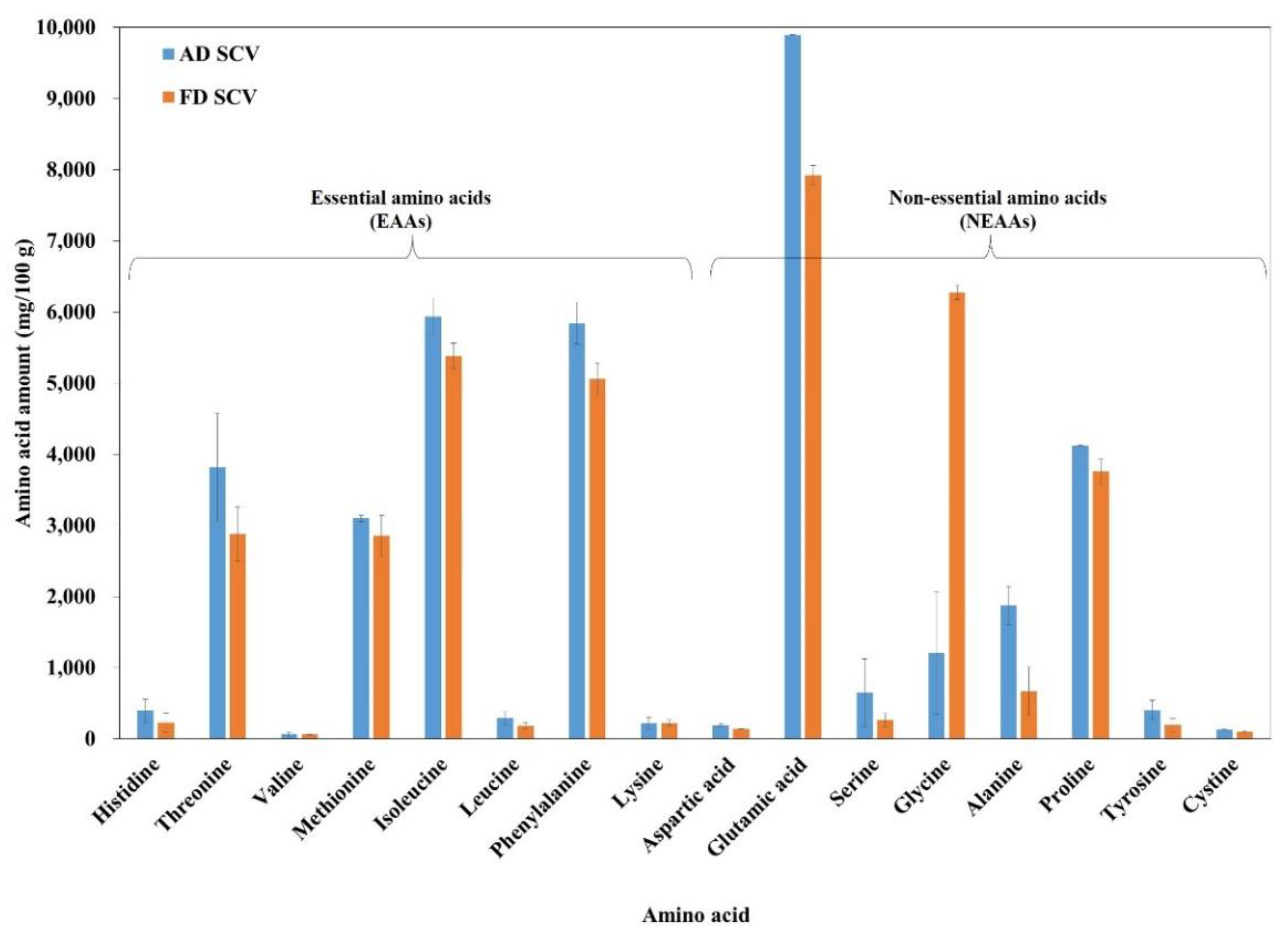
| Type | AD SCV (mg/100 g) | AD SCV (%) | FD SCV (mg/100 g) | FD SCV (%) |
|---|---|---|---|---|
| Essential amino acids (EAAs) | ||||
| Histidine | 396.18 ± 163.86 | 1.04 | 225.99 ± 135.30 | 0.62 |
| Threonine | 3815.16 ± 766.14 | 10.00 | 2878.91 ± 377.70 | 7.95 |
| Valine | 64.00 ± 35.21 | 0.17 | 62.26 ± 6.95 | 0.17 |
| Methionine | 3100.43 ± 44.01 | 8.12 | 2854.84 ± 290.10 | 7.88 |
| Isoleucine | 5938.32 ± 263.54 | 15.56 | 5385.95 ± 181.72 | 14.87 |
| Leucine | 295.86 ± 92.82 | 0.78 | 185.63 ± 44.80 | 0.51 |
| Phenylalanine | 5841.59 ± 295.02 | 15.31 | 5056.60 ± 229.27 | 13.96 |
| Lysine | 224.32 ± 82.05 | 0.59 | 223.01 ± 42.83 | 0.62 |
| Total EAAs | 19,675.86 | 51.57 | 16,873.19 | 46.58 |
| Non-essential amino acids (NEAAs) | ||||
| Aspartic acid | 191.85 ± 22.15 | 0.50 | 140.87 ± 5.67 | 0.39 |
| Glutamic acid | 9897.19 ± 4.90 | 25.93 | 7926.57 ± 136.11 | 21.89 |
| Serine | 647.22 ± 479.46 | 1.70 | 263.47 ± 91.24 | 0.73 |
| Glycine | 1210.25 ± 861.02 | 3.17 | 6275.05 ± 98.92 | 17.33 |
| Alanine | 1877.57 ± 270.81 | 4.92 | 673.43 ± 342.47 | 1.86 |
| Proline | 4121.35 ± 11.38 | 10.80 | 3755.68 ± 184.38 | 10.37 |
| Tyrosine | 407.29 ± 136.74 | 1.07 | 196.90 ± 95.39 | 0.54 |
| Cystine | 134.40 ± 4.06 | 0.35 | 105.48 ± 9.33 | 0.29 |
| Total NEAAs | 18,487.12 | 48.44 | 19,337.45 | 53.40 |
| EAAs/NEAAs | 1.06 | 0.87 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Dave, D.; Trenholm, S.; Ramakrishnan, V.V.; Murphy, W. Effect of Drying on Nutritional Composition of Atlantic Sea Cucumber (Cucumaria frondosa) Viscera Derived from Newfoundland Fisheries. Processes 2021, 9, 703. https://doi.org/10.3390/pr9040703
Liu Y, Dave D, Trenholm S, Ramakrishnan VV, Murphy W. Effect of Drying on Nutritional Composition of Atlantic Sea Cucumber (Cucumaria frondosa) Viscera Derived from Newfoundland Fisheries. Processes. 2021; 9(4):703. https://doi.org/10.3390/pr9040703
Chicago/Turabian StyleLiu, Yi, Deepika Dave, Sheila Trenholm, Vegneshwaran V. Ramakrishnan, and Wade Murphy. 2021. "Effect of Drying on Nutritional Composition of Atlantic Sea Cucumber (Cucumaria frondosa) Viscera Derived from Newfoundland Fisheries" Processes 9, no. 4: 703. https://doi.org/10.3390/pr9040703
APA StyleLiu, Y., Dave, D., Trenholm, S., Ramakrishnan, V. V., & Murphy, W. (2021). Effect of Drying on Nutritional Composition of Atlantic Sea Cucumber (Cucumaria frondosa) Viscera Derived from Newfoundland Fisheries. Processes, 9(4), 703. https://doi.org/10.3390/pr9040703







