Effect of Cryoconcentration Assisted by Centrifugation-Filtration on Bioactive Compounds and Microbiological Quality of Aqueous Maqui (Aristotelia chilensis (Mol.) Stuntz) and Calafate (Berberis microphylla G. Forst) Extracts Pretreated with High-Pressure Homogenization
Abstract
1. Introduction
2. Materials and Methods
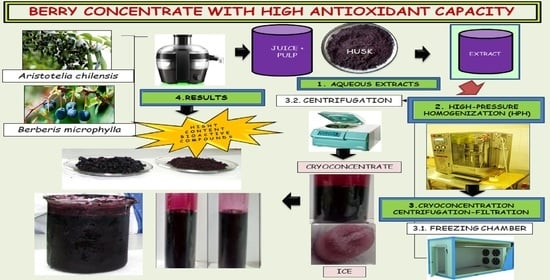
2.1. Raw Materials
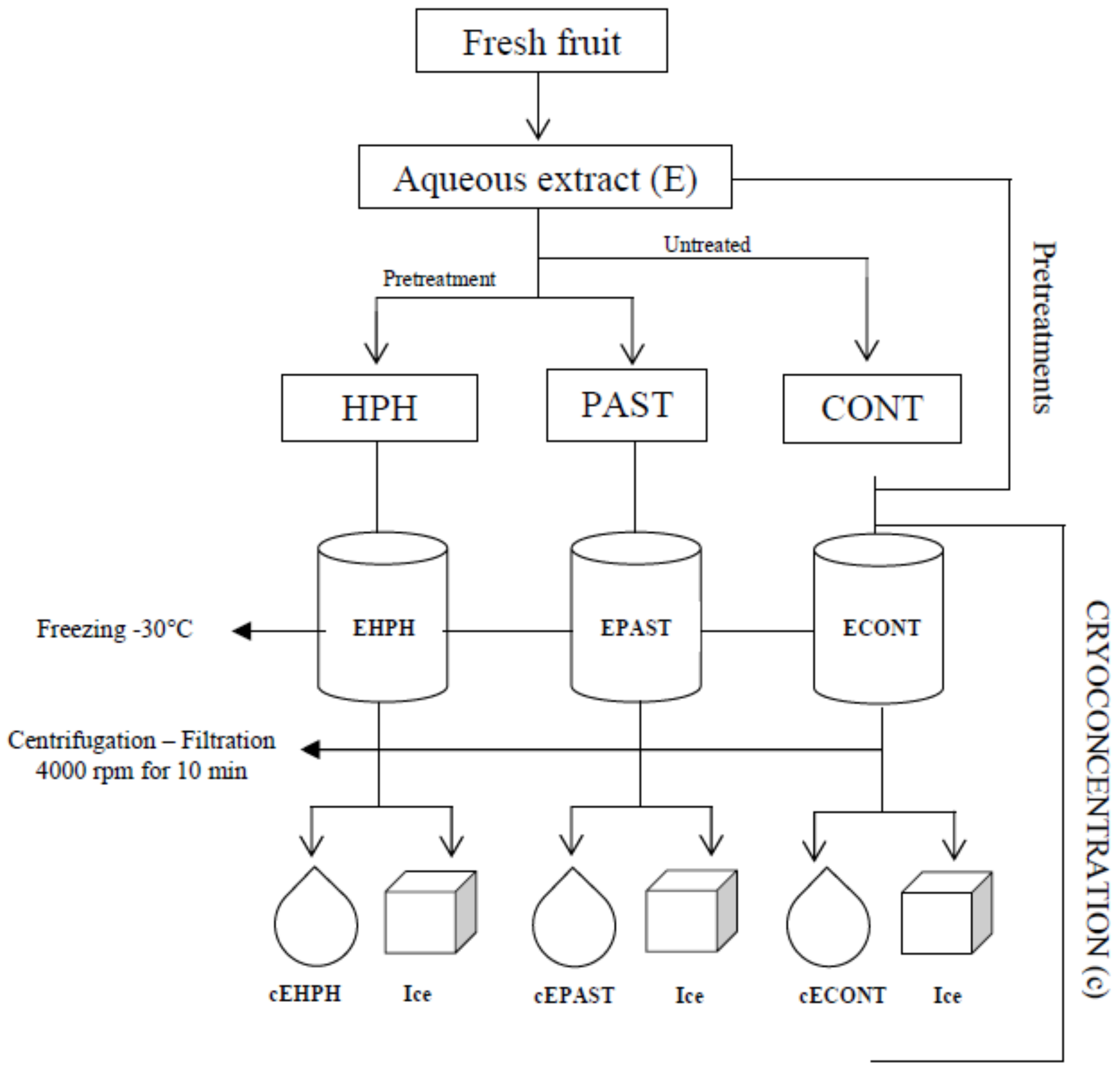
2.2. General Experimental Procedure
2.3. Production of Juice and Aqueous Extract
2.4. High-Pressure Homogenization (HPH) and Pasteurization (PAST)
2.5. Cryoconcentration by Centrifugation-Filtration
2.6. Microbiological Parameters
2.7. Analysis of Total Soluble Solids (TSS)
2.8. Process Parameter Calculations
2.8.1. Concentration Efficiency
2.8.2. Solute Yield
2.8.3. Percentage of Impurities (I)
2.8.4. Validation of Results
2.9. Quantification of Bioactive Compounds
2.9.1. Total Polyphenol Content (TPC)
2.9.2. Total Anthocyanin Content (TAC)
2.10. Statistical Analysis
3. Results and Discussion
3.1. Optimization of Process Parameters of High-Pressure Homogenization (HPH) in Maqui and Calafate Extracts
3.2. Parameters of Cryoconcentration Process of Maqui and Calafate Cryoconcentrated Products
3.2.1. Soluble Solids Content in Maqui and Calafate Extracts and Concentrates
3.2.2. Freezing Curves for Aqueous Maqui and Calafate Extracts
3.2.3. Cryoconcentration Parameters (Separation Efficiency, Recovered Solute Yield, Impurities, and Validation of Results) in Maqui and Calafate Cryoconcentrates
3.3. Bioactive Compounds (Total Polyphenols, Total Anthocyanins, and Antioxidant Capacity) Pre- and Post-Treatment by High–Pressure Homogenization (HPH) and Cryoconcentration of Aqueous Maqui and Calafate Extracts
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Céspedes, C.L.; El-Hafidi, M.; Pavon, N.; Alarcon, J. Antioxidant and cardioprotective activities of phenolic extracts from fruits of Chilean blackberry Aristotelia chilensis (Elaeocarpaceae), Maqui. Food Chem. 2008, 107, 820–829. [Google Scholar] [CrossRef]
- Fredes, C.; Montenegro, G.; Zoffoli, P.; Santander, F.; Robert, P. Comparison of the phenolic content, total anthocyanin content and antioxidant capacity of polyphenol-rich fruits grown in Chile. Cienc. Investig. Agrar. 2014, 41, 49–61. [Google Scholar]
- Genskowsky, E.; Puente, L.; Pérez-Álvarez, J.; Fernandez-Lopez, J.; Muñoz, L.; Viuda-Martos, M. Assessment of antibacterial and antioxidant properties of chitosan edible films incorporated with maqui berry (Aristotelia chilensis). LWT 2015, 64, 1057–1062. [Google Scholar] [CrossRef]
- Rodríguez, K.; Ah-Hen, K.S.; Vega-Gálvez, A.; Vásquez, V.; Quispe-Fuentes, I.; Rojas, P.; Lemus-Mondaca, R. Changes in bioactive components and antioxidant capacity of maqui, Aristotelia chilensis [Mol] Stuntz, berries during drying. LWT 2016, 65, 537–542. [Google Scholar] [CrossRef]
- Araneda, X.; Quilaman, E.; Martinez, M.; Morales, D. Elaboración y evaluación de jugo de maqui (Aristotelia chilensis (Mol.) Stuntz) por arrastre de vapor. Sci. Agropecu. 2014, 5, 149–156. [Google Scholar] [CrossRef][Green Version]
- Bastías-Montes, J.M.; Monterrosa, K.; Muñoz-Fariña, O.; García, O.; Acuña-Nelson, S.M.; Martín, C.V.-S.; Quevedo-Leon, R.; Kubo, I.; Avila-Acevedo, J.G.; Domiguez-Lopez, M.; et al. Chemoprotective and antiobesity effects of tocols from seed oil of Maqui-berry: Their antioxidative and digestive enzyme inhibition potential. Food Chem. Toxicol. 2020, 136, 111036. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, A.; Hermosín-Gutiérrez, I.; Mardones, C.; Vergara, C.; Herlitz, E.; Vega, M.; Dorau, C.; Winterhalter, P.; Von Baer, D. Polyphenols and Antioxidant Activity of Calafate (Berberis microphylla) Fruits and Other Native Berries from Southern Chile. J. Agric. Food Chem. 2010, 58, 6081–6089. [Google Scholar] [CrossRef]
- Hoffmann, J.A.; Jullian, F.A. Flora Silvestre de Chile, 2nd ed.; Ediciones Fundación Claudio Gay: Santiago, Chile, 1991; p. 246. [Google Scholar]
- Pascual-Teresa, S.; Sánchez-Ballesta, M. Anthocyanins: From plant to health. Phytochem. Rev. 2008, 7, 281–299. [Google Scholar] [CrossRef]
- Fredes, C.; Osorio, M.J.; Parada, J.; Robert, P. Stability and bioaccessibility of anthocyanins from maqui (Aristotelia chilensis [Mol.] Stuntz) juice microparticles. LWT 2018, 91, 549–556. [Google Scholar] [CrossRef]
- Sánchez, J.; Ruiz, Y.; Auleda, J.; Hernández, E.; Raventós, M. Review. Freeze Concentration in the Fruit Juices Industry. Food Sci. Technol. Int. 2009, 15, 303–315. [Google Scholar] [CrossRef]
- Ávila, R.; Bullón, J. La concentración de jugos de fruta: Aspectos básicos de los procesos sin y con membrana. Rev. Fac. Ing. Univ. Cent. Venez. 2013, 28, 3. [Google Scholar]
- Petzold, G.; Orellana, P.; Moreno, J.; Junod, J.; Bugueño, G. Freeze concentration as a technique to protect valuable heat-labile components of foods. In Innovative Processing Technologies for Foods with Bioactive Compounds, 1st ed.; Moreno, J.J., Ed.; CRC Press: Boca Raton, FA, USA, 2016; pp. 184–190. [Google Scholar]
- Orellana, P.; Petzold, G.; Lissage, P.; Pensaben, J. Protection of polyphenols in blueberry juice by vacuum-assisted block freeze concentration. Food Chem. Toxicol. 2017, 109, 1093–1102. [Google Scholar] [CrossRef]
- Bastías-Montes, J.M.; Martín, C.V.-S.; Muñoz-Fariña, O.; Petzold-Maldonado, G.; Quevedo-León, R.; Wang, H.; Yi, Y.; Céspedes-Acuña, C.L. Cryoconcentration procedure for aqueous extracts of maqui fruits prepared by centrifugation and filtration from fruits harvested in different years from the same localities. J. Berry Res. 2019, 9, 377–394. [Google Scholar] [CrossRef]
- Raventós, M.; Hernández, E.; Auleda, J. Freeze Concentration Applications in Fruit Processing. In Advances in Fruit Processing Technologies; Rodríguez, S., Fernandes, F.A.N., Eds.; CRC Press: Boca Raton, FL, USA, 2012; pp. 263–286. [Google Scholar]
- Aider, M.; de Halleux, D. Passive and microwave-assisted thawing in maple sap cryoconcentration technology. J. Food Eng. 2008, 85, 65–72. [Google Scholar] [CrossRef]
- Aider, M.; De Halleux, D. Production of concentrated cherry and apricot juices by cryoconcentration technology. LWT 2008, 41, 1768–1775. [Google Scholar] [CrossRef]
- Amran, N.A.; Samsuri, S.; Safiei, N.Z.; Zakaria, Z.Y.; Jusoh, M. Review: Parametric Study on the Performance of Progressive Cryoconcentration System. Chem. Eng. Commun. 2015, 203, 957–975. [Google Scholar] [CrossRef]
- Adorno, W.; Rezzadori, K.; Arend, G.; Chaves, V.; Reginatto, F.; Di Luccio, M.; Petrus, J. Mejora del contenido de compuestos fenólicos y la actividad antioxidante del jugo de fresa (Fragaria × ananassa) mediante tecnología de concentración de congelación de bloques. Rev. Int. Cienc. Tecnol. Aliment. 2017, 52, 781–787. [Google Scholar]
- Orellana-Palma, P.; Petzold, G.; Guerra-Valle, M.; Astudillo-Lagos, M. Impact of block cryoconcentration on polyphenol retention in blueberry juice. Food Biosci. 2017, 20, 149–158. [Google Scholar] [CrossRef]
- Yang, Z.; Han, Y.; Gu, Z.; Fan, G.; Chen, Z. Termal degradation kinetics of aqueous anthocyanins and visual color of purple corn (Zea mays L.). Innov. Food Sci. Emerg. Technol. 2007, 9, 341–347. [Google Scholar] [CrossRef]
- Kranz, S.; Hartman, T.; Siega-Riz, A.M.; Herring, A.H. A Diet Quality Index for American Preschoolers Based on Current Dietary Intake Recommendations and an Indicator of Energy Balance. J. Am. Diet. Assoc. 2006, 106, 1594–1604. [Google Scholar] [CrossRef]
- Pérez-Grijalva, B.; Herrera-Sotero, M.; Mora-Escobedo, R.; Zebadúa-García, J.C.; Silva-Hernández, E.; Oliart-Ros, R.; Pérez-Cruz, C.; Guzmán-Gerónimo, R. Effect of microwaves and ultrasound on bioactive compounds and microbiological quality of blackberry juice. LWT 2018, 87, 47–53. [Google Scholar] [CrossRef]
- Suárez-Jacobo, A.; Gervilla, R.; Guamis, B.; Roig-Sagués, A.X.; Saldo, J. Effect of UHPH on indigenous microbiota of apple juice: A preliminary study of microbial shelf-life. Int. J. Food Microbiol. 2010, 136, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Xu, Y.; Wu, J.; Xiao, G.; Fu, M.; Zhang, Y. Effect of ultra-high pressure homogenisation processing on phenolic compounds, antioxidant capacity and anti-glucosidase of mulberry juice. Food Chem. 2014, 153, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Wibowo, S.; Grauwet, T.; Santiago, J.S.; Tomic, J.; Vervoort, L.; Hendrickx, M.; Van Loey, A. Quality changes of pasteurised orange juice during storage: A kinetic study of specific parameters and their relation to colour instability. Food Chem. 2015, 187, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Fleschhut, J.; Kratzer, F.; Rechkemmer, G.; Kulling, S.E. Stability and biotransformation of various dietary anthocyanins in vitro. Eur. J. Nutr. 2006, 45, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Aadil, R.M.; Zeng, X.-A.; Han, Z.; Sun, D.-W. Effects of ultrasound treatments on quality of grapefruit juice. Food Chem. 2013, 141, 3201–3206. [Google Scholar] [CrossRef]
- Tiwari, B.K.; O’Donnell, C.P.; Patras, A.; Brunton, N.; Cullen, P.J. Stability of anthocyanins and ascorbic acid in sonicated strawberry juice during storage. Eur. Food Res. Technol. 2008, 228, 717–724. [Google Scholar] [CrossRef]
- Velázquez-Estrada, R.; Hernández-Herrero, M.; Rüfer, C.; Guamis-López, B.; Roig-Sagués, A. Influence of ultra high pressure homogenization processing on bioactive compounds and antioxidant activity of orange juice. Innov. Food Sci. Emerg. Technol. 2013, 18, 89–94. [Google Scholar] [CrossRef]
- Amador-Espejo, G.; Suàrez-Berencia, A.; Juan, B.; Bárcenas, M.; Trujillo, A. Effect of moderate inlet temperatures in ultra-high-pressure homogenization treatments on physicochemical and sensory characteristics of milk. J. Dairy Sci. 2014, 97, 659–671. [Google Scholar] [CrossRef]
- Sentandreu, E.; Stinco, C.M.; Vicario, I.M.; Mapelli-Brahm, P.; Navarro, J.L.; Meléndez-Martínez, A.J. High-pressure homogenization as compared to pasteurization as a sustainable approach to obtain mandarin juices with improved bioaccessibility of carotenoids and flavonoids. J. Clean. Prod. 2020, 262, 121325. [Google Scholar] [CrossRef]
- Stinco, C.M.; Sentandreu, E.; Mapelli-Brahm, P.; Navarro, J.L.; Vicario, I.M.; Meléndez-Martínez, A.J. Influence of high pressure homogenization and pasteurization on the in vitro bioaccessibility of carotenoids and flavonoids in orange juice. Food Chem. 2020, 331, 127259. [Google Scholar] [CrossRef]
- Guan, Y.; Zhou, L.; Bi, J.; Yi, J.; Liu, X.; Chen, Q.; Wu, X.; Zhou, M. Change of microbial and quality attributes of mango juice treated by high pressure homogenization combined with moderate inlet temperatures during storage. Innov. Food Sci. Emerg. Technol. 2016, 36, 320–329. [Google Scholar] [CrossRef]
- Martínez-Monteagudo, S.I.; Yan, B.; Balasubramaniam, V.M. Engineering Process Characterization of High-Pressure Homogenization—From Laboratory to Industrial Scale. Food Eng. Rev. 2016, 9, 143–169. [Google Scholar] [CrossRef]
- Bastías-Montes, J.M. Dispositivo Para Crioconcentrar Jugos. Instituto Nacional de Propiedad Industrial (INAPI) Patent N°60801, 28 September 2018. [Google Scholar]
- Mena, P.; Vegara, S.; Martí, N.; García-Viguera, C.; Saura, D.; Valero, M. Changes on indigenous microbiota, color, bioactive compounds and antioxidant activity of pasteurized pomegranate juice. Food Chem. 2013, 141, 2122–2129. [Google Scholar] [CrossRef] [PubMed]
- Ramaswamy, H.; Marcotte, M. Food Processing; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Zahran, D.A. Combinations of nisin and γ irradiation to improve microbiological quality of minced chicken during refrigerated storage. Life Sci. J. 2015, 12, 147–152. [Google Scholar]
- Moreno, F.; Raventós, M.; Hernández, E.; Ruiz, Y. Block freeze-concentration of coffee extract: Effect of freezing and thawing stages on solute recovery and bioactive compounds. J. Food Eng. 2014, 120, 158–166. [Google Scholar] [CrossRef]
- Petzold, G.; Moreno, J.; Lastra, P.; Rojas, K.; Orellana, P. Block freeze concentration assisted by centrifugation applied to blueberry and pineapple juices. Innov. Food Sci. Emerg. Technol. 2015, 30, 192–197. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M.; Lester, P. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Lee, J.; Durst, R.W.; Wrolstad, R.E.; Eisele, T.; Giusti, M.M.; Hofsommer, H.; Koswig, S.; Krueger, D.A.; Kupina, S.; Martin, S.K.; et al. Determination of Total Monomeric Anthocyanin Pigment Content of Fruit Juices, Beverages, Natural Colorants, and Wines by the pH Differential Method: Collaborative Study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Geciova, J.; Bury, D.; Jelen, P. Methods for disruption of microbial cells for potential use in the dairy industry—A review. Int. Dairy J. 2002, 12, 541–553. [Google Scholar] [CrossRef]
- Bevilacqua, A.; Sinigaglia, M.; Corbo, M.R. Effects of pH, cinnamaldehyde and heat-treatment time on spore viability of Alicyclobacillus acidoterrestris. Int. J. Food Sci. Technol. 2009, 44, 380–385. [Google Scholar] [CrossRef]
- Tribst, A.A.; Franchi, M.A.; Cristianini, M.; De Massaguer, P.R. Inactivation of Aspergillus niger in Mango Nectar by High-Pressure Homogenization Combined with Heat Shock. J. Food Sci. 2009, 74, M509–M514. [Google Scholar] [CrossRef]
- Welti-Chanes, J.; Ochoa-Velasco, C.; Guerrero-Beltrán, J. High-pressure homogenization of orange juice to inactivate pectinmethylesterase. Innov. Food Sci. Emerg. Technol. 2009, 10, 457–462. [Google Scholar] [CrossRef]
- Raventós, M.; Auleda, J.M.; HernándeZ, E. Modelo Para la Predicción de Puntos de Congelación de Zumos. In Proceedings of the II Congreso Iberoamericano Sobre Seguridad Alimentaria, V Congreso Español de Ingeniería de Alimentos, Barcelona, Spain, 5–7 November 2008. [Google Scholar]
- Petzold, G.; Orellana, P.; Moreno, J.; Cerda, E.; Parra, P. Vacuum-assisted block freeze concentration applied to wine. Innov. Food Sci. Emerg. Technol. 2016, 36, 330–335. [Google Scholar] [CrossRef]
- Lewicki, P.P. Raoult’s law based food water sorption isotherm. J. Food Eng. 2000, 43, 31–40. [Google Scholar] [CrossRef]
- Orellana-Palma, P.; Tobar-Bolaños, G.; Casas-Forero, N.; Zúñiga, R.N.; Petzold, G. Quality Attributes of Cryoconcentrated Calafate (Berberis microphylla) Juice during Refrigerated Storage. Foods 2020, 9, 1314. [Google Scholar] [CrossRef]
- Brauch, J.; Buchweitz, M.; Schweiggert, R.M.; Carle, R. Detailed analyses of fresh and dried maqui (Aristotelia chilensis (Mol.) Stuntz) berries and juice. Food Chem. 2016, 190, 308–316. [Google Scholar] [CrossRef]
- Suárez-Jacobo, Á.; Rüfer, C.E.; Gervilla, R.; Guamis, B.; Roig-Sagués, A.X.; Saldo, J. Influence of ultra-high pressure homogenisation on antioxidant capacity, polyphenol and vitamin content of clear apple juice. Food Chem. 2011, 127, 447–454. [Google Scholar] [CrossRef]
- Saldo, J.; Suárez-Jacobo, Á.; Gervilla, R.; Guamis, B.; Roig-Sagués, A.X. Use of ultrahigh-pressure homogenization to preserve apple juice without heat damage. High Press. Res. 2009, 29, 52. [Google Scholar] [CrossRef]
- Klopotek, Y.; Otto, K.; Böhm, V. Processing Strawberries to Different Products Alters Contents of Vitamin C, Total Phenolics, Total Anthocyanins, and Antioxidant Capacity. J. Agric. Food Chem. 2005, 53, 5640–5646. [Google Scholar] [CrossRef] [PubMed]
- Kader, F.; Rovel, B.; Girardin, M.; Metche, M. Mechanism of browning in fresh highbush blueberry fruit (Vaccinium corymbosum L.). Role of blueberry polyphenol oxidase, chlorogenic acid and anthocyanins. J. Sci. Food Agric. 1997, 74, 31–34. [Google Scholar] [CrossRef]
- Skrede, G.; Wrolstad, R.E.; Durst, R.W. Changes in anthocyanins and polyphenolics during juice processing of highbush blueberries (Vaccinium corymbosum L.). J. Food Sci. 2000, 65, 357–364. [Google Scholar] [CrossRef]
- Patras, A.; Brunton, N.P.; Da Pieve, S.; Butler, F. Impact of high pressure processing on total antioxidant activity, phenolic, ascorbic acid, anthocyanin content and colour of strawberry and blackberry purées. Innov. Food Sci. Emerg. Technol. 2009, 10, 308–313. [Google Scholar] [CrossRef]
- Frank, K.; Köhler, K.; Schuchmann, H.P. Stability of anthocyanins in high pressure homogenisation. Food Chem. 2012, 130, 716–719. [Google Scholar] [CrossRef]
- Buckow, R.; Kastell, A.; Terefe, N.S.; Versteeg, C. Pressure and Temperature Effects on Degradation Kinetics and Storage Stability of Total Anthocyanins in Blueberry Juice. J. Agric. Food Chem. 2010, 58, 10076–10084. [Google Scholar] [CrossRef] [PubMed]
- Martynenko, A.; Chen, Y. Degradation kinetics of total anthocyanins and formation of polymeric color in blueberry hydrothermodynamic (HTD) processing. J. Food Eng. 2016, 171, 44–51. [Google Scholar] [CrossRef]
- Marszałek, K.; Woźniak, L.; Skąpska, S. The application of high pressure–mild temperature processing for prolonging the shelf -life of strawberry purée. High Press. Res. 2016, 36, 220–234. [Google Scholar] [CrossRef]
- Castañeda-Ovando, A.; Pacheco-Hernández, M.D.L.; Páez-Hernández, M.E.; Rodríguez, J.A.; Galán-Vidal, C.A. Chemical studies of anthocyanins: A review. Food Chem. 2009, 113, 859–871. [Google Scholar] [CrossRef]
- Picard, C. Efecto de La Trehalosa y de Las Altas Presiones de Homogeneización Sobre Las Propiedades Físico-Químicas y Funcionales del Zumo de Mandarina; Trabajo Fin de Grado; Universitat Politècnica de València: Valencia, Spain, July 2015. [Google Scholar]
- Kubo, M.T.K.; Augusto, P.E.; Cristianini, M. Effect of high pressure homogenization (HPH) on the physical stability of tomato juice. Food Res. Int. 2013, 51, 170–179. [Google Scholar] [CrossRef]
- Cao, X.; Zhang, Y.; Zhang, F.; Wang, Y.; Yi, J.; Liao, X. Effects of high hydrostatic pressure on enzymes, phenolic compounds, anthocyanins, polymeric color and color of strawberry pulps. J. Sci. Food Agric. 2011, 91, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Grimi, N.; Mamouni, F.; Lebovka, N.; Vorobiev, E.; Vaxelaire, J. Impact of apple processing modes on extracted juice quality: Pressing assisted by pulsed electric fields. J. Food Eng. 2011, 103, 52–61. [Google Scholar] [CrossRef]



| Sample/ Treatment | Maqui | Calafate | ||
|---|---|---|---|---|
| AMM (Log10 CFU/mL) | M & Y (Log10 CFU/mL) | AMM (Log10 CFU/mL) | M & Y (Log10 CFU/mL) | |
| Control | 2.92 ± 1.98 a | 3.69 ± 1.23 a | 1.94 ± 0.07 a | 4.14 ± 0.002 a |
| Control HPH W/P | 2.71 ± 1.68 a | 3.83 ± 1.00 a | 1.98 ± 0.55 a | 1.12 ± 0.01 b |
| 100 MPa/1 pass | 1.76 ± 2.49 a | ND | 1.26 ± 0.30 a | ND |
| 100 MPa/2 passes | 0.76 ± 1.08 a | ND | 1.21 ± 0.06 a | ND |
| 200 MPa/1 pass | ND | ND | ND | ND |
| 200 MPa/2 passes | ND | ND | ND | ND |
| 300 MPa/1 pass | ND | ND | ND | ND |
| 300 MPa/2 passes | ND | ND | ND | ND |
| Sample | Maqui | Calafate | ||
|---|---|---|---|---|
| Soluble Solids/ Treatment | Sample (°Bx) | Ice (°Bx) | Sample (°Bx) | Ice (°Bx) |
| Extract | 10.54 ± 0.42 a | - | 6.82 ± 0.19 a | - |
| cCONT | 53.41 ± 2.99 b | 2.11 ± 0.07 a | 39.84 ± 6.39 b | 1.57 ± 0.52 a |
| cHPH | 49.89 ± 0.19 b | 1.95 ± 0.06 a | 43.48 ± 6.17 b,c | 1.68 ± 0.57 a |
| cPAST | 49.72 ± 2.76 b | 2.01 ± 0. 35 a | 49.46 ± 3.50 c | 2.10 ± 0.40 a |
| Sample | Maqui | Calafate | ||||
|---|---|---|---|---|---|---|
| Parameters/ Treatments | Efficiency (%) | Solute Yield * | Impurities (%) | Efficiency (%) | Solute Yield * | Impurities (%) |
| cCONT | 96.08 ± 0.32 a | 0.166 ± 0.01 a | 3.92 ± 0.32 a | 96.15 ± 0.68 a | 0.150 ± 0.03 a | 3.85 ± 0.68 a |
| cHPH | 96.11 ± 0.12 a | 0.192 ± 0.01 a | 3.89 ± 0.12 a | 96.23 ± 1.04 a | 0.137 ± 0.04 a | 3.77 ± 1.04 a |
| cPAST | 95.97 ± 0.48 a | 0.193 ± 0.02 a | 4.03 ± 0.48 a | 95.76 ± 0.71 b | 0.107 ± 0.02 b | 4.24 ± 0.71 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vidal-San Martín, C.; Bastías-Montes, J.M.; Villagra-Jorquera, C.; Salinas-Huenchulao, G.; Flores-Ríos, A.; Gonzáles-Díaz, N.; Tamarit-Pino, Y.; Muñoz-Fariña, O.; Quevedo-León, R. Effect of Cryoconcentration Assisted by Centrifugation-Filtration on Bioactive Compounds and Microbiological Quality of Aqueous Maqui (Aristotelia chilensis (Mol.) Stuntz) and Calafate (Berberis microphylla G. Forst) Extracts Pretreated with High-Pressure Homogenization. Processes 2021, 9, 692. https://doi.org/10.3390/pr9040692
Vidal-San Martín C, Bastías-Montes JM, Villagra-Jorquera C, Salinas-Huenchulao G, Flores-Ríos A, Gonzáles-Díaz N, Tamarit-Pino Y, Muñoz-Fariña O, Quevedo-León R. Effect of Cryoconcentration Assisted by Centrifugation-Filtration on Bioactive Compounds and Microbiological Quality of Aqueous Maqui (Aristotelia chilensis (Mol.) Stuntz) and Calafate (Berberis microphylla G. Forst) Extracts Pretreated with High-Pressure Homogenization. Processes. 2021; 9(4):692. https://doi.org/10.3390/pr9040692
Chicago/Turabian StyleVidal-San Martín, Carla, José Miguel Bastías-Montes, Constanza Villagra-Jorquera, Gheldred Salinas-Huenchulao, Abigail Flores-Ríos, Natalia Gonzáles-Díaz, Yanara Tamarit-Pino, Ociel Muñoz-Fariña, and Roberto Quevedo-León. 2021. "Effect of Cryoconcentration Assisted by Centrifugation-Filtration on Bioactive Compounds and Microbiological Quality of Aqueous Maqui (Aristotelia chilensis (Mol.) Stuntz) and Calafate (Berberis microphylla G. Forst) Extracts Pretreated with High-Pressure Homogenization" Processes 9, no. 4: 692. https://doi.org/10.3390/pr9040692
APA StyleVidal-San Martín, C., Bastías-Montes, J. M., Villagra-Jorquera, C., Salinas-Huenchulao, G., Flores-Ríos, A., Gonzáles-Díaz, N., Tamarit-Pino, Y., Muñoz-Fariña, O., & Quevedo-León, R. (2021). Effect of Cryoconcentration Assisted by Centrifugation-Filtration on Bioactive Compounds and Microbiological Quality of Aqueous Maqui (Aristotelia chilensis (Mol.) Stuntz) and Calafate (Berberis microphylla G. Forst) Extracts Pretreated with High-Pressure Homogenization. Processes, 9(4), 692. https://doi.org/10.3390/pr9040692