Influence of Packaging Material on Polyphenol Content and Antioxidant Activity in Some Commercial Beers
Abstract
1. Introduction
- (i)
- evaluation and comparison of the phenolic profiles and antioxidant activities of commercial beers packaged in three different types of materials;
- (ii)
- evaluation of the influence of packaging material on the antioxidant activity and polyphenols in beers, using a statistical analysis.
2. Materials and Methods
2.1. Beer Samples
2.2. Chemicals
2.3. Determination of Total Phenolic Content
2.4. Determination of Phenolic Profile by HPLC
2.5. Antioxidant Capacity
2.5.1. Ferric Reducing Antioxidant Power (FRAP) Assay
2.5.2. DPPH Radical Scavenging Activity
2.6. Statistical Analysis
3. Results and Discussion
3.1. Total Phenolic Contents
3.2. Individual Phenolic Acids
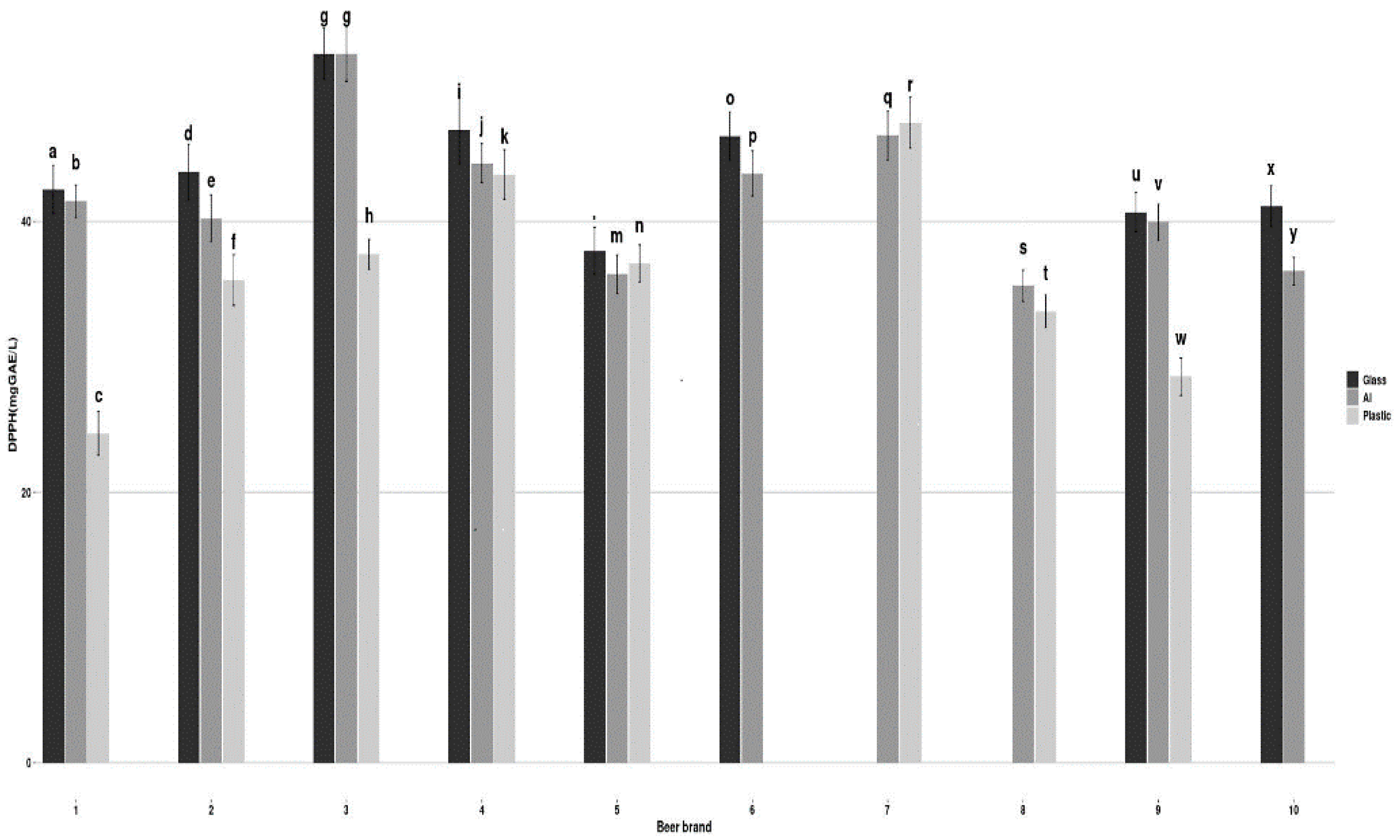
3.3. Antioxidant Activity
3.4. Effect of the Packaging Material
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arranz, S.; Chiva-Blanch, G.; Valderas-Martínez, P.; Medina-Remón, A.; Lamuela-Raventós, R.M.; Estruch, R. Wine, Beer, Alcohol and Polyphenols on Cardiovascular Disease and Cancer. Nutrients 2012, 4, 759–781. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-López, L.; Vecino, X.; Barbosa-Pereira, L.; Moldes, A.B.; Cruz, J.M. A multifunctional extract from corn steep liquor: Antioxidant and surfactant activities. Food Funct. 2016, 7, 3724–3732. [Google Scholar] [CrossRef] [PubMed]
- Hajji, T.; Mansouri, S.; Vecino, X.; Cruz-Freire, J.M.; Rezgui, S.; Ferchichi, A. Identification and characterization of phenolic compounds extracted from barley husks by LC-MS and antioxidant activity in vitro. J. Cereal Sci. 2018, 81, 83–90. [Google Scholar] [CrossRef]
- Tapia-Quirós, P.; Montenegro-Landívar, M.F.; Reig, M.; Vecino, X.; Alvarino, T.; Cortina, J.L.; Saurina, J.; Granados, M. Olive Mill and Winery Wastes as Viable Sources of Bioactive Compounds: A Study on Polyphenols Recovery. Antioxidants 2020, 9, 1074. [Google Scholar] [CrossRef]
- Habschied, K.; Lončarić, A.; Mastanjević, K. Screening of Polyphenols and Antioxidative Activity in Industrial Beers. Foods 2020, 9, 238. [Google Scholar] [CrossRef]
- Rasouli, H.; Farzaei, M.H.; Khodarahmi, R. Polyphenols and their benefits: A review. Int. J. Food Prop. 2017, 20, 1700–1741. [Google Scholar] [CrossRef]
- Quifer-Rada, R.; Vallverdú-Queralt, A.; Martínez-Huélamo, M.; Chiva-Blanch, G.; Jáuregui, O.; Estruch, R.; Lamuela-Raventós, R. A comprehensive characterisation of beer polyphenols by high resolution mass spectrometry (LC-ESI-LTQ-Orbitrap-MS). Food Chem. 2015, 169, 336–343. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, W.; Lu, J.; Zhao, M. Phenolic profiles and antioxidant activities of commercial beers. Food Chem. 2010, 119, 1150–1158. [Google Scholar] [CrossRef]
- Tafulo, P.A.R.; Barbosa Queiros, R.; Delerue-Matos, C.M.; Ferreira Sales, M.G. Control and comparison of the antioxidant capacity of beers. Food Res. Int. 2010, 43, 1702–1709. [Google Scholar] [CrossRef]
- Gerhauser, C. Beer constituents as potential cancer chemopreventive agents. Eur. J. Cancer 2005, 41, 1941–1954. [Google Scholar] [CrossRef]
- Nardini, M.; Natella, F.; Scaccini, C.; Ghiselli, A. Phenolic acids from beer are absorbed and extensively metabolized in humans. J. Nutr. Biochem. 2006, 17, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Gorinstein, S.; Caspi, A.; Libman, I.; Leontowicz, H.; Leontowicz, M.; Tashma, Z.; Katrich, E.; Jastrzebski, Z.; Trakhtenberg, S. Bioactivity of beer and its influence on human metabolism. Int. J. Food Sci. Nutr. 2007, 58, 94–107. [Google Scholar] [CrossRef] [PubMed]
- Brunazzi, G.; Parisi, S.; Pereno, A. The Importance of Packaging Design for the Chemistry of Food Products; Springer: Berlin/Heidelberg, Germany, 2014; pp. 13–15, 61–65. ISBN 978-3-319-08452-7. [Google Scholar]
- Sendon, R.; Sanches-Silva, A.; Bustos, J.; Martin, P.; Martinez, N.; Cirugeda, M.E. Detection of migration of phthalates from agglomerated crock stoppers using HPLC-MS/MS. J. Sep. Sci. 2012, 35, 1319–1326. [Google Scholar] [CrossRef]
- Carnol, L.; Schummer, C.; Moris, G. Quantification of six phthalates and one adipate in Luxembourgish beer using HS-SPME-GC/MS. Food Anal. Methods 2017, 10, 298–309. [Google Scholar] [CrossRef]
- Müller, J.P.; Steinegger, A.; Schlatter, C. Contribution of aluminum from packaging materials and cooking utensils to the daily aluminum intake. Zeitschrift für Lebensmittel-Untersuchung und Forschung 1993, 197, 332–341. [Google Scholar] [CrossRef]
- Stahl, T.; Taschan, H.; Brunn, H. Aluminium content of selected foods and food products. Environ. Sci. Eur. 2011, 23–37. [Google Scholar] [CrossRef]
- Pereira da Silva, C.H.T.; Pereira da Silva, T.P.S.; Nobre de Almeida, C.V.T.; Lima, D.C.A.; Cavalcanti de Amorim, E.L. Antioxidant capacity and phenolic content of Caesalpinia pyramidalis Tul and Sapium glandulosum (L.), Morong from Northeastern Brazil. Molecules 2011, 16, 4728–4739. [Google Scholar] [CrossRef] [PubMed]
- USP 30—NF 25 Supplement 1. United States Pharmacopeial Convention; USP: North Bethesda, MD, USA, 2007. [Google Scholar]
- Benzie, I.F.F.; Szeto, Y.T. Total antioxidant capacity of tea by ferric reducing antioxidant power assay. J. Agric. Food Chem. 1999, 47, 633–636. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Tech. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- R Version 3.4.3—“Kite-Eating Tree”; Copyright (C) 2017 The R Foundation for Statistical Computing; Platform: x86_64-pc-linux-gnu (64-bit); Sourced via CRAN—Mirror. Vienna, Austria. 2017. Available online: https://cran.wu.ac.at/ (accessed on 1 March 2021).
- Callemien, D.; Collin, S. Structure, Organoleptic Properties, Quantification Methods, and Stability of Phenolic Compounds in Beer—A Review Food. Food Rev. Int. 2010, 2, 1–84. [Google Scholar]
- Piazzon, A.; Forte, M.; Nardini, M. Characterization of phenolics content and antioxidant activity of different beer types. J. Agric. Food Chem. 2010, 58, 10677–10683. [Google Scholar] [CrossRef] [PubMed]
- Nardini, M.; Ghiselli, A. Determination of free and bound phenolic acid in beer. Food Chem. 2004, 84, 137–143. [Google Scholar] [CrossRef]
- Krofta, K.; Mikyska, A.; Haskova, D. Antioxidant characteristics of hops and hop products. J. Inst. Brew. 2008, 114, 160–166. [Google Scholar] [CrossRef]
- Szwajgier, D.; Pielecki, J.; Targonska, Z. The release of ferulic acid and feruloylated oligosaccharides during wort and beer production. J. Inst. Brew. 2005, 111, 372–379. [Google Scholar] [CrossRef]
- Socha, R.; Pająk, P.; Fortuna, T.; Buksa, K. Antioxidant activity and the most abundant phenolics in commercial dark beers. Int. J. Food Prop. 2017, 20, S595–S609. [Google Scholar] [CrossRef]
- Stewart, G.G.; Borthwick, R.; Bryce, J.H.; Cooper, D.; Cunningham, S.; Hart, C.; Rees, E. Recent developments in high gravity brewing. Tech. Q. Master Brew. Assoc. Am. 1998, 34, 264–270. [Google Scholar]
- Cook, J.M.; Karelitz, R.L.; Dalsis, D.E. Measurement of Oxygen, Nitrogen, and Carbon Dioxide in beverage headspace. J. Chromatogr. Sci. 1985, 23, 57–63. [Google Scholar] [CrossRef]
- Dávalos, A.; Gómez-Cordovés, C.; Bartolomé, B. Commercial dietary antioxidant supplements assayed for their antioxidant activity by different methodologies. J. Agric. Food Chem. 2003, 51, 2512–2519. [Google Scholar] [CrossRef]
- Rahman, M.M.; Islam, M.B.; Biswas, M.; Khurshid Alam, A.H. In vitro antioxidant and free radical scavenging activity of different parts of Tabebuia pallida growing in Bangladesh. BMC Res Notes. 2015, 8, 621. [Google Scholar] [CrossRef]
- Chen, T.S.; Liou, S.Y.; Wu, H.C.; Tsai, F.J.; Tsai, C.H.; Huang, C.Y.; Chang, Y.L. New analytical method for investigating the antioxidant power of food extracts on the basis of their electron-donating ability: Comparison to the ferric reducing/antioxidant power (FRAP) assay. J. Agric. Food Chem. 2010, 58, 8477–8480. [Google Scholar] [CrossRef]
- Bamforth, C.W. Beer quality: Oxidation. Brew. Guard. 2000, 4, 31–34. [Google Scholar]
- Vanderhaegen, B.; Neven, H.; Verachtert, H.; Derdelinckx, G. The chemistry of beer aging—A critical review. Food Chem. 2006, 95, 357–381. [Google Scholar] [CrossRef]
- Youn, J.S.; Kim, Y.J.; Na, H.Y.; Jung, H.R.; Song, C.K.; Kang, S.Y.; Kim, J.Y. Antioxidant activity and contents of leaf extracts obtained from Dendropanax morbifera LEV are dependent on the collection season and extraction conditions. Food Sci. Biotechnol. 2019, 28, 201–207. [Google Scholar] [CrossRef]
- Kareem, H.S.; Ariffin, A.; Nordin, N.; Heidelberg, T.; Abdul-Aziz, A.; Kong, K.W.; Yehye, W. Correlation of antioxidant activities with theoretical studies for new hydrazone compounds bearing a 3,4,5-trimethoxy benzyl moiety. Eur. J. Med. Chem. 2015, 103, 497–505. [Google Scholar] [CrossRef]




| Packing Mateial | Beer Brand | Gallic Acid | Ferulic Acid | Caffeic Acid | P-Coumaic Acid | Vanillic Acid | Other Acids | Sum of Individual Phenols |
|---|---|---|---|---|---|---|---|---|
| Glass | 1 | 17.32 ± 0.27 | 4.41 ± 0.06 | 3.36 ± 0.12 | 0.85 ± 0.02 | 1.29 ± 0.05 | 1.11 ±0.04 | 28.34 ± 0.30 |
| 2 | 20.54 ± 0.11 | 6.98 ± 0.09 | 5.77 ± 0.07 | 0.58 ± 0.01 | 0.57 ± 0.01 | 1.24 ± 0.01 | 35.68 ± 1.47 | |
| 3 | 10.42 ± 0.12 | 4.36 ± 0.11 | 4.24 ± 0.05 | 1.26 ± 0.01 | 0.27 ± 0.01 | 3.30 ± 0.24 | 23.85 ± 1.07 | |
| 4 | 22.03 ± 0.67 | 3.78 ± 0.24 | 1.49 ± 0.03 | 0.74 ± 0.02 | 0.72 ± 0.02 | 2.41 ± 0.09 | 31.17 ± 1.12 | |
| 5 | 21.40 ± 0.45 | 4.56 ± 0.07 | 5.85 ± 0.13 | 0.65 ± 0.01 | 1.05 ± 0.10 | 2.55 ± 0.12 | 36.06 ± 3.19 | |
| 6 | 20.33 ± 0.23 | 2.95 ± 0.04 | 3.63 ± 0.08 | 0.86 ± 0.03 | 0.98 ± 0.04 | 1.31 ± 0.22 | 30.06 ± 1.28 | |
| 7 | - | - | - | - | - | - | - | |
| 8 | - | - | - | - | - | - | - | |
| 9 | 11.13 ± 0.10 | 7.89 ± 0.21 | 1.79 ± 0.02 | 2.41 ± 0.01 | 1.63 ± 0.05 | 3.48 ± 0.08 | 28.33 ± 2.01 | |
| 10 | 20.02 ± 0.42 | 2.04 ± 0.08 | 3.91 ± 0.01 | 1.55 ± 0.02 | 0.71 ± 0.01 | 0.70 ± 0.01 | 28.93 ± 1.14 | |
| Al | 1 | 15.07 ± 0.23 | 3.59 ± 0.10 | 1.75 ± 0.04 | 1.10 ± 0.01 | 0.41 ± 0.01 | 1.68 ± 0.13 | 23.60 ± 0.89 |
| 2 | 18.49 ± 0.40 | 4.12 ± 0.14 | 4.74 ± 0.10 | 0.87 ± 0.01 | 0.54 ± 0.02 | 4.32 ± 0.25 | 33.07 ± 1.50 | |
| 3 | 11.06 ± 0.12 | 3.02 ± 0.16 | 3.59 ± 0.07 | 1.25 ± 0.01 | 0.88 ± 0.12 | 2.52 ± 0.14 | 22.32 ± 1.13 | |
| 4 | 22.28 ± 0.51 | 3.89 ± 0.20 | 1.18 ± 0.02 | 1.20 ± 0.05 | 1.08 ± 0.06 | 2.20 ± 0.15 | 31.83 ± 1.70 | |
| 5 | 19.57 ± 1.03 | 4.78 ± 0.09 | 3.94 ± 0.01 | 0.76 ± 0.02 | 0.67 ± 0.01 | 2.70 ± 0.11 | 32.42 ± 1.22 | |
| 6 | 19.13 ± 0.82 | 3.90 ± 0.04 | 1.19 ± 0.01 | 0.41 ± 0.07 | 1.14 ± 0.10 | 1.63 ± 0.01 | 27.30 ± 0.89 | |
| 7 | 18.75 ± 0.47 | 2.73 ± 0.01 | 1.04 ± 0.01 | 0.58 ± 0.05 | 1.05 ± 0.02 | 2.67 ± 0.10 | 26.82 ± 1.04 | |
| 8 | 16.14 ± 0.15 | 3.77 ± 0.10 | 2.46 ± 0.02 | 1.52 ± 0.11 | 0.68 ± 0.01 | 1.29 ± 0.05 | 25.86 ± 1.20 | |
| 9 | 12.56 ± 0.30 | 6.54 ± 0.12 | 1.34 ± 0.01 | 1.46 ± 0.08 | 1.84 ± 0.11 | 2.47 ± 0.15 | 29.21 ± 1.56 | |
| 10 | 19.37 ± 0.50 | 3.89 ± 0.20 | 2.54 ± 0.01 | 1.52 ± 0.10 | 0.64 ± 0.01 | 0.41 ± 0.02 | 28.37 ± 1.23 | |
| Plastic | 1 | 17.12 ± 0.71 | 3.97 ± 0.12 | 1.46 ± 0.03 | 1.98 ± 0.02 | 1.17 ± 0.10 | 1.51 ± 0.05 | 27.21 ± 1.50 |
| 2 | 19.64 ± 0.52 | 4.16 ± 0.07 | 2.55 ± 0.02 | 1.56 ± 0.01 | 0.75 ± 0.01 | 3.47 ± 0.18 | 32.13 ± 2.02 | |
| 3 | 10.08 ± 0.20 | 3.47 ± 0.25 | 2.26 ± 0.12 | 1.55 ± 0.01 | 1.09 ± 0.04 | 2.20 ± 0.10 | 20.65 ± 1.21 | |
| 4 | 20.54 ± 1.21 | 3.12 ± 0.67 | 1.23 ± 0.04 | 0.82 ± 0.01 | 0.67 ± 0.01 | 2.40 ± 0.05 | 28.70 ± 1.47 | |
| 5 | 19.37 ± 0.90 | 5.08 ± 0.04 | 3.85 ± 0.09 | 0.94 ± 0.01 | 0.83 ± 0.12 | 3.13 ± 0.12 | 33.20 ± 2.26 | |
| 6 | - | - | - | - | - | - | - | |
| 7 | 18.18 ± 1.25 | 3.42 ± 0.20 | 1.51 ± 0.10 | 0.29 ± 0.01 | 1.42 ± 0.15 | 4.11 ± 0.17 | 28.93 ± 0.86 | |
| 8 | 18.02 ± 0.81 | 2.09 ± 0.05 | 1.96 ± 0.22 | 1.06 ± 0.05 | 0.67 ± 0.01 | 3.22 ± 0.09 | 27.02 ± 1.16 | |
| 9 | 11.21 ± 0.54 | 7.78 ± 0.82 | 1.29 ± 0.04 | 1.38 ± 0.02 | 1.07 ± 0.05 | 2.43 ± 0.15 | 25.16 ± 1.80 | |
| 10 | - | - | - | - | - | - | - | |
| p-value | 0.8516 | 0.7096 | 0.0312 | 0.8651 | 0.9292 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bratu, M.M.; Birghila, S.; Popescu, A.; Negreanu-Pirjol, B.-S.; Radu, M.; Birghila, C. Influence of Packaging Material on Polyphenol Content and Antioxidant Activity in Some Commercial Beers. Processes 2021, 9, 620. https://doi.org/10.3390/pr9040620
Bratu MM, Birghila S, Popescu A, Negreanu-Pirjol B-S, Radu M, Birghila C. Influence of Packaging Material on Polyphenol Content and Antioxidant Activity in Some Commercial Beers. Processes. 2021; 9(4):620. https://doi.org/10.3390/pr9040620
Chicago/Turabian StyleBratu, Mihaela Mirela, Semaghiul Birghila, Antoanela Popescu, Bogdan-Stefan Negreanu-Pirjol, Marius Radu, and Corina Birghila. 2021. "Influence of Packaging Material on Polyphenol Content and Antioxidant Activity in Some Commercial Beers" Processes 9, no. 4: 620. https://doi.org/10.3390/pr9040620
APA StyleBratu, M. M., Birghila, S., Popescu, A., Negreanu-Pirjol, B.-S., Radu, M., & Birghila, C. (2021). Influence of Packaging Material on Polyphenol Content and Antioxidant Activity in Some Commercial Beers. Processes, 9(4), 620. https://doi.org/10.3390/pr9040620






