Abstract
In this study, we examined the effect of ethanolic extract of Salicornia herbacea (ESH), isorhamnetin 3-O-glucoside (I3G), quercetin 3-O-glucoside (Q3G), quercetin, and isorhamnetin on α-glucosidase activity and glucose-stimulated insulin secretion (GSIS) in insulin-secreting rat insulinoma (INS-1) cells. A portion of the ethyl acetate fraction of ESH was chromatographed on a silica gel by a gradient elution with chloroform and methanol to provide Q3G and I3G. ESH, Q3G, and quercetin inhibited α-glucosidase activity, and quercetin (IC50 value was 29.47 ± 3.36 μM) inhibited the activity more effectively than Q3G. We further demonstrated that ESH, Q3G, quercetin, I3G, and isorhamnetin promote GSIS in INS-1 pancreatic β-cells without inducing cytotoxicity. Among them, I3G was the most effective in enhancing GSIS. I3G enhanced the phosphorylation of total extracellular signal-regulated kinase (ERK), insulin receptor substrate-2 (IRS-2), phosphatidylinositol 3-kinase (PI3K), Akt, and activated pancreatic and duodenal homeobox-1 (PDX-1), which are associated with insulin secretion and β-cell function. As components of ESH, Q3G has the potential to regulate blood glucose by inhibiting α-glucosidase activity, and I3G enhances the insulin secretion, but its bioavailability should be considered in determining biological importance.
1. Introduction
Diabetes mellitus (DM) is one of the most prevalent and costly health conditions impairing patients’ quality of life worldwide [1]. It is characterized by hyperglycemia that is the consequence of insufficient secretion of insulin or insulin resistance [2]. Therefore, control of postprandial hyperglycemia is an important target in DM. α-Glucosidase inhibitors such as miglitol, acarbose, and voglibose are antidiabetic drugs that reduce postprandial blood glucose by delaying the process of carbohydrate absorption in the small intestine. However, miglitol, acarbose, and voglibose are known to induce side effects such as stomach pain, diarrhea, and bloating, known as a side effect of any compound that blocks this enzyme [3].
Sulfonylureas such as glibenclamide and gliclazide are widely used for the treatment of type 2 DM. These sulfonylureas potentiate insulin secretion from pancreatic β-cells, but are currently being largely replaced by inhibitors of dipeptidyl peptidase-4 (DPP-4) and glucosurics [4]. At the same time, side effects such as skin reactions, nausea, and dizziness have been reported [5].
Salicornia herbacea is a halophyte that can grow in salt fields or salt marshes along seashores in Korea. It has been used as a culinary vegetable as well as a traditional medicine by people living in coastal areas [6]. Quercetin 3-O-glucoside (Q3G) and isorhamnetin 3-O-glucoside (I3G). were isolated and identified from this plant and quercetin and isorhamnetin were prepared. It has been reported that Q3G has antidiabetic effects via synthesis and secretion of insulin [7] and inhibition of the sodium glucose co-transporter in rats [8]. It is already well known that quercetin is α-glucosidase inhibitor, and it has been isolated from Forsythia suspensa (Thunb) Vahl [9], Matricaria recutita L. [10], and Eucommia ulmoides [11]. In contrast, little is known about the biological activities of I3G and isorhamnetin. We therefore evaluated the effects of Q3G, I3G, quercetin, and isorhamnetin on α-glucosidase inhibitory activity and insulin secretion in insulin-secreting rat insulinoma (INS-1) cells. Inhibition of α-glucosidase, an intestinal enzyme, is a therapeutic approach in DM patients because α-glucosidase inhibitors can prevent postprandial hyperglycemia by slowing the absorption of glucose [12]. Measuring the activity of glucosidase will provide scientific information on the biochemical potency of Q3G, I3G, quercetin, isorhamnetin and the ethanol extract of S. herbacea (ESH) in the development of novel α-glucosidase inhibitors.
Impaired glucose-stimulated insulin secretion (GSIS) is one of the consequences of β-cell dysfunction in diabetes [13]. Quercetin potentiates pancreatic GSIS that enhances the phosphorylation of total extracellular signal-regulated kinase (ERK), playing a key role in β-cell signaling and function [14]. An increase in activated duodenal homeobox-1 (PDX-1) and Akt after treatment with quercetin contributes to increased pancreatic β-cell mass and insulin secretion [15]. Effects of ESH, Q3G, I3G, quercetin, and isorhamnetin on α-glucosidase activity, GSIS from pancreatic β-cells, and the mechanism of action were evaluated as a treatment strategy for DM. This study demonstrates that ESH has the potential to both inhibit α-glucosidase activity and increase insulin secretion. Q3G was found to inhibit α-glucosidase activity and I3G was found to increase GSIS; these effects were associated with overexpression of ERK, PI3K, Akt, insulin receptor substrate-2 (IRS-2), and PDX-1 involved in enhanced insulin secretion and β-cell function.
2. Materials and Methods
2.1. Plant Material
S. herbacea was collected from Suncheonman Byeolryang Yeomjeon, Suncheon, Korea (2019). A voucher specimen was deposited at the Department of Plant Science and Technology, Chung-Ang University (Anseong, Korea).
2.2. Extraction and Isolation of Q3G and I3G
The air-dried powdered samples were extracted with ethanol under reflux. After removal of the solvent, the residue was suspended in water and then fractionated with n-hexane, ethyl acetate, chloroform, and n-butanol. A portion of the ethyl acetate fraction was eluted on a silica gel chromatography by a gradient elution with chloroform and methanol to afford Q3G and I3G (Figure 1).
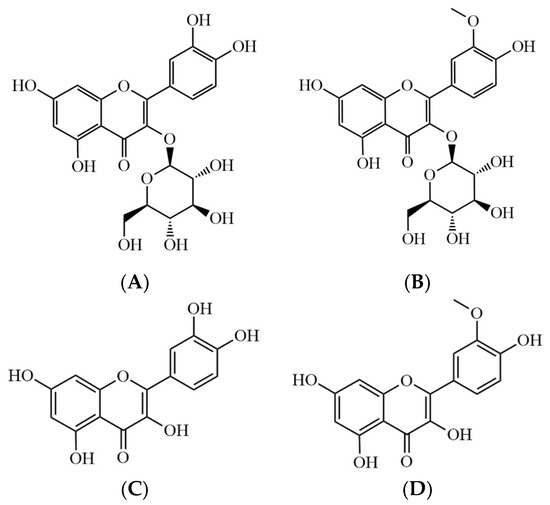
Figure 1.
Chemical structures of (A) Q3G, (B) I3G, (C) quercetin, and (D) isorhamnetin. Quercetin 3-O-glucoside (Q3G), isorhamnetin 3-O-glucoside (I3G).
2.3. α-Glucosidase Inhibitory Activity Assay
Previously reported methods with slight modifications were used to determine the α-glucosidase inhibitory activities [16,17]. For the α-glucosidase inhibitory activity assay, the α-glucosidase inhibitor acarbose was used as the positive control. Acarbose, ESH, Q3G, I3G, quercetin, and isorhamnetin were prepared by dissolution in 1% dimethyl sulfoxide (DMSO) in water (the final concentration of DMSO in the reactional mixture was 0.2%). Next, 100 μL of 0.5 U/mL α-glucosidase was mixed with 80 μL of sample in 120 μL of 0.1 M phosphate buffer (pH 6.8) and incubated at 37 °C for 15 min. Then, 100 μL of 5 mM p-NPG as the substrate was added and incubated at 37 °C for 15 min, after which the reaction was stopped by adding 100 μL of 0.2 M sodium carbonate solution. The absorbance values of p-nitrophenol (pNP) released from p-nitrophenyl-β-D-glucopyranoside (pNPG) were measured at 405 nm using a PowerWave XS microplate reader (Bio-Tek Instruments, Winooski, VT, USA). Inhibitory effects of the samples on α-glucosidase were calculated as follows: α-glucosidase inhibitory activity (%) = [(A0 − A1)/A0] × 100, where A0 is the absorbance without the sample (control) and A1 is the absorbance with the sample.
2.4. Cell Culture and Cell Viability
INS-1 rat insulin-secreting β-cells (Biohermes, Shanghai, China) were maintained in RPMI-1640 medium (Cellgro, Manassas, VA, USA) as described in a published method [18]. Cells were plated on 96-well plates to determine the non-toxic concentration ranges of ESH, Q3G, I3G, quercetin, and isorhamnetin using Ez-Cytox reagent containing water-soluble tetrazolium salts (WSTs) (Daeil Lab Service Co., Seoul, Korea). In viable cells, WSTs produce a highly water-soluble formazan (orange color that absorbs at 450 nm) through mitochondrial electron transport systems. After 24 h of incubation, cells were treated with ESH, Q3G, I3G, quercetin, and isorhamnetin for 24 h. Subsequently, 10 μL of Ez-Cytox reagent was added and incubated for 2 h. A microplate reader (PowerWave XS, Bio-Tek Instruments, Winooski, VT, USA) was used to measure the absorbance values at 450 nm, as described in published methods [19,20].
2.5. GSIS Assay
INS-1 cells were plated on 12-well plates to measure the effects of Q3G, I3G, quercetin, and isorhamnetin isolated from ESH on GSIS in INS-1 cells. After 24 h of incubation, the cells were washed twice with Krebs–Ringer bicarbonate HEPES buffer (KRBB) and 2.8 mM glucose. After starvation in fresh KRBB for 2 h, the cells were treated with ESH, Q3G, I3G, quercetin, isorhamnetin, and gliclazide as the positive control. After 2 h, cells were simultaneously treated with 2.8 mM (hypoglycemia) or 16.7 mM glucose (hyperglycemia) and incubated for 1 h. GSIS was assessed using a rat insulin ELISA kit (Gentaur, Shibayagi Co. Ltd., Shibukawa, Gunma, Japan), as described previously [18], and then expressed as GSI = insulin concentration in 16.7 mM glucose/insulin concentration in 2.8 mM glucose.
2.6. Western Blot Analysis
INS-1 cells were plated on 6-well plates to measure the effect of I3G isolated from ESH on protein expression changes of P-ERK, ERK, PI3K, Akt, P-IRS-2 (Ser731), IRS-2, P-PI3K, P-Akt (Ser473), and PDX-1 in INS-1 cells. After 24 h of incubation, the cells were treated with I3G for 24 h. Next, the protein extraction and western blot analysis were performed to assess the changes in protein expression, following published methods [21,22].
2.7. Statistical Analysis
All analyses were conducted using SPSS Statistics ver. 19.0 (SPSS Inc., Chicago, IL, USA). Nonparametric comparisons of samples were conducted with the Kruskal–Wallis test to analyze the results. A value of p < 0.05 was considered to be statistically significant.
3. Results
3.1. Preparation of Q3G, I3G, Quercetin, and Isorhamnetin
The structures of Q3G and I3G were elucidated by spectral analysis and comparison with the published literature [23,24]. In brief, Q3G and I3G were obtained as yellow crystals from methanol recrystallization. They responded to the Shinoda and Molisch tests. In the 1H- and 13C-NMR spectra, the typical flavonoid signals were observed. The characteristic fragment ion peaks showed the retro-Diels Alder fragmentation of flavonoids. Quercetin and isorhamnetin were obtained from Natural Product Institute of Science and Technology (www.nist.re.kr (accessed on 8 March 2021)), Anseong, Korea. The concentrations of Q3G and I3G as active compounds in the ESH were 0.54 and 1.06 mg/g, respectively.
3.2. α-Glucosidase Inhibitory Activities of ESH, Q3G, I3G, Quercetin, and Isorhamnetin
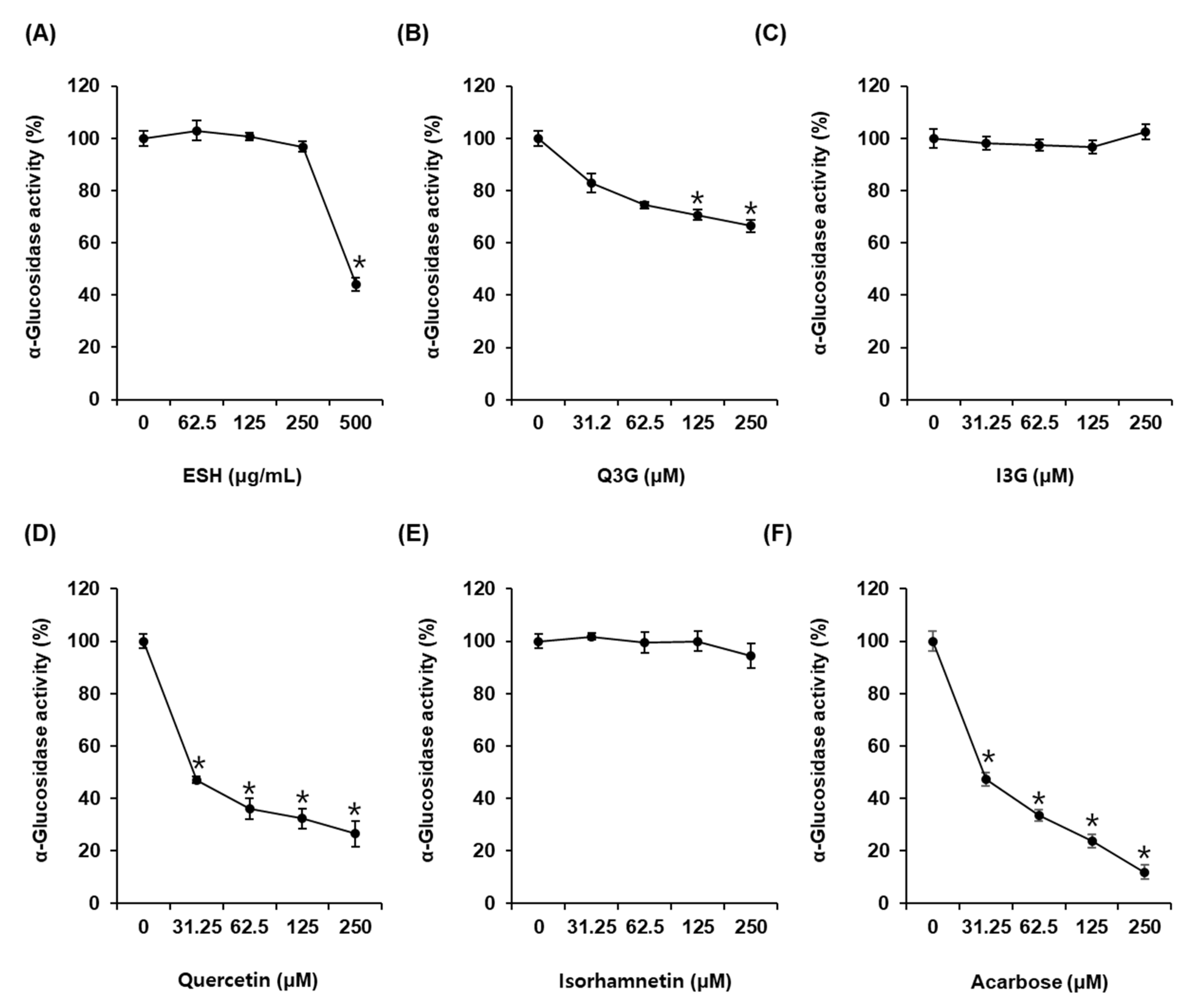
As shown in Figure 2A, α-glucosidase activity was 44.03 ± 2.45% after incubation with ESH at 500 μg/mL. α-Glucosidase activity was 81.22 ± 3.55%, 75.11 ± 4.84%, 71.83 ± 4.69%, and 61.97 ± 2.16% after incubation with Q3G at 31.25 μM, 62.5 μM, 125 μM, and 250 μM, respectively, compared with that of the control (0 μM) (Figure 2B). However, I3G showed no α-glucosidase inhibitory activity (Figure 2C). α-Glucosidase activity was 46.98 ± 2.86%, 36.03 ± 0.91%, 32.31 ± 1.37%, and 26.47 ± 2.18% after incubation with quercetin at 31.25 μM, 62.5 μM, 125 μM, and 250 μM, respectively, compared with that of the control (0 μM). Its IC50 value was 29.47 ± 3.36 μM (Figure 2D), whereas isorhamnetin showed no α-glucosidase inhibitory activity (Figure 2E). Acarbose as the positive control exhibited an IC50 value of 29.81 ± 1.31 μM. α-Glucosidase activity was 47.34 ± 2.06% after incubation with acarbose at 31.25 μM compared with that of the control (0 μM) (Figure 2F).
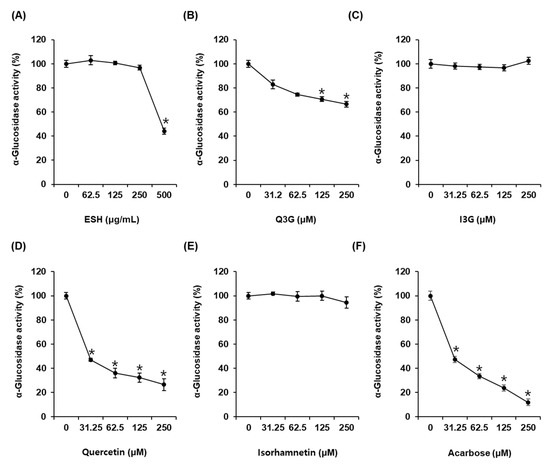
Figure 2.
α-Glucosidase inhibitory activities of (A) ethanolic extract of Salicornia herbacea (ESH), (B) quercetin 3-O-glucoside (Q3G) (C) isorhamnetin 3-O-glucoside (I3G), (D) quercetin, (E) isorhamnetin, and (F) acarbose (positive control) compared with that of the control (0 μM) by α-glucosidase inhibitory activity assay (n = 3 independent experiments, * p < 0.05, Kruskal–Wallis nonparametric test). Data are expressed as the mean ± SEM.
3.3. Effect of ESH, Q3G, I3G, Quercetin, and Isorhamnetin on GSIS
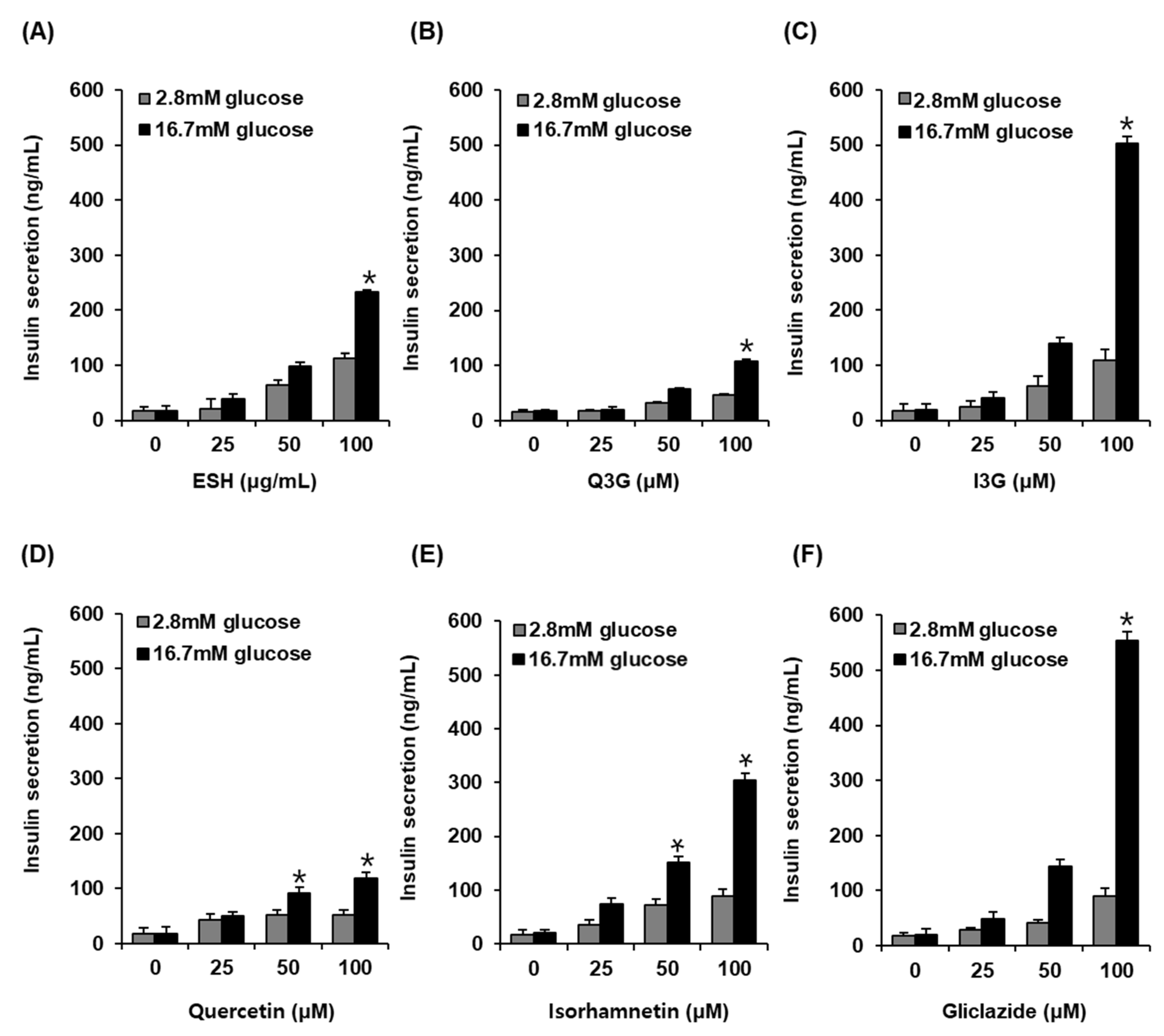
As shown in Figure 3, at 2.8 mM glucose (hypoglycemia), no significant insulin secretion was observed on treatment with ESH, Q3G, I3G, and gliclazide. However, ESH at 100 μg/mL enhanced GSIS (232.85 ± 3.83 ng/mL) as compared to high glucose concentration at 16.7 mM alone (17.92 ± 8.73 ng/mL) (Figure 3A). In addition, Q3G at 100 μM enhanced insulin secretion (107.01 ± 4.85 ng/mL) as compared to high glucose concentration at 16.7 mM alone (17.92 ± 1.45 ng/mL) (Figure 3B). Interestingly, I3G at 100 μM significantly increased insulin secretion (502.11 ± 12.36 ng/mL) as compared to high glucose concentration at 16.7 mM alone (18.97 ± 11.04 ng/mL) (Figure 3C). Quercetin at 100 μM increased insulin secretion (119.38 ± 10.38 ng/mL) as compared to high glucose concentration at 16.7 mM alone (17.31 ± 10.36 ng/mL) (Figure 3D), whereas isorhamnetin at 100 μM significantly increased insulin secretion (304.37 ± 13.36 ng/mL) as compared to high glucose concentration at 16.7 mM alone (20.23 ± 5.52 ng/mL). In these results, the effect of I3G was similar to that of 100 μM gliclazide (554.26 ± 15.36 ng/mL) in which case also insulin secretion increased as compared to high glucose concentration at 16.7 mM alone (20.07 ± 10.43 ng/mL) (Figure 3D).
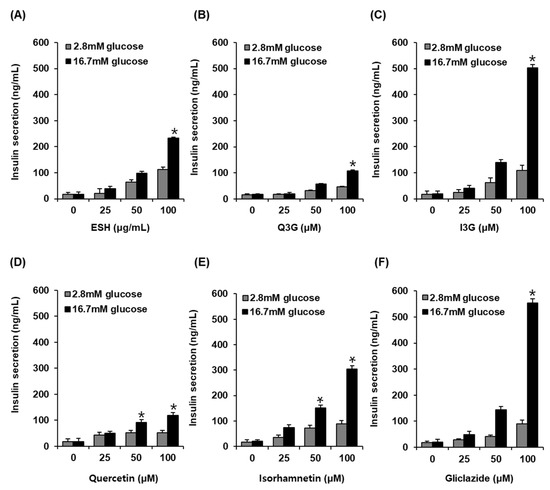
Figure 3.
Effect of (A) ethanolic extract of Salicornia herbacea (ESH), (B) quercetin 3-O-glucoside (Q3G) (C) isorhamnetin 3-O-glucoside (I3G), (D) quercetin, (E) isorhamnetin, and (F) positive control (gliclazide) compared with that of the control (0 μM) on glucose-stimulated insulin secretion (GSIS) in INS-1 rat insulin-secreting β-cells for 1 h by insulin secretion assay (n = 3 independent experiments, * p < 0.05, Kruskal–Wallis nonparametric test). Data are expressed as the mean ± SEM.
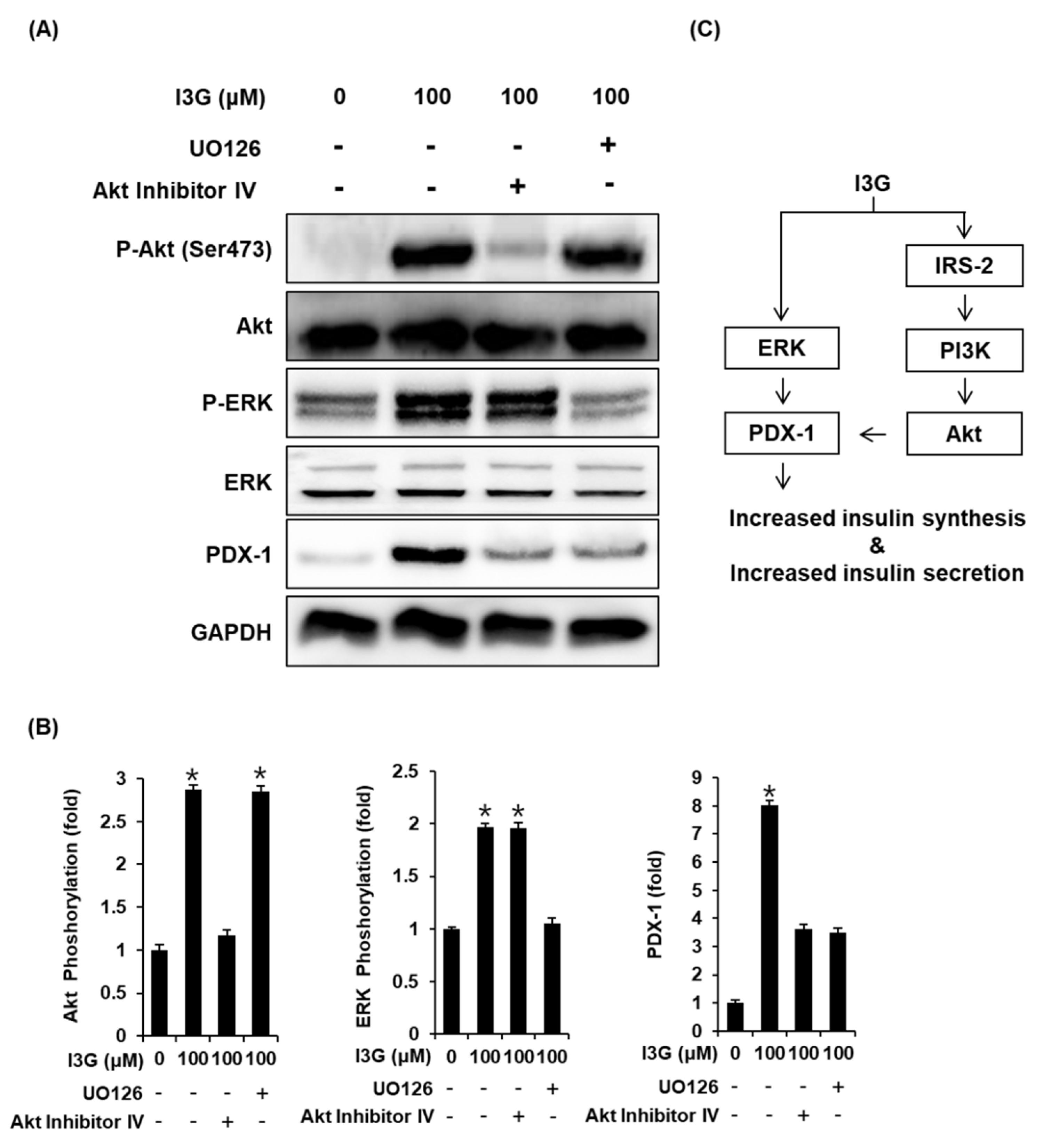
3.4. Effect of I3G on the Protein Expression of P-ERK, ERK, P-PI3K, PI3K, P-IRS-2, IRS-2 (Ser731), Akt, P-Akt (Ser473), and PDX-1
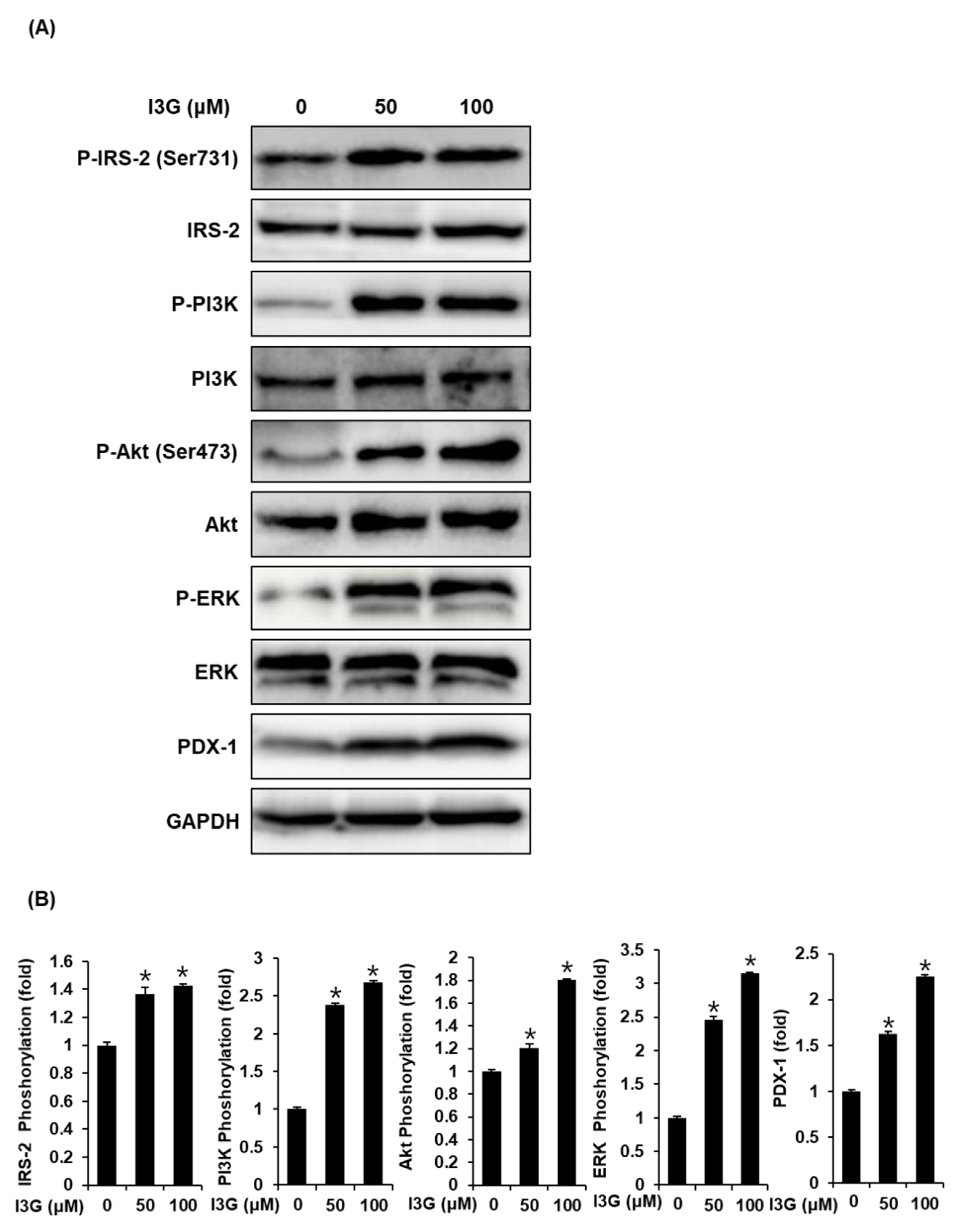
To further validate the mechanistic insights on the effect of I3G on GSIS, we measured the expression of proteins including ERK, IRS-2, Akt, PI3K, and PDX-1, which are related to pancreatic β-cell metabolism. As shown in Figure 4A,B, upon treatment with I3G at 50 and 100 μM, protein expression levels of P-ERK, ERK, P-IRS-2 (Ser731), P-Akt (Ser473), P-PI3K, and PDX-1 were increased, compared with the control (0 μM). As shown in Figure 5A,B, increased expression level of Akt upon treatment with I3G at 100 μM was decreased after co-treatment with Akt inhibitor IV (Akt inhibitor), in which case, PDX-1 expression was also decreased. In addition, increased expression level of P-ERK upon treatment with I3G at 100 μM was decreased after co-treatment with U0126 (ERK inhibitor), in which case, PDX-1 expression was also decreased.
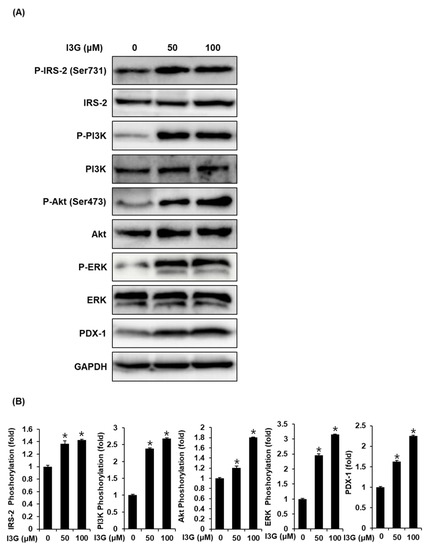
Figure 4.
(A) Protein expression levels of phospho-insulin receptor substrate-2 (P-IRS-2) (Ser731), IRS-2, phospho-extracellular signal-regulated kinase (P-ERK), ERK, phospho-phosphatidylinositol 3-kinase (P-PI3K), PI3K, phospho-Akt (P-Akt) (Ser473), Akt, pancreatic and duodenal homeobox-1 (PDX-1), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) in INS-1 cells treated with 50 or 100 μM isorhamnetin 3-O-glucoside (I3G), or untreated for 24 h. (B) Bar graphs present the densitometric quantification of western blot bands (n = 3 independent experiments, * p < 0.05, Kruskal–Wallis nonparametric test). Data are expressed as the mean ± SEM.
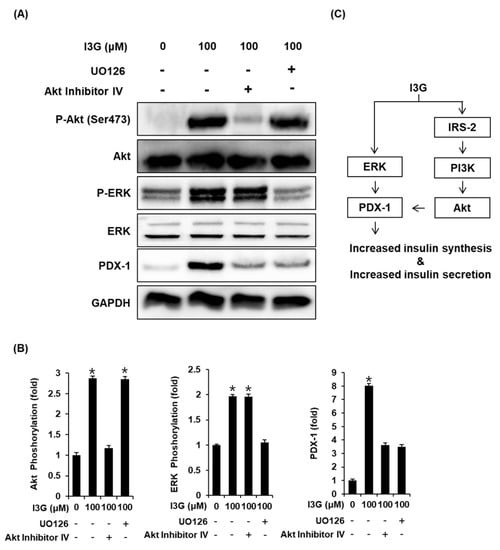
Figure 5.
(A) Protein expression levels of phospho-Akt (P-Akt) (Ser473), Akt, phospho-extracellular signal-regulated kinase (P-ERK), ERK, pancreatic and duodenal homeobox-1 (PDX-1), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) in INS-1 cells treated with 100 μM isorhamnetin 3-O-glucoside (I3G), and/or (B) U0126 (ERK inhibitor, 10 μM) or Akt Inhibitor IV (Akt inhibitor, 500 nM) for 24 h. (B) Bar graphs present the densitometric quantification of western blot bands (n = 3 independent experiments, * p < 0.05, Kruskal–Wallis nonparametric test). Data are expressed as the mean ± SEM. (C) Schematic illustration of the effects of I3G isolated from ethanolic extract of Salicornia herbacea (ESH) on the protein expression levels related to pancreatic β-cell metabolism.
4. Discussion
This study demonstrates that ESH has the potential to both inhibit α-glucosidase activity and increase GSIS. In addition, Q3G, quercetin, and isorhamnetin were found to inhibit α-glucosidase activity, and I3G, quercetin, and isorhamnetin enhanced GSIS. Dietary carbohydrates are finally hydrolyzed to glucose in the intestine by α-glucosidase located in the mucosal brush-border surface of the membrane of intestinal cells [25]. Effective α-glucosidase inhibitors from natural products, with great structural diversity, have been shown to be a good source for antidiabetic therapies to delay glucose absorption, achieving better glycemic control [26]. To explore biologically active compounds from ESH, we tested the inhibitory activities of Q3G and I3G isolated from ESH against α-glucosidase from Saccharomyces cerevisiae. p-Nitrophenyl-α-D-glucopyranoside (p-NPG) is used as a substrate for the α-glucosidase inhibitory activity assay [16,17].
ESH inhibited the α-glucosidase activity by 44% at a high concentration of 500 μg/mL. It was reported that extract of S. herbacea at the same concentration show α-glucosidase inhibitory activity, where the activity is 10% [27]. I3G and isorhamnetin have no statistically significant α-glucosidase inhibitory activity, whereas Q3G and quercetin inhibited α-glucosidase activity. Quercetin inhibited the activity more effectively than Q3G. These results showed that the sugar moiety of the quercetin glycoside does not enhance the α-glucosidase inhibitory activity. Similarly, it was found that quercetin and Q3G isolated from tartary buckwheat bran have α-glucosidase inhibitory activity, and the inhibitory activity of quercetin is stronger than that of Q3G [28]. The absorption of quercetin glycosides depends on the position and nature of the sugar moiety [29]. In general, after eating quercetin-rich foods, the concentration of quercetin reaching human plasma is in the low range [30]. It can only reach levels of up to 5 µM in plasma [31]. In the present study, the concentrations of quercetin and Q3G required for the α-glucosidase inhibitory activity is too high, so, bioavailability should be considered in determining biological importance of ESH components.
INS-1 pancreatic β-cells have been used to study the effects of GSIS [32]. This method has been used in many previous studies to evaluate insulin secretion capacity. In the previous study, genistein, a soy isoflavone, alone do not affect insulin secretion in INS-1 cells, but significantly enhances in response to glucose stimulation [33]. Importantly, the GSIS assay proceeds after determination of the highest non-cytotoxic concentration. A previous study reported that oleuropein, a phenolic compound, promotes GSIS without inducing cytotoxicity in INS-1 cells [34]. In the present study, ESH enhanced GSIS without inducing cytotoxicity in INS-1 cells. In contrast to the results for α-glucosidase, I3G and isorhamnetin had a better effect than Q3G and quercetin on insulin secretion in response to high-glucose stimulation, without inducing cytotoxicity in INS-1 cells. I3G enhanced the GSIS more effectively than isorhamnetin. These results showed that the sugar moiety of the isorhamnetin glycoside had effect on enhancing GSIS.
Impaired insulin secretion is a feature of type 2 DM which could be due to a reduction in pancreatic β-cell size and number [13]. In an experimental model of type 2 DM, PDX-1, a homeodomain-containing transcription factor for PDX-1 itself, is a primary regulator of insulin secretion and glucose metabolism related to β-cell function [35]. In vitro studies have shown that PDX-1 suppression can lead to a decrease in insulin synthesis [36]. In the present study, I3G resulted in the overexpression of PDX-1. PDX-1 is phosphorylated by phosphorylation of ERK, PI3K, and Akt [37,38]. Enhanced proliferation of INS-1 pancreatic β-cells by ERK and Akt promotes insulin secretion [39]. Oleuropein promotes GSIS and ERK phosphorylation in INS-1 cells in a concentration-dependent manner [34]. In agreement with earlier studies, we found that ERK phosphorylation was increased by I3G. In addition, PI3K-dependent phosphorylation of Akt at Ser473 in the presence of I3G was increased. In both INS-1 cells and high fat diet-fed rats, serine protease inhibitor from visceral adipose improves pancreatic β-cell function though PI3K-dependent Akt phosphorylation at Thr308 and IRS-2 phosphorylation at Ser731, which leads to increases in insulin secretion [40]. Akt phosphorylation can be activated by tyrosine phosphorylation of IRS-2, linking insulin receptors in pancreatic β cells [41]. In the present study, I3G enhanced IRS-2 phosphorylation at Ser731. In addition, treatment with specific Akt and ERK inhibitor attenuated the effects of I3G on expression levels of P-ERK and Akt, respectively, and both Akt and ERK inhibitor attenuated the effects of I3G on the expression level of PDX-1. Together, these data indicated a role of Akt and ERK in the control of PDX-1 expression after treatment with I3G. Consequently, I3G improved insulin secretion by regulating the expression of ERK, PI3K, Akt, IRS-2, and PDX-1. However, bioavailability should be considered in determining clinical importance since I3G is not well absorbed in its parent form [42].
5. Conclusions
Q3G and quercetin inhibited α-glucosidase activity, but in micro molar concentrations. Quercetin inhibited the activity more effectively than Q3G. In addition, I3G was the most effective in enhancing GSIS by regulating the expression of ERK, PI3K, Akt, IRS-2, and PDX-1. Bioavailability should be considered in determining the clinical importance of these compounds.
Author Contributions
K.S.K., J.Y.P., and S.L. conceived and designed the experiments; D.L. and J.Y.P. performed the experiments; S.L. and J.Y.P. analyzed the data; S.L. and K.S.K. contributed reagents, materials, and analytical tools; and D.L., J.Y.P., and K.S.K. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research was a part of a project titled ‘Development of functional food material containing antidiabetic effects by Salicornia herbacea,’ funded by the Ministry of Oceans and Fisheries, Korea (20190112). This research was also funded by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2019R1F1A1059173).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors thank B. K. Min for the sample of S. herbacea supported by Suncheonman Byeolryang Yeomjeon, Suncheon, Korea.
Conflicts of Interest
There is no conflict of interest.
References
- Trikkalinou, A.; Papazafiropoulou, A.K.; Melidonis, A. Type 2 diabetes and quality of life. World J. Diabetes 2017, 8, 120. [Google Scholar] [CrossRef]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2013, 36 (Suppl. S1), S67–S74. [Google Scholar] [CrossRef] [PubMed]
- Kumar, Y.; Goyal, R.; Thakur, A. Pharmacotherapeutics of miglitol: An α-glucosidase inhibitor. J. Anal. Pharm. Res. 2018, 7, 617–619. [Google Scholar] [CrossRef]
- Loubani, M.; Fowler, A.; Standen, N.B.; Galinanes, M. The effect of gliclazide and glibenclamide on preconditioning of the human myocardium. Eur. J. Pharmacol. 2005, 515, 142–149. [Google Scholar] [CrossRef]
- Holmes, B.; Heel, R.; Brogden, R.; Speight, T.; Avery, G. Gliclazide. Drugs 1984, 27, 301–327. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.S.; Tae, G.S. Ecological studies on the halophyto communities at western and southern coasts in Korea (IV)-The halophyte communities at the different salt marsh habitats. Korean J. Ecol. 1983, 6, 167–176. [Google Scholar]
- Panda, S.; Kar, A. Antidiabetic and antioxidative effects of Annona squamosa leaves are possibly mediated through quercetin-3-O-glucoside. Biofactors 2007, 31, 201–210. [Google Scholar] [CrossRef]
- Hamouda, N.N.; Qureshi, M.A.; Alkaabi, J.M.; Oz, M.; Howarth, F.C. Reduction in the amplitude of shortening and Ca2+ transient by phlorizin and quercetin-3-O-glucoside in ventricular myocytes from Streptozotocin-induced diabetic rats. Physiol. Res. 2016, 65, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.; Wang, J.; Zhang, L. α-Glucosidase inhibitors from Forsythia suspense (Thunb) Vahl. China J. Chin. Matern. Med. 2010, 35, 1156–1159. [Google Scholar]
- Ichiki, H.; Takeda, O.; Sakakibara, I.; Terabayashi, S.; Takeda, S.; Sasaki, H. Inhibitory effects of compounds from Anemarrhenae Rhizoma on α-glucosidase and aldose reductase and its contents by drying conditions. J. Nat. Med. 2007, 61, 146–153. [Google Scholar] [CrossRef]
- Watanabe, J.; Kawabata, J.; Kurihara, H.; Niki, R. Isolation and identification of α-glucosidase inhibitors from tochu-cha (Eucommia ulmoides). Biosci. Biotechnol. Biochem. 1997, 61, 177–178. [Google Scholar] [CrossRef]
- Van De Laar, F.A.; Lucassen, P.L.; Akkermans, R.P.; Van De Lisdonk, E.H.; Rutten, G.E.; Van Weel, C. α-Glucosidase inhibitors for patients with type 2 diabetes: Results from a cochrane systematic review and meta-analysis. Diabetes Care 2005, 28, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Cantley, J.; Ashcroft, F.M. Q&A: Insulin secretion and type 2 diabetes: Why do β-cells fail? BMC Biol. 2015, 13, 33. [Google Scholar]
- Youl, E.; Bardy, G.; Magous, R.; Cros, G.; Sejalon, F.; Virsolvy, A.; Richard, S.; Quignard, J.; Gross, R.; Petit, P. Quercetin potentiates insulin secretion and protects INS-1 pancreatic β-cells against oxidative damage via the ERK1/2 pathway. Br. J. Pharmacol. 2010, 161, 799–814. [Google Scholar] [CrossRef]
- Li, J.-M.; Wang, W.; Fan, C.-Y.; Wang, M.-X.; Zhang, X.; Hu, Q.-H.; Kong, L.-D. Quercetin preserves β-cell mass and function in fructose-induced hyperinsulinemia through modulating pancreatic Akt/FoxO1 activation. Evid. Based Complement. Altern. Med. 2013, 2013, 303902. [Google Scholar]
- Sheng, Z.; Dai, H.; Pan, S.; Wang, H.; Hu, Y.; Ma, W. Isolation and characterization of an α-glucosidase inhibitor from Musa spp. (Baxijiao) flowers. Molecules 2014, 19, 10563–10573. [Google Scholar] [CrossRef]
- Thao, N.P.; Binh, P.T.; Luyen, N.T.; Hung, T.M.; Dang, N.H.; Dat, N.T. α-Amylase and α-glucosidase inhibitory activities of chemical constituents from Wedelia chinensis (Osbeck.) Merr. leaves. J. Anal. Methods Chem. 2018, 2018, 2794904. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Lee, D.H.; Choi, S.; Lee, J.S.; Jang, D.S.; Kang, K.S. Identification and isolation of active compounds from Astragalus membranaceus that improve insulin secretion by regulating pancreatic β-cell metabolism. Biomolecules 2019, 9, 618. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Lee, D.-S.; Kim, C.-E.; Shin, M.-S.; Seo, C.-S.; Shin, H.-K.; Hwang, G.S.; An, J.M.; Kim, S.-N.; Kang, K.S. Effects of fermented black ginseng on wound healing mediated by angiogenesis through the mitogen-activated protein kinase pathway in human umbilical vein endothelial cells. J. Ginseng Res. 2018, 42, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Lee, D.-S.; Jung, K.; Hwang, G.S.; Lee, H.L.; Yamabe, N.; Lee, H.-J.; Eom, D.-W.; Kim, K.H.; Kang, K.S. Protective effect of ginsenoside Rb1 against tacrolimus-induced apoptosis in renal proximal tubular LLC-PK1 cells. J. Ginseng Res. 2018, 42, 75–80. [Google Scholar] [CrossRef]
- Abdul, Q.A.; Seong, S.H.; Ahn, B.R.; Islam, M.N.; Jung, H.A.; Choi, J.S. Anti-inflammatory potential of Artemisia capillaris and its constituents in LPS-induced RAW264. 7 cells. Nat. Prod. Sci. 2018, 24, 171–180. [Google Scholar] [CrossRef][Green Version]
- Chun, J.; Song, K.; Kim, Y.S. Anti-inflammatory activity of standardized fraction from Inula helenium L. via suppression of NF-κB pathway in RAW 264.7 cells. Nat. Prod. Sci. 2019, 25, 16–22. [Google Scholar] [CrossRef][Green Version]
- Lee, Y.S.; Lee, H.S.; Shin, K.H.; Kim, B.K.; Lee, S. Constituents of the halophyte Salicornia herbacea. Arch. Pharm. Res. 2004, 27, 1034–1036. [Google Scholar] [CrossRef]
- Kim, J.Y.; Cho, J.-Y.; Ma, Y.-K.; Park, K.Y.; Lee, S.-H.; Ham, K.-S.; Lee, H.J.; Park, K.-H.; Moon, J.-H. Dicaffeoylquinic acid derivatives and flavonoid glucosides from glasswort (Salicornia herbacea L.) and their antioxidative activity. Food Chem. 2011, 125, 55–62. [Google Scholar] [CrossRef]
- Hooton, D.; Lentle, R.; Monro, J.; Wickham, M.; Simpson, R. The secretion and action of brush border enzymes in the mammalian small intestine. Rev. Physiol. Biochem. Pharmacol. 2015, 168, 59–118. [Google Scholar] [PubMed]
- Kumar, S.; Narwal, S.; Kumar, V.; Prakash, O. α-Glucosidase inhibitors from plants: A natural approach to treat diabetes. Pharmacogn. Rev. 2011, 5, 19. [Google Scholar] [CrossRef]
- Hwang, J.-Y.; Lee, S.-K.; Jo, J.-R.; Kim, M.-E.; So, H.-A.; Cho, C.-W.; Seo, Y.-W.; Kim, J.-I. Hypolipidemic effect of Salicornia herbacea in animal model of type 2 diabetes mellitus. Nutr. Res. Pract. 2007, 1, 371–375. [Google Scholar] [CrossRef]
- Li, Y.Q.; Zhou, F.C.; Gao, F.; Bian, J.S.; Shan, F. Comparative evaluation of quercetin, isoquercetin and rutin as inhibitors of α-glucosidase. J. Agric. Food Chem. 2009, 57, 11463–11468. [Google Scholar] [CrossRef] [PubMed]
- Materska, M. Quercetin and its derivatives: Chemical structure and bioactivity-a review. Pol. J. Food Nutr. Sci. 2008, 58, 4. [Google Scholar]
- Lee, J.; Mitchell, A.E. Pharmacokinetics of quercetin absorption from apples and onions in healthy humans. J. Agric. Food Chem. 2012, 60, 3874–3881. [Google Scholar] [CrossRef] [PubMed]
- Hollman, P.C. Absorption, bioavailability, and metabolism of flavonoids. Pharm. Biol. 2004, 42, 74–83. [Google Scholar] [CrossRef]
- Llanos, P.; Contreras-Ferrat, A.; Barrientos, G.; Valencia, M.; Mears, D.; Hidalgo, C. Glucose-dependent insulin secretion in pancreatic β-cell islets from male rats requires Ca2+ release via ROS-stimulated ryanodine receptors. PLoS ONE 2015, 10, e0129238. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhen, W.; Yang, Z.; Carter, J.D.; Si, H.; Reynolds, K.A. Genistein acutely stimulates insulin secretion in pancreatic β-cells through a cAMP-dependent protein kinase pathway. Diabetes 2006, 55, 1043–1050. [Google Scholar] [CrossRef]
- Wu, L.; Velander, P.; Liu, D.; Xu, B. Olive component oleuropein promotes β-cell insulin secretion and protects β-cells from amylin amyloid-induced cytotoxicity. Biochemistry 2017, 56, 5035–5039. [Google Scholar] [CrossRef]
- Sacco, F.; Seelig, A.; Humphrey, S.J.; Krahmer, N.; Volta, F.; Reggio, A.; Marchetti, P.; Gerdes, J.; Mann, M. Phosphoproteomics reveals the GSK3-PDX1 axis as a key pathogenic signaling node in diabetic islets. Cell Metab. 2019, 29, 1422–1432.e3. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Yu, J.; Wang, A.; Liu, S.H.; Sinnett-Smith, J.; Wu, J.; Sanchez, R.; Nemunaitis, J.; Ricordi, C.; Rozengurt, E.; et al. Metformin restrains pancreatic duodenal homeobox-1 (PDX-1) function by inhibiting ERK signaling in pancreatic ductal adenocarcinoma. Curr. Mol. Med. 2016, 16, 83–90. [Google Scholar] [CrossRef]
- Khoo, S.; Griffen, S.C.; Xia, Y.; Baer, R.J.; German, M.S.; Cobb, M.H. Regulation of insulin gene transcription by ERK1 and ERK2 in pancreatic β cells. J. Biol. Chem. 2003, 278, 32969–32977. [Google Scholar] [CrossRef]
- Zhou, G.; Sinnett-Smith, J.; Liu, S.-H.; Yu, J.; Wu, J.; Sanchez, R.; Pandol, S.J.; Abrol, R.; Nemunaitis, J.; Rozengurt, E. Down-regulation of pancreatic and duodenal homeobox-1 by somatostatin receptor subtype 5: A novel mechanism for inhibition of cellular proliferation and insulin secretion by somatostatin. Front. Physiol. 2014, 5, 226. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Lee, J.; Jung, H.; Ha, T.; Kim, S.; Han, N.; Lee, E.; Kim, T.; Kwon, M.; Lee, S. Triiodothyronine induces proliferation of pancreatic β-cells through the MAPK/ERK pathway. Exp. Clin. Endocrinol. Diabetes 2014, 226, 240–245. [Google Scholar] [CrossRef]
- Liu, S.; Li, X.; Wu, Y.; Duan, R.; Zhang, J.; Du, F.; Zhang, Q.; Li, Y.; Li, N. Effects of vaspin on pancreatic β cell secretion via PI3K/Akt and NF-κB signaling pathways. PLoS ONE 2017, 12, e0189722. [Google Scholar] [CrossRef]
- Norman, B.M.; de Plata Cecilia, A. Role of AKT/mTORC1 pathway in pancreatic β-cell proliferation. Colomb. Méd. 2012, 43, 235–243. [Google Scholar]
- Du, L.-Y.; Zhao, M.; Xu, J.; Qian, D.-W.; Jiang, S.; Shang, E.-X.; Guo, J.-M.; Duan, J.-A. Analysis of the metabolites of isorhamnetin 3-O-glucoside produced by human intestinal flora in vitro by applying ultraperformance liquid chromatography/quadrupole time-of-flight mass spectrometry. J. Agric. Food Chem. 2014, 62, 2489–2495. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).