Abstract
The valorization of Spirulina as a potential biosorption material to treat contaminated wastewater was evaluated. Batch experiments were conducted to study the influence of pH value and ionic strength on the biosorption capacity of Spirulina. Higher removal capacity was observed at pH 5.2, while higher ionic strength was found to result in lower adsorption capacity, which suggests that ion exchange is a relevant mechanism for Pb (II) adsorption on Spirulina. The immobilization of Spirulina on alginate beads was found not only to increase the adsorption capacity, but also to overcome limitations such as unacceptable pressure drops on column systems. The Langmuir model was the most appropriate model to describe the biosorption equilibrium of lead by free and immobilized Spirulina. The experimental breakthrough curves were evaluated using the Thomas, Bohart-Adams, and dose-response models. The experimental results were most properly described by the dose-response model, which is consistent with previous results. The adsorption capacity of Spirulina was found to increase linearly with the influent lead concentration (in the range 4–20 mg L−1) at 1.6 mL min−1 flow rate. Batch and column experiments were compared to better understand the biosorption process. The promising results obtained indicate the potential use of Spirulina immobilized on alginate beads to treat industrial wastewater polluted with toxic metals.
1. Introduction
The increasing pollution of the environment by heavy metals due to industrial activity poses serious risks for human health and living organisms. Lead, which is non-biodegradable and toxic even at low concentrations, is one of the most serious environmental pollutants [1]. The presence of lead in various types of wastewater should be controlled according to the standard for the permitted amount of metal determined by the Environmental Protection Agency [2].
Several conventional technologies have been proposed to remove lead from aquatic environments. Recently, biosorption has been proposed as an emerging and low-cost alternative based on the sorption of dissolved pollutants on a biomaterial. This technology overcomes the most relevant drawbacks of conventional methods, mainly the indirect disposal of toxic metal sludge and the limited adsorption efficiency at low metal concentrations [3]. Biomaterials, such as bacteria, algae, fungi, and agricultural wastes, have been proposed as low-cost biosorbents for the removal of heavy metals from wastewater [4]. Among these biomaterials, algae have been widely suggested as ideal biosorbents for the removal of toxic metals from water effluents [5]. The presence of negatively charged groups on its surface, such as amino, hydroxyl, carboxyl, sulfhydryl, and sulfonate, allows the binding of heavy metals on algal biomass [6].
Brouers and Al-Musawi proposed the use of a fresh mixture of green and blue-green algae as a biosorbent for lead removal contained in aqueous solutions. They concluded that algal biomass is a promising biosorbent with a maximum removal efficiency of 98% at 40 °C and pH 3 [7]. Ecklonia radiata, a brown marine algae, was found to have a much higher value of adsorption capacity for lead than other conventional adsorbents such as powder-activated carbon and natural zeolites [8]. A green marine alga, Ulva lactuca, has also been proposed as an effective and natural biosorbent for heavy metal removal under acidic pH conditions [9]. Verma et al. evaluated the role of the brown marine alga Sargassum filipendula in the removal of lead from industrial wastewater. They associated the efficiency of the brown algae with its high content in acidic polysaccharide, usually Ca and Na alginates [10].
Spirulina (a blue-green algae) has been also proposed as an ideal biosorbent not only due to its fast growth, but also because it contains a wide range of functional groups, i.e., carboxyl, hydroxyl, sulfate, and other charged groups [11,12,13,14,15,16,17]. Şeker et al. evaluated the competitive biosorption of Pb, Cd and Ni, concluding that Spirulina has a higher selectivity toward Pb (II) ions. They suggested the application of Spirulina as a biosorbent to be used in large-scale batch biosorption systems [18]. Several studies focused on biosorbents have concluded their higher affinity for Pb biosorption in multi-metal systems associated with its ionic properties, i.e., electronegativity, ionic radius, and redox potential [19]. In recent studies, the chemical modification of Spirulina using sulfuric acid was proven to allow a strong bond between the metal ions and the modified biosorbent [20,21]. The chemical treatments, such as surface modification through exposing the biosorbent to acid solutions, were focused on improving metal biosorption efficiency and removing soluble organic compounds [22].
The usage of algae as a biosorbent presents some limitations in separating the biomass from the effluent, which produces unacceptable pressure drops. These problems are associated with the physical characteristics of the biomass (i.e., small particle size with low density and poor rigidity) [23]. The use of immobilization or cross-linking technologies could overcome these limitations by providing mechanical strength, rigidity, ideal size, and porous characteristics. The selection of a suitable carrier material is crucial to an effective immobilization. Due to their low cost, simplicity, and high biosorption capacity, alginate-based systems have been widely proposed as immobilization matrixes [24,25,26,27].
In this study, Spirulina was tested as a biosorbent to remove lead from an aqueous solution. The influence of relevant operational parameters, such as initial pH value of the aqueous solution and ionic strength, was evaluated at batch system. Adsorption isotherm models were applied to experimental data obtained from the biosorption of Pb (II) on free and alginate-immobilized biomass. The assessment of the biosorption process of Pb (II) on Spirulina immobilized in calcium alginate beads was performed in a laboratory scale fixed-bed column. The breakthrough curve was used to study the efficiency of the continuous process with the proposed biosorbent.
2. Materials and Methods
2.1. Cultivation of Spirulina sp.
A biosorbent for the removal of lead from aqueous solutions was prepared from the microalga Arthrospira (Spirulina) platensis, which was obtained from the Spanish Bank of Algae (BEA 0007B), University of Las Palmas de Gran Canaria. The isolation and cultivation of Spirulina was carried out at laboratory scale using Zarrouk medium [28]. The fresh biomass obtained, after filtration through 20 μm mesh, was washed with deionized water and frozen at −80 °C before lyophilization.
Lead solutions were obtained using Pb(NO3)2 in deionized water. The pH value and the ionic strength were controlled by adding diluted solutions of 0.1 M HNO3 and 0.01 and 0.1 M NaNO3, respectively.
2.2. Immobilization of Spirulina in Alginate Gel
The immobilization of Spirulina was carried out following the method described by Lu and Wilkins [29]. A Spirulina biomass (1 g) and deionized water (25 mL) were mixed with the same mass of sodium alginate (from Macrocystis pyrifera) (Sigma-Aldrich, St. Louis, MO, USA) dissolved in deionized water (25 mL). The resulting solution was heated to 85 °C for 15 min. With the aim of obtaining beads with diameters ranging between 1.5 and 2.0 mm, the mixture was dropped through a syringe with an internal diameter of 2 mm into a solution of 0.5 M CaCl2. After 24 h, the solution was rinsed with deionized water and immersed in a solution of 0.5 M HCl for more than 24 h. Finally, the beads were washed again with deionized water.
2.3. Batch Biosorption Studies
Batch experiments were proposed to evaluate the adsorption of Pb (II) on free and immobilized Spirulina. The experiments with free biosorbent were conducted by adding a known mass of Spirulina to 1 L of aqueous Pb(NO3)2 solutions of different concentrations. Following the guidelines published by the Organization for Economic Cooperation and Development (OECD) [30], the solid to liquid ratio (S/L) was 50 mg L−1. The samples were stirred in an orbital shaker at 120 rpm at 25 °C. After completion, the mixture was filtered using Whatman GF/C filters (pore size 0.6 µm, Whatman, Maidstone, UK) through vacuum filtration. Each experiment was performed in triplicate using the same operational conditions. The effect of the initial pH of the solutions, i.e., 2, 3, 4, 4.5, and 5.23 (the pH value of the original Pb solution, not adjusted), was evaluated using Pb (II) solutions with initial concentrations in the range 0.6 to 5.6 mg L−1. For isotherm studies, the initial Pb (II) concentration was varied from 0.6 to 5.6 mg L−1 at pH 5.23 for 72 h. The effect of ionic strength on the biosorption of Pb (II) by Spirulina was evaluated by adding 0.01 and 0.1 M NaNO3. The concentration of Pb (II) was varied from 0.6 to 5.6 mg L−1 to obtain isotherms with different ionic strengths.
The behavior of Spirulina immobilized on alginate gel was also evaluated by performing batch experiments. The essays were conducted by immersing 35 mg of alginate or Spirulina immobilized on alginate gel in 100 mL of aqueous Pb(NO3)2 solutions of different concentrations (from 20 to 150 mg L−1) until the equilibrium was reached. The final concentration of Pb (II) in the aqueous solution was analyzed by atomic absorption spectroscopy (AAS) (Varian SpectrAA-110, Varian, Palo Alto, CA, USA).
With the aim of determining the total content of Pb (II) in solid samples, microwave-assisted acid digestion was conducted. The concentration of Pb (II) was also determined by AAS (Varian SpectrAA-110). Each experiment was performed in triplicate under the same experimental conditions.
The biosorbent capacity and the removal percentage of Pb (II) were obtained as follows:
where q (mg g−1) is the amount of adsorbed lead ions, C0 (mg L−1) is the initial concentration of lead, Cf (mg L−1) is the final lead concentration, V (L) is the volume of the metal solution, and W (g) is the weight of the biosorbent.
2.4. Column Biosorption Experiments
Experiments in continuous systems, shown in Figure 1, were carried out using a glass column with a length of 3.7 cm and an internal diameter of 3.1 cm. The alginate-immobilized Spirulina mass contained in the column was of 10 g. The synthetic solutions containing Pb was circulated with a flow rate of 1.6 mL min−1 through the column using a peristaltic pump. Samples were collected at selected times until bed saturation.
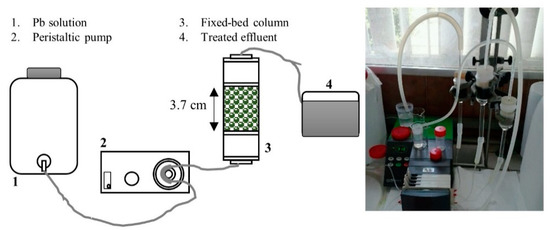
Figure 1.
Setup of the experimental system.
The adsorption capacity was calculated from the breakthrough curve as:
where C (mg L−1) is the amount of metal ions in the treated solution, Q (mL min−1) is the volumetric flow rate, W (g) is the dry weight of the biosorbent, and te (min) is the operation time of the column when C/C0 = 1.
The initial concentration of Pb was varied from 4 to 20 mg L−1 to evaluate the influence of the metal concentration on column performance. The initial and final concentrations of Pb in aqueous solutions were determined by AAS (Varian SpectrAA-110).
2.5. Mathematical Models
2.5.1. Adsorption Isotherms
The Freundlich [31], Langmuir [32], and Dubinin-Radushkevich [33] isotherms (Equations (4)–(6), respectively) were fitted to equilibrium data by non-linear regression.
where qe (mg g−1) is the amount of lead adsorbed per unit weight of biosorbent at equilibrium with a given solution concentration Ce (mg L−1), kf (mg(n−1)/n L1/n g−1) is the adsorption capacity, and n (-) is a parameter related to the adsorption intensity.
where qmax_L (mg g−1) indicates the maximum amount of metal ions per unit mass of biomass at equilibrium, and kL (L mg−1) is the equilibrium adsorption constant.
where qmax_DB (mg g−1) is the maximum adsorption capacity, β (mol2 J−2) is a constant associated with the adsorption energy, and ε (J mol−1) is Polanyi potential obtained as:
where R is the universal gas constant (8.314 J mol−1 K−1).
The value of the mean adsorption energy, E (J mol−1), is obtained as:
The value of E is widely used to make a prediction about the nature of the adsorption processes, either physical or chemical [34].
2.5.2. Sorption Dynamics in Fixed-Bed Columns
The understanding of the sorption phenomena in a fixed column is required for design and operation of a full-scale adsorption process. It should be noted that the description of the sorption in a continuous process is not straightforward due to the importance of axial dispersion, sorption kinetics, intraparticle diffusion resistance, and mass transfer. Therefore, several mathematical models have been reported to predict breakthrough behaviors and to calculate the design parameters for fixed-bed column adsorption.
The Thomas and Bohart-Adams models are frequently used to predict the dynamic behavior in fixed-column processes. Thomas’ equation is obtained from a Langmuir adsorption equilibrium system with a pseudo second-order reaction kinetic law and without axial dispersion [35]:
where C and C0 (mg L−1) are the effluent and influent lead concentration, kTH (mL mg−1 min−1) is the Thomas rate constant, qTH (mg g−1) is the adsorption capacity, W (g) is the mass of biosorbent, Q (mL min−1) is the flow rate, and Veff (L) the volume of the effluent.
The Bohart-Adams model was founded on the surface rate theory and supposes that equilibrium is not achieved instantaneously. This model is usually applied to describe the initial part of the breakthrough curve [36]. The equation can be expressed as:
where kAB (L mg−1 min−1) is the rate constant, N0 (mg L−1) is the saturation concentration, Z (cm) is the bed depth, and ν (cm min−1) is the linear velocity obtained by dividing the flow rate by the column section area.
The modified dose–response model [37] was also implemented to evaluate experimental values. This empirical model focuses on the description of the kinetics of metal removal in a biosorption column and minimizes the error generated by previous models, especially for lower and higher times of the breakthrough curve.
where a (-) and b (L) are the model parameters.
2.5.3. Mathematical Model Parameter Determination
With the aim of determining all model parameters, a non-linear square method was applied using Solver-add MS-Excel. The differences between values predicted by the model and the experimental values were estimated by the root mean square errors (RMSEs) as:
where m is the number of data points and Xexp and Xmodel are the experimental and the predicted values, respectively, of the adsorption capacity, q (mg g−1), or the fractional concentration, C/C0, for isotherm or fixed-bed adsorption models, respectively.
The minimization of RMSE values with simultaneous variation of model parameters allowed the fit of the experimental data to the non-linear forms of the equations. With the purpose of comparing the results obtained from different models, the coefficient of determination (R2) of parity plots (i.e., experimental versus model values) was determined.
3. Results and Discussion
3.1. Effect of Initial pH of the Solution
The effect of the pH value of the aqueous solution on the biosorption of Pb (II) ions on Spirulina was evaluated. The changes of Pb (II) adsorption capacity at different initial pH values (from 2 to 5.2) are depicted in Figure 2. As can be seen, the Pb (II) biosorption capacity of Spirulina is strongly dependent on pH value. When the solution pH was below 3, the biosorption capacity was significantly lower. These results are in agreement with the competition between protons and Pb (II) for adsorption sites, and with the increase in electrostatic repulsion between positively charged species. At these pH values, Pb is mainly present as Pb2+ [38]. With increasing pH, the biosorption capacity of Pb (II) on Spirulina increased. These results have been previously related to the occurrence of more ligands carrying negative charges, such as carboxyl groups, which entails higher attraction of metal ions and biosorption onto biosorbent surfaces [39]. According to the experimental results, no significant differences in Pb (II) removal were observed for pH values from 4.5 to 5.2. As 5.2 was the pH of the original aqueous Pb (II) solution, this value was selected for further experiments. Previous studies dealing with the biosorption of Pb (II) have found similar optimal pH values [40,41,42].
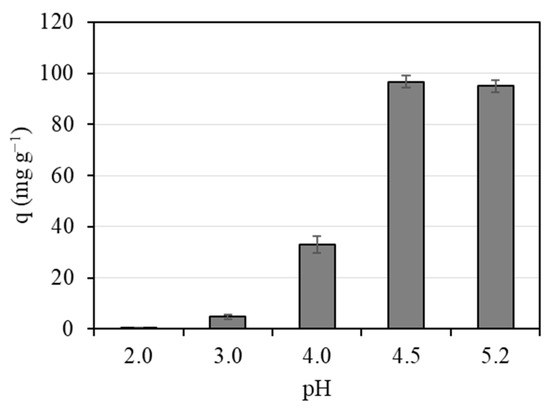
Figure 2.
Effect of pH on adsorption capacity of Pb (II) by Spirulina (C0 = 5.63 mg L−1, S/L = 50 mg L−1).
3.2. Effect of Ionic Strength on Pb (II) Biosorption
The influence of ionic strength on Pb (II) biosorption was evaluated by adding solutions of 0.01 and 0.1 M NaNO3 at pH 5.2. The experimental results, presented in Figure 3, show the importance of the ionic strength on biosorption of lead by Spirulina. The adsorption capacity of Pb (II) was 95.07 mg g−1 for an initial Pb concentration of 5.63 mg L−1 and pH 5.2. The adsorption capacity decreased linearly by increasing ionic strength, obtaining 90.2 and 36.7 mg kg−1 at ionic strength 0.01 and 0.1 M, respectively, under the same experimental conditions.
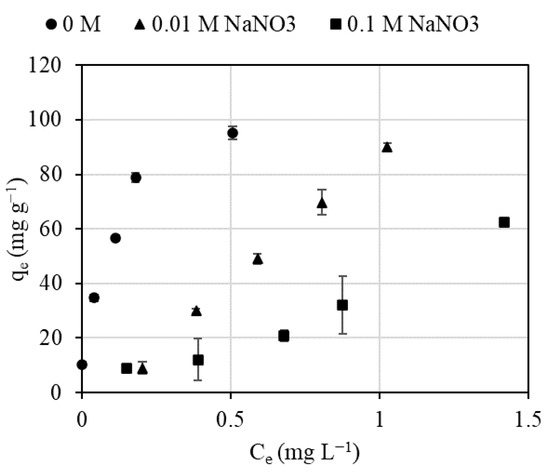
Figure 3.
Evolution of adsorbed Pb (II) concentration on Spirulina for different ionic strengths (experimental conditions: S/L: 50 mg L−1, contact time: 72 h, pH: 5.2).
Changes in the ionic strength not only affect the interfacial potential and, subsequently, the activity of electrolyte ions, but also the competition between the electrolyte ions. According to the experimental results, it could be concluded that Na ions displace Pb ions. Previous studies have explained the effect of ionic strength as an electrostatic competition of cations added as salts with heavy metals. In other words, for high ionic strength, adsorption sites are covered by counter-ions, which entail a loss of charge, and, consequently, this weakens the binding forces due to electrostatic interactions [39]. These results suggest that ion exchange is the most relevant mechanism for Pb (II) biosorption on Spirulina, as discussed in previous work carried out with other algae [43]. Other interactions, i.e., complexation/coordination, electrostatic interactions, chemisorption, physisorption, microprecipitation, and reduction, can also coexist with ion exchange [4].
3.3. Adsorption Isotherms
The Freundlich, Langmuir, and Dubinin-Radushkevich isotherm models were applied to study the equilibrium data. The experimental results were simulated with non-linear forms of these model equations, as presented in Figure 4. As can be seen in Table 1, these three models properly describe the biosorption process with high correlation coefficients and low RSME values. The Langmuir isotherm shows a slightly better fit with a correlation coefficient (R2) of 0.992. Pb (II) biosorption can be regarded as more likely with monolayer adsorption, as concluded in previous studies dealing with biosorption of metals onto algae [44,45,46]. The maximum adsorption capacity according to the Langmuir model was 114.47 mg g−1, which is comparable to the results from other studies (Table 2). The Dubinin-Radushkevich isotherm allows the description of the nature of the biosorption process through the estimation of the free energy of sorption. From the value of β, the value of the free energy obtained was higher than 8 kJ mol−1, which has been associated with chemical adsorption of Pb on Spirulina’s surface [34]. These results are consistent with previous studies dealing with Spirulina as a biosorbent [18].
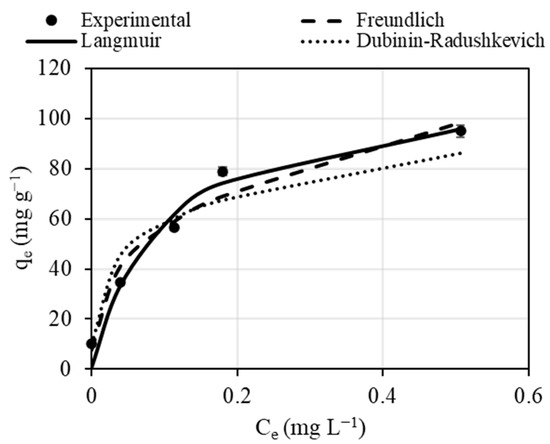
Figure 4.
Equilibrium adsorption isotherm for the adsorption of Pb (II) on Spirulina (pH = 5.23 (not adjusted), S/L = 50 mg L−1).

Table 1.
Equilibrium isotherm parameters for biosorption of Pb (II) on Spirulina. RMSE, root mean square error.

Table 2.
Adsorption capacity of Pb (II) by different biosorbents.
3.4. Immobilized Biomass
3.4.1. Isotherm Studies
Batch experiments were carried out with alginate gel and alginate-immobilized Spirulina to evaluate the isotherm models, i.e., Freundlich, Langmuir, and Dubinin-Radushkevich. The equilibrium isotherm parameters for the biosorption of Pb (II) under these experimental conditions are presented in Table 3. As can be seen, the three selected models fit properly to the experimental data. According to the Langmuir model, the maximum adsorption capacity was 303.94 and 282.17 mg g−1 for alginate and immobilized Spirulina, respectively. These values are consistent with other studies dealing with alginate as a matrix of a natural polymer to immobilize algal biomass [25]. The higher values obtained for biosorption capacity corroborate the potential of alginate as biosorbent in Pb (II) removal. Regarding the value of the free energy obtained from the Dubinin-Radushkevich parameters, it could be concluded that the adsorption of Pb is of a chemical nature in agreement with the results obtained for free Spirulina.

Table 3.
Equilibrium isotherm parameters for the biosorption of Pb (II) on alginate and Spirulina immobilized in alginate.
3.4.2. Fixed-Bed Column Studies
The breakthrough curve obtained for a flow rate of 1.6 mL min−1 and for an inlet metal ion concentration of 20 mg L−1 is presented in Figure 5. Initially, most of the Pb (II) bound close to the inlet of the column, while the downstream sections were not exposed to the metal. The shape of this curve depends on several experimental factors, such as inlet flow rates, concentrations, and other properties of the column, i.e., diameter and bed height. The section of the bed in which the concentration of Pb (II) changes importantly is known as the mass transfer zone (MTZ). The shape of this section depends on the equilibrium equation, the axial dispersion in the reactor, and the kinetics of adsorption. A square-wave curve is the ideal shape of a breakthrough, since the column would allow the largest volume of contaminated water to be treated, and the maximal amount of metal would be removed during operation. Therefore, the shape of the breakthrough curve experimentally obtained could indicate that the equilibrium between the adsorbent and the surrounding solution was not reached instantaneously [50].
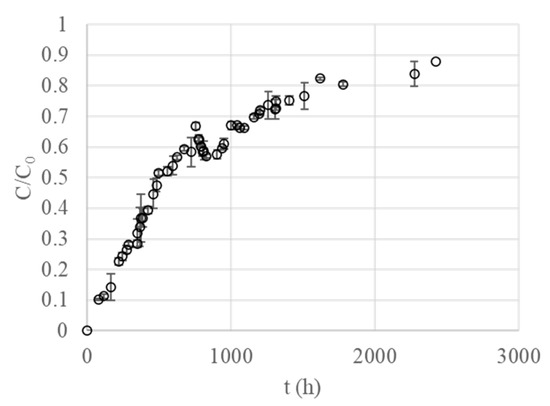
Figure 5.
Breakthrough curve for the biosorption of Pb (II) on Spirulina (C0 = 20 mg L−1, Q = 1.6 mL min−1, W = 10 g).
The mathematical description of this curve is required not only for designing a fixed-bed adsorber, but also to predict the behavior of the dynamic system. The estimated parameters obtained by non-linear regression analysis are presented in Table 4. Neither the Thomas nor the Bohart-Adams model adequately reproduced the experimental data, giving R2 values of 0.907 and 0.849, respectively.

Table 4.
Model parameters obtained by non-linear regression analysis for column studies.
As can be concluded from the RSME and R2 values (Table 4), the dose-response model shows the best fit to the experimental data. These results are in accordance with previous studies that found that this model minimizes the most relevant errors obtained from other models, particularly at low and high values of the operation time [51].
The dependence of the adsorption capacity of immobilized Spirulina on alginate on the initial metal concentration of Pb (II) is shown in Figure 6. According to the experimental results, the biosorption capacity increased linearly with an increase in the initial Pb (II) concentration. As the system is characterized by a Langmuir isotherm, the maximum value of adsorption capacity could be determined from model parameters presented in Table 3. The maximum adsorption capacities (q*) for initial concentrations of 4 and 20 mg L−1 were 243.91 and 273.51 mg g−1, respectively, while the experimental adsorption capacity (q) was 47.98 and 202.85 mg g−1, respectively. These results indicate that sorption was controlled by the mass transfer for the flow rate studied. In other words, the metal was not able to reach all positions of the biosorbent surface in a time shorter than residence time, in agreement with the conclusions from the breakthrough curve shape (Figure 5).
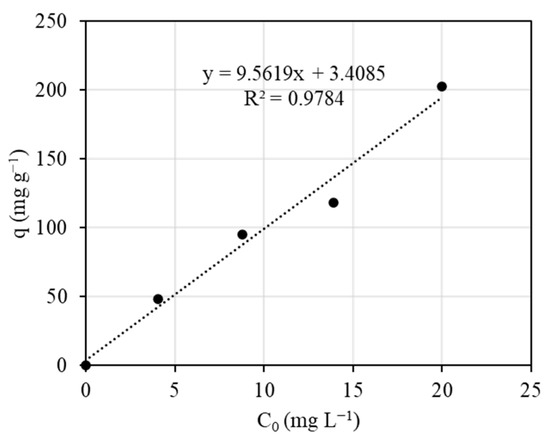
Figure 6.
Dependence of the adsorption capacity of Spirulina on the initial metal concentration of Pb (II).
4. Conclusions
Immobilized Spirulina on alginate beads was proposed as an effective biosorbent for lead removal in batch and fixed-bed columns. From batch experiments, the biosorption capacity of Spirulina was concluded to be strongly dependent on pH value. The experimental results suggested that ion exchange is probably the most relevant mechanism for Pb (II) sorption on Spirulina. The Langmuir model properly described the isotherm data of lead biosorption by free and immobilized Spirulina, indicating a monolayer sorption onto the biomass surface. The potential of Pb (II) adsorption from Spirulina using immobilized biomass in a continuous fixed-bed column was assessed. The breakthrough curve was applied to study the efficiency of the column using immobilized Spirulina. The dose-response model described better the experimental data than both the Thomas and the Bohart-Adams models, which is associated with the minimization of relevant errors obtained at low and high values of the operation time. The importance of the influent metal concentration was also evaluated, concluding that the adsorption capacity of Spirulina increased linearly with the initial metal concentration. From the comparison of batch and column studies, it was found that equilibrium between Spirulina and the solution was not instantaneously reached in the dynamic studies. The promising results obtained for immobilized Spirulina on alginate beads could be the first step of the valorization of algal biomass for the treatment of industrial wastewater contaminated by toxic metals such as Pb.
Author Contributions
Conceptualization, all authors; methodology, C.J. and J.M.R.-M.; software, J.M.R.-M. and M.V.-G.; validation, C.J. and J.M.R.-M.; investigation, all authors; resources, all authors; data curation, all authors; writing—original draft preparation, M.V.-G.; writing—review and editing, all authors; supervision, C.J. and J.M.R.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The University of Malaga is acknowledged for the financial support from the “Plan Propio de Investigación”.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy Metal Toxicity and the Environment. Mol. Clin. Environ. Toxicol. 2012, 101, 133–164. [Google Scholar] [CrossRef]
- Environmental Protection Agency. Lead and Copper Rule Revisions White Paper; Environmental Protection Agency: Washington, DC, USA, 2016.
- Beni, A.A.; Esmaeili, A. Biosorption, an Efficient Method for Removing Heavy Metals from Industrial Effluents: A Review. Environ. Technol. Innov. 2020, 17, 100503. [Google Scholar] [CrossRef]
- Torres, E. Biosorption: A Review of the Latest Advances. Processes 2020, 8, 1584. [Google Scholar] [CrossRef]
- Ubando, A.T.; Africa, A.D.M.; Maniquiz-Redillas, M.C.; Culaba, A.B.; Chen, W.-H.; Chang, J.-S. Microalgal Biosorption of Heavy Metals: A Comprehensive Bibliometric Review. J. Hazard. Mater. 2021, 402, 123431. [Google Scholar] [CrossRef]
- Davis, T.A.; Volesky, B.; Mucci, A. A Review of the Biochemistry of Heavy Metal Biosorption by Brown Algae. Water Res. 2003, 37, 4311–4330. [Google Scholar] [CrossRef]
- Brouers, F.; Al-Musawi, T.J. On the Optimal Use of Isotherm Models for the Characterization of Biosorption of Lead onto Algae. J. Mol. Liq. 2015, 212, 46–51. [Google Scholar] [CrossRef]
- Matheickal, J.T.; Yu, Q. Biosorption of Lead from Aqueous Solutions by Marine Algae Ecklonia radiata. Water Sci. Technol. 1996, 34, 1–7. [Google Scholar] [CrossRef]
- Areco, M.M.; Hanela, S.; Duran, J.; dos Santos Afonso, M. Biosorption of Cu(II), Zn(II), Cd(II) and Pb(II) by Dead Biomasses of Green Alga Ulva lactuca and the Development of a Sustainable Matrix for Adsorption Implementation. J. Hazard. Mater. 2012, 213, 123–132. [Google Scholar] [CrossRef]
- Verma, A.; Kumar, S.; Kumar, S. Biosorption of Lead Ions from the Aqueous Solution by Sargassum filipendula: Equilibrium and Kinetic Studies. J. Environ. Chem. Eng. 2016, 4, 4587–4599. [Google Scholar] [CrossRef]
- Li, Z.-Y.; Guo, S.-Y.; Li, L. Study on the Process, Thermodynamical Isotherm and Mechanism of Cr(III) Uptake by Spirulina Platensis. J. Food Eng. 2006, 75, 129–136. [Google Scholar] [CrossRef]
- Rangabhashiyam, S.; Balasubramanian, P. Characteristics, Performances, Equilibrium and Kinetic Modeling Aspects of Heavy Metal Removal Using Algae. Bioresour. Technol. Rep. 2019, 5, 261–279. [Google Scholar] [CrossRef]
- Zinicovscaia, I.; Safonov, A.; Zelenina, D.; Ershova, Y.; Boldyrev, K. Evaluation of Biosorption and Bioaccumulation Capacity of Cyanobacteria Arthrospira (Spirulina) Platensis for Radionuclides. Algal Res. 2020, 51, 102075. [Google Scholar] [CrossRef]
- Lebron, Y.A.R.; Moreira, V.R.; Santos, L.V.S.; Jacob, R.S. Remediation of Methylene Blue from Aqueous Solution by Chlorella Pyrenoidosa and Spirulina Maxima Biosorption: Equilibrium, Kinetics, Thermodynamics and Optimization Studies. J. Environ. Chem. Eng. 2018, 6, 6680–6690. [Google Scholar] [CrossRef]
- Gong, R.; Ding, Y.; Liu, H.; Chen, Q.; Liu, Z. Lead Biosorption and Desorption by Intact and Pretreated Spirulina Maxima Biomass. Chemosphere 2005, 58, 125–130. [Google Scholar] [CrossRef]
- Chojnacka, K.; Chojnacki, A.; Górecka, H. Biosorption of Cr3+, Cd2+ and Cu2+ Ions by Blue–Green Algae Spirulina Sp.: Kinetics, Equilibrium and the Mechanism of the Process. Chemosphere 2005, 59, 75–84. [Google Scholar] [CrossRef]
- Solisio, C.; Lodi, A.; Torre, P.; Converti, A.; Del Borghi, M. Copper Removal by Dry and Re-Hydrated Biomass of Spirulina Platensis. Bioresour. Technol. 2006, 97, 1756–1760. [Google Scholar] [CrossRef]
- Şeker, A.; Shahwan, T.; Eroğlu, A.E.; Yılmaz, S.; Demirel, Z.; Dalay, M.C. Equilibrium, Thermodynamic and Kinetic Studies for the Biosorption of Aqueous Lead(II), Cadmium(II) and Nickel(II) Ions on Spirulina Platensis. J. Hazard. Mater. 2008, 154, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Mahamadi, C. On the Dominance of Pb during Competitive Biosorption from Multi-Metal Systems: A Review. Cogent Environ. Sci. 2019, 5, 1635335. [Google Scholar] [CrossRef]
- Almomani, F.; Bohsale, R.R. Bio-Sorption of Toxic Metals from Industrial Wastewater by Algae Strains Spirulina Platensis and Chlorella Vulgaris: Application of Isotherm, Kinetic Models and Process Optimization. Sci. Total Environ. 2020, 755, 142654. [Google Scholar] [CrossRef]
- Sayadi, M.H.; Rashki, O.; Shahri, E. Application of Modified Spirulina Platensis and Chlorella Vulgaris Powder on the Adsorption of Heavy Metals from Aqueous Solutions. J. Environ. Chem. Eng. 2019, 7, 103169. [Google Scholar] [CrossRef]
- Ramrakhiani, L.; Ghosh, S.; Majumdar, S. Surface Modification of Naturally Available Biomass for Enhancement of Heavy Metal Removal Efficiency, Upscaling Prospects, and Management Aspects of Spent Biosorbents: A Review. Appl. Biochem. Biotechnol. 2016, 180, 41–78. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.; Edyvean, R.G.J. Biosorption of Lead, Copper and Zinc Ions on Loofa Sponge Immobilized Biomass of Phanerochaete Chrysosporium. Miner. Eng. 2004, 17, 217–223. [Google Scholar] [CrossRef]
- De Araujo, L.G.; de Borba, T.R.; de Pádua Ferreira, R.V.; Canevesi, R.L.S.; da Silva, E.A.; Dellamano, J.C.; Marumo, J.T. Use of Calcium Alginate Beads and Saccharomyces Cerevisiae for Biosorption of 241Am. J. Environ. Radioact. 2020, 223, 106399. [Google Scholar] [CrossRef]
- Bayramoğlu, G.; Tuzun, I.; Celik, G.; Yilmaz, M.; Arica, M.Y. Biosorption of Mercury(II), Cadmium(II) and Lead(II) Ions from Aqueous System by Microalgae Chlamydomonas Reinhardtii Immobilized in Alginate Beads. Int. J. Miner. Process. 2006, 81, 35–43. [Google Scholar] [CrossRef]
- McHale, A.P.; McHale, S. Microbial Biosorption of Metals: Potential in the Treatment of Metal Pollution. Biotechnol. Adv. 1994, 12, 647–652. [Google Scholar] [CrossRef]
- Petrovič, A.; Simonič, M. Removal of Heavy Metal Ions from Drinking Water by Alginate-Immobilised Chlorella Sorokiniana. Int. J. Environ. Sci. Technol. 2016, 13, 1761–1780. [Google Scholar] [CrossRef]
- Zarrouk, C. Université de Paris Contribution à l’Étude d’une Cyanophycée: Influence de Divers Facteurs Physiques et Chimiques sur la Croissance et la Photosynthèse de Spirulina Maxima (Setch et Gardner) Geitler; Faculté des Sciences de l’Université de Paris: Paris, France, 1966. [Google Scholar]
- Lu, Y.; Wilkins, E. Heavy Metal Removal by Caustic-Treated Yeast Immobilized in Alginate. J. Hazard. Mater. 1996, 49, 165–179. [Google Scholar] [CrossRef]
- OCDE. Test. No. 106: Adsorption—Desorption Using a Batch Equilibrium Method; OECD Publishing: Paris, France, 2000; ISBN 92-64-06960-7. [Google Scholar]
- Freundlich, H.M. F Over the Adsorption in Solution. J. Phys. Chem. 1906, 57, 385–471. [Google Scholar]
- Langmuir, I. The Constitution and Fundamental Properties of Solids and Liquids. Part I. Solids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef]
- Dubinin, M.M. The Potential Theory of Adsorption of Gases and Vapors for Adsorbents with Energetically Nonuniform Surfaces. Chem. Rev. 1960, 60, 235–241. [Google Scholar] [CrossRef]
- Hu, Q.; Zhang, Z. Application of Dubinin–Radushkevich Isotherm Model at the Solid/Solution Interface: A Theoretical Analysis. J. Mol. Liq. 2019, 277, 646–648. [Google Scholar] [CrossRef]
- Thomas, H.C. Heterogeneous Ion Exchange in a Flowing System. J. Am. Chem. Soc. 1944, 66, 1664–1666. [Google Scholar] [CrossRef]
- Bohart, G.S.; Adams, E.Q. Some Aspects of the Behavior of Charcoal with Respect to Chlorine. J. Am. Chem. Soc. 1920, 42, 523–544. [Google Scholar] [CrossRef]
- Yan, G.; Viraraghavan, T.; Chen, M. A New Model for Heavy Metal Removal in a Biosorption Column. Adsorpt. Sci. Technol. 2001, 19, 25–43. [Google Scholar] [CrossRef]
- Ford, R.G.; Wilkin, R.T.; Puls, R.W. Monitored Natural Attenuation of Inorganic Contaminants in Ground Water; Naiontal Risk Management Research Laboratory, U.S. Environmental Protection Agency: Washington, DC, USA, 2007; Volume 2.
- Vilar, V.J.P.; Botelho, C.M.S.; Boaventura, R.A.R. Influence of PH, Ionic Strength and Temperature on Lead Biosorption by Gelidium and Agar Extraction Algal Waste. Process. Biochem. 2005, 40, 3267–3275. [Google Scholar] [CrossRef]
- Ajitha, P.; Kumar, V.; Madhavan, S.; Gomathi, T.; Kannan, R.; Sudha, P.N.; Sukumaran, A. Removal of Toxic Heavy Metal Lead (II) Using Chitosan Oligosaccharide-Graft-Maleic Anhydride/Polyvinyl Alcohol/Silk Fibroin Composite. Int. J. Biol. Macromol. 2017, 104, 1469–1482. [Google Scholar] [CrossRef]
- Nadeem, R.; Manzoor, Q.; Iqbal, M.; Nisar, J. Biosorption of Pb(II) onto Immobilized and Native Mangifera Indica Waste Biomass. J. Ind. Eng. Chem. 2016, 35, 185–194. [Google Scholar] [CrossRef]
- Zhou, K.; Yang, Z.; Liu, Y.; Kong, X. Kinetics and Equilibrium Studies on Biosorption of Pb(II) from Aqueous Solution by a Novel Biosorbent: Cyclosorus Interruptus. J. Environ. Chem. Eng. 2015, 3, 2219–2228. [Google Scholar] [CrossRef]
- Li, Y.-H.; Du, Q.; Peng, X.; Wang, D.; Wang, Z.; Xia, Y.; Wei, B. Physico-Chemical Characteristics and Lead Biosorption Properties of Enteromorpha Prolifera. Colloids Surf. Biointerfaces 2011, 85, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, H. Biosorption of Chromium by Using Spirulina Sp. Arab. J. Chem. 2016, 9, 846–853. [Google Scholar] [CrossRef]
- Solisio, C.; Lodi, A.; Soletto, D.; Converti, A. Cadmium Biosorption on Spirulina Platensis Biomass. Bioresour. Technol. 2008, 99, 5933–5937. [Google Scholar] [CrossRef]
- Tavana, M.; Pahlavanzadeh, H.; Zarei, M.J. The Novel Usage of Dead Biomass of Green Algae of Schizomeris Leibleinii for Biosorption of Copper(II) from Aqueous Solutions: Equilibrium, Kinetics and Thermodynamics. J. Environ. Chem. Eng. 2020, 8, 104272. [Google Scholar] [CrossRef]
- Jalali, R.; Ghafourian, H.; Asef, Y.; Davarpanah, S.J.; Sepehr, S. Removal and Recovery of Lead Using Nonliving Biomass of Marine Algae. J. Hazard. Mater. 2002, 92, 253–262. [Google Scholar] [CrossRef]
- Liu, X.; Bai, X.; Dong, L.; Liang, J.; Jin, Y.; Wei, Y.; Li, Y.; Huang, S.; Qu, J. Composting Enhances the Removal of Lead Ions in Aqueous Solution by Spent Mushroom Substrate: Biosorption and Precipitation. J. Clean. Prod. 2018, 200, 1–11. [Google Scholar] [CrossRef]
- Gupta, V.K.; Rastogi, A. Biosorption of Lead from Aqueous Solutions by Green Algae Spirogyra Species: Kinetics and Equilibrium Studies. J. Hazard. Mater. 2008, 152, 407–414. [Google Scholar] [CrossRef]
- Benjamin, M.M.; Lawler, D.F. Water Quality Engineering: Physical/Chemical Treatment Processes; John Wiley & Sons: Hoboken, NJ, USA, 2013; ISBN 1-118-16965-4. [Google Scholar]
- Calero, M.; Hernáinz, F.; Blázquez, G.; Tenorio, G.; Martín-Lara, M.A. Study of Cr (III) Biosorption in a Fixed-Bed Column. J. Hazard. Mater. 2009, 171, 886–893. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).