Using Peptidomics and Machine Learning to Assess Effects of Drying Processes on the Peptide Profile within a Functional Ingredient
Abstract
1. Introduction
2. Materials and Methods
2.1. Hydrolysate Preparation and Drying
2.2. Liquid Chromatography Tandem Mass Spectrometry (LC-MS/MS) Analysis
2.3. Bioactivity Prediction
2.4. Visualisation
2.5. Statistics
3. Results
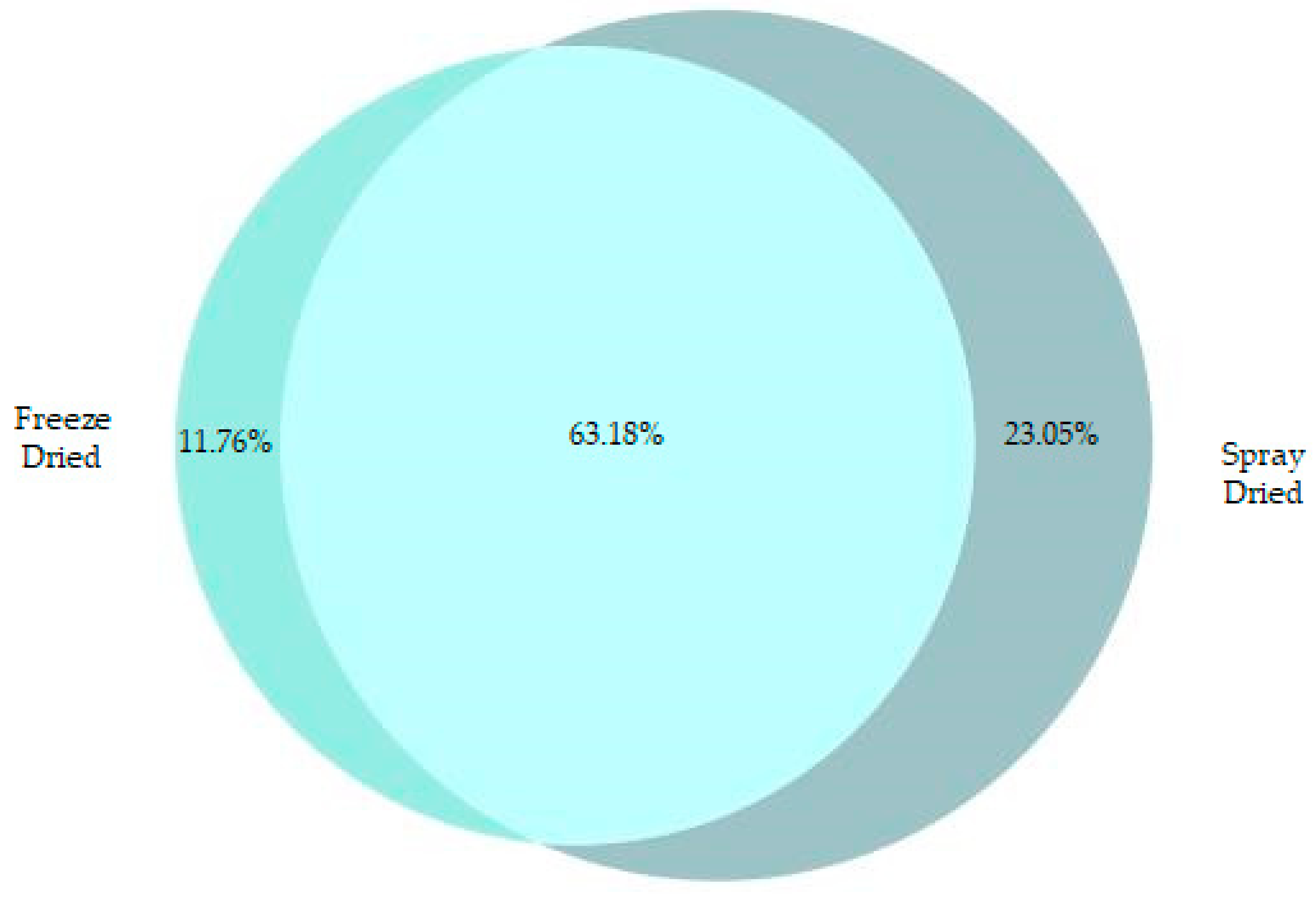
3.1. Peptide Count and Physical Characteristics of FD and SD Samples
3.2. Effects of FD and SD on Constituent Peptide Characteristics
3.2.1. Amino Acid Distribution
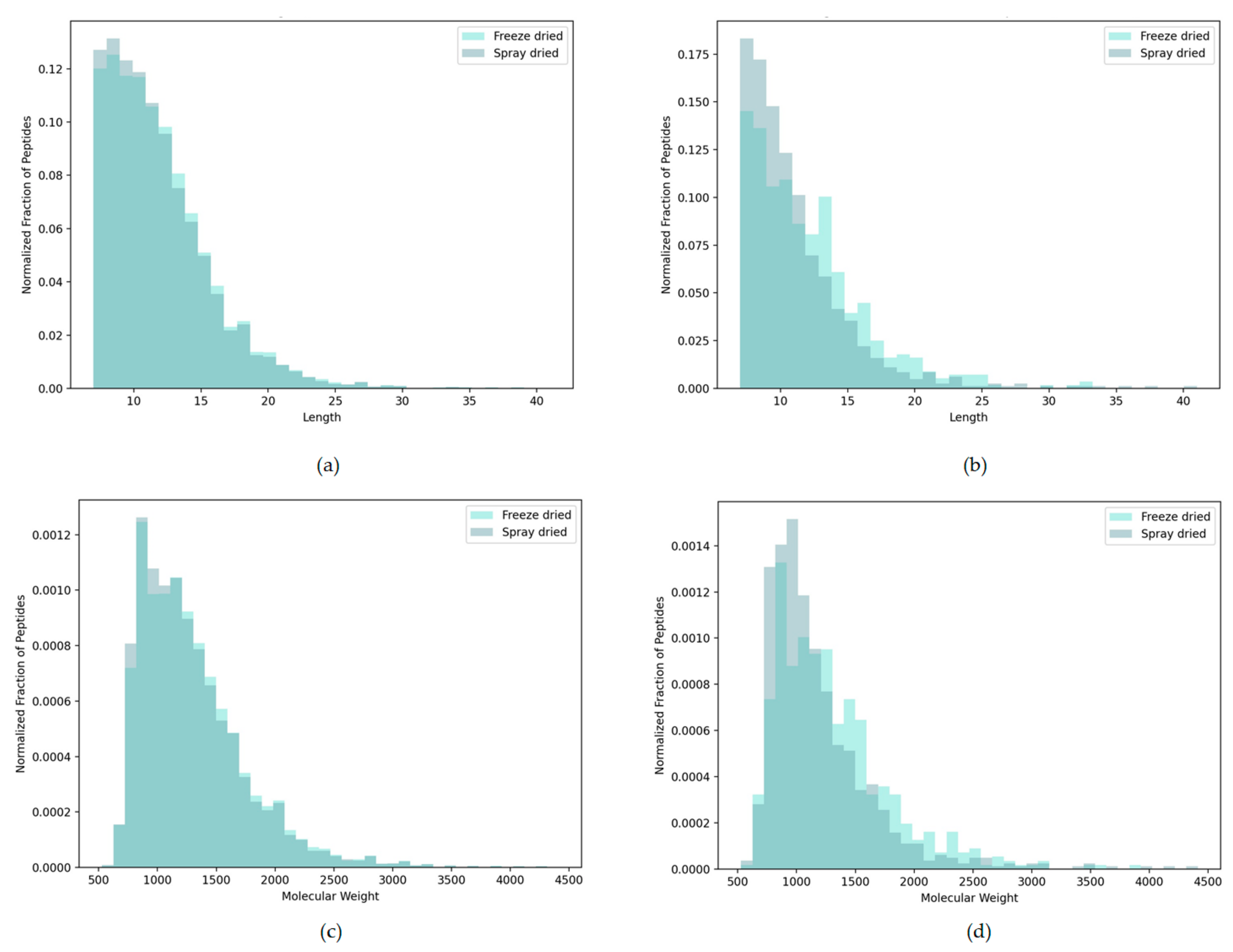
3.2.2. Peptide Length and Weight
3.2.3. Peptide Charge
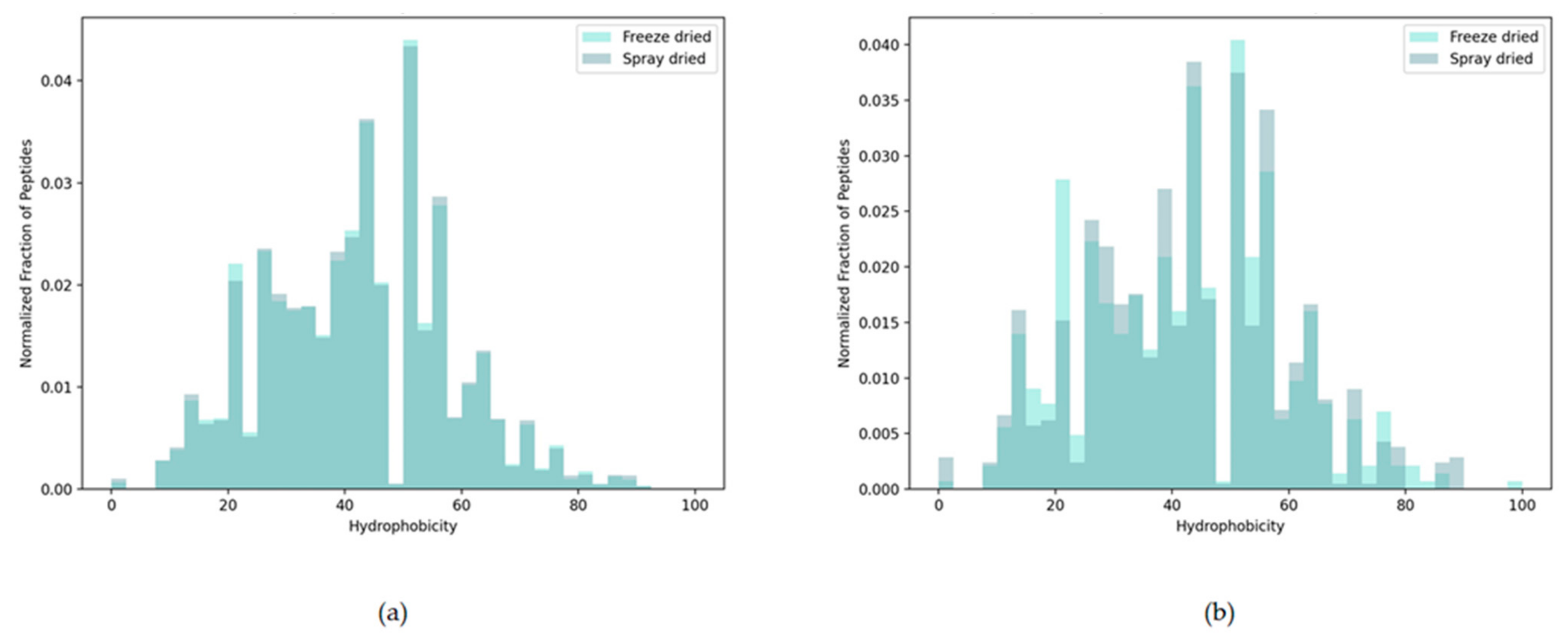
3.2.4. Peptide Hydrophobicity
3.3. Key Bioactive Peptide Retention
3.4. Predicted Anti-Inflammatory Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tunick, M. Whey protein production and utilization. In Whey Processing, Functionality and Health Benefits; Onwulata, C., Huth, P.J., Eds.; Blackwell Publishing: Oxford, UK; IFT Press: Ames, IA, USA, 2008; pp. 169–184. [Google Scholar]
- Rivera del Rio, A.; Opazo-Navarrete, M.; Cepero-Betancourt, Y.; Tabilo-Munizaga, G.; Boom, R.M.; Janssen, A.E.M. Heat-induced changes in microstructure of spray-dried plant protein isolates and its implications on in vitro gastric digestion. LWT 2020, 118, 108795. [Google Scholar] [CrossRef]
- Östbring, K.; Sjöholm, I.; Rayner, M.; Erlanson-Albertsson, C. Effects of storage conditions on degradation of chlorophyll and emulsifying capacity of thylakoid powders produced by different drying methods. Foods 2020, 9, 669. [Google Scholar] [CrossRef]
- Silva, J.; Freixo, R.; Gibbs, P.; Teixeira, P. Spray-drying for the production of dried cultures. Int. J. Dairy Technol. 2011, 64, 321–335. [Google Scholar] [CrossRef]
- Barbosa, J.; Borges, S.; Amorim, M.; Pereira, M.J.; Oliveira, A.; Pintado, M.E.; Teixeira, P. Comparison of spray drying, freeze drying and convective hot air drying for the production of a probiotic orange powder. J. Funct. Foods 2015, 17, 340–351. [Google Scholar] [CrossRef]
- Chong, S.Y.; Wong, C.W. Production of Spray-Dried Sapodilla (Manilkara zapota) Powder from Enzyme-Aided Liquefied Puree. J. Food Process. Preserv. 2015, 39, 2604–2611. [Google Scholar] [CrossRef]
- Schmitz-Schug, I.; Foerst, P.; Kulozik, U. Impact of the spray drying conditions and residence time distribution on lysine loss in spray dried infant formula. Dairy Sci. Technol. 2013, 93, 443–462. [Google Scholar] [CrossRef]
- Li, X.; Feng, Y.; Ting, S.; Jiang, J.; Liu, Y. Effect of processing conditions on the physiochemical properties and nutrients retention of spray-dried microcapsules using mixed protein system. CYTA J. Food 2019, 17, 25–35. [Google Scholar] [CrossRef]
- Haque, M.A.; Adhikari, B. Proteins in Spray Drying Process. Handb. Ind. Dry. 2015, 33, 971–983. [Google Scholar]
- Castro-Albarrán, J.; Aguilar-Uscanga, B.R.; Calon, F.; St-Amour, I.; Solís-Pacheco, J.; Saucier, L.; Ratti, C. Spray and freeze drying of human milk on the retention of immunoglobulins (IgA, IgG, IgM). Dry. Technol. 2016, 34, 1801–1809. [Google Scholar] [CrossRef]
- Ma, J.J.; Mao, X.Y.; Wang, Q.; Yang, S.; Zhang, D.; Chen, S.W.; Li, Y.H. Effect of spray drying and freeze drying on the immunomodulatory activity, bitter taste and hygroscopicity of hydrolysate derived from whey protein concentrate. LWT Food Sci. Technol. 2014, 56, 296–302. [Google Scholar] [CrossRef]
- Wang, W. Lyophilization and development of solid protein pharmaceuticals. Int. J. Pharm. 2000, 203, 1–60. [Google Scholar] [CrossRef]
- Fang, Z.; Bhandari, B. Spray Drying, Freeze Drying and Related Processes for Food Ingredient and Nutraceutical Encapsulation; Woodhead Publishing: Cambridge, UK, 2012; pp. 73–109. [Google Scholar]
- Li-Chan, E.C.Y. Bioactive peptides and protein hydrolysates: Research trends and challenges for application as nutraceuticals and functional food ingredients. Curr. Opin. Food Sci. 2015, 1, 28–37. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Fogacci, F.; Colletti, A. Potential role of bioactive peptides in prevention and treatment of chronic diseases: A narrative review. Br. J. Pharmacol. 2017, 174, 1378–1394. [Google Scholar] [CrossRef] [PubMed]
- Möller, N.P.; Scholz-Ahrens, K.E.; Roos, N.; Schrezenmeir, J. Bioactive peptides and proteins from foods: Indication for health effects. Eur. J. Nutr. 2008, 47, 171–182. [Google Scholar] [CrossRef]
- Udenigwe, C.C. Mohan Aishwarya Mechanisms of food protein-derived antihypertensive peptides other than ACE inhibition. J. Funct. Foods 2014, 8, 45–52. [Google Scholar] [CrossRef]
- Zhang, S.S.; Han, L.W.; Shi, Y.P.; Li, X.B.; Zhang, X.M.; Hou, H.R.; Lin, H.W.; Liu, K.C. Two novel multi-functional peptides from meat and visceral mass of marine snail Neptunea arthritica cumingii and their activities in vitro and in vivo. Mar. Drugs 2018, 16, 473. [Google Scholar] [CrossRef] [PubMed]
- Samantha, S.; Bruna, A.; Martin, A.; Fabio, B.; Sandro, A.; Aline, R. Drying by spray drying in the food industry: Micro-encapsulation, process parameters and main carriers used. Afr. J. Food Sci. 2015, 9, 462–470. [Google Scholar] [CrossRef]
- Wang, H.; Tong, X.; Yuan, Y.; Peng, X.; Zhang, Q.; Zhang, S.; Xie, C.; Zhang, X.; Yan, S.; Xu, J.; et al. Effect of Spray-Drying and Freeze-Drying on the Properties of Soybean Hydrolysates. J. Chem. 2020, 2020, 9201457. [Google Scholar] [CrossRef]
- Kanwate, B.W.; Ballari, R.V.; Kudre, T.G. Influence of spray-drying, freeze-drying and vacuum-drying on physicochemical and functional properties of gelatin from Labeo rohita swim bladder. Int. J. Biol. Macromol. 2019, 121, 135–141. [Google Scholar] [CrossRef]
- Chen, C.; Chi, Y.J.; Xu, W. Comparisons on the Functional Properties and Antioxidant Activity of Spray-Dried and Freeze-Dried Egg White Protein Hydrolysate. Food Bioprocess Technol. 2012, 5, 2342–2352. [Google Scholar] [CrossRef]
- Udenigwe, C.C.; Aluko, R.E. Food Protein-Derived Bioactive Peptides: Production, Processing, and Potential Health Benefits. J. Food Sci. 2012, 77, R11–R24. [Google Scholar] [CrossRef]
- Kennedy, K.; Cal, R.; Casey, R.; Lopez, C.; Adelfio, A.; Molloy, B.; Wall, A.M.; Holton, T.A.; Khaldi, N. The anti-ageing effects of a natural peptide discovered by Artificial Intelligence. Int. J. Cosmet. Sci. 2020, 42, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, K.; Keogh, B.; Lopez, C.; Adelfio, A.; Molloy, B.; Kerr, A.; Wall, A.M.; Jalowicki, G.; Holton, T.A.; Khaldi, N. An Artificial Intelligence Characterized Functional Ingredient, Derived from Rice, Inhibits TNF-α and Significantly Improves Physical Strength in an Inflammaging Population. Foods 2020, 9, 1147. [Google Scholar] [CrossRef] [PubMed]
- Rein, D.; Ternes, P.; Demin, R.; Gierke, J.; Helgason, T.; Schön, C. Artificial intelligence identified peptides modulate inflammation in healthy adults. Food Funct. 2019, 10, 6030–6041. [Google Scholar] [CrossRef] [PubMed]
- Corrochano, A.R.; Cal, R.; Kennedy, K.; Wall, A.; Murphy, N.; Trajkovic, S.; O’Callaghan, S.; Adelfio, A.; Khaldi, N. Characterising the efficacy and bioavailability of bioactive peptides identified for attenuating muscle atrophy within a Vicia faba-derived functional ingredient. Curr. Res. Food Sci. 2021. in review. [Google Scholar]
- Wickham, H. Use R! ggplot2—Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2009; ISBN 9780387938363. [Google Scholar]
- Lopez, C.; Adelfio, A.; Wall, A.M.; Molloy, B.; Holton, T.A.; Khaldi, N. Human milk and infant formulae: Peptide differences and the opportunity to address the functional gap. Curr. Res. Food Sci. 2020. [Google Scholar] [CrossRef] [PubMed]
- Caparino, O.A.; Tang, J.; Nindo, C.I.; Sablani, S.S.; Powers, J.R.; Fellman, J.K. Effect of drying methods on the physical properties and microstructures of mango (Philippine ‘Carabao’ var.) powder. J. Food Eng. 2012, 111, 135–148. [Google Scholar] [CrossRef]
- Saifullah, M.; Yusof, Y.A.; Chin, N.L.; Aziz, M.G. Physicochemical and flow properties of fruit powder and their effect on the dissolution of fast dissolving fruit powder tablets. Powder Technol. 2016, 301, 396–404. [Google Scholar] [CrossRef]
- Zhao, Q.; Xiong, H.; Selomulya, C.; Chen, X.D.; Huang, S.; Ruan, X.; Zhou, Q.; Sun, W. Effects of Spray Drying and Freeze Drying on the Properties of Protein Isolate from Rice Dreg Protein. Food Bioprocess Technol. 2013, 6, 1759–1769. [Google Scholar] [CrossRef]
- Tang, W.L.; Zhang, M.; Adhikari, B.; Mujumdar, A.S. Effects of Preparation and Drying Methods on the Antioxidant Activity of Enzymatically Hydrolyzed Porcine Placenta Hydrolysates. Dry. Technol. 2013, 31, 1600–1610. [Google Scholar] [CrossRef]
- Blaise, K.; Nicolas, N.Y.; Clémence, B.; Richard, K. Influence of spray-drying temperature on physicochemical and functional properties of protein isolates of three leguminous plants (Canavalia ensiformis, Vigna unguiculata and Glycine max) from Cameroon. Cogent Chem. 2017, 3. [Google Scholar] [CrossRef]
- Sharma, A.; Singla, D.; Rashid, M.; Raghava, G.P.S. Designing of peptides with desired half-life in intestine-like environment. BMC Bioinform. 2014, 15, 1–8. [Google Scholar] [CrossRef]
- Yakimov, A.P.; Afanaseva, A.S.; Khodorkovskiy, M.A.; Petukhov, M.G. Design of Stable a-Helical Peptides and Thermostable Proteins in Biotechnology and Biomedicine. Acta Nat. 2016, 8, 70–81. [Google Scholar] [CrossRef]
- Huang, M.; Zhang, H.; Zhao, C.; Chen, G.; Zou, Y. Amino acid content in rice grains is affected by high temperature during the early grain-filling period. Sci Rep. 2019, 9, 2700. [Google Scholar] [CrossRef] [PubMed]
- Panja, A.S.; Maiti, S.; Bandyopadhyay, B. Protein stability governed by its structural plasticity is inferred by physicochemical factors and salt bridges. Sci. Rep. 2020, 10, 1822. [Google Scholar] [CrossRef] [PubMed]
- Kramer, R.M.; Shende, V.R.; Motl, N.; Pace, C.N.; Scholtz, J.M. Toward a molecular understanding of protein solubility: Increased negative surface charge correlates with increased solubility. Biophys. J. 2012, 102, 1907–1915. [Google Scholar] [CrossRef]
- Wang, L.; Ding, L.; Du, Z.; Liu, J. Effects of hydrophobicity and molecular weight on the transport permeability of oligopeptides across Caco-2 cell monolayers. J. Food Biochem. 2020, 44, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, K.; Cal, R.; Casey, R.; Holton, T.; Adelfio, A.; Wall, A.; Khaldi, N. pep_35E7UW, a natural peptide with cutaneous anti-ageing effects discovered within the Oryza sativa proteome through machine learning. J. Dermatol. Cosmetol. 2020, 4, 109–116. [Google Scholar] [CrossRef]
- Cal, R.; Davis, H.; Kerr, A.; Wall, A.; Molloy, B.; Chauhan, S.; Trajkovic, S.; Holyer, I.; Adelfio, A.; Khaldi, N. Preclinical Evaluation of a Food-Derived Functional Ingredient to Address Skeletal Muscle Atrophy. Nutrients 2020, 12, 2274. [Google Scholar] [CrossRef] [PubMed]



| Peptide Sequence | Bioactivity | FD | SD |
|---|---|---|---|
| TVFDGVLRPGQL | Anti-Inflammatory | Yes | Yes |
| FYNEGDAPVVAL + | Anti-Inflammatory | Yes | Yes |
| IYGPDTGVDYKDNQMR | Anti-Inflammatory | Yes | Yes |
| GYYGEQQQQPGMTR | Anti-Inflammatory | Yes | Yes |
| IDGYDTPVEGR | Anti-Inflammatory | Yes | Yes |
| NGVLRPGQL | Anti-Inflammatory | Yes | Yes |
| SEEGYYGEQQQQPGMTR | Anti-Inflammatory | Yes | Yes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chauhan, S.; O’Callaghan, S.; Wall, A.; Pawlak, T.; Doyle, B.; Adelfio, A.; Trajkovic, S.; Gaffney, M.; Khaldi, N. Using Peptidomics and Machine Learning to Assess Effects of Drying Processes on the Peptide Profile within a Functional Ingredient. Processes 2021, 9, 425. https://doi.org/10.3390/pr9030425
Chauhan S, O’Callaghan S, Wall A, Pawlak T, Doyle B, Adelfio A, Trajkovic S, Gaffney M, Khaldi N. Using Peptidomics and Machine Learning to Assess Effects of Drying Processes on the Peptide Profile within a Functional Ingredient. Processes. 2021; 9(3):425. https://doi.org/10.3390/pr9030425
Chicago/Turabian StyleChauhan, Sweeny, Sean O’Callaghan, Audrey Wall, Tomasz Pawlak, Ben Doyle, Alessandro Adelfio, Sanja Trajkovic, Mark Gaffney, and Nora Khaldi. 2021. "Using Peptidomics and Machine Learning to Assess Effects of Drying Processes on the Peptide Profile within a Functional Ingredient" Processes 9, no. 3: 425. https://doi.org/10.3390/pr9030425
APA StyleChauhan, S., O’Callaghan, S., Wall, A., Pawlak, T., Doyle, B., Adelfio, A., Trajkovic, S., Gaffney, M., & Khaldi, N. (2021). Using Peptidomics and Machine Learning to Assess Effects of Drying Processes on the Peptide Profile within a Functional Ingredient. Processes, 9(3), 425. https://doi.org/10.3390/pr9030425








