Creating Values from Biomass Pyrolysis in Sweden: Co-Production of H2, Biocarbon and Bio-Oil
Abstract
1. Introduction
2. Materials and Methods
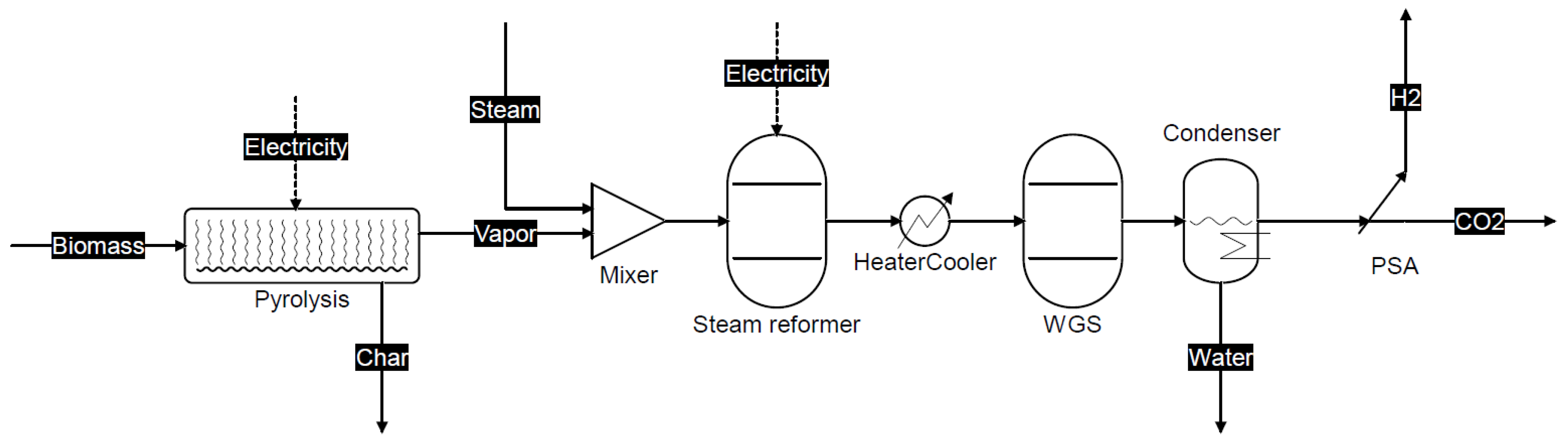
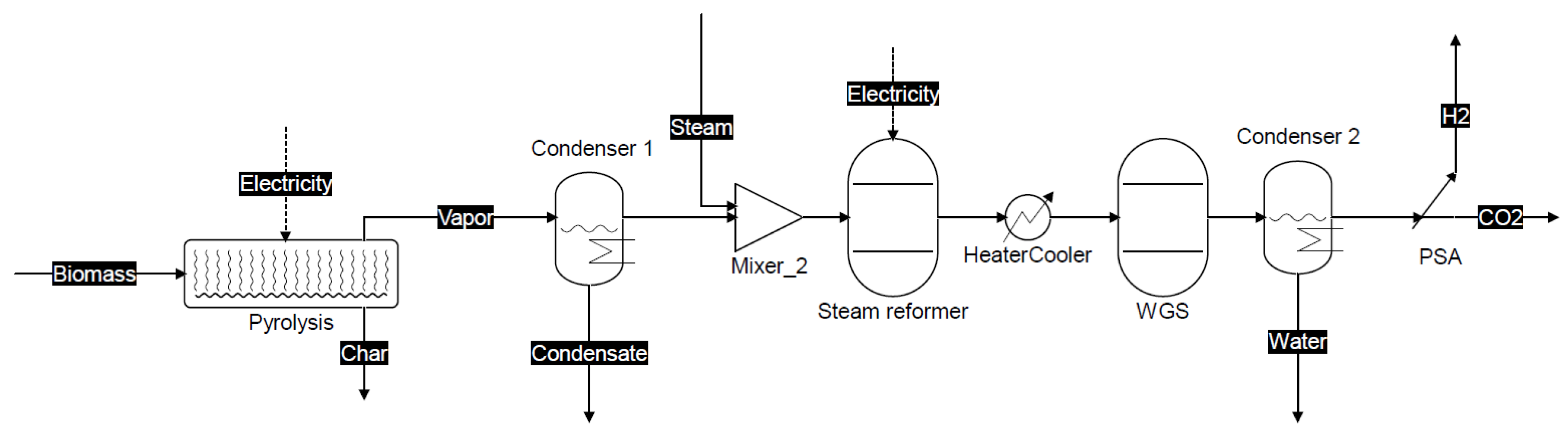
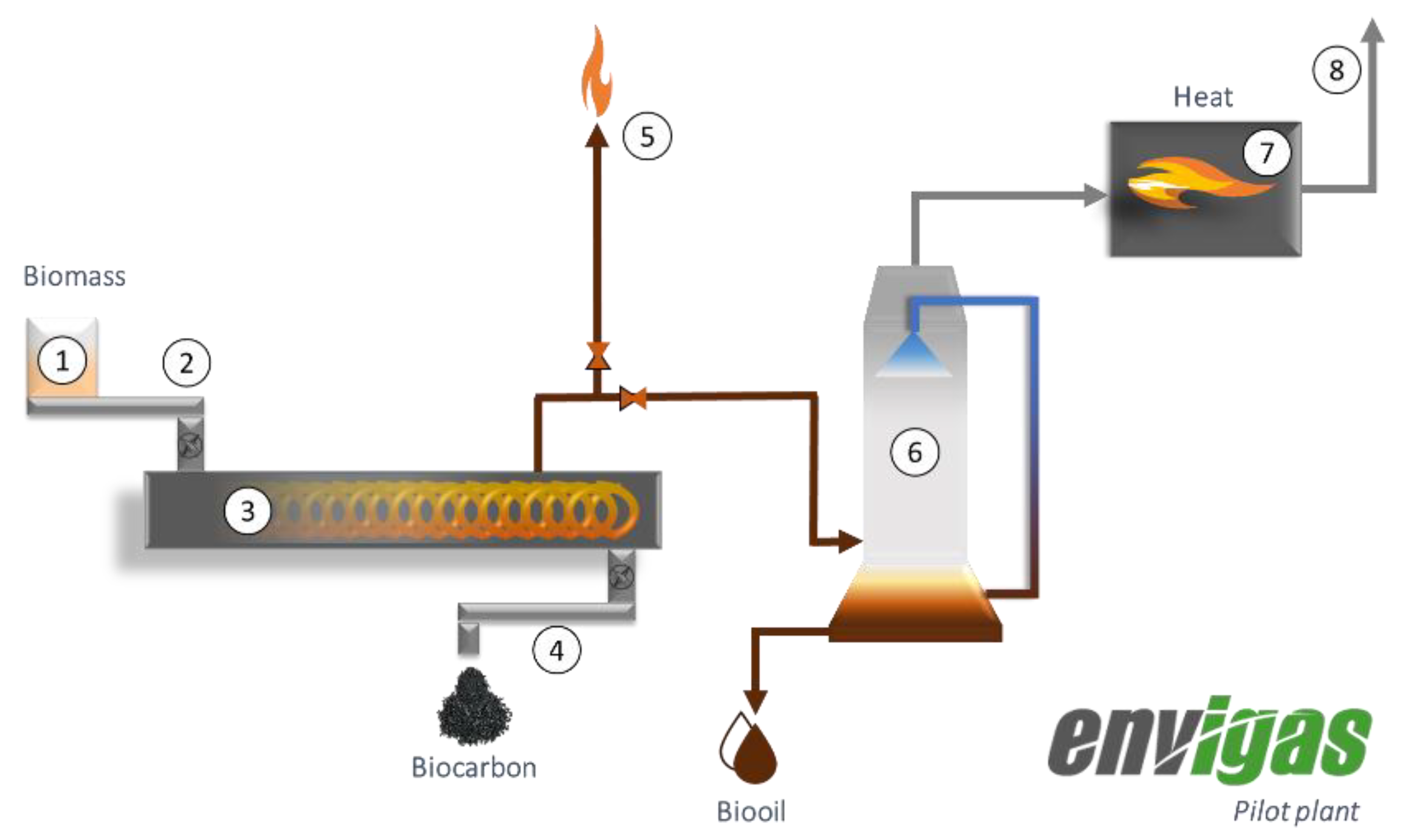
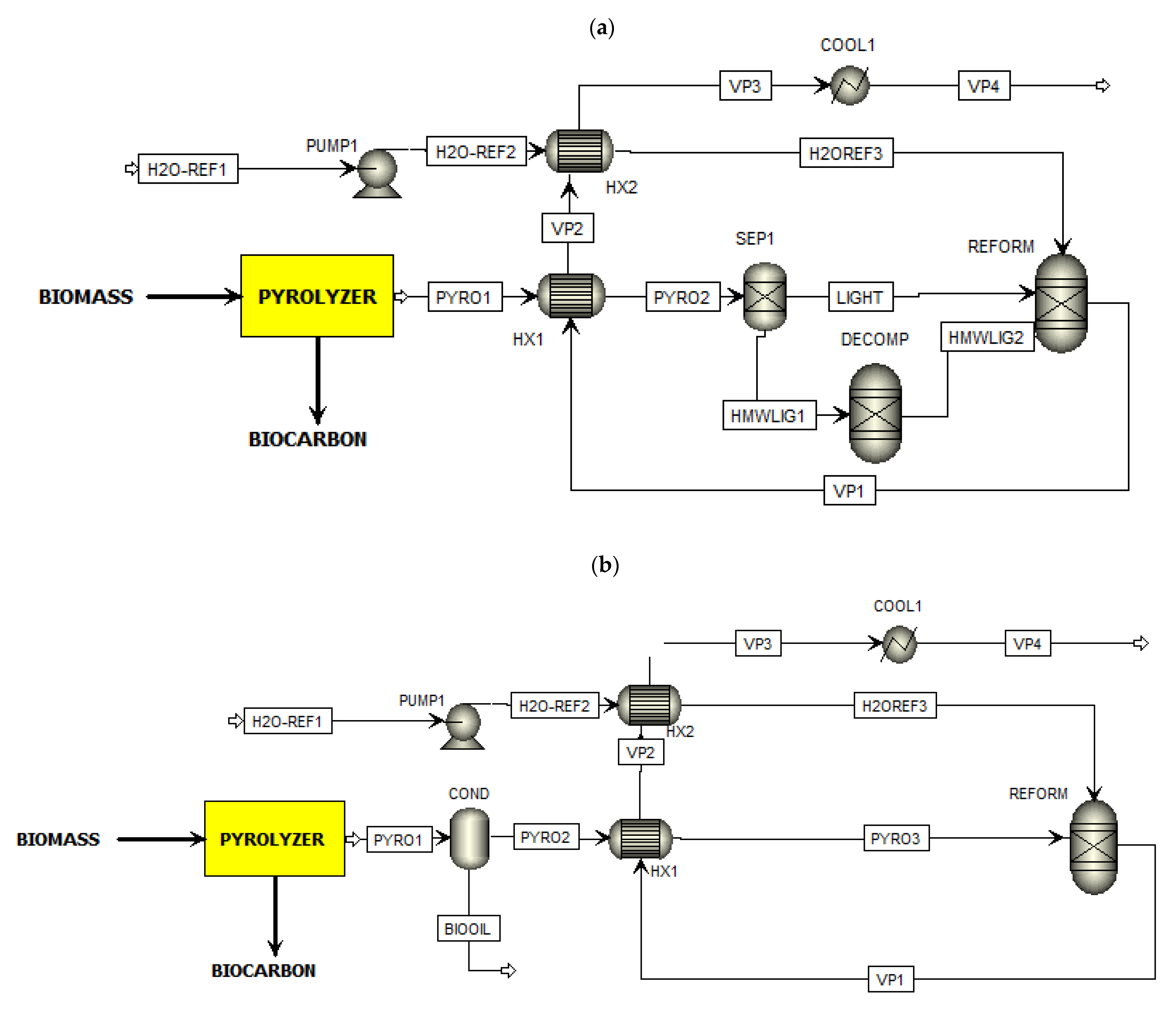
2.1. Process Description of the Proposed Co-Production Systems
2.2. Materials and Products Analysis
2.3. Calculation/Simulation
- The biomass feed input is 1000 kg/h.
- The process is operated under steady-state conditions.
- Gases are treated as ideal gases, and the ambient pressure is 101.325 kPa.
- The property method selected is Peng-Robinson for the all-reactor modules.
- The efficiency of the compressor and pump are 90 and 75%, respectively [36].
2.4. Assumptions and Basis for Calculations
2.4.1. Pyrolysis Yields
2.4.2. Liquid and Gas Compositions
2.4.3. Bio-Oil Condenser
2.4.4. Steam Reformer
2.4.5. WGS Reactor
2.4.6. Pressure Swing Absorber (PSA)
2.5. System Evaluation
3. Results and Discussion
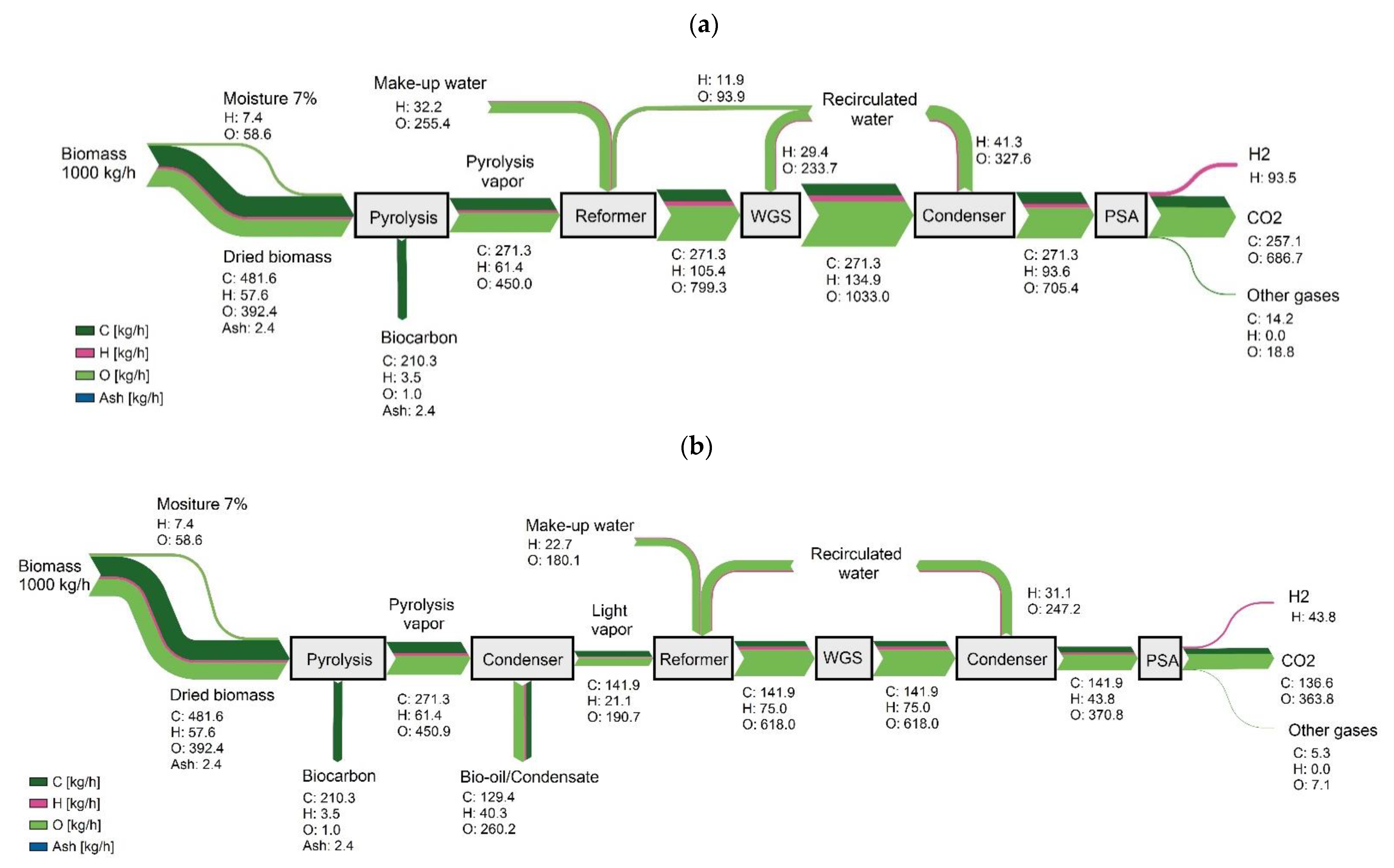
3.1. Mass and Energy Flow
3.1.1. Scheme 1
3.1.2. Scheme 2
3.2. Sensitivity Analysis
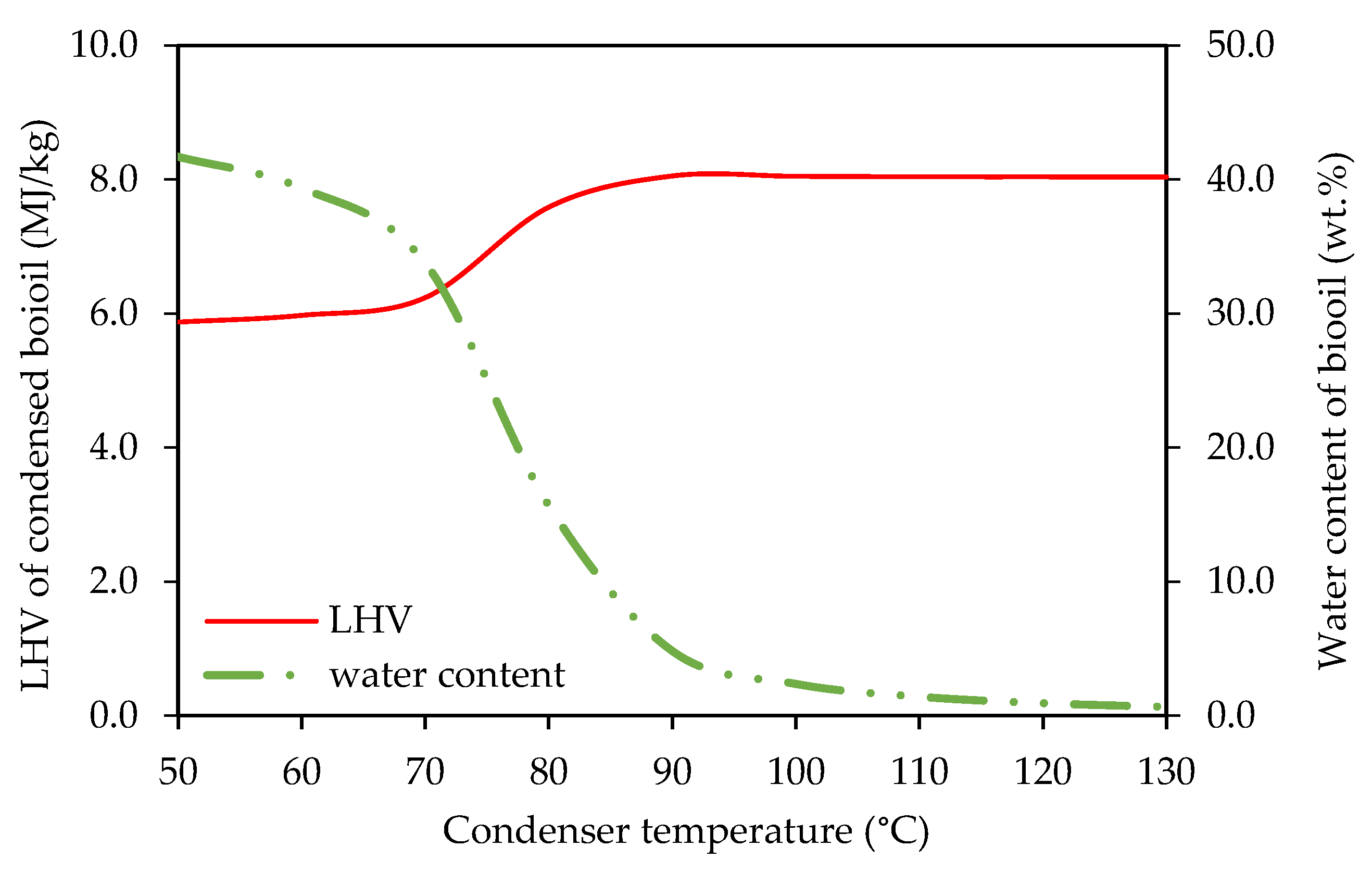
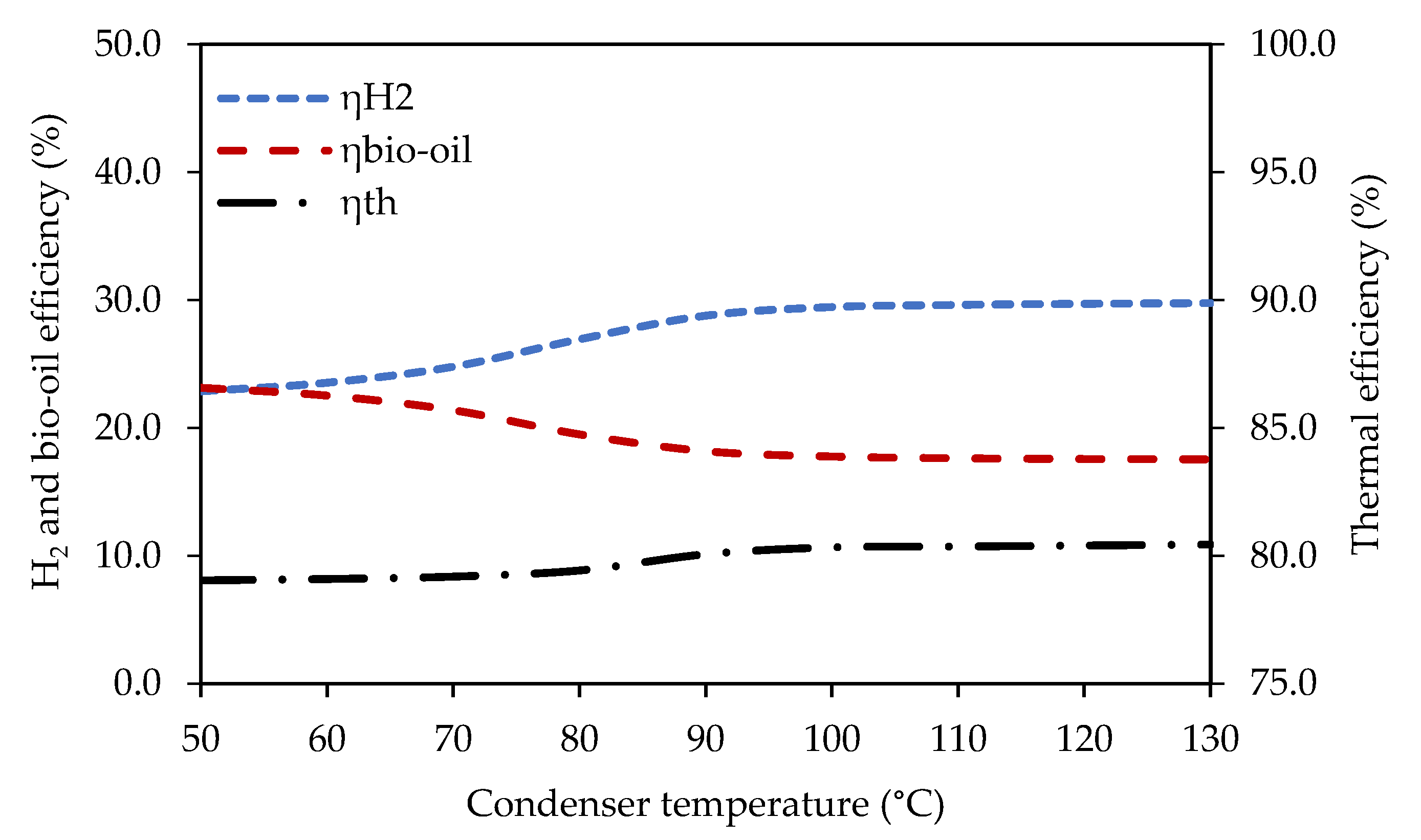
3.2.1. Effect of Temperature of the Bio-Oil Condenser
3.2.2. Effect of Moisture Content of Biomass
3.3. Operating Expense Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sveriges Geologiska Undersökning (SGU) Bergverksstatistik 2019: Statistics of the Swedish Mining Industry. 2019. Available online: http://resource.sgu.se/bergsstaten/bergverksstatistik-2019.pdf (accessed on 11 November 2020).
- Sandberg, H.; Lagneborg, R.; Lindblad, B.; Axelsson, H.; Bentell, L. CO2 emissions of the Swedish steel industry. Scand. J. Metall. 2001, 30, 420–425. [Google Scholar] [CrossRef]
- Suopajärvi, H.; Umeki, K.; Mousa, E.; Hedayati, A.; Romar, H.; Kemppainen, A.; Wang, C.; Phounglamcheik, A.; Tuomikoski, S.; Norberg, N.; et al. Use of biomass in integrated steelmaking—Status quo, future needs and comparison to other low-CO2 steel production technologies. Appl. Energy 2018, 213, 384–407. [Google Scholar] [CrossRef]
- Babich, A.; Senk, D. Coke in the iron and steel industry. In New Trends in Coal Conversion; Elsevier: Amsterdam, The Netherlands, 2019; pp. 367–404. [Google Scholar]
- Pei, M.; Petäjäniemi, M.; Regnell, A.; Wijk, O. Toward a fossil free future with hybrit: Development of iron and steelmaking technology in Sweden and Finland. Metals 2020, 10, 972. [Google Scholar] [CrossRef]
- Mousa, E.; Wang, C.; Riesbeck, J.; Larsson, M. Biomass applications in iron and steel industry: An overview of challenges and opportunities. Renew. Sustain. Energy Rev. 2016, 65, 1247–1266. [Google Scholar] [CrossRef]
- Sveaskog Brief Facts 1: What is Swedish Forestry? Available online: https://www.sveaskog.se/en/forestry-the-swedish-way/short-facts/brief-facts-1/ (accessed on 26 January 2021).
- Swedish Bioenergy Assn (Svebio). Roadmap Bioenergy—Meeting the Demand for Bioenergy in a Fossil Free SWEDEN. 2020. Available online: https://www.svebio.se/app/uploads/2020/03/Roadmap-Bioenergy-2020.pdf (accessed on 26 January 2021).
- Phounglamcheik, A.; Wang, L.; Romar, H.; Kienzl, N.; Broström, M.; Ramser, K.; Skreiberg, Ø.; Umeki, K. Effects of Pyrolysis Conditions and Feedstocks on the Properties and Gasification Reactivity of Charcoal from Woodchips. Energy Fuels 2020, 34, 8353–8365. [Google Scholar] [CrossRef]
- Sophonrat, N.; Brink, T.; Sjöblom, K. PyNe 45 Newsletter—IEA Bioenergy Task 34. 2019, pp. 13–17. Available online: https://task34.ieabioenergy.com/wp-content/uploads/sites/3/2019/12/PyNe-45_final.pdf (accessed on 30 November 2020).
- Arregi, A.; Amutio, M.; Lopez, G.; Bilbao, J.; Olazar, M. Evaluation of thermochemical routes for hydrogen production from biomass: A review. Energy Convers. Manag. 2018, 165, 696–719. [Google Scholar] [CrossRef]
- Shiva Kumar, S.; Himabindu, V. Hydrogen production by PEM water electrolysis—A review. Mater. Sci. Energy Technol. 2019, 2, 442–454. [Google Scholar] [CrossRef]
- Zaini, I.N.; Nurdiawati, A.; Aziz, M. Cogeneration of power and H2 by steam gasification and syngas chemical looping of macroalgae. Appl. Energy 2017, 207, 134–145. [Google Scholar] [CrossRef]
- Zaini, I.N.; Gomez-Rueda, Y.; García López, C.; Ratnasari, D.K.; Helsen, L.; Pretz, T.; Jönsson, P.G.; Yang, W. Production of H2-rich syngas from excavated landfill waste through steam co-gasification with biochar. Energy 2020, 207, 118208. [Google Scholar] [CrossRef]
- Nurdiawati, A.; Zaini, I.N.; Irhamna, A.R.; Sasongko, D.; Aziz, M. Novel configuration of supercritical water gasification and chemical looping for highly-efficient hydrogen production from microalgae. Renew. Sustain. Energy Rev. 2019, 112, 369–381. [Google Scholar] [CrossRef]
- Czernik, S.; Evans, R.; French, R. Hydrogen from biomass-production by steam reforming of biomass pyrolysis oil. Catal. Today 2007, 129, 265–268. [Google Scholar] [CrossRef]
- Zhang, S.P.; Li, X.J.; Li, Q.Y.; Xu, Q.L.; Yan, Y.J. Hydrogen production from the aqueous phase derived from fast pyrolysis of biomass. J. Anal. Appl. Pyrolysis 2011, 92, 158–163. [Google Scholar] [CrossRef]
- Norouzi, O.; Jafarian, S.; Safari, F.; Tavasoli, A.; Nejati, B. Promotion of hydrogen-rich gas and phenolic-rich bio-oil production from green macroalgae Cladophora glomerata via pyrolysis over its bio-char. Bioresour. Technol. 2016, 219, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Adrados, A.; Lopez-Urionabarrenechea, A.; Solar, J.; Requies, J.; De Marco, I.; Cambra, J.F. Upgrading of pyrolysis vapours from biomass carbonization. J. Anal. Appl. Pyrolysis 2013, 103, 293–299. [Google Scholar] [CrossRef]
- Adrados, A.; Lopez-Urionabarrenechea, A.; Acha, E.; Solar, J.; Caballero, B.M.; de Marco, I. Hydrogen rich reducing gases generation in the production of charcoal from woody biomass carbonization. Energy Convers. Manag. 2017, 148, 352–359. [Google Scholar] [CrossRef]
- Arregi, A.; Lopez, G.; Amutio, M.; Barbarias, I.; Bilbao, J.; Olazar, M. Hydrogen production from biomass by continuous fast pyrolysis and in-line steam reforming. RSC Adv. 2016, 6, 25975–25985. [Google Scholar] [CrossRef]
- Chen, F.; Wu, C.; Dong, L.; Vassallo, A.; Williams, P.T.; Huang, J. Characteristics and catalytic properties of Ni/CaAlOx catalyst for hydrogen-enriched syngas production from pyrolysis-steam reforming of biomass sawdust. Appl. Catal. B Environ. 2016, 183, 168–175. [Google Scholar] [CrossRef]
- Efika, C.E.; Wu, C.; Williams, P.T. Syngas production from pyrolysis-catalytic steam reforming of waste biomass in a continuous screw kiln reactor. J. Anal. Appl. Pyrolysis 2012, 95, 87–94. [Google Scholar] [CrossRef]
- Xiao, X.; Meng, X.; Le, D.D.; Takarada, T. Two-stage steam gasification of waste biomass in fluidized bed at low temperature: Parametric investigations and performance optimization. Bioresour. Technol. 2011, 102, 1975–1981. [Google Scholar] [CrossRef]
- Ochoa, A.; Arregi, A.; Amutio, M.; Gayubo, A.G.; Olazar, M.; Bilbao, J.; Castaño, P. Coking and sintering progress of a Ni supported catalyst in the steam reforming of biomass pyrolysis volatiles. Appl. Catal. B Environ. 2018, 233, 289–300. [Google Scholar] [CrossRef]
- Situmorang, Y.A.; Zhao, Z.; An, P.; Yu, T.; Rizkiana, J.; Abudula, A.; Guan, G. A novel system of biomass-based hydrogen production by combining steam bio-oil reforming and chemical looping process. Appl. Energy 2020, 268, 115122. [Google Scholar] [CrossRef]
- Jones, S.; Meyer, P.; Snowden-Swan, L.; Susanne, K.J.; Pimphan, M.; Asanga, P.; Eric, T.; Abhijit, D.; Jacob, J. Process Design and Economics for the Conversion of Lignocellulosic Biomass to Hydrocarbon Fuels: Fast Pyrolysis and Hydrotreating Bio-Oil Pathway. U.S. Department of Energy Bioenergy Technologies Office. 2013. Available online: https://www.nrel.gov/docs/fy14osti/61178.pdf (accessed on 1 May 2020).
- Larsson, M.; Görling, M.; Grönkvist, S.; Alvfors, P. Bio-methane upgrading of pyrolysis gas from charcoal production. Sustain. Energy Technol. Assess. 2013, 3, 66–73. [Google Scholar] [CrossRef]
- Arregi, A.; Lopez, G.; Amutio, M.; Artetxe, M.; Barbarias, I.; Bilbao, J.; Olazar, M. Role of operating conditions in the catalyst deactivation in the in-line steam reforming of volatiles from biomass fast pyrolysis. Fuel 2018, 216, 233–244. [Google Scholar] [CrossRef]
- Lónyi, F.; Valyon, J.; Someus, E.; Hancsók, J. Steam reforming of bio-oil from pyrolysis of MBM over particulate and monolith supported Ni/γ-Al2O3 catalysts. Fuel 2013, 112, 23–30. [Google Scholar] [CrossRef]
- Wu, C.; Huang, Q.; Sui, M.; Yan, Y.; Wang, F. Hydrogen production via catalytic steam reforming of fast pyrolysis bio-oil in a two-stage fixed bed reactor system. Fuel Process. Technol. 2008, 89, 1306–1316. [Google Scholar] [CrossRef]
- Wang, D.; Czernik, S.; Montané, D.; Mann, M.; Chornet, E. Biomass to Hydrogen via Fast Pyrolysis and Catalytic Steam Reforming of the Pyrolysis Oil or Its Fractions. Ind. Eng. Chem. Res. 1997, 36, 1507–1518. [Google Scholar] [CrossRef]
- Remiro, A.; Valle, B.; Aguayo, A.T.; Bilbao, J.; Gayubo, A.G. Steam reforming of raw bio-oil in a fluidized bed reactor with prior separation of pyrolytic lignin. Energy Fuels 2013, 27, 7549–7559. [Google Scholar] [CrossRef]
- Guo, W.; Lim, C.J.; Bi, X.; Sokhansanj, S.; Melin, S. Determination of effective thermal conductivity and specific heat capacity of wood pellets. Fuel 2013, 103, 347–355. [Google Scholar] [CrossRef]
- Haseli, Y.; Van Oijen, J.A.; De Goey, L.P.H. Modeling biomass particle pyrolysis with temperature-dependent heat of reactions. J. Anal. Appl. Pyrolysis 2011, 90, 140–154. [Google Scholar] [CrossRef]
- Nurdiawati, A.; Zaini, I.N.; Amin, M.; Sasongko, D.; Aziz, M. Microalgae-based coproduction of ammonia and power employing chemical looping process. Chem. Eng. Res. Des. 2019, 146, 311–323. [Google Scholar] [CrossRef]
- Pinheiro Pires, A.P.; Arauzo, J.; Fonts, I.; Domine, M.E.; Fernández Arroyo, A.; Garcia-Perez, M.E.; Montoya, J.; Chejne, F.; Pfromm, P.; Garcia-Perez, M. Challenges and opportunities for bio-oil refining: A review. Energy Fuels 2019, 33, 4683–4720. [Google Scholar] [CrossRef]
- Li, B.; Lv, W.; Zhang, Q.; Wang, T.; Ma, L. Pyrolysis and catalytic upgrading of pine wood in a combination of auger reactor and fixed bed. Fuel 2014, 129, 61–67. [Google Scholar] [CrossRef]
- Ningbo, G.; Baoling, L.; Aimin, L.; Juanjuan, L. Continuous pyrolysis of pine sawdust at different pyrolysis temperatures and solid residence times. J. Anal. Appl. Pyrolysis 2015, 114, 155–162. [Google Scholar] [CrossRef]
- Goteti, A. Experimental Investigation and Systems Modeling of Fractional Catalytic Pyrolysis of Pine. Ph.D. Dissertation, Georgia Institute of Technology, Atlanta, GA, USA, 2010; p. 218. [Google Scholar]
- Voss, B.; Madsen, J.; Hansen, J.B.; Andersson, K.J. Topsøe Tar Reforming in Skive—The tough get going. Catal. Rev. 2016, 29, 7–14. [Google Scholar]
- Kraussler, M.; Binder, M.; Hofbauer, H. 2250-H Long Term Operation of a Water Gas Shift Pilot Plant Processing Tar-Rich Product Gas From an Industrial Scale Dual Fluidized Bed Biomass Steam Gasification Plant. Int. J. Hydrogen Energy 2016, 41, 6247–6258. [Google Scholar] [CrossRef]
- Yao, J.; Kraussler, M.; Benedikt, F.; Hofbauer, H. Techno-economic assessment of hydrogen production based on dual fluidized bed biomass steam gasification, biogas steam reforming, and alkaline water electrolysis processes. Energy Convers. Manag. 2017, 145, 278–292. [Google Scholar] [CrossRef]
- Nugrahany, F. Modelling of Biomass Pyrolysis with Ex-situ Catalytic Upgrading for Bio-crude Production. Master’s Thesis, KTH Royal Institute of Technology, Stockholm, Sweden, 2018. [Google Scholar]
- Yang, H.; Kudo, S.; Kuo, H.P.; Norinaga, K.; Mori, A.; Mašek, O.; Hayashi, J.I. Estimation of enthalpy of bio-oil vapor and heat required for pyrolysis of biomass. Energy Fuels 2013, 27, 2675–2686. [Google Scholar] [CrossRef]
- Storaenso Köp Pellets i Bulk. Available online: https://www.storaenso.com/sv-se/products/pellets/pellets-for-residential-heating/buy-pellets-bulk?gclid=Cj0KCQjw-O35BRDVARIsAJU5mQWPi4PVUc_o2b5eQWinuRbbl7q-kiDdlMGuMGIG_g3Yo9QKJ-WOSkoaAndNEALw_wcB (accessed on 29 November 2020).
- Avfall Sverige. Swedish Waste Management 2018. 2018. Available online: https://www.avfallsverige.se/fileadmin/user_upload/Publikationer/Avfallshantering_2018_EN.pdf (accessed on 29 November 2020).
- Glenk, G.; Reichelstein, S. Economics of converting renewable power to hydrogen. Nat. Energy 2019, 4, 216–222. [Google Scholar] [CrossRef]
- BOC Industrial Gases Price List (ex. VAT). 1 January 2020. Available online: https://www.boconline.co.uk/en/images/industrial-gases-price-list-uk_tcm410-512096.pdf (accessed on 29 November 2020).
- Sönnichsen, N. Prices of Electricity for Industry in Sweden from 1996 to 2018. Available online: https://www.statista.com/statistics/596262/electricity-industry-price-sweden/ (accessed on 29 November 2020).
- Prisjakt Grillkol—Grilltillbehör. Available online: https://www.prisjakt.nu/c/grilltillbehor?98091=31484 (accessed on 5 January 2021).
- IEA Coal 2020 Analysis and Forecast to 2025. Available online: https://www.iea.org/reports/coal-2020/prices-and-costs (accessed on 5 January 2021).
- Setra Pyrocell. Available online: http://www.setragroup.com/en/pyrocell/ (accessed on 5 January 2021).
- Jarboe, L.R.; Wen, Z.; Choi, D.; Brown, R.C. Hybrid thermochemical processing: Fermentation of pyrolysis-derived bio-oil. Appl. Microbiol. Biotechnol. 2011, 91, 1519–1523. [Google Scholar] [CrossRef]



| Configuration and Operating Parameters | Pyrolysis Reactor/Steam Reforming Reactor | Catalyst | H2 Yield (wt.%) | H2 Concentration (vol.%) | CO Concentration after Reformer (vol.%) | Liquid Yield (wt.%) | Ref. |
|---|---|---|---|---|---|---|---|
| Wood sawdust, Pyrolysis (500 °C) + steam reformer (600 °C, 20 gcat min/gvolatile, S/B = 4 (S/C~8)) | Spouted bed/fluidized bed, Lab-scale | Ni commercial | 11 | 66 (94% of stoic) | 3 | 0 (100% conversion) 1 | [21] |
| Wood sawdust, Pyrolysis (500 °C) + steam reformer (800 °C, S/B ~4) | Fixed bed/fixed bed, Lab-scale | Ni/CaAlOx | 2.8 | 45 | 44 | 1–5 | [22] |
| Wood pellet, Pyrolysis (500 °C) + steam reformer (760 °C, S/B = NA) | Screw reactor/fixed bed, Bench-scale | NiO/Al2O3 | 44 | 30 | 27 | [23] | |
| Wood chip, Pyrolysis (600 °C) + steam reformer (650 °C, S/C=3) | Fluidized bed/fixed bed, Lab-scale | Ni/Al2O3, Ni-brown coal char | Approx. 10 | 60 | Approx. 10 | <1 | [24] |
| Olive tree cuttings, Pyrolysis (750 °C) + catalytic reactor (900 °C, no additional steam) | Non-stirred batch/fixed bed, Lab-scale | Biochar + Ni/Al2O3-CeO2 | 17.6 | 47.4 | 33.8 | [19,20] |
| Biomass | Char | ||
|---|---|---|---|
| Moisture | wt.%, as * | 6.6 | <2 |
| Ash | wt.%, as | 0.24 | 1.2 |
| C | wt.%, daf ** | 51.7 ± 2.6 | |
| H | wt.%, daf | 6.18 ± 0.62 | |
| O # | wt.%, daf | 42.12 ± 2.66 | |
| C | wt.%, waf ‡ | 95.24 ± 4.76 | |
| H | wt.%, waf | 1.32 ± 0.13 | |
| O # | wt.%, waf | 3.44 ± 4.76 | |
| LHV | MJ/kg, af | 19.1 ± 1.0 | 34.5 ± 1.7 |
| Heat of formation † | MJ/kg | −5.274 | 1.688 |
| Heat capacity | J/kgK | 1190 [34] | 1300 [35] |
| Compounds | Ref. [37] | Values Used in This Study (wt.% of Bio-Oil) |
|---|---|---|
| Water | (19–30 wt.%) | 35 |
| C2-C4 light molecules | (10–22 wt.%) | |
| Acetic acid | 2–6 | 2 |
| Hydroxyacetaldehyde | 1–14 | 7 |
| Hydroxyacetone (Acetol) | 5–9 | 7 |
| Formic acid | <1 | |
| 2-Butenoic acid | 2 | |
| Monofurans | (1 wt.%) | |
| Furfural | 0.1–0.2 | 1 |
| 2(5H)-Furanone | 0.2–0.8 | |
| Monophenols | (1–5 wt.%) | |
| Phenol | 0.1 | |
| Guaiacol | 0.2–0.5 | |
| Cresol | 0.1–0.4 | 1 |
| Syringol | 0.4 | |
| Isoeugenol | 0.1–0.2 | 1 |
| Anhydrosugar | (10–20 wt.%) | |
| Levoglucosan | NA | 10 |
| Cellobiosan | NA | |
| Maltose | 10 | |
| Pyrolytic lignin | (15–27 wt.%) | |
| Hybrid oligomers | (11–18 wt.%) | |
| Humins | (3–7 wt.%) | |
| High molecular weight lignin and hybrid oligomers (HMWLIG) | 24 | |
| Total | 100 |
| High MW Lignin-Derived Compound | wt.% | Formula (mol) |
|---|---|---|
| C | 67.1 | 20 |
| H | 11.0 | 38.935 |
| O | 21.9 | 4.913 |
| LHV * | MJ/kg | 33.25 |
| Heat of formation † | MJ/kg | −6.72 |
| Heat capacity ‡ | J/kgK | 1970 [40] |
| Gas Compositions | wt.% |
|---|---|
| H2 | 1.2 |
| CO | 45.00 |
| CO2 | 35.00 |
| CH4 | 12.00 |
| C2H6 | 2.00 |
| C3H8 | 4.80 |
| Abbreviations | Type of Block | Function |
|---|---|---|
| Blocks | ||
| COOL1 | Heater | Syngas cooling before being fed to the WGS reactor. |
| COOL2 | Heater | Cooling of the WGS reactor’s H2-rich gas products. |
| COOL3 | Heater | An intra-cooling unit for the PSA module. |
| COMP1 | Compressor | Compression of the H2-rich vapor to 1 MPa prior to the PSA module. |
| COND | Flash2 | Bio-oil condenser prior to the reforming process. |
| DECOMP | RYield | Conversion of the non-conventional bio-oil’s high molecular weight lignin fraction to its basic molecules (C, H2, and O2). Only for Scheme 1. |
| HX1 | HeatX | Recovering the heat of reformer’s syngas to preheat the pyrolysis vapor stream. |
| HX2 | HeatX | Further heat recovery of reformer’s syngas to generate steam input for the reforming process. |
| HX3 | HeatX | Generation of WGS steam input by recovering the sensible heat of the WGS outlet stream. |
| MIX1 | Mixer | Mixing of the condensed water and the make-up water. |
| REFORM | RGibbs | Catalytic steam reforming of pyrolysis vapor at 850 °C. |
| SEP1 | Sep | Separation of pyrolysis vapor stream into a light fraction (conventional) and a high molecular weight lignin fraction (non-conventional). |
| SEP2 | Sep | Condenser to separate water from the H2-rich dry gases. |
| PSA | Sep | PSA module operated at 1 MPa and 50 °C. |
| PUMP1 | Pump | Water supply to the steam reformer. |
| PUMP2 | Pump | Water supply to the WGS reactor. |
| WGS | RStoic | WGS reactor operated at 425 °C and ambient pressure. |
| Streams | ||
| HMWLIG1 | The non-conventional bio-oil’s high molecular weight lignin fraction. | |
| HMWLIG2 | Basic molecules of the non-conventional bio-oil’s high molecular weight lignin fraction. | |
| H2 | Final H2 product from the PSA module. | |
| H2O-COND | Condensed water separated from the WGS reactor product. | |
| H2O-MAKE | Make-up water needed for the co-production system. | |
| H2O-REF1 | Water supply for the reforming process. | |
| H2O-REF2 | Pumped water supply for the reforming process. | |
| H2O-REF3 | Steam input for the reforming process. | |
| H2O-SPLY | Water supply for the entire co-production system. | |
| H2O-WGS1 | Water supply for the WGS reactor. | |
| H2O-WGS2 | Pumped water supply for the WGS reactor. | |
| H2O-WGS3 | Steam input for the WGS reactor. | |
| LIGHT | Separated light fraction of the pyrolysis vapor. Contains all the uncondensed gases and bio-oil compounds except the high molecular weight lignin fraction. | |
| PYRO1 | Pyrolysis vapor produced from the biomass pyrolysis. The content of this stream is determined based on the experimental and calculation results. | |
| PYRO2 | In Scheme 1: Preheated pyrolysis vapor going into the reformer.In Scheme 2: Uncondensed pyrolysis vapor going into the heat exchanger. | |
| PYRO3 | Preheated pyrolysis vapor going into the reformer. Only in Scheme 2. | |
| REST | Contains of mostly CO2 with minor fraction of CO and CH4. | |
| VP1 | High-temperature syngas (850 °C) produced from the reformer. The stream consists of H2, CO, CO2, and CH4. | |
| VP2 | Cooled syngas after heat exchanger “HX1”. | |
| VP3 | Cooled syngas after heat exchanger “HX2”. | |
| VP4 | Syngas input for the WGS reactor. | |
| VP5 | H2-rich gases produced by the WGS reactor. | |
| VP6 | Cooled H2-rich gases. | |
| VP7 | Cooled H2-rich gases. | |
| VP8 | Dry H2-rich gases. | |
| VP9 | Compressed dry H2-rich gases. | |
| VP10 | Dry H2-rich gases input for PSA module. |
| Parameters | Scheme 1 | Scheme 2 * |
|---|---|---|
| H2 yield (wt.% dry biomass) | 10.0 | 4.7 |
| CO2 yield (wt.% dry biomass) | 101.1 | 53.6 |
| Bio-oil yield (wt.% dry biomass) | - | 46.0 |
| (%) | 47.1 | 22.9 |
| (%) | - | 23.1 |
| (%) | 79.0 | 79.0 |
| Parameters | Moisture Content of Biomass (wt.%) | |
|---|---|---|
| 7 | 14 | |
| H2 yield (wt.% dry biomass) | 4.7 | 4.7 |
| CO2 yield (wt.% dry biomass) | 53.6 | 53.7 |
| H2 yield (wt.% wet biomass) | 4.4 | 4.0 |
| CO2 yield (wt.% wet biomass) | 50.0 | 46.1 |
| (%) | 23.1 | 21.2 |
| (%) | 22.9 | 21.0 |
| (%) | 79.0 | 72.6 |
| Parameter | Assumption | References |
|---|---|---|
| Duration of operation | 8000 h/yr | |
| Raw materials | ||
| Biomass pellet | 6.6 wt.% moisture content | |
| 3 SEK/kg | Retail price [46] | |
| Reformer catalyst | Monolith catalyst | [41] |
| No make-up catalyst required | ||
| WGS catalyst | 0.215 SEK/kg | [27] |
| 0.02 wt.% hourly make-up | ||
| Solid disposal | 3.9 SEK/kg | [47] |
| (spent catalyst) | ||
| Products | ||
| H2 | 33 SEK/kg | [48] |
| Biocarbon | 8 SEK/kg | Envigas |
| Bio-oil crude | 9 SEK/kg dry oil | Envigas |
| CO2 | 11.1 | Retail price [49] |
| Utilities | ||
| Electricity | 0.7 SEK/kWh | [50] |
| Water | 9.75 SEK/m3 | |
| Personnel | ||
| Plant manager (1 person) | 70,000 SEK/person/month | |
| Plant engineer (1 person) | 50,000 SEK/person/month | |
| Shift operators (12 person) | 30,000 SEK/person/month | |
| Overheads | 90% of total salaries |
| Parameter | Case 1 | Case 2 | Case 3 |
|---|---|---|---|
| Pyrolysis | |||
| Biomass pellet (kg/h) | 1000 | 1000 | 1000 |
| Reactor heating (kW) | 420.3 | 420.3 | 420.3 |
| Bio-oil condenser | |||
| Recirculation pump (kW) | - | 7.5 | 7.5 |
| Reformer | |||
| Reactor heating (kW) | 699.70 | 553.80 | 464.30 |
| Water supply (kg/h) | 287.60 | 350.57 | 202.88 |
| WGS reactor | |||
| Make-up catalyst (kg/h) | 0.71 | 0.37 | 0.47 |
| Water supply (kg/h) | 0.00 | 0.00 | 0.00 |
| Solid disposal (kg/h) | 0.71 | 0.37 | 0.47 |
| PSA | |||
| Compressor and utilities (kW) | 178.50 | 86.40 | 111.10 |
| Products yields | |||
| Biocarbon (kg/h) | 217.20 | 217.20 | 217.20 |
| H2 (kg/h) | 93.55 | 43.83 | 57.00 |
| Bio-oil (kg/h) | 0.00 | 429.69 | 178.15 |
| CO2 (kg/h) | 944.39 | 500.32 | 628.18 |
| Bio-oil price | |||
| Water content in bio-oil (wt.%) | 41.70% | 0.64% | |
| Bio-oil price based on water content | - | 5.25 | 8.94 |
| Parameter | Case 1 | Case 2 | Case 3 |
|---|---|---|---|
| Variable operating cost (MSEK/year) | |||
| Pyrolysis | |||
| Biomass pellet | 24.00 | 24.00 | 24.00 |
| Reactor heating | 2.35 | 2.35 | 2.35 |
| Bio-oil condenser | |||
| Recirculation pump | - | 0.04 | 0.04 |
| Reformer | |||
| Reactor heating | 3.92 | 3.10 | 2.60 |
| Water supply | 0.02 | 0.03 | 0.02 |
| WGS reactor | |||
| Make-up catalyst | 0.001 | 0.001 | 0.001 |
| Solid disposal | 0.022 | 0.011 | 0.015 |
| PSA | |||
| Compressor and utilities | 1.00 | 0.48 | 0.62 |
| Fixed operating cost (MSEK/year) | |||
| Salary | 5.76 | 5.76 | 5.76 |
| Overhead | 5.184 | 5.184 | 5.184 |
| Products values (MSEK/year) | |||
| Biocarbon | 13.90 | 13.90 | 13.90 |
| H2 | 24.70 | 11.57 | 15.05 |
| Bio-oil | 0.00 | 17.19 | 12.83 |
| CO2 | 83.86 | 44.43 | 55.78 |
| Total OPEX (MSEK/year) | 42.26 | 40.96 | 40.59 |
| Total revenue (MSEK/year) | 38.60 | 42.66 | 41.78 |
| Total revenue including CO2 (MSEK/year) | 122.46 | 87.09 | 97.56 |
| Gross production cost 1 (SEK/kg-biomass) | 5.28 | 5.12 | 5.07 |
| Loss/Gain based on OPEX 2 (SEK/kg-biomass) | −0.46 | 0.32 | 0.14 |
| Loss/Gain based on OPEX2 with CO2 (SEK/kg-biomass) | 23.85 | 13.97 | 16.92 |
| Minimum biocarbon selling price 3 (SEK/kg) | 10.11 | ||
| Minimum bio-oil selling price 4 (SEK/kg) | 3.44 | 5.59 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaini, I.N.; Sophonrat, N.; Sjöblom, K.; Yang, W. Creating Values from Biomass Pyrolysis in Sweden: Co-Production of H2, Biocarbon and Bio-Oil. Processes 2021, 9, 415. https://doi.org/10.3390/pr9030415
Zaini IN, Sophonrat N, Sjöblom K, Yang W. Creating Values from Biomass Pyrolysis in Sweden: Co-Production of H2, Biocarbon and Bio-Oil. Processes. 2021; 9(3):415. https://doi.org/10.3390/pr9030415
Chicago/Turabian StyleZaini, Ilman Nuran, Nanta Sophonrat, Kurt Sjöblom, and Weihong Yang. 2021. "Creating Values from Biomass Pyrolysis in Sweden: Co-Production of H2, Biocarbon and Bio-Oil" Processes 9, no. 3: 415. https://doi.org/10.3390/pr9030415
APA StyleZaini, I. N., Sophonrat, N., Sjöblom, K., & Yang, W. (2021). Creating Values from Biomass Pyrolysis in Sweden: Co-Production of H2, Biocarbon and Bio-Oil. Processes, 9(3), 415. https://doi.org/10.3390/pr9030415







