Effect of Choline-Based Deep Eutectic Solvent Pretreatment on the Structure of Cellulose and Lignin in Bagasse
Abstract
1. Introduction
2. Materials and Methods
2.1. Material and Reagents
2.2. Compositional Analysis of Bagasse
2.3. Preparation of DES
2.4. Pretreatment of Bagasse Using DES
2.5. Materials Characterizations
2.5.1. Amount of Lignin Dissolved in DES
2.5.2. Scanning Electron Microscopy (SEM)
2.5.3. X-ray Diffraction (XRD)
2.5.4. Fourier Transform Infrared (FTIR) Spectroscopy
2.5.5. NMR Determination of Lignin Dissolved in DES
2.5.6. Thermogravimetric Analysis (TGA)
3. Results
3.1. Chemical Compositions of Bagasse Fiber
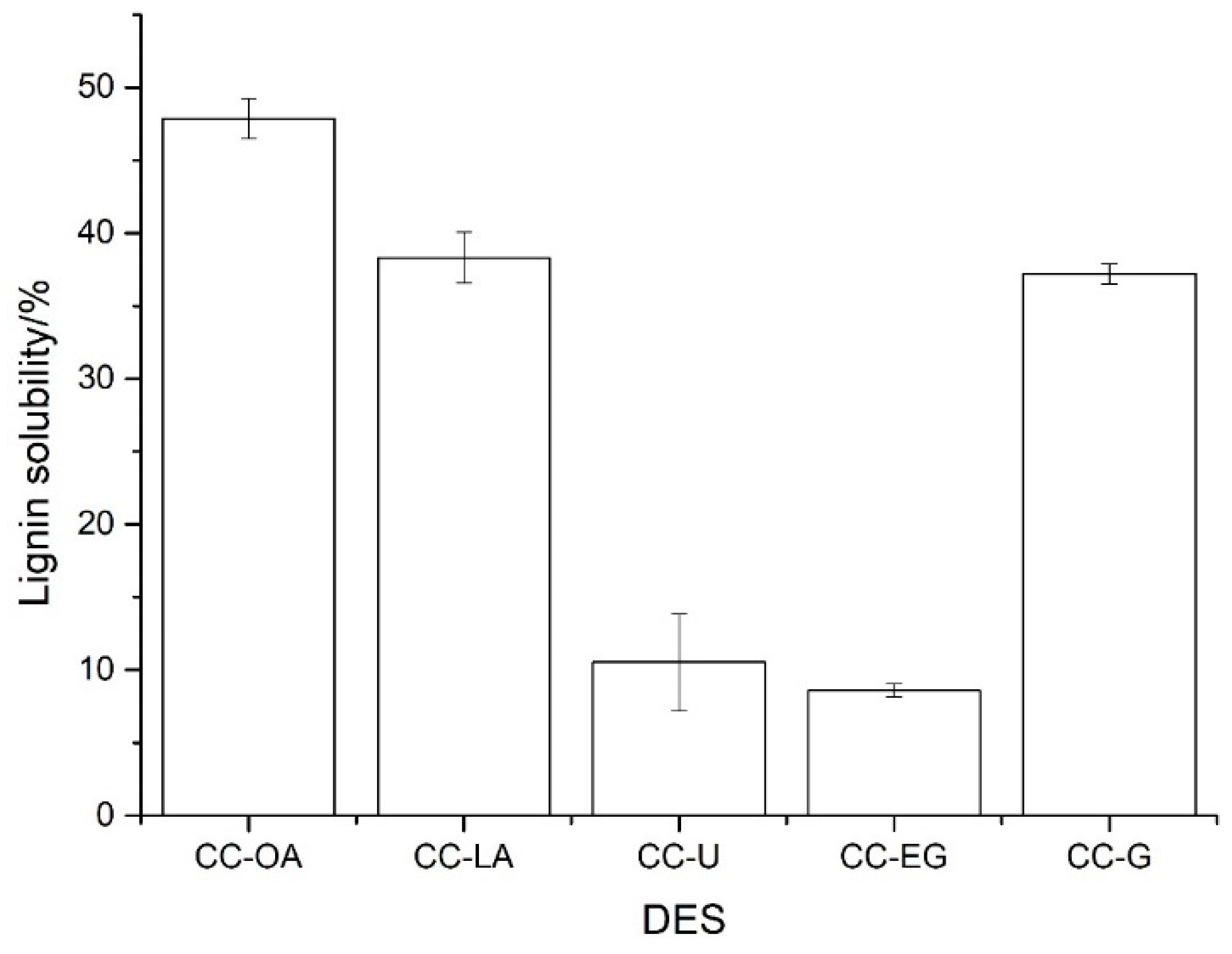
3.2. Solubility Analysis of Lignin in Different DESs
3.3. Analysis of Surface Morphology of Bagasse
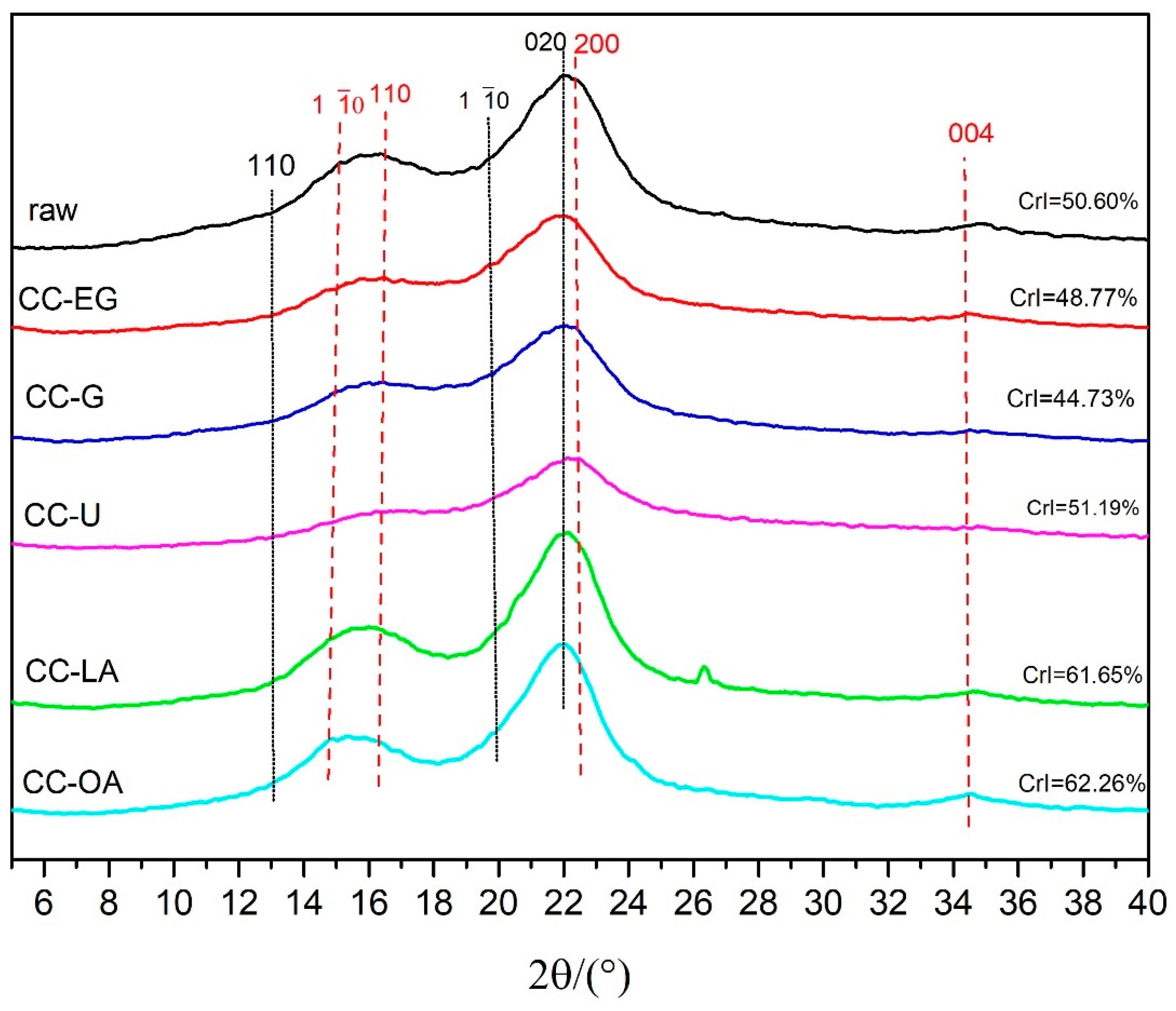
3.4. Crystallinity Analysis
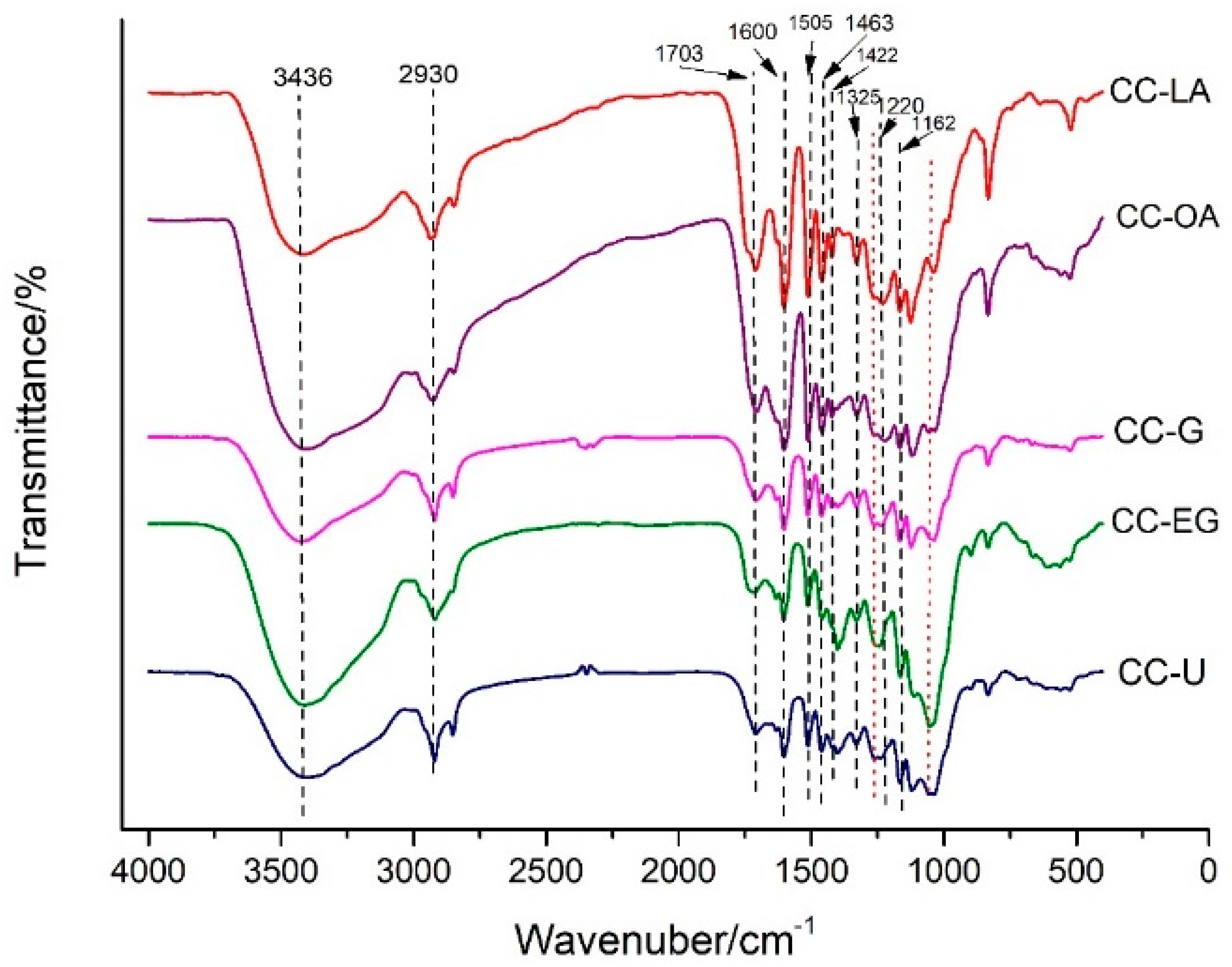
3.5. Chemical Composition Analysis of Bagasse
3.6. NMR Analysis of Lignin
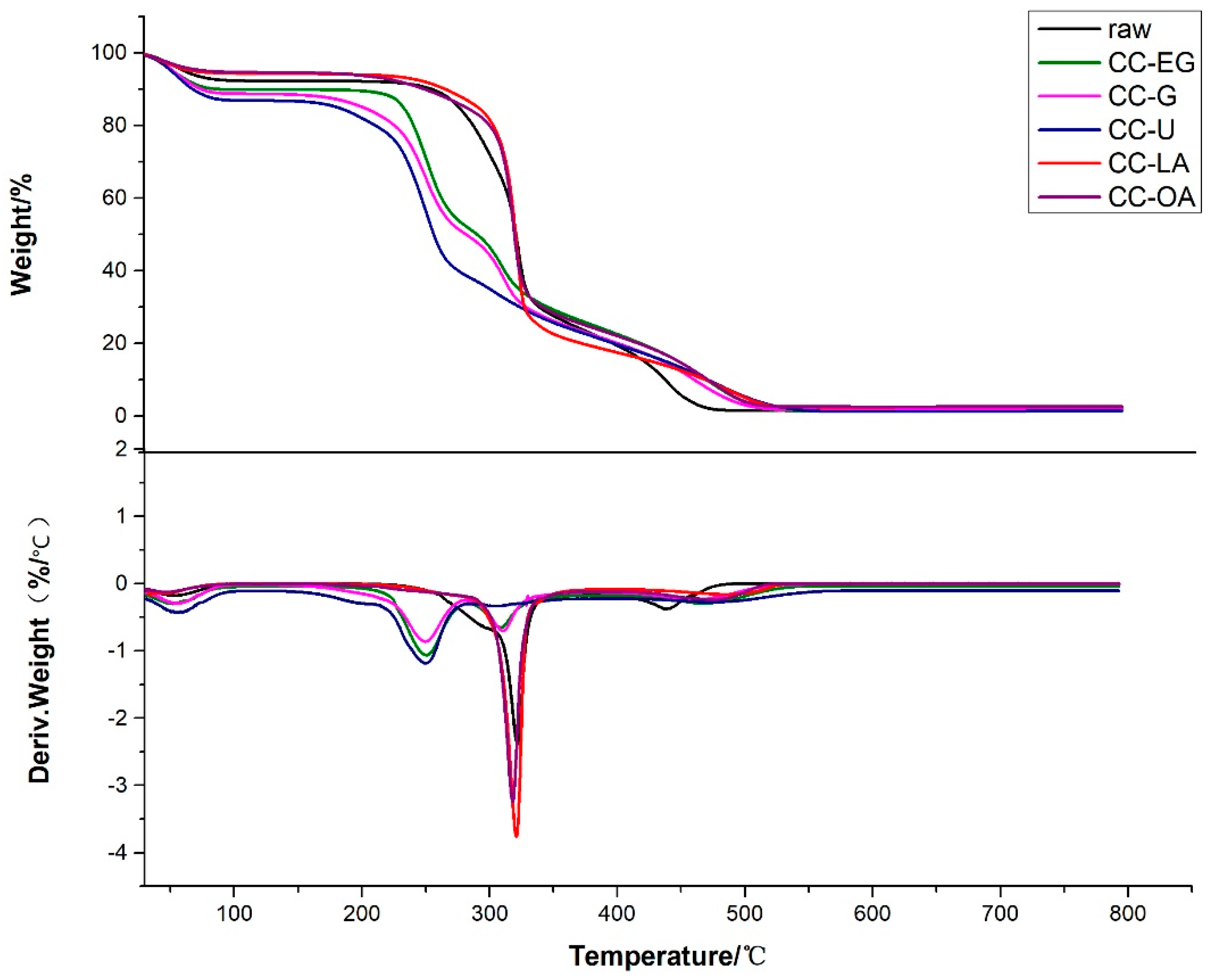
3.7. Thermal Stability
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sinclair, A.; Jiang, L.; Bajwa, D.; Bajwa, S.; Tangpong, S.; Wang, X. Cellulose Nanofibers Produced from Various Agricultural Residues and Their Reinforcement Effects in Polymer Nanocomposites. J. Appl. Polym. Sci. 2018, 135, 46304–46305. [Google Scholar] [CrossRef]
- Ming, L.G.; Luo, Y.Z.; Xiang, Y.Z.; Zhi, X.J. Research progress of grafting technology for sugar cane bagasse. Chem. Industr. Eng. Progr. 2014, 33, 2350–2355, 2379. [Google Scholar]
- Shu, L.S.; Fu, W.H. Analysis and Detection of Pulp and Paper Making; China Light Industry Press: Beijing, China, 2003; p. 35. [Google Scholar]
- Chao, L.; Xiao, G.Y.; Yi, L.; Li, G.D.; Deng, J.L. Progress in modification and application of bagasse cellulose. Guangzhou Chem. 2017, 42, 71–76. [Google Scholar]
- Hao, Q.H.; Quan, N.G.; Chu, H.Q.; Hao, L.; Hai, Y.Z. Effect of microstructure of bagasse on the structure and properties of polylactic acid / bagasse composites. Mat. Rev. 2018, 32 (Suppl. 1), 220–225. [Google Scholar]
- Zdanowicz, M.; Wilpiszewska, K.; Spychaj, T. Deep Eutectic Solvents for Polysaccharides Processing. Rev. Carbohydr. Polym. 2018, 200, 361–380. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.S.; Xin, W.J.; Ling, C.; Qu, H.; Hai, L.L. Extraction and separation of cellulose from loofa sponge with two types of choline-based deep eutectic solvents. Tran. China Puip. Paper 2018, 2, 6–12. [Google Scholar]
- Jin, M.Z.; Da, L.G.; Yun, P.G.; Guo, X.X. Research progress in lignin separation with deep eutectic solven. China Puip. Paper 2019, 38, 53–58. [Google Scholar]
- Zhang, C.W.; Xia, S.Q.; Ma, P.S. Facile Pretreatment of Lignocellulosic Biomass Using Deep Eutectic Solvents. Bioresour. Technol. 2016, 219, 1–5. [Google Scholar] [CrossRef]
- Song, Y.L.; Shi, X.Y.; Ma, S.; Yang, X.; Zhang, X. A Novel Aqueous Gallic Acid-based Natural Deep Eutectic Solvent for Delignification of Hybrid Poplar and Enhanced Enzymatic Hydrolysis of Treated Pulp. Cellulose 2020, 27, 8301–8315. [Google Scholar] [CrossRef]
- Carrion, P.P.; Martin, R.P.; Hernandez, N.S.; Sanchez, S.L.; LuisMarcos, R.J.; Martin, G.J. Crystallinity of Cellulose Microfibers Derived From Cistus Ladanifer and Erica Arborea Shrubs. Maderas Ciencia y Tecnologia 2019, 21, 447–456. [Google Scholar]
- Guo, Z.W.; Zhang, Q.L.; You, T.T.; Zhang, X.; Xu, F.; Wu, Y.Y. Short-time Deep Eutectic Solvent Pretreatment for Enhanced Enzymatic Saccharification and Lignin Valorization. Green Chem. 2019, 21, 3099–3108. [Google Scholar] [CrossRef]
- Gong, W.H.; Ma, Y.; Lu, X. Structural features and antioxidant activities of lignin extracted from sunflower seed shell. Food Sci. 2017, 38, 23–28. [Google Scholar]
- Liu, L.Y.; Niu, K.; Liu, C.G. Effect of ionic liquid pretreatment on lignocellulosic biomass from oilseeds. Chem. Eng. J. 2013, 64 (Suppl. 1), 104–110. [Google Scholar]
- Jie, B.B.; Lian, H.L.; Sun, X. Modification of lignin phenol formaldehyde adhesive with ChCl/Glycerol deep eutectic solvent. J. For. Eng. 2016, 1, 107–113. [Google Scholar]
- Tan, Y.T.; Ngoh, G.C.; Chua, A.S.M. Effect of Functional Groups in Acid Constituent of Deep Eutectic Solvent for Extraction of Reactive Lignin. Bioresour. Technol. 2019, 281, 359–366. [Google Scholar] [CrossRef]
- Li, L.F.; Yu, L.P.; Wu, Z.G.; Hu, Y.C. Delignification of Poplar Wood with Lactic Acid-based Deep Eutectic Solvents. Wood Res-Slovak. 2019, 64, 507–522. [Google Scholar]
- Soares, B.; Silvestre, A.J.D.; Rodrigues, P.P.C.; Freire, C.S.R.; Coutinho, J.A.P. Hydrotropy and Cosolvency in Lignin Solubilization with Deep Eutectic Solvents. ACS Sustain. Chem. Eng. 2019, 7, 12485–12493. [Google Scholar] [CrossRef]
- Shen, X.J.; Wen, J.L.; Mei, Q.Q.; Chen, X.; Sun, D.; Yuan, T.Q.; Sun, R.C. Facile Fractionation of Lignocelluloses by Biomass-derived Deep Eutectic Solvent (DES) Pretreatment for Cellulose Enzymatic Hydrolysis and Lignin Valorization. Green Chem. 2019, 21, 275–283. [Google Scholar] [CrossRef]
- Ling, J. Structural Change of Aggregation State during Cellulose Pretreatment and Nanocrystalline Preparation. Master’s Thesis, Beijing Forestry University, Beijing, China, 2019. [Google Scholar]
- New, E.K.; Wu, T.Y.; Lee, C.B.T.L.; Poon, Z.Y.; Loow, Y.L.; Foo, L.Y.W.; Procentese, A.; Siow, L.F.; Teoh, W.H.; Daud, N.N.N.; et al. Potential Use of Pure and Diluted Choline Chloride-based Deep Eutectic Solvent in Delignification of Oil Palm Fronds. Proc. Safet. Envir. Prot. 2019, 123, 190–198. [Google Scholar] [CrossRef]
- Yang, S.M.; Jiang, Z.H.; Ren, H.Q.; Fei, B.H. Determination of crystallinity of bamboo cellulose by X-ray diffraction. J. Northe. Forestr. Univ. 2010, 38, 75–77. [Google Scholar]
- Li, F.; Huang, Y.H.; Ye, C.Y.; Feng, Q.M. The structure and physicochemical properties of phloem of ‘72 poplar’. J. For. Eng. 2021, 6, 28–33. [Google Scholar]
- Bai, Y.C. Highly Efficient Isolation of Lignocellulose Components by Deep Eutectic Solvents and Cellulose Utilization. Master’s Thesis, South China University of Technology, Guangzhou, China, 2018. [Google Scholar]
- Liu, J.K.; Yang, G.H.; Qi, L.T.; Xue, Y.; Chen, J.C. Selective extraction of poplar lignin with choline -based deep eutectic solvents. China Puip. Paper 2020, 39, 1–9. [Google Scholar]
- Cui, T. The effects of various pretreatment methods on cellulose crystalline structure. Tran. China Puip. Paper 2020, 35, 9–15. [Google Scholar]
- Wu, Z.H. Preparation, Characterization and Acetylation of Different Crystalline Nano Cellulose. Master’s Thesis, South China University of Technology, Guangzhou, China, 2019. [Google Scholar]
- Liu, Z.G.; Gao, Y.; Jin, H.; Yu, S.H. Study on natural cellulose crystallinity determinated by the technology of XRD peak separation. China Test. 2015, 41, 38–41. [Google Scholar]
- Ruesgas, R.M.; Figueroa, E.M.C.; Durand, E. Application of Deep Eutectic Solvents (DES) for Phenolic Compounds Extraction: Overview, Challenges, and Opportunities. J. Agric. Food Chem. 2017, 65, 3591–3601. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.L.; Chandra, R.P.; Zhang, X.; Saddler, J.N. Non-productive Celluase Binding onto Deep Eutectic Solvent (DES) Extracted Lignin from Willow and Corn Stover with Inhibitory Effects on Enzymatic Hydrolysis of Cellulose. Carbohydr. Polym. 2020, 250, 116956. [Google Scholar] [CrossRef]
- Tian, D.; Guo, Y.J.; Hu, J.G.; Yang, G.; Zhang, J.; Luo, L.; Xiao, Y.L.; Deng, S.H.; Deng, D.P.; Zhou, W.; et al. Acidic Deep Eutectic Solvents Pretreatment for Selective Lignocellulosic Biomass Fractionation with Enhanced Cellulose Reactivity. Int. J. Biol. Macromol. 2020, 142, 288–297. [Google Scholar] [CrossRef]
- Lee, M.K.; Liu, Y.Y.; Cui, J.H. Effect of Molar Ratios of Des on Lignin Contents and Handsheets Properties of Thermomechanical Pulp. J. Korea Tappi. 2016, 48, 28–33. [Google Scholar]
- Lee, M.K.; Liu, Y.Y.; Cui, J.H.; Li, Z.X. Effects of Pre-treatment By Betaine-oxalic Acid Based Deep Eutectic Solvent on Manufacturing Efficiency of Microfibrillated Cellulose. J. Korea Tappi. 2016, 48, 203–210. [Google Scholar]
- Chirila, O.; Totolin, M.; Cazacu, G.; Dobromir, M.; Vasile, C. Lignin Modification with Carboxylic Acids and Butyrolactone under Cold Plasma Conditions. Ind. Eng. Chem. Res. 2013, 52, 13264–13271. [Google Scholar] [CrossRef]
- Wen, J. Structure Analysis of Biomass Lignin and Its Pre-Treatment Dissolution Mechanism; Beijing Forestry University: Beijing, China, 2014. [Google Scholar]
- An, Y.X. Cholinium Ionic Liquid-Based Pretreatment and Enzymatic Hydrolysis of Lignocellulosic Biomass, and Their Mechanisms; South China University of Technology: Guangzhou, China, 2016. [Google Scholar]
- Giummarella, N.; Linden, P.A.; Areskogh, D. Fractional Profiling of Kraft Lignin Structure: Unravelling Insights on Lignin Reaction Mechanisms. ACS Sustain. Chem. Eng. 2020, 8, 1112–1120. [Google Scholar] [CrossRef]
- Levdansky, A.V.; Kondrasenko, A.A.; Malyar, Y.N.; Levdansky, V.A.; Kuznetsov, B.N. Study of Organosolv Lignins by Methods of Ftir and Nmr Spectroscopy. J. Sib. Fed. Univ. Chem. 2019, 12, 201–220. [Google Scholar] [CrossRef]
- Hou, Q.Q. Preparation and Properties of Soybean Dregs Microfibrillated Cellulose. Master’s Thesis, Shaanxi University of Science & Technology, Xi’an, China, 2018. [Google Scholar]
- Li, P.P.; Sirvio, J.A.; Haapala, A.; Liimatainen, H. Cellulose Nanofibrils from Nonderivatizing Urea-based Deep Eutectic Solvent Pretreatments. ACS Appl. Mater. Inter. 2017, 9, 2846–2855. [Google Scholar] [CrossRef] [PubMed]
- Loow, Y.L.; Wu, T.Y.; Yang, G.H.; Ang, L.Y.; New, E.K.; Siow, L.F.; Jahim, J.M.; Mohammad, A.W.; Teoh, W.H. Deep Eutectic Solvent and Inorganic Salt Pretreatment of Lignocellulosic Biomass for Improving Xylose Recovery. Bioresour. Technol. 2018, 249, 818–825. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Bai, X.L.; Lusi, A.; Zhang, H.W.; Wan, C.X. Insights into structural changes of lignin toward tailored properties during deep Eutectic solvent pretreatment. ACS Sustain. Chem. Eng. 2020, 8, 9783–9793. [Google Scholar] [CrossRef]




| Cellulose | Hemicellulose | Lignin | Ash | Moisture | Extract |
|---|---|---|---|---|---|
| 55.07 | 25.19 | 19.84 | 0.89 | 8.36 | 1.53 |
| ±1.27 | ±0.43 | ±0.17 | ±0.01 | ±0.12 | ±0.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Huang, C.; Zhao, Y.; Zheng, C.; Su, H.; Zhang, L.; Luo, W.; Zhao, H.; Wang, S.; Huang, L.-J. Effect of Choline-Based Deep Eutectic Solvent Pretreatment on the Structure of Cellulose and Lignin in Bagasse. Processes 2021, 9, 384. https://doi.org/10.3390/pr9020384
Li C, Huang C, Zhao Y, Zheng C, Su H, Zhang L, Luo W, Zhao H, Wang S, Huang L-J. Effect of Choline-Based Deep Eutectic Solvent Pretreatment on the Structure of Cellulose and Lignin in Bagasse. Processes. 2021; 9(2):384. https://doi.org/10.3390/pr9020384
Chicago/Turabian StyleLi, Cuicui, Chongxing Huang, Yuan Zhao, Chaojian Zheng, Hongxia Su, Lanyu Zhang, Wanru Luo, Hui Zhao, Shuangfei Wang, and Li-Jie Huang. 2021. "Effect of Choline-Based Deep Eutectic Solvent Pretreatment on the Structure of Cellulose and Lignin in Bagasse" Processes 9, no. 2: 384. https://doi.org/10.3390/pr9020384
APA StyleLi, C., Huang, C., Zhao, Y., Zheng, C., Su, H., Zhang, L., Luo, W., Zhao, H., Wang, S., & Huang, L.-J. (2021). Effect of Choline-Based Deep Eutectic Solvent Pretreatment on the Structure of Cellulose and Lignin in Bagasse. Processes, 9(2), 384. https://doi.org/10.3390/pr9020384








