Abstract
In this work was evaluated the effect of sequential inoculum of Hanseniaspora uvarum AS27 strain and a commercial Saccharomyces cerevisiae yeast on the physical–chemical and organoleptic features of Aglianico, a traditional red wine of Southern Italy. Four fermentation treatments on a pilot scale were performed. In fermentation treatment A, the alcoholic fermentation was spontaneously conducted by the indigenous yeasts present in grape must. In the fermentation treatments B and C were inoculated respectively S. cerevisiae FE and H. uvarum AS27 strains, as a single starter. The fermentation treatment D was initially inoculated with H. uvarum AS27, and S. cerevisiae strain was added after 72 h (sequential inoculation). Microbiological, physical–chemical parameters and sensory profiles of the wines have been defined. The results showed that the use of H. uvarum AS27, in sequential inoculum with S. cerevisiae FE, influenced the wine composition, enriching it in polyphenolic and volatile compounds. Further, the sensory evaluation showed that the use of H. uvarum AS27 strain, in co-culture with S. cerevisiae, gives the wine more pleasant characteristics. Therefore, the results have highlighted how the use of particular non-Saccharomyces yeasts can represent a biotechnological resource in red wine production.
1. Introduction
In recent years, the use of commercial starters has allowed vignerons to enhance wine quality [1]. Consumers, who are increasingly more demanding, are looking for distinctive characteristics in wines and this encourages producers to develop biotechnological strategies to improve the aromatic complexity of wines. The metabolic activities of yeast, as well as of lactic acid bacteria, determine the production of several compounds that significantly influence wines’ aroma [2]. In particular, the use of commercial yeasts ensures complete and linear fermentations and allows vignerons to obtain wines without defects free from off-flavors. However, at the same time, the exclusive use of Saccharomyces yeasts could reduce wine diversification [3]. Recently, the role of non-Saccharomyces yeasts in winemaking has been re-evaluated based on their enzymatic pool, which can be important in the valorization of wines [4]. The fermentation conducted by multiple starters, composed of Saccharomyces and non-Saccharomyces yeasts, turned out to be an advantageous biotechnological strategy that allows emulating, as far as possible, what happens in spontaneous fermentation; this technique produces wines without defects and with more complex and distinctive aromatic characteristics [5]. The limited use of non-Saccharomyces yeasts has often been conditioned by their low resistance to alcohol and the production of undesirable compounds such as acetic acid, sulfur compounds, etc.; today, however, the technologies and equipment used make it possible to work in conditions that limit the production of unwanted volatile compounds [6,7,8].
Unlike Saccharomyces species, non-Saccharomyces yeasts possess different enzymes: glycosidase, pectinases, proteases β-glucanases, lichenases, β-glucosidases, cellulases, xylanases, amylases, lipases, esterases, etc [9]. These can play an important role both in the technological extractive phase and during the fermentation, in the release or production, of terpenoids, fatty acid esters, higher alcohols, esters, etc [10,11]. The use of these yeasts in fermentation could constitute a valid alternative to the use of enzymes produced by filamentous bacteria and fungi [12]. Numerous aromatic compounds are present in the grapes as glycosidic precursors without sensory properties [13,14]. The enzyme glucosidase hydrolyzes the β-D-glucosidic bond, favoring the formation of volatile compounds such as norisoprenoids, benzenoids, aliphatic alcohols, and terpenes that contribute to the definition of the organoleptic characteristics of the wine. The esterase of the yeast can have a significant effect on the fruity flavors of the wine. Non-Saccharomyces yeasts in possession of proteolytic and pectinolytic enzymes can be useful in different stages of the winemaking process [9]. Additionally, the pectic enzymes can accelerate the extraction of juice from grapes and promote the release of phenolic compounds during the maceration phase [15,16]. To maximize the metabolic activity of non-Saccharomyces yeasts, various methods of use have been evaluated, but sequential inoculation has proved to be the best option. This technique allows non-Saccharomyces yeasts to play their role without the competition of Saccharomyces which, inoculated in a second phase, complete the fermentation process [7]. Several researchers have shown that with this biotechnological strategy it is possible to produce wines with distinct aromatic profiles and improve the complexity of the wine [17]. In recent years, the genus Hanseniaspora has been the subject of numerous studies and it has been shown that several oenological characteristics belonging to this genus can positively influence the color, taste, aromas, and stability of wines [18,19,20,21,22,23,24]. Based on the above considerations, in this study we evaluated the oenological potential, as a starter, of the H. uvarum AS27 strain in Aglianico wine production. Aglianico (Vitis vinifera L.) is a renowned red grape cultivar widespread in Southern Italy [25] rich in polyphenolic compounds [26,27,28].
2. Materials and Methods
2.1. Yeasts and Growth Conditions
In this study, a commercial yeast, S. cerevisiae FE (Fermol Elegance, AEB, San Polo, BS; Italy) and H. uvarum AS27 strain have been used. This non-Saccharomyces yeast was isolated from grape must [29] and belongs to the culture collection of the DiAAA (Department of Agricultural, Environmental and Food Sciences, University of Molise).
H. uvarum AS27 strain was chosen for its strong enzymatic activities (β-glucosidase, esterase and pectolytic activity) and good oenological properties, such as low production of acetic acid, good alcohol production, and good sulphite tolerance [29]. The S. cerevisiae strain was rehydrated before use according to the manufacturer’s instructions and a pre-culture of the H. uvarum AS27 strain, grown in YPD medium (1% w/v yeast extract, 2% w/v peptone and 2% w/v dextrose) at 20 °C under aerobic condition for 48 h, was used. The cultures were centrifuged at 5000 rpm for 10 min at 4 °C, washed twice with sterile physiological solution (0.9% NaCl) before use, and inoculated in Aglianico grape juice to a concentration of 6.0 log CFU/mL.
2.2. Winemaking Design
For the fermentation experiments, red grapes (Vitis vinifera cv. Aglianico) were harvested during the 2018 vintage and transported to Mastroberardino winery (Atripalda, AV-Italy). The grapes were de-stemmed, potassium metabisulfite (70 mg/L) was added and the must was used for four different fermentation treatments (FT): FT-A, spontaneous fermentation; FT-B, inoculated with S. cerevisiae FE; FT-C, inoculated with H. uvarum AS27 strain; FT-D, initially inoculated with H. uvarum AS27 and after 72 h (sequential inoculum), with S. cerevisiae FE. For every FT, carried out in triplicate, we used stainless steel tanks (1 hL) containing 80 L of grape must with skins and the punching-down was done three times a day until the end of alcoholic fermentation. The grape must used for the experiments showed the following chemical composition: pH 3.06; sugar content 22.9° Brix, titratable acidity 9.50 g/L (as tartaric acid), malic acid 3.90 g/L, total polyphenols 940 mg/L (as gallic acid equivalent), anthocyanins 65 mg/L (as malvidin-3-glucoside), catechins 32 mg/L, YAN (yeast assimilable nitrogen) 146 mg/L. The physical–chemical analyses of the grape must were carried out in duplicate and were performed according to the corresponding European Community (EC) methods [30]. To maintain an optimal YAN level for yeasts, after 72 h of fermentation, in all the fermentation treatments 25 mg/L of ammonium phosphate was added. The alcoholic fermentation was monitored, assessing the reducing sugars and ethanol content, considering the variation during fermentation time. The temperature was set at 23–24 °C. At the end of alcoholic fermentation from every fermentation treatment, skins were pressed and the wines obtained were subjected to chemical and sensory analysis.
2.3. Microbiological Analysis
Samples were taken under aseptic conditions during alcoholic fermentation (0, 3, 6, 9, 12, 15, and 18 days) and subjected to yeast enumeration. Viable cell counts were evaluated by the plate-counting technique using WL agar (Oxoid, Hampshire, UK), containing 100 mg/L chloramphenicol (Sigma-Aldrich, St. Louis, USA) for bacterial growth inhibition.
The colony color and colony topography parameters were adopted to differentiate the Saccharomyces from non-Saccharomyces [31]. The various macroscopic colonies formed were counted, and representative colony forms were isolated and maintained on YPD agar slopes at 4 °C until phenotypic and genotypic characterization. The identification of typical colonies was performed using morphological, physiological, and biochemical tests according to the scheme of Barnett et al. [32]. The presence and predominance of the inoculated S. cerevisiae FE and H. uvarum AS27 starter cultures were assessed by RAPD-PCR [33]. After yeasts counting, from each different Petri plate (WL medium), 10 colonies were randomly picked and subjected to genetic characterization. Two milliliters of overnight cultures in YPD broth medium were centrifuged at 14,000 rpm for 10 min at 4 °C to pellet the cells and the pellet was subjected to DNA extraction using a yeast genomic DNA isolation kit (Norgen Biotek, Thorold, Canada). One hundred nanograms of the DNA extracted was subjected to RAPD-PCR with primer M13 (5′-GAGGGTGGCGGTTCT-3′) [34]. The amplification products were separated by electrophoresis on 1.5% (w/v) agarose gel (Sigma-Aldrich, Steinheim, Germany) in 0.5× TBE buffer and then subjected to ethidium bromide staining. RAPD-PCR gels were digitally captured and analyzed by GEL DOC XR System (Bio-Rad, Hercules, CA, USA) using the software Quantity One Analysis (Bio-Rad) and analyzed with the pattern analysis software package, Gel Compare II Version 2.0 (Applied Maths, Kortrijk, Belgium). The calculation of similarities in the profiles of bands was based on Pearson product-moment correlation coefficient. Dendrograms were obtained using the Unweighted Pair Group Method using Arithmetic Average (UPGMA) clustering algorithm [33].
2.4. Chemical Analytical Methods
Chemical analyses were performed according to the corresponding European Community (EC) methods [30]. The malic acid and lactic acid were determined spectrophotometrically (BioSpectrometer Eppendorf, Hamburg, Germany) using an enzymatic kit (Boehringer Mannheim, GmbH, Mannheim, Germany). Volatile compounds (µg/L) were determined by gas chromatography (GC) (Thermoquest Mod. 8000, Rodano, Milan, Italy) and flame ionization detection equipped with a fused capillary column ZB-Wax (30 m × 0.32 mm i.d., 0.50 µm film thickness, Phenomenex, Torrance, CA, USA), according to International Organization of Vine and Wine (OIV) [35]. After the addition of the internal standard (Butan-2-ol; 0.1 mg/mL in water), 1 µL of the sample was injected directly in split mode (1:50); injection port at 250 °C; the oven temperature was increased from 40 °C (5 min) to 240 °C at a rate of 7 °C/min; carrier gas helium was used with a flow rate of 60 kPa [29,36,37,38]. These analyses were carried out in duplicate and all the reagents were purchased from Sigma-Aldrich.
2.5. Sensory Profile of Wines
To evaluate the different sensorial characteristics of the wines, the samples were tasted by a panel of 20 judges (10 females and 10 males), between 22 and 61 years of age, recruited from the National Organization of Wine Tasters (ONAV, Italy) [39] according to Italian ministerial disciplines [40]. Three replicates of each FT were considered for the sensory evaluation. Wine profile evaluations took place in three sessions. In each session, the panelists evaluated four wines, obtained by the different FT, presented in a randomized order. The samples (30 mL) were presented at room temperature (18 °C) in black tulip-shaped glasses, covered with glass Petri dishes, and coded with random three-digit codes. Unsalted crackers and room temperature water were provided to rinse the mouth between samples [41]. Previous to the tasting sessions, the studied parameters were established by consensus according to ONAV methodology. The panelists were asked to rate the wines according to an unstructured scale from 0 (absent) to 9 (very intense), to rate the intensity of the following parameters: overall judgment, spiciness, herbal characters, acidity, astringency, softness, sweet cherry, red fruits, retro olfactory spiciness (ro-spiciness), retro olfactory red fruits (ro-red fruits), and color.
2.6. Statistical Analysis
The wine chemistry and the sensorial analysis data represent three biological replicates for each different FT (n = 3). The data obtained were analyzed using the software R (v 4.0.3). Analysis of variance (ANOVA) was performed. Statistical significance was attributed to values of p ≤ 0.05.
3. Results and Discussion
3.1. Fermentation Kinetics
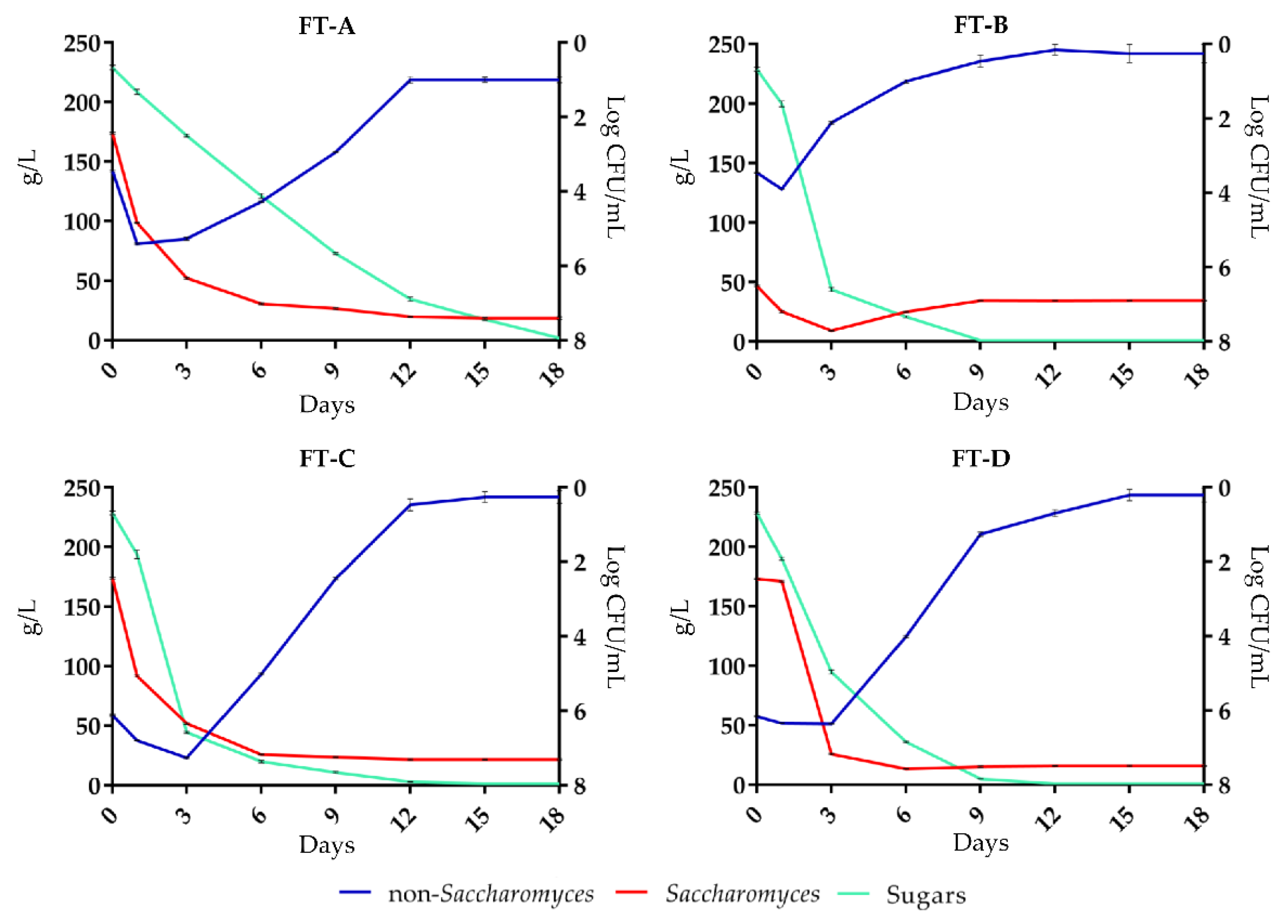
The different “yeast populations” and “fermentative kinetics” are shown in Figure 1. In all FT, sugars were completely fermented although with different dynamics. In FT-A the non-Saccharomyces yeasts were predominant in the first two days of fermentation with a cellular concentration of 5.2 log CFU/mL, and on the 6th day it was about 4.2 log CFU/mL, after which a progressive decrease was observed until reaching a concentration of about 1.0 log CFU/mL on the 12th day. Saccharomyces yeasts, initially present with a concentration of about 2.4 log CFU/mL, from the sixth day until the end of the alcoholic fermentation retained a concentration of about 7.3 log CFU/mL.
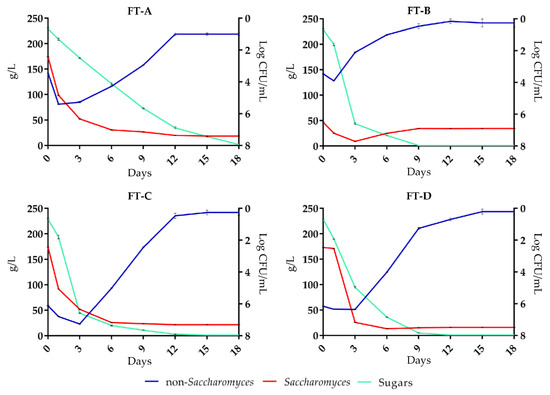
Figure 1.
Evolution of Saccharomyces and non-Saccharomyces yeasts, and sugars consumption in the four fermentation treatments: FT-A (spontaneous fermentation); FT-B (S. cerevisiae FE); FT-C (H. uvarum AS27); FT-D (H. uvarum AS27 + S. cerevisiae FE).
In FT-A, non-Saccharomyces yeasts were predominant after the first two days of fermentation with a cell concentration of 5.2 log CFU/mL, which gradually decreased to a concentration of about 1.0 log CFU/mL after 12 days.
In FT-B, fermentation was started and completed by the S. cerevisiae FE strain, inoculated at a concentration of 6.5 log CFU/mL, reached the maximum growth rate on the 3rd day of fermentation (7.7 log CFU/mL); the density of non-Saccharomyces yeast, initially about 3.4 log CFU/mL, quickly decreased after the addition of the starter S. cerevisiae FE, which instead maintained a cell concentration of 6.9 log CFU/mL at the end of alcoholic fermentation (8th day).
In FT-C, where the starter H. uvarum AS27 was inoculated, non-Saccharomyces yeasts reached a cell concentration of 7.2 log CFU mL on the 3rd day of fermentation, after which their cell density had a gradual decline. Saccharomyces yeasts reached their maximum cell density after six days (about 7.0 log CFU/mL) which was similar until the end of alcoholic fermentation.
In FT-D, in which H. uvarum AS27 yeast was initially inoculated, the non-Saccharomyces yeasts counts showed a cellular concentration of about 6.1 log CFU/mL until the 3rd day, which corresponds to the inoculation of the S. cerevisiae FE. From this point on, the population of the non-Saccharomyces yeasts had a rapid decrease, while Saccharomyces yeasts maintained a cellular concentration of about 7.5 log CFU/mL until the end of the alcoholic fermentation (12th day). RAPD-PCR analysis confirmed the presence and predominance of the starter of S. cerevisiae FE and H. uvarum AS27 in FT-B, FT-C, and FT-D treatments (data not shown). The data showed (Figure 1) that the best fermentation performances were detected when S. cerevisiae FE inoculum (FT-B) or sequential inoculum of H. uvarum AS27 and S. cerevisiae FE (FT-D) were applied. In fact, in tanks B and D, the alcohol fermentation progressed regularly and sugars were fully fermented after nine days. In FT-C, where the must was inoculated with H. uvarum AS27, as a single starter, the fermentation was completed, in 12 days, by indigenous Saccharomyces. In FT-D, the non-Saccharomyces were the predominant yeast until the 3rd day of fermentation when S. cerevisiae FE was co-inoculated.
3.2. Wine Chemistry
Table 1 shows the chemical characteristics of the wines at the end of alcoholic fermentation. The results of volatile acidity emphasize the compatibility of H. uvarum AS27 with the winemaking process, as also evidenced by other authors [19,29], and the use of selected apiculate yeasts in co-culture with S. cerevisiae did not affect the volatile acidity. In fact, in the wine obtained in FT-D, the volatile acidity value was less than 0.5 g/L. Instead, a greater increase in volatile acidity (0.97 g/L) was detected in wine obtained in FT-A (spontaneous fermentation).

Table 1.
Chemical parameters of Aglianico wines obtained by different fermentation treatments: FT-A (spontaneous fermentation); FT-B (S. cerevisiae FE); FT-C (H. uvarum AS27); FT-D (H. uvarum AS27 + S. cerevisiae FE).
The concentrations of glycerol in the wines, obtained in FT-B and FT-D, 8.36 g/L and 8.76 g/L respectively, were not significantly different from each other. As already reported in literature [42], certain S. cerevisiae strains could affect glycerol content and the presence of H. uvarum AS27 in the early stages of the alcoholic fermentation did not affect the total production of this compound. In contrast, in FT-A and FT-C the glycerol amounts, 7.0 g/L, and 7.23 g/L respectively, were significantly different from amounts detected in the wines produced in FT-B and FT-D.
Further, significant differences were found in the amounts of polyphenolic compounds in the wines. In FT-D the highest amounts of total polyphenols, anthocyanins, and catechins were detected. This result could be attributable to the more intense enzymatic activity of the H. uvarum AS27 during the grape maceration phase; this would also explain the highest color intensity (IC) in wine produced in FT-D. The results of the analysis of the volatile compounds are reported in Table 2.
The use of H. uvarum AS27 starter, in FT-C and FT-D, regardless of combination with S. cerevisiae, contributed to obtaining wines with lower amounts of acetaldehyde and ethyl acetate than wine obtained by spontaneous fermentation (FT-A). Several studies reported the negative effects of these compounds on wine quality. In detail, high values in acetaldehyde could result in the appearance of oxidation off-flavor [43,44].
Some species of yeasts have a propensity to produce more ethyl acetate than others.
Ethyl acetate is the major ester produced by yeast and at low levels it imparts a fruity character to the wine. The aroma threshold in wine is around 18 mg/L and levels up to around 60 mg/L are considered to have a positive effect on the wine [45].
In the wines obtained in the four fermentation treatments, significantly different amounts of the higher alcohols were detected, except for the compounds 2-methyl-1-butanol, 2-phenylethanol, and methanol, whose amounts were similar. Although the amount of all individual alcohols was not higher than the respective threshold levels, the overall content of the higher alcohols influences the organoleptic quality of the wines and, if not more than 300 mg/L, contributes to a positive impact on the aroma and flavor of wine [46,47]. Additionally, the higher alcohols can be esterified with acetic acid to produce low-threshold aromatic esters (e.g., 2-phenylethyl acetate, isoamyl acetate, isobutyl acetate), most of them with floral or fruity descriptors [48]. As regards terpenic compounds, in the wines produced in FT-C and FT-D were detected higher amounts of geraniol, linalool, nerol, nonanol, and 4-terpineol than in the wines obtained by spontaneous fermentation (FT-A) and using S. cerevisiae as a single starter (FT-B). This relation was also reported by other authors [49] and could be attributable to the metabolism of H. uvarum that through its β-glucosidase activity can favor the increase in amounts of volatile terpenes [7,19]. Linanol was the only terpene found in concentrations above the olfactory threshold level and our data confirm that this compound particularly characterizes Aglianico wines [50]. The amounts of the other terpenic compounds were not higher than their olfactory thresholds. However, the aroma of the wine is the sum of all the volatile compounds, and as a consequence of the synergistic character, some volatile compounds can be potentiated by the presence of others [51]. Therefore, even terpenic alcohols, that have concentrations below their odor threshold, could contribute to aromatic complexity.

Table 2.
Volatile composition of the wines obtained by different fermentation treatments: FT-A (spontaneous fermentation); FT-B (S. cerevisiae FE); FT-C (H. uvarum AS27); FT-D (H. uvarum AS27 + S. cerevisiae FE).
Table 2.
Volatile composition of the wines obtained by different fermentation treatments: FT-A (spontaneous fermentation); FT-B (S. cerevisiae FE); FT-C (H. uvarum AS27); FT-D (H. uvarum AS27 + S. cerevisiae FE).
| Compounds | FT-A | FT-B | FT-C | FT-D | Threshold (µg/L) | Odor Descriptor | Ref. |
|---|---|---|---|---|---|---|---|
| Acetaldehyde ** | 11.5 ± 0.1 b | 9.61 ± 0.12 a | 9.52 ± 0.31 a | 9.27 ± 0.12 a | 500 | Green leaves, fruity | [52] |
| Ethyl acetate ** | 118 ± 1 d | 44.8 ± 1.1 a | 68.5 ± 1.8 c | 55.5 ± 1.9 b | 18,000 | Solvent, fruity, sweetish | [53] |
| 1-Heptanol | 186 ± 11 c | 111 ± 7 b | 82.4 ± 5.1 a | 109 ± 12 b | 200–300 | Lemon, orange, copper | [54] |
| 2-Methyl propanol | 24.4 ± 0.1 a | 27.8 ± 0.1 c | 26.6 ± 0.2 b | 24.5 ± 0.4 a | 40,000 | Wine, solvent, bitter | [55] |
| 1-Octanol | 237 ± 2 c | 207 ± 2 b | 198 ± 2 a | 212 ± 3 b | 820 | Coconut, walnut, oily | [52] |
| 1-Pentanol | 63.1 ± 1.1 c | 35.1 ± 0.9 a | 47.8 ± 1.2 b | 58.9 ± 2.6 c | 64,430 | Alcohol, medicinal | [52] |
| 1-Propanol | 18.9 ± 1.3 b | 31.8 ± 1.2 d | 13.7 ± 1.5 a | 22.8 ± 1.6 c | 830 | Pungent, harsh, ripe fruit | [55] |
| 2,3-Butanediol | 412 ± 12 a | 598 ± 14 c | 551 ± 12 b | 618 ± 17 c | 120,000 | Butter, creamy | [54] |
| 2-Methyl-1-butanol | 156 ± 12 a | 173. ± 12 a | 164 ± 14 a | 203 ± 13 b | 32,000 | Alcohol, banana | [52] |
| 2-Pentanol | 46.4 ± 1.1 a | 55.4 ± 1.1 b | 82.4 ± 1.6 c | 81.4 ± 1.6 c | - | Green | [56] |
| 2-Phenylethanol | 89.8 ± 11.3 a | 69.1 ± 4.1 a | 78.7 ± 14.2 a | 112 ± 12 b | 14,000 | Roses | [57] |
| 3-Methyl-1-butanol | 86.8 ± 1.2 a | 108 ± 11 a | 101 ± 12 a | 119 ± 12 b | 30,000 | Cheese | [57] |
| Hexanoic acid | 64.8 ± 1.6 a | 83.2 ± 1.6 b | 66.4 ± 1.9 a | 78.6 ± 2.7 b | 420 | Cheese, rancid | [54] |
| Methanol | 119 ± 1 | 112 ± 13 | 107 ± 4 | 105 ± 6 | 100,000 | Sweet | [58] |
| Methionol | 163 ± 2 b | 104 ± 2 a | 99.2 ± 3.6 a | 98.4 ± 6.1 a | 500 | Boiled potato, rubber | [59] |
| Linalool | 32.2 ± 0.3 b | 23.2 ± 1.4 a | 69.3 ± 0.7 c | 68.6 ± 0.8 c | 25.2 | Muscat, flowery, fruit | [54] |
| 4-Terpineol | 19.7 ± 0.1 a | 28.5 ± 0.3 b | 45.8 ± 0.6 d | 43.5 ± 0.5 c | 110–400 | Light aroma, wood, soil | [54] |
| α-Terpineol | 8.4 ± 0.7 a | 14.9 ± 0.8 b | 37.5 ± 0.4 d | 32.8 ± 0.8 c | 250 | Anise | [57] |
| Nerol | 34.5 ± 0.7 a | 42.6 ± 1.1 b | 77.8 ± 0.5 c | 98.6 ± 1.0 d | 400 | Rose-like aromas | [60] |
| Nonanol | 12.5 ± 0.9 a | 29.53 ± 1.4 b | 68.2 ± 1.5 c | 66.5 ± 0.9 c | 310 | Coconut, walnut, oily | [52] |
| Geraniol | 10.4 ± 0.2 b | 5.62 ± 0.13 a | 10.9 ± 0.2 b | 12.5±0.2 c | 130 | Floral | [60] |
Data are expressed as mean values ± standard deviations (n = 3); a–d: within a row, different letters indicate significant differences (p ≤ 0.05). All data are expressed as µg/L, except where otherwise indicated (** value was expressed in mg/L).
Several authors reported the ability of non-Saccharomyces yeasts to enhance the quality and aromatic complexity of wines, affecting both the primary and the secondary aroma [48,61]. The wide variety of aromas is greatly demanded and desired in traditional red wine [29,38]. In this context, it should be considered that the exclusive use of S. cerevisiae strains as fermentative yeasts could flatten the aromatic profiles. Conversely, the co-inoculum or sequential inoculum of Hanseniaspora spp. and Saccharomyces yeasts could highly affect the wine quality, releasing varietal aromas from precursors such as glycosylated terpenes or bonded thiols employing β-glucosidase or C-S-lyase activities [48,62,63].
Our results eliminate several doubts related to the use of Hanseniaspora selected strains in winemaking, confirming, as also reported by other authors, that the use of Saccharomyces and non-Saccharomyces in sequential inoculum produces wines with aromatic complexity greater than that of wines obtained with the use of S. cerevisiae as a single starter [3]. However, greater aromatic complexity does not axiomatically mean a better sensory quality of wines. Therefore, sensory analysis is important to evaluate the wine bouquet [64].
3.3. Sensory Analysis
Chemical analysis, while allowing the recognition of the individual components responsible for sensory characteristics, fails to provide information on the real sensory perception as a whole, which is also the result of synergistic or antagonistic effects of the various chemical compounds contained in the wine [65].
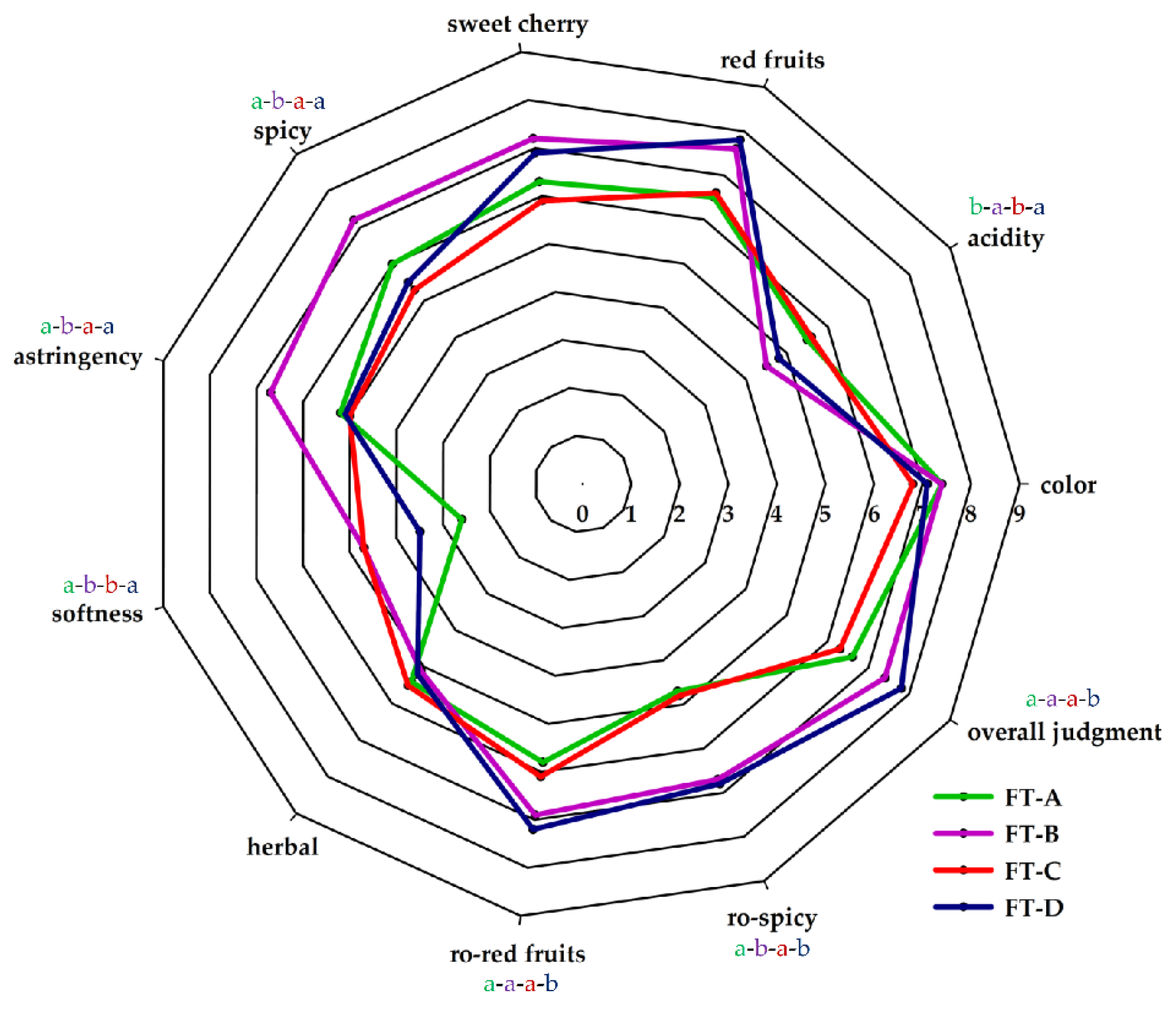
Therefore, to assess the impacts of all compounds, a sensory evaluation of the wines was carried out with a group of sensory experts. The results of the sensory analysis of the four wines are reported in Figure 2. Significant differences were obtained in the scores awarded in the evaluation of acidity, spiciness, astringency, softness, ro-red fruits, ro-spiciness, and overall judgment (Table S1; supplementary material).
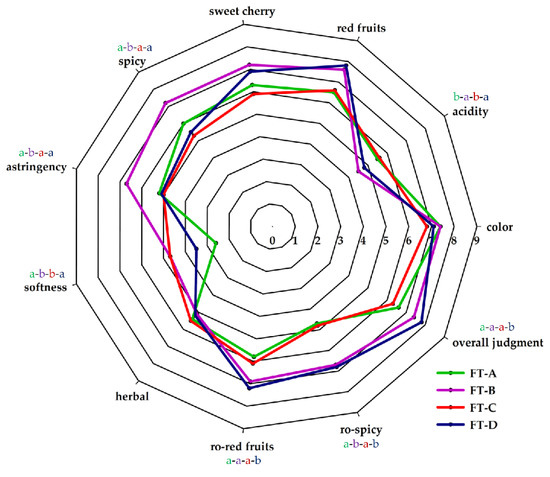
Figure 2.
Sensory profiles of Aglianico wines obtained by different fermentation treatments: FT-A (spontaneous fermentation); FT-B (S. cerevisiae FE); FT-C (H. uvarum AS27); FT-D (H. uvarum AS27 + S. cerevisiae FE).
The wines obtained in FT-B and FT-D scored best in overall judgment. The score of this parameter has been awarded on the basis of an overall evaluation of the wine that includes the visual, taste, and olfactory aspects of the wine. The sensory color analysis did not show significant differences between the four wines produced, although chemical analyses had detected significant differences in the amounts of total polyphenols, anthocyanins, catechins, and in IC and CT values. The sensory evaluation of color is based on the perception of the following characters: intensity, hue, vivacity, clarity; therefore, it is not entirely related to chemical–physical analysis. The assessment of astringency was significantly different between the wine produced in FT-B and the other wines. It is not easy to justify this result because astringency is influenced by the concentration of several compounds. The class of molecules that contributes most significantly to overall red wine astringency is tannins [66]. However, the astringent sensation can also be modulated by other compounds contained in wine such as acids, sugars, ethanol, anthocyanins, and polysaccharides [67,68,69]. The fraction of anthocyanin, unlike polysaccharides, can increase perceived astringency, along with the presence of some tannins [70,71]. Different studies have revealed that several polysaccharide families can interact with tannins, so they could reduce astringency by limitation of available proanthocyanidins [72,73]. Some polysaccharides, such as mannoproteins, are released from yeast cell walls during fermentation and later, during contact of wine with yeast lees [74]. In some research, it has been shown that the non-Saccharomyces yeasts have a greater capacity to release polysaccharides compared to S. cerevisiae and that this feature is also strain-dependent [75,76]. On the basis of this consideration, the ability to release these compounds by the H. uvarum AS27 strain should also be investigated in the future.
Polysaccharides, and especially mannoproteins, also have an important sensory level on softness sensory perception. The softness is a tactile sensation related to the presence of various substances such as glycerol, alcohols, and polysaccharides. It is perceived as an embracing and roundness sensation on the tongue [77]. Glycerol is a non-volatile compound that has no aromatic properties, but which significantly contributes to wine quality by providing sweetness, fullness, and softness [78]. Therefore, the amounts of polysaccharides and glycerol contained in the four different wines may have influenced this sensory parameter. In the sensory panel, the judges attributed significantly higher scores in sensory perception of acidity to wines obtained in FT-A and FT-C; the reason may be due to the higher volatile acidity values found in these wines. Some acids in lower concentrations such as succinic acid, pyruvic acid, citric acid, etc., without significant repercussions on pH values, may affect sensory perception [48]. Besides, as far as the acidity is concerned, the indirect impact of the yeast strain is linked to its ability to produce and release polysaccharides, which increase the softness of the wines, causing their acidic sensation to decrease. Among the other parameters examined by the taster judges, significant differences were found in spiciness, ro-red fruits and ro-spiciness. These sensory perceptions are mainly due to compounds originating from the grape varieties [79].
4. Conclusions
The results showed that the use of H. uvarum AS27 as the selected strain, in sequential inoculum with a commercial Saccharomyces yeast, could be a biotechnological resource to enhance Aglianico wine. The use of mixed starters represents an alternative both to spontaneous fermentation and to the use of Saccharomyces strains as a single starter, taking advantage of the positive role that non-Saccharomyces yeasts, suitably selected, can have in the definition of the chemical and organoleptic characteristics of wine [80]. Future investigations will be carried out on the vinification of Aglianico and other grape varieties using H. uvarum AS27 in co-culture with S. cerevisiae strains.
Moreover, using better performing analytical techniques such as liquid or gas chromatography/mass spectrometry, it will be possible to have more information on the composition of the wines produced and their evolution during ripening and aging.
Supplementary Materials
The following are available online at https://www.mdpi.com/2227-9717/9/2/326/s1. Table S1. Sensory analysis data of Aglianico wines.
Author Contributions
Conceptualization, M.I., M.S. and M.D.R.; methodology, F.C., M.D.R.; software, F.L.; validation, M.I., S.J.L.; formal analysis, F.C., B.T.; investigation, M.I.; resources, P.T.; data curation, F.L., B.T.; writing—original draft preparation, M.I., S.J.L.; writing—review and editing, M.I., B.T., F.L.; visualization, S.J.L.; supervision, M.I., P.T., M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wolfe, B.E.; Dutton, R.J. Fermented Foods as Experimentally Tractable Microbial Ecosystems. Cell 2015, 161, 49–55. [Google Scholar] [CrossRef]
- Belda, I.; Ruiz, J.; Esteban-Fernández, A.; Navascués, E.; Marquina, D.; Santos, A.; Moreno-Arribas, M.V. Microbial Contribution to Wine Aroma and Its Intended Use for Wine Quality Improvement. Molecules 2017, 22, 189. [Google Scholar] [CrossRef] [PubMed]
- Andorrà, I.; Berradre, M.; Rozès, N.; Mas, A.; Guillamón, J.M.; Esteve-Zarzoso, B. Effect of Pure and Mixed Cultures of the Main Wine Yeast Species on Grape Must Fermentations. Eur. Food Res. Technol. 2010, 231, 215–224. [Google Scholar] [CrossRef]
- Benito, Á.; Calderón, F.; Benito, S. The Influence of Non-Saccharomyces Species on Wine Fermentation Quality Parameters. Fermentation 2019, 5, 54. [Google Scholar] [CrossRef]
- Padilla, B.; Zulian, L.; Ferreres, À.; Pastor, R.; Esteve-Zarzoso, B.; Beltran, G.; Mas, A. Sequential Inoculation of Native Non-Saccharomyces and Saccharomyces Cerevisiae Strains for Wine Making. Front. Microbiol. 2017, 8, 1293. [Google Scholar] [CrossRef]
- Moreira, N.; Pina, C.; Mendes, F.; Couto, J.A.; Hogg, T.; Vasconcelos, I. Volatile Compounds Contribution of Hanseniaspora Guilliermondii and Hanseniaspora Uvarum during Red Wine Vinifications. Food Control 2011, 22, 662–667. [Google Scholar] [CrossRef]
- Ciani, M.; Comitini, F.; Mannazzu, I.; Domizio, P. Controlled Mixed Culture Fermentation: A New Perspective on the Use of Non-Saccharomyces Yeasts in Winemaking. FEMS Yeast Res. 2010, 10, 123–133. [Google Scholar] [CrossRef]
- Comitini, F.; Gobbi, M.; Domizio, P.; Romani, C.; Lencioni, L.; Mannazzu, I.; Ciani, M. Selected Non-Saccharomyces Wine Yeasts in Controlled Multistarter Fermentations with Saccharomyces Cerevisiae. Food Microbiol. 2011, 28, 873–882. [Google Scholar] [CrossRef]
- Fernández, M.; Úbeda, J.F.; Briones, A.I. Typing of Non-Saccharomyces Yeasts with Enzymatic Activities of Interest in Wine-Making. Int. J. Food Microbiol. 2000, 59, 29–36. [Google Scholar] [CrossRef]
- Mendes Ferreira, A.; Clímaco, M.C.; Mendes Faia, A. The Role of Non-Saccharomyces Species in Releasing Glycosidic Bound Fraction of Grape Aroma Components—A Preliminary Study. J. Appl. Microbiol. 2001, 91, 67–71. [Google Scholar] [CrossRef]
- López, S.; Mateo, J.J.; Maicas, S. Screening of Hanseniaspora Strains for the Production of Enzymes with Potential Interest for Winemaking. Fermentation 2016, 2, 1. [Google Scholar] [CrossRef]
- Haight, K.G.; Gump, B.H. The Use of Macerating Enzymes in Grape Juice Processing. Am. J. Enol. Vitic. 1994, 45, 113. [Google Scholar]
- Winterhalter, P.; Skouroumounis, G.K. Glycoconjugated aroma compounds: Occurrence, role and biotechnological transformation. In Biotechnology of Aroma Compounds; Berger, R.G., Babel, W., Blanch, H.W., Cooney, C.L., Enfors, S.-O., Eriksson, K.-E.L., Fiechter, A., Klibanov, A.M., Mattiasson, B., Primrose, S.B., et al., Eds.; Springer: Berlin/Heidelberg, Germany, 1997; pp. 73–105. ISBN 978-3-540-68602-6. [Google Scholar]
- Barbagallo, R.N.; Spagna, G.; Palmeri, R.; Restuccia, C.; Giudici, P. Selection, Characterization and Comparison of β-Glucosidase from Mould and Yeasts Employable for Enological Applications. Enzym. Microb. Technol. 2004, 35, 58–66. [Google Scholar] [CrossRef]
- Medina, K.; Boido, E.; Dellacassa, E.; Carrau, F. Effects of Non-Saccharomyces Yeasts on Color, Anthocyanin, and Anthocyanin-Derived Pigments of Tannat Grapes during Fermentation. Am. J. Enol. Vitic. 2018, 69, 148. [Google Scholar] [CrossRef]
- Mendoza, L.; Farias, M.E. Improvement of Wine Organoleptic Characteristics by Non-Saccharomyces Yeasts. In Current Research, Technology and Education Topics in Applied Microbiology and Microbial Biotechnology; Formatex Research Center: Badajoz, Spain, 2010; Volume 2, pp. 908–919. [Google Scholar]
- Chen, K.; Escott, C.; Loira, I.; del Fresno, J.M.; Morata, A.; Tesfaye, W.; Calderon, F.; Suárez-Lepe, J.A.; Han, S.; Benito, S. Use of Non-Saccharomyces Yeasts and Oenological Tannin in Red Winemaking: Influence on Colour, Aroma and Sensorial Properties of Young Wines. Food Microbiol. 2018, 69, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Moreira, N.; Mendes, F.; Guedes de Pinho, P.; Hogg, T.; Vasconcelos, I. Heavy Sulphur Compounds, Higher Alcohols and Esters Production Profile of Hanseniaspora Uvarum and Hanseniaspora Guilliermondii Grown as Pure and Mixed Cultures in Grape Must. Int. J. Food Microbiol. 2008, 124, 231–238. [Google Scholar] [CrossRef]
- Lombardi, S.J.; Pannella, G.; Iorizzo, M.; Moreno-Arribas, M.V.; Tremonte, P.; Succi, M.; Sorrentino, E.; Macciola, V.; Di Renzo, M.; Coppola, R. Sequential Inoculum of Hanseniaspora Guilliermondii and Saccharomyces Cerevisiae for Winemaking Campanino on an Industrial Scale. World J. Microbiol. Biotechnol. 2018, 34, 161. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Dou, G.; Guo, H.; Zhang, Q.; Qin, X.; Yu, W.; Jiang, C.; Xiao, H. Volatile Organic Compounds of Hanseniaspora Uvarum Increase Strawberry Fruit Flavor and Defense during Cold Storage. Food Sci. Nutr. 2019, 7, 2625–2635. [Google Scholar] [CrossRef]
- López, S.; Mateo, J.; Maicas, S. Characterisation of Hanseniaspora Isolates with Potential Aroma-Enhancing Properties in Muscat Wines. S. Afr. J. Enol. Vitic. 2014, 35, 292–303. [Google Scholar] [CrossRef][Green Version]
- Capozzi, V.; Berbegal, C.; Tufariello, M.; Grieco, F.; Spano, G. Impact of Co-Inoculation of Saccharomyces Cerevisiae, Hanseniaspora Uvarum and Oenococcus Oeni Autochthonous Strains in Controlled Multi Starter Grape Must Fermentations. LWT 2019, 109, 241–249. [Google Scholar] [CrossRef]
- Medina, K.; Boido, E.; Fariña, L.; Gioia, O.; Gomez, M.E.; Barquet, M.; Gaggero, C.; Dellacassa, E.; Carrau, F. Increased Flavour Diversity of Chardonnay Wines by Spontaneous Fermentation and Co-Fermentation with Hanseniaspora Vineae. Food Chem. 2013, 141, 2513–2521. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Jin, G.-J.; Xu, Y.-H.; Tao, Y.-S. Wine Aroma Response to Different Participation of Selected Hanseniaspora Uvarum in Mixed Fermentation with Saccharomyces Cerevisiae. Food Res. Int. 2018, 108, 119–127. [Google Scholar] [CrossRef]
- De Lorenzis, G.; Imazio, S.; Biagini, B.; Failla, O.; Scienza, A. Pedigree Reconstruction of the Italian Grapevine Aglianico (Vitisvinifera L.) from Campania. Mol. Biotechnol. 2013, 54, 634–642. [Google Scholar] [CrossRef]
- Gambuti, A.; Strollo, D.; Erbaggio, A.; Lecce, L.; Moio, L. Effect of Winemaking Practices on Color Indexes and Selected Bioactive Phenolics of Aglianico Wine. J. Food Sci. 2007, 72, S623–S628. [Google Scholar] [CrossRef]
- Bonfante, A.; Alfieri, S.M.; Albrizio, R.; Basile, A.; De Mascellis, R.; Gambuti, A.; Giorio, P.; Langella, G.; Manna, P.; Monaco, E.; et al. Evaluation of the Effects of Future Climate Change on Grape Quality through a Physically Based Model Application: A Case Study for the Aglianico Grapevine in Campania Region, Italy. Agric. Syst. 2017, 152, 100–109. [Google Scholar] [CrossRef]
- Picariello, L.; Rinaldi, A.; Forino, M.; Errichiello, F.; Moio, L.; Gambuti, A. Effect of Different Enological Tannins on Oxygen Consumption, Phenolic Compounds, Color and Astringency Evolution of Aglianico Wine. Molecules 2020, 25, 4607. [Google Scholar] [CrossRef]
- Testa, B.; Lombardi, S.J.; Iorizzo, M.; Letizia, F.; Di Martino, C.; Di Renzo, M.; Strollo, D.; Tremonte, P.; Pannella, G.; Ianiro, M.; et al. Use of Strain Hanseniaspora Guilliermondii BF1 for Winemaking Process of White Grapes Vitis Vinifera Cv Fiano. Eur. Food Res. Technol. 2020, 246, 549–561. [Google Scholar] [CrossRef]
- Regulation, H.A.T. Commission Regulation (EEC) No. 2676/90 Determining Community Methods for the Analysis of Wines. Off. J. L 1990, 272, 1–192. [Google Scholar]
- Pallmann, C.L.; Brown, J.A.; Olineka, T.L.; Cocolin, L.; Mills, D.A.; Bisson, L.F. Use of WL Medium to Profile Native Flora Fermentations. Am. J. Enol. Vitic. 2001, 52, 198. [Google Scholar]
- Sugiyama, J. Yeasts: Characteristics and Identification, Third Edition, by J.A. Barnett, R.W. Payne, and D. Yarrow. Mycopathologia 2001, 149, 159–160. [Google Scholar] [CrossRef]
- Urso, R.; Rantsiou, K.; Dolci, P.; Rolle, L.; Comi, G.; Cocolin, L. Yeast Biodiversity and Dynamics during Sweet Wine Production as Determined by Molecular Methods. FEMS Yeast Res. 2008, 8, 1053–1062. [Google Scholar] [CrossRef]
- Testa, B.; Lombardi, S.J.; Tremonte, P.; Succi, M.; Tipaldi, L.; Pannella, G.; Sorrentino, E.; Iorizzo, M.; Coppola, R. Biodiversity of Lactobacillus Plantarum from Traditional Italian Wines. World J. Microbiol. Biotechnol. 2014, 30, 2299–2305. [Google Scholar] [CrossRef]
- OIV. Compendium of International Methods of Analysis of Spirituous Beverages of Vitivinicultural Origin; OIV: Paris, France, 2014. [Google Scholar]
- Iorizzo, M.; Macciola, V.; Testa, B.; Lombardi, S.J.; De Leonardis, A. Physicochemical and Sensory Characteristics of Red Wines from the Rediscovered Autochthonous Tintilia Grapevine Grown in the Molise Region (Italy). Eur. Food Res. Technol. 2014, 238, 1037–1048. [Google Scholar] [CrossRef]
- Arcari, S.G.; Caliari, V.; Sganzerla, M.; Godoy, H.T. Volatile Composition of Merlot Red Wine and Its Contribution to the Aroma: Optimization and Validation of Analytical Method. Talanta 2017, 174, 752–766. [Google Scholar] [CrossRef]
- Lombardi, S.J.; Pannella, G.; Iorizzo, M.; Testa, B.; Succi, M.; Tremonte, P.; Sorrentino, E.; Di Renzo, M.; Strollo, D.; Coppola, R. Inoculum Strategies and Performances of Malolactic Starter Lactobacillus Plantarum M10: Impact on Chemical and Sensorial Characteristics of Fiano Wine. Microorganisms 2020, 8, 516. [Google Scholar] [CrossRef]
- Presidential decree DPR 563 8 Luglio 1981-Riconoscimento Della Personalità Giuridica dell’O.N.A.V; G.U., 1981; Volume 280.
- Ministerial decree DM 25 Luglio 2003-Disciplina Degli Esami Chimico-Fisici ed Organolettici e Dell’attività Delle Commissioni di Degustazione dei vini D.O.C.G. e D.O.C.; G.U., 2003; Volume 210.
- Lisanti, M.T.; Gambuti, A.; Genovese, A.; Piombino, P.; Moio, L. Partial Dealcoholization of Red Wines by Membrane Contactor Technique: Effect on Sensory Characteristics and Volatile Composition. Food Bioprocess Technol. 2013, 6, 2289–2305. [Google Scholar] [CrossRef]
- Ciani, M.; Maccarelli, F. Oenological Properties of Non-Saccharomyces Yeasts Associated with Wine-Making. World J. Microbiol. Biotechnol. 1997, 14, 199–203. [Google Scholar] [CrossRef]
- Carlton, W.K.; Gump, B.; Fugelsang, K.; Hasson, A.S. Monitoring Acetaldehyde Concentrations during Micro-Oxygenation of Red Wine by Headspace Solid-Phase Microextraction with On-Fiber Derivatization. J. Agric. Food Chem. 2007, 55, 5620–5625. [Google Scholar] [CrossRef]
- Picariello, L.; Gambuti, A.; Picariello, B.; Moio, L. Evolution of Pigments, Tannins and Acetaldehyde during Forced Oxidation of Red Wine: Effect of Tannins Addition. LWT 2017, 77, 370–375. [Google Scholar] [CrossRef]
- Plata, C.; Millán, C.; Mauricio, J.C.; Ortega, J.M. Formation of Ethyl Acetate and Isoamyl Acetate by Various Species of Wine Yeasts. Food Microbiol. 2003, 20, 217–224. [Google Scholar] [CrossRef]
- Lambrechts, M.; Pretorius, I. Yeast and Its Importance to Wine Aroma-a Review. S. Afr. J. Enol. Vitic. 2000, 21, 97–129. [Google Scholar] [CrossRef]
- Swiegers, J.H.; Pretorius, I.S. Yeast Modulation of Wine Flavor. In Advances in Applied Microbiology; Academic Press: Cambridge, MA, USA, 2005; pp. 131–175. Volume 57, ISBN 0065-2164. [Google Scholar]
- Morata, A.; Escott, C.; Bañuelos, M.A.; Loira, I.; del Fresno, J.M.; González, C.; Suárez-Lepe, J.A. Contribution of Non-Saccharomyces Yeasts to Wine Freshness. A Review. Biomolecules 2020, 10, 34. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.; López, R.; Cacho, J.F. Quantitative Determination of the Odorants of Young Red Wines from Different Grape Varieties. J. Sci. Food Agric. 2000, 80, 1659–1667. [Google Scholar] [CrossRef]
- Genovese, A.; Dimaggio, R.; Lisanti, M.T.; Piombino, P.; Moio, L. Aroma Composition of Red Wines by Different Extraction Methods and Gas Chromatography-SIM/Mass Spectrometry Analysis. Ann. Chim. 2005, 95, 383–394. [Google Scholar] [CrossRef]
- Styger, G.; Prior, B.; Bauer, F.F. Wine Flavor and Aroma. J. Ind. Microbiol. Biotechnol. 2011, 38, 1145. [Google Scholar] [CrossRef]
- Miller, G.H. Back Matter. In Whisky Science; Springer: Berlin/Heidelberg, Germany, 2019; pp. 421–467. [Google Scholar]
- Selfridge, T.B.; Amerine, M.A. Odor Thresholds and Interactions of Ethyl Acetate and Diacetyl in an Artificial Wine Medium. Am. J. Enol. Vitic. 1978, 29, 1. [Google Scholar]
- Tao, Y.-S.; Li, H. Active Volatiles of Cabernet Sauvignon Wine from Changli County. Health 2009, 1, 176. [Google Scholar] [CrossRef]
- Cortés-Diéguez, S.; Rodriguez-Solana, R.; Domínguez, J.M.; Díaz, E. Impact Odorants and Sensory Profile of Young Red Wines from Four Galician (NW of Spain) Traditional Cultivars. J. Inst. Brew. 2015, 121, 628–635. [Google Scholar] [CrossRef]
- Niu, Y.; Kong, J.; Xiao, Z.; Chen, F.; Ma, N.; Zhu, J. Characterization of Odor-Active Compounds of Various Chinese “Wuliangye” Liquors by Gas Chromatography–Olfactometry, Gas Chromatography–Mass Spectrometry and Sensory Evaluation. Int. J. Food Prop. 2017, 20, S735–S745. [Google Scholar] [CrossRef]
- Culleré, L.; Escudero, A.; Cacho, J.; Ferreira, V. Gas Chromatography−Olfactometry and Chemical Quantitative Study of the Aroma of Six Premium Quality Spanish Aged Red Wines. J. Agric. Food Chem. 2004, 52, 1653–1660. [Google Scholar] [CrossRef] [PubMed]
- Leonardos, G.; Kendall, D.; Barnard, N. Odor Threshold Determinations of 53 Odorant Chemicals. J. Air Pollut. Control Assoc. 1969, 19, 91–95. [Google Scholar] [CrossRef]
- Jiang, B.; Zhang, Z.-W. A Preliminary Study of Aroma Composition and Impact Odorants of Cabernet Franc Wines under Different Terrain Conditions of the Loess Plateau Region (China). Molecules 2018, 23, 1096. [Google Scholar] [CrossRef]
- Čuš, F.; Jenko, M. The Influence of Yeast Strains on the Composition and Sensory Quality of Gewürztraminer Wine. Food Technol. Biotechnol. 2013, 51, 547–553. [Google Scholar]
- Giorello, F.; Valera, M.J.; Martin, V.; Parada, A.; Salzman, V.; Camesasca, L.; Fariña, L.; Boido, E.; Medina, K.; Dellacassa, E.; et al. Genomic and Transcriptomic Basis of Hanseniaspora Vineae’s Impact on Flavor Diversity and Wine Quality. Appl. Environ. Microbiol. 2019, 85, e01959-18. [Google Scholar] [CrossRef]
- Ciani, M.; Beco, L.; Comitini, F. Fermentation Behaviour and Metabolic Interactions of Multistarter Wine Yeast Fermentations. Int. J. Food Microbiol. 2006, 108, 239–245. [Google Scholar] [CrossRef]
- Fleet, G.H. Yeast Interactions and Wine Flavour. Int. J. Food Microbiol. 2003, 86, 11–22. [Google Scholar] [CrossRef]
- De Mets, G.; Goos, P.; Hertog, M.; Peeters, C.; Lammertyn, J.; Nicolaï, B.M. Sensory Quality of Wine: Quality Assessment by Merging Ranks of an Expert-Consumer Panel. Aust. J. Grape Wine Res. 2017, 23, 318–328. [Google Scholar] [CrossRef]
- Genovese, A.; Lisanti, M.T.; Gambuti, A.; Piombino, P.; Moio, L. Relationship between Sensory Perception and Aroma Compounds of Monovarietal Red Wines. Acta Hortic. 2007, 754, 549–556. [Google Scholar] [CrossRef]
- García-Estévez, I.; Ramos-Pineda, A.M.; Escribano-Bailón, M.T. Interactions between Wine Phenolic Compounds and Human Saliva in Astringency Perception. Food Funct. 2018, 9, 1294–1309. [Google Scholar] [CrossRef]
- Diako, C.; McMahon, K.; Mattinson, S.; Evans, M.; Ross, C. Alcohol, Tannins, and Mannoprotein and Their Interactions Influence the Sensory Properties of Selected Commercial Merlot Wines: A Preliminary Study. J. Food Sci. 2016, 81, S2039–S2048. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, E.; Mateus, N.; Plet, B.; Pianet, I.; Dufourc, E.; De Freitas, V. Influence of Wine Pectic Polysaccharides on the Interactions between Condensed Tannins and Salivary Proteins. J. Agric. Food Chem. 2006, 54, 8936–8944. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, A.; Gambuti, A.; Moio, L. Precipitation of Salivary Proteins After the Interaction with Wine: The Effect of Ethanol, PH, Fructose, and Mannoproteins. J. Food Sci. 2012, 77, C485–C490. [Google Scholar] [CrossRef]
- Gawel, R.; Godden, P.; Williamson, P.; Francis, L.; Smith, P.; Waters, L.; Herderich, M.; Johnson, D. Influence of Phenolics on White Wine Quality and Style. Wine Vitic. J. 2014, V29N3. [Google Scholar]
- Vidal, S.; Francis, L.; Williams, P.; Kwiatkowski, M.; Gawel, R.; Cheynier, V.; Waters, E. The Mouth-Feel Properties of Polysaccharides and Anthocyanins in a Wine like Medium. Food Chem. 2004, 85, 519–525. [Google Scholar] [CrossRef]
- Troszyńska, A.; Narolewska, O.; Robredo, S.; Estrella, I.; Hernández, T.; Lamparski, G.; Amarowicz, R. The Effect of Polysaccharides on the Astringency Induced by Phenolic Compounds. Food Qual. Prefer. 2010, 21, 463–469. [Google Scholar] [CrossRef]
- Brandão, E.; Silva, M.S.; García-Estévez, I.; Williams, P.; Mateus, N.; Doco, T.; de Freitas, V.; Soares, S. The Role of Wine Polysaccharides on Salivary Protein-Tannin Interaction: A Molecular Approach. Carbohydr. Polym. 2017, 177, 77–85. [Google Scholar] [CrossRef]
- Lombardi, S.J.; De Leonardis, A.; Lustrato, G.; Testa, B.; Iorizzo, M. Yeast Autolysis in Sparkling Wine Aging: Use of Killer and Sensitive Saccharomyces Cerevisiae Strains in Co-Culture. Recent Pat. Biotechnol. 2015, 9, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Domizio, P.; Liu, Y.; Bisson, L.F.; Barile, D. Use of Non-Saccharomyces Wine Yeasts as Novel Sources of Mannoproteins in Wine. Food Microbiol. 2014, 43, 5–15. [Google Scholar] [CrossRef]
- Giovani, G.; Rosi, I.; Bertuccioli, M. Quantification and Characterization of Cell Wall Polysaccharides Released by Non-Saccharomyces Yeast Strains during Alcoholic Fermentation. Int. J. Food Microbiol. 2012, 160, 113–118. [Google Scholar] [CrossRef]
- Gawel, R.; Smith, P.A.; Waters, E.J. Influence of Polysaccharides on the Taste and Mouthfeel of White Wine. Aust. J. Grape Wine Res. 2016, 22, 350–357. [Google Scholar] [CrossRef]
- Lubbers, S.; Verret, C.; Voilley, A. The Effect of Glycerol on the Perceived Aroma of a Model Wine and a White Wine. LWT-Food Sci. Technol. 2001, 34, 262–265. [Google Scholar] [CrossRef]
- Gambuti, A.; Genovese, A.; Lamorte, S.; Capuano, R.; Lisanti, M.T.; Piombino, P.; Moio, L. Study of the Influence of Grape Ripeness Degree on Aroma Characteristics of Aglianico Wines by Instrumental and Sensory Analysis. Acta Hortic. 2007, 754, 533–540. [Google Scholar] [CrossRef]
- Padilla, B.; Gil, J.V.; Manzanares, P. Past and Future of Non-Saccharomyces Yeasts: From Spoilage Microorganisms to Biotechnological Tools for Improving Wine Aroma Complexity. Front. Microbiol. 2016, 7, 411. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).