Ultrafast Electron/Energy Transfer and Intersystem Crossing Mechanisms in BODIPY-Porphyrin Compounds
Abstract
1. Introduction
2. Experiment
2.1. Materials and Equipment
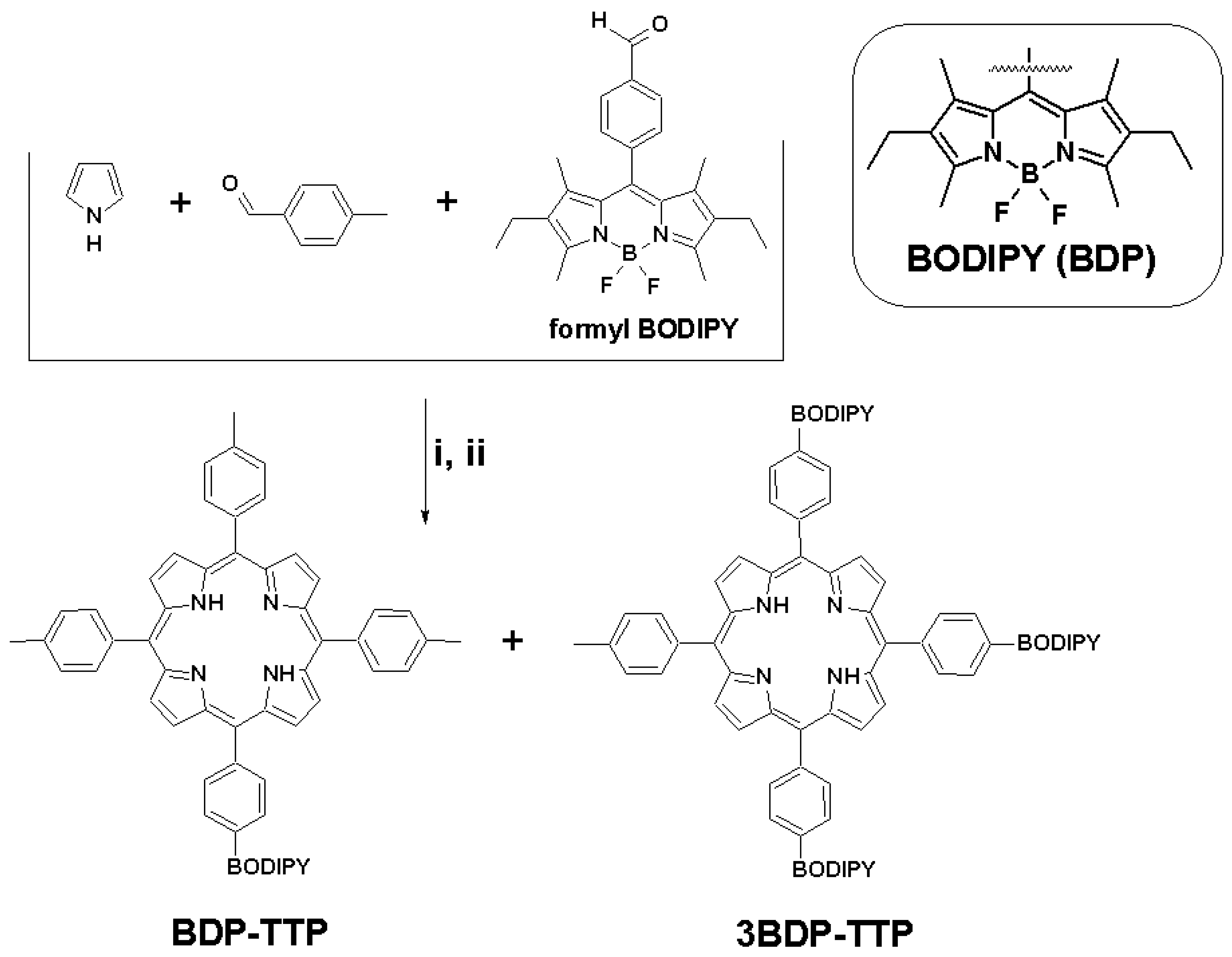
2.2. Synthesis
2.3. Optical Measurement
3. Results and Discussion
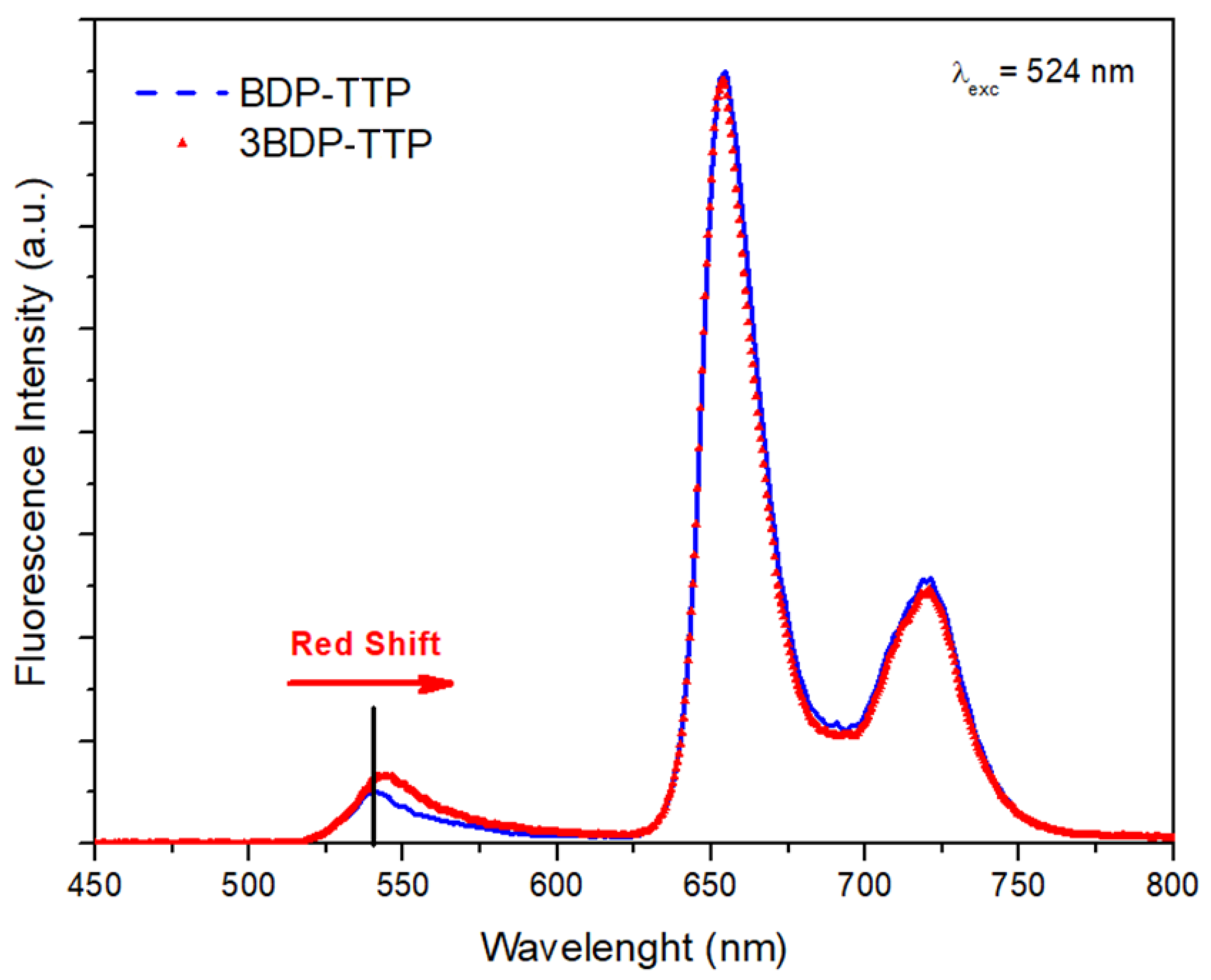
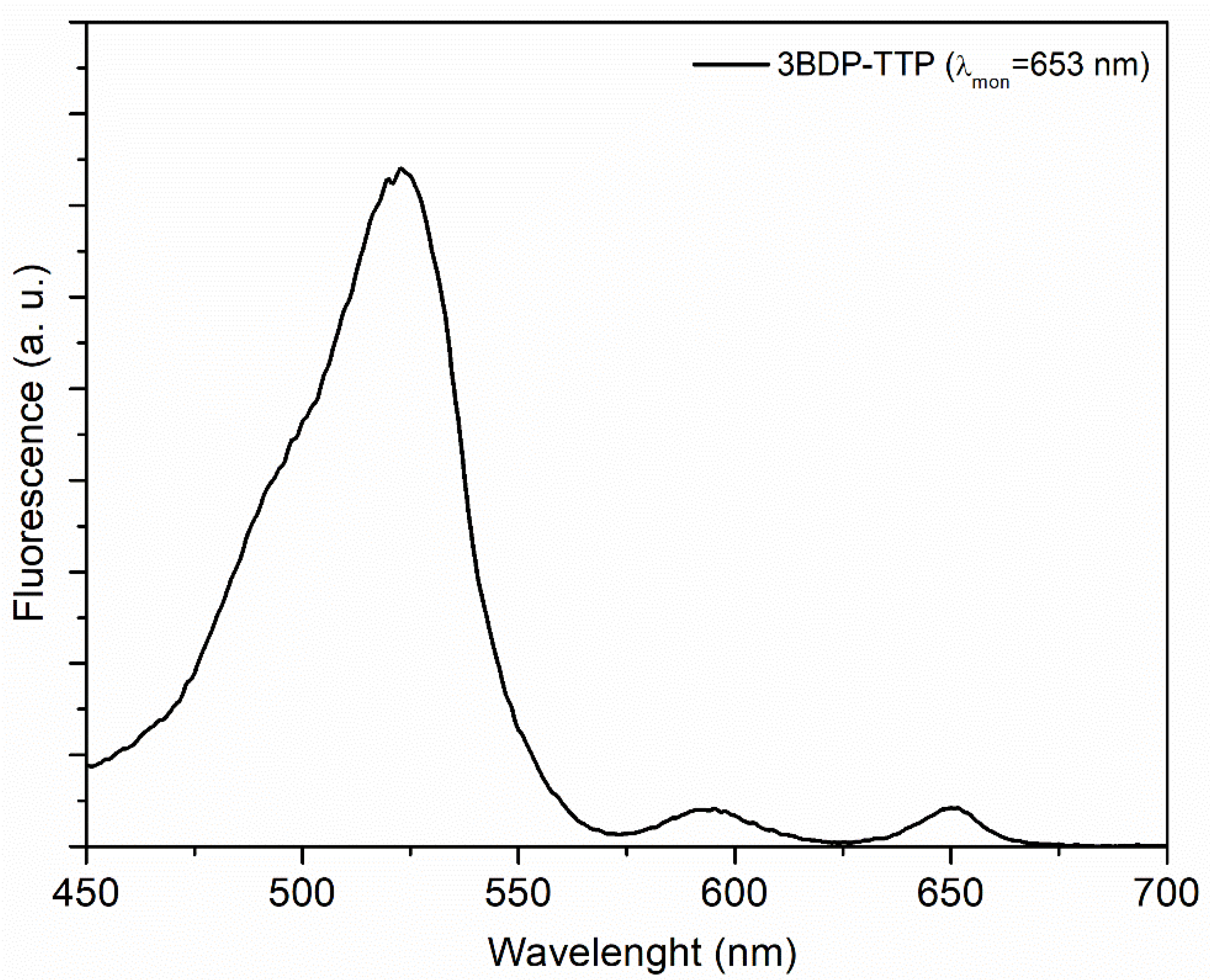
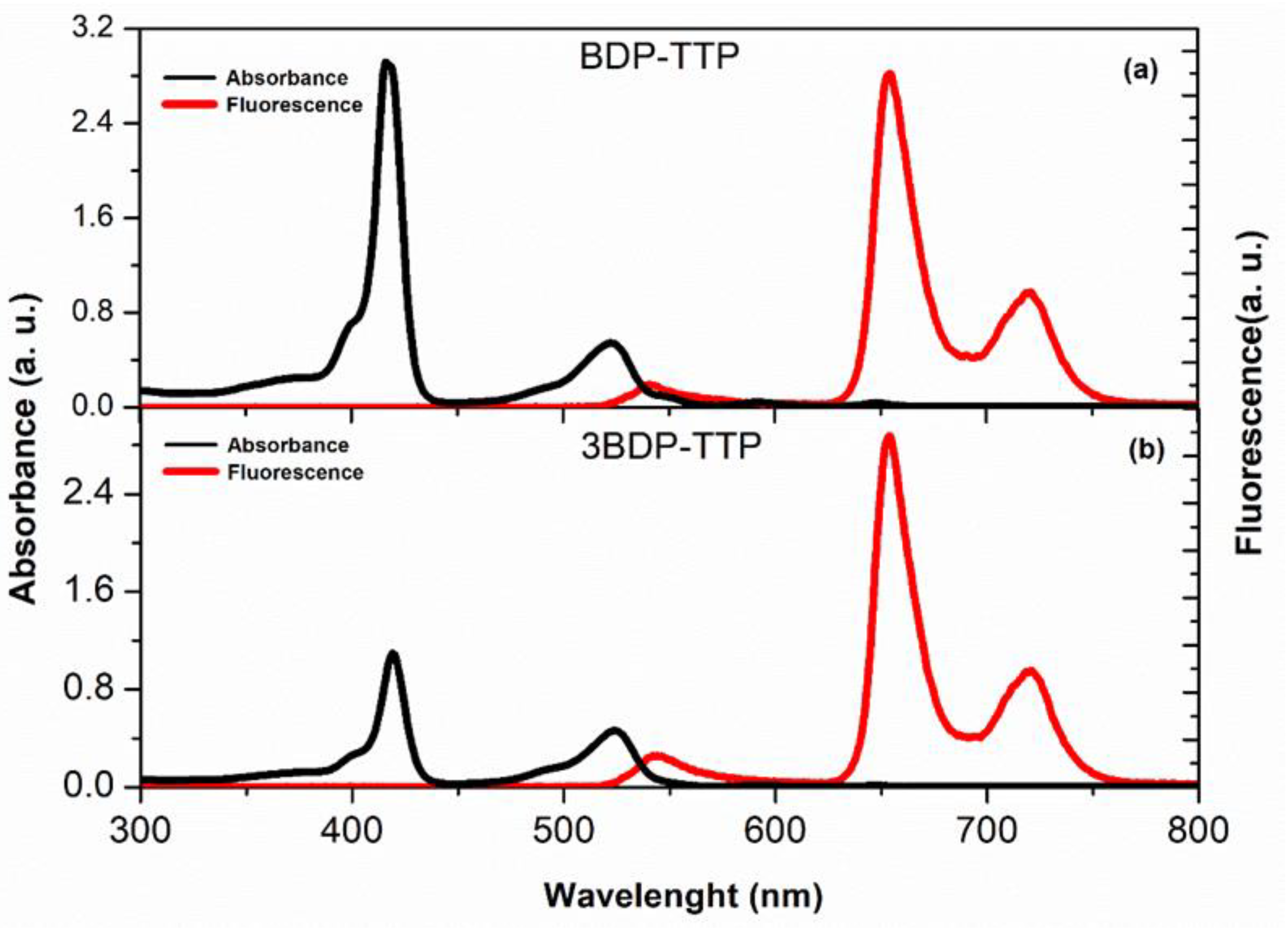
3.1. Steady-State Absorption and Fluorescence Measurements
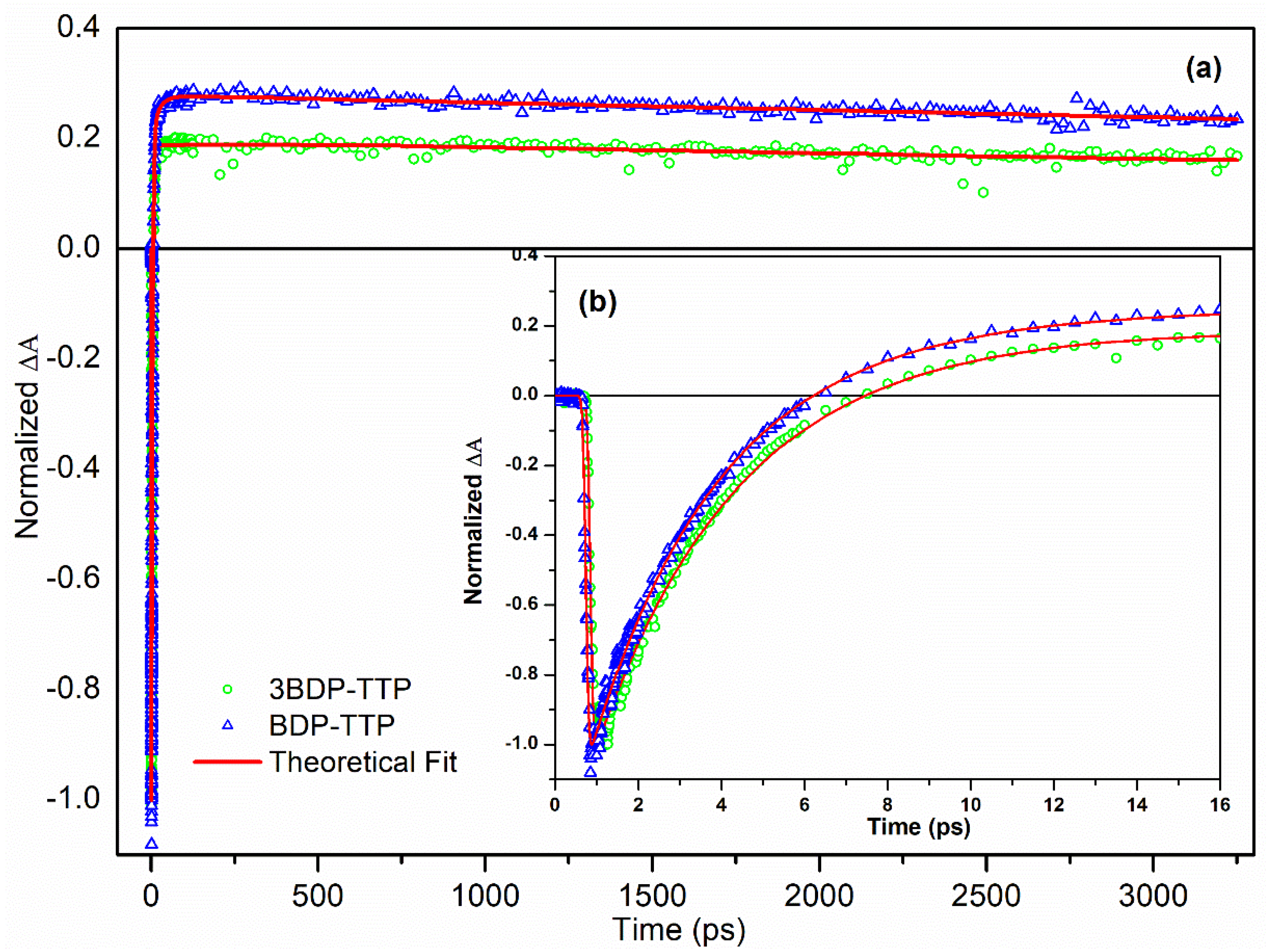
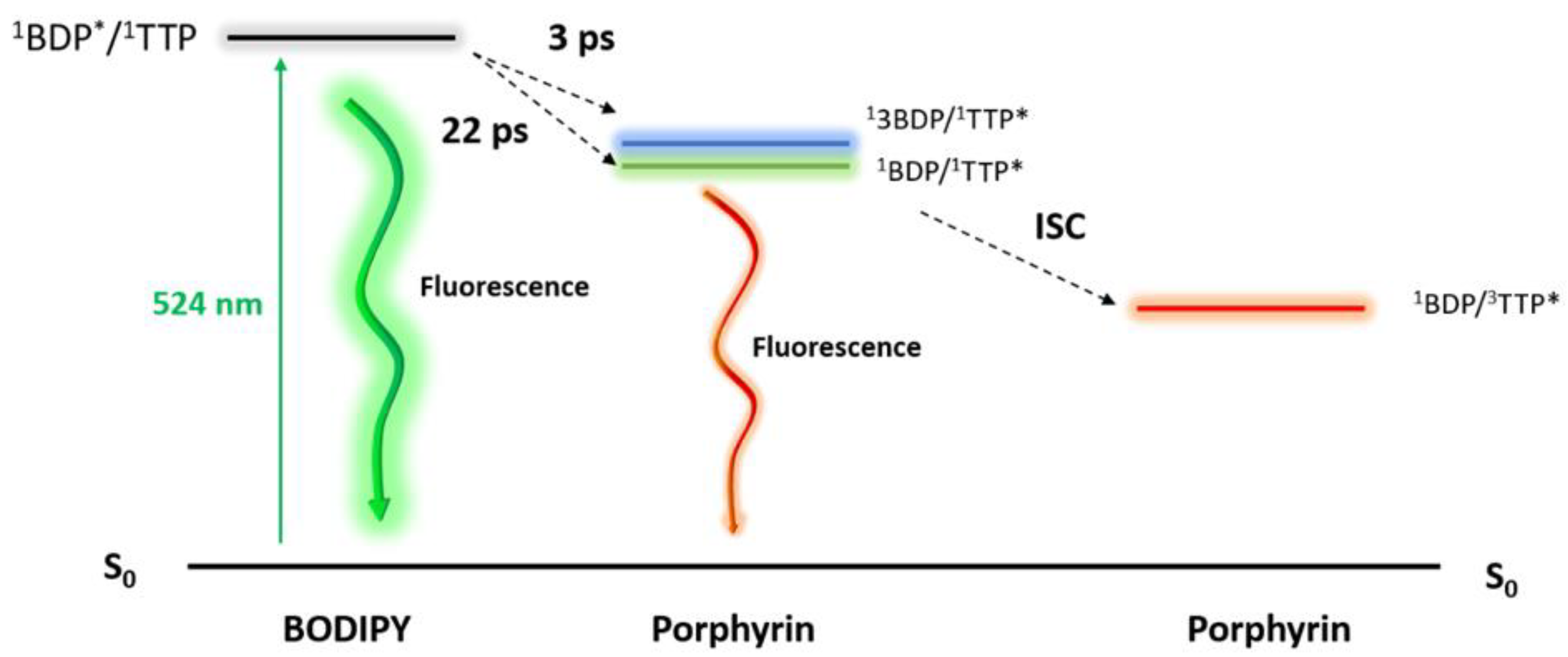
3.2. Ultrafast Pump-Probe Spectroscopy Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Khan, T.K.; Ravikanth, M. Synthesis of covalently linked boron-dipyrromethene-chromophore conjugates using 3-bromo boron-dipyrromethene as a key precursor. Tetrahedron 2011, 67, 5816–5824. [Google Scholar] [CrossRef]
- Ziessel, R.; Ulrich, G.; Harriman, A. The chemistry of Bodipy: A new El Dorado for fluorescence tools. N. J. Chem. 2007, 31, 496–501. [Google Scholar] [CrossRef]
- Ulrich, G.; Ziessel, R.; Harriman, A. The chemistry of fluorescent bodipy dyes: Versatility unsurpassed. Angew. Chem. Int. Ed. 2008, 47, 1184–1201. [Google Scholar] [CrossRef]
- Benstead, M.; Mehl, G.H.; Boyle, R.W. 4,4’-Difluoro-4-bora-3a,4a-diaza-s-indacenes (BODIPYs) as components of novel light active materials. Tetrahedron 2011, 67, 3573–3601. [Google Scholar] [CrossRef]
- Loudet, A.; Burgess, K. BODIPY dyes and their derivatives: Syntheses and spectroscopic properties. Chem. Rev. 2007, 107, 4891–4932. [Google Scholar] [CrossRef]
- Guliyev, R.; Coskun, A.; Akkaya, E.U. Design strategies for ratiometric chemosensors: Modulation of excitation energy transfer at the energy donor site. J. Am. Chem. Soc. 2009, 131, 9007–9013. [Google Scholar] [CrossRef]
- Sevinç, G.; Küçüköz, B.; Yilmaz, H.; Şirikçi, G.; Yaglioglu, H.G.; Hayvali, M.; Elmali, A. Explanation of pH probe mechanism in borondipyrromethene-benzimidazole compound using ultrafast spectroscopy technique. Sens. Actuators B Chem. 2014, 193, 737–744. [Google Scholar] [CrossRef]
- Kobayashi, H.; Ogawa, M.; Alford, R.; Choyke, P.L.; Urano, Y. New Strategies for Fluorescent Probe Design in Medical Diagnostic Imaging. Chem. Rev. 2010, 110, 2620–2640. [Google Scholar] [CrossRef]
- Lai, R.Y.; Bard, A.J. Electrogenerated chemiluminescence 71. Photophysical, electrochemical, and electrogenerated chemiluminescent properties of selected dipyrromethene-BF2 dyes. J. Phys. Chem. B 2003, 107, 5036–5042. [Google Scholar] [CrossRef]
- Baruah, M.; Qin, W.; Basarić, N.; De Borggraeve, W.M.; Boens, N. BODIPY-based hydroxyaryl derivatives as fluorescent pH probes. J. Org. Chem. 2005, 70, 4152–4157. [Google Scholar] [CrossRef]
- Rurack, K.; Kollmannsberger, M.; Daub, J. Molecular switching in the near infrared (NIR) with a functionalized boron-dipyrromethene dye. Angew. Chem. Int. Ed. 2001, 40, 385–387. [Google Scholar] [CrossRef]
- Ziessel, R.; Bonardi, L.; Retailleau, P.; Ulrich, G. Isocyanate-, Isothiocyanate-, Urea-, and Thiourea-Substituted Boron Dipyrromethene Dyes as Fluorescent Probes. J. Org. Chem. 2006, 71, 3093–3102. [Google Scholar] [CrossRef]
- Baruah, M.; Qin, W.; Vallée, R.A.L.; Beljonne, D.; Rohand, T.; Dehaen, W.; Boens, N. A highly potassium-selective ratiometric fluorescent indicator based on BODIPY azacrown ether excitable with visible light. Org. Lett. 2005, 7, 4377–4380. [Google Scholar] [CrossRef]
- Turfan, B.; Akkaya, E.U. Modulation of boradiazaindacene emission by cation-mediated oxidative PET. Org. Lett. 2002, 4, 2857–2859. [Google Scholar] [CrossRef]
- Li, F.; Yang, S.I.; Ciringh, Y.; Seth, J.; Martin, C.H.; Singh, D.L.; Kim, D.; Birge, R.R.; Bocian, D.F.; Holten, D.; et al. Design, synthesis, and photodynamics of light-harvesting arrays comprised of a porphyrin and one, two, or eight boron-dipyrrin accessory pigments. J. Am. Chem. Soc. 1998, 120, 10001–10017. [Google Scholar] [CrossRef]
- Lammi, R.K.; Ambroise, A.; Balasubramanian, T.; Wagner, R.W.; Bocian, D.F.; Holten, D.; Lindsey, J.S. Structural control of photoinduced energy transfer between adjacent and distant sites in multiporphyrin arrays. J. Am. Chem. Soc. 2000, 122, 7579–7591. [Google Scholar] [CrossRef]
- Ambroise, A.; Kirmaier, C.; Wagner, R.W.; Loewe, R.S.; Bocian, D.F.; Holten, D.; Lindsey, J.S. Weakly coupled molecular photonic wires: Synthesis and excited-state energy-transfer dynamics. J. Org. Chem. 2002, 67, 3811–3826. [Google Scholar] [CrossRef]
- Zhong, F.; Karatay, A.; Zhao, L.; Zhao, J.; He, C.; Zhang, C.; Yaglioglu, H.G.; Elmali, A.; Küçüköz, B.; Hayvali, M. Broad-Band N∧N Pt(II) Bisacetylide Visible Light Harvesting Complex with Heteroleptic Bodipy Acetylide Ligands. Inorg. Chem. 2015, 54, 7803–7817. [Google Scholar] [CrossRef]
- Golovkova, T.A.; Kozlov, D.V.; Neckers, D.C. Synthesis and properties of novel fluorescent switches. J. Org. Chem. 2005, 70, 5545–5549. [Google Scholar] [CrossRef]
- Guo, S.; Xu, L.; Xu, K.; Zhao, J.; Küçüköz, B.; Karatay, A.; Yaglioglu, H.G.; Hayvali, M.; Elmali, A. Bodipy-C60 triple hydrogen bonding assemblies as heavy atom-free triplet photosensitizers: Preparation and study of the singlet/triplet energy transfer. Chem. Sci. 2015, 6, 3724–3737. [Google Scholar] [CrossRef] [PubMed]
- Dumanoğulları, F.M.; Tutel, Y.; Küçüköz, B.; Sevinç, G.; Karatay, A.; Yılmaz, H.; Hayvali, M.; Elmali, A. Investigation of ultrafast energy transfer mechanism in BODIPY-Porphyrin dyad system. J. Photochem. Photobiol. A Chem. 2019, 373, 116–121. [Google Scholar] [CrossRef]
- Kumaresan, D.; Gupta, I.; Ravikanth, M. Synthesis of 21-oxoporphyrin building blocks and energy donor appended systems. Tetrahedron Lett. 2001, 42, 8547–8550. [Google Scholar] [CrossRef]
- Wagner, R.W.; Lindsey, J.S. A molecular photonic wire. J. Am. Chem. Soc. 1994, 116, 9759–9760. [Google Scholar] [CrossRef]
- Goeb, S.; Ziessel, R. Synthesis of novel tetrachromophoric cascade-type Bodipy dyes. Tetrahedron Lett. 2008, 49, 2569–2574. [Google Scholar] [CrossRef]
- Ma, J.; Yuan, X.; Küçüköz, B.; Li, S.; Zhang, C.; Majumdar, P.; Karatay, A.; Li, X.; Gul Yaglioglu, H.; Elmali, A.; et al. Resonance energy transfer-enhanced rhodamine-styryl Bodipy dyad triplet photosensitizers. J. Mater. Chem. C 2014, 2, 3900–3913. [Google Scholar] [CrossRef]
- Kim, T.G.; Castro, J.C.; Loudet, A.; Jiao, J.G.S.; Hochstrasser, R.M.; Burgess, K.; Topp, M.R. Correlations of structure and rates of energy transfer for through-bond energy-transfer cassettes. J. Phys. Chem. A 2006, 110, 20–27. [Google Scholar] [CrossRef]
- Wan, C.W.; Burghart, A.; Chen, J.; Bergström, F.; Johansson, L.B.; Wolford, M.F.; Kim, T.G.; Topp, M.R.; Hochstrasser, R.M.; Burgess, K. Anthracene—BODIPY cassettes: Syntheses and energy transfer. Chem. A Eur. J. 2003, 9, 4430–4441. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Kurganskii, I.; Elmali, A.; Zhang, H.; Gao, Y.; Lv, L.; Zhao, J.; Karatay, A.; Luo, L.; Fedin, M. Electronic coupling and spin-orbit charge transfer intersystem crossing (SOCT-ISC) in compact BDP-carbazole dyads with different mutual orientations of the electron donor and acceptor. J. Chem. Phys. 2020, 152. [Google Scholar] [CrossRef] [PubMed]
- Xiaolin, Z.; Yi, X.; Xuhong, Q. Highly efficient energy transfer in the light harvesting system composed of three kinds of boron-dipyrromethene derivatives. Org. Lett. 2008, 10, 29–32. [Google Scholar] [CrossRef]
- Bröring, M.; Krüger, R.; Link, S.; Kleeberg, C.; Köhler, S.; Xie, X.; Ventura, B.; Flamigni, L. Bis(BF2)-2,2′-bidipyrrins (BisBODIPYs): Highly fluorescent BODIPY dimers with large stokes shifts. Chem. A Eur. J. 2008, 14, 2976–2983. [Google Scholar] [CrossRef] [PubMed]
- Benniston, A.C.; Copley, G.; Elliott, K.J.; Harrington, R.W.; Clegg, W. Redox-controlled fluorescence modulation in a BODIPY-quinone dyad. Eur. J. Org. Chem. 2008, 2705–2713. [Google Scholar] [CrossRef]
- Bura, T.; Retailleau, P.; Ziessel, R. Efficient synthesis of panchromatic dyes for energy concentration. Angew. Chem. Int. Ed. 2010, 49, 6659–6663. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, J.; Barbon, A.; Toffoletti, A.; Liu, Y.; An, Y.; Xu, L.; Karatay, A.; Yaglioglu, H.G.; Yildiz, E.A.; et al. Radical-Enhanced Intersystem Crossing in New Bodipy Derivatives and Application for Efficient Triplet-Triplet Annihilation Upconversion. J. Am. Chem. Soc. 2017, 139, 7831–7842. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Ma, L.; Zhao, J.; Küçüköz, B.; Karatay, A.; Hayvali, M.; Yaglioglu, H.G.; Elmali, A. BODIPY triads triplet photosensitizers enhanced with intramolecular resonance energy transfer (RET): Broadband visible light absorption and application in photooxidation. Chem. Sci. 2014, 5, 489–500. [Google Scholar] [CrossRef]
- Ghosh, A.; Vangberg, T. Comparative Thermochemistry of Metalloporphyrin Isomers as a Function of Metal Ion Size. A Possible Insight into Nature’s Choice of Porphyrin over Isomeric Ligands. Inorg. Chem. 1998, 37, 6276–6280. [Google Scholar] [CrossRef]
- Sisemore, M.F.; Selke, M.; Burstyn, J.N.; Valentine, J.S. Metalloporphyrin Peroxo Complexes of Iron(III), Manganese(III), and Titanium(IV). Comparative Studies Demonstrating That the Iron(III) Complex Is Extremely Nucleophilic. Inorg. Chem. 1997, 36, 979–984. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Huang, X.; Phan, H.L.N.; Emge, T.J.; Li, J.; Wang, X. 1-D infinite array of metalloporphyrin cages. Inorg. Chem. 2004, 43, 6878–6880. [Google Scholar] [CrossRef]
- Giribabu, L.; Rao, T.A.; Maiya, B.G. “Axial-bonding”-type hybrid porphyrin arrays: Synthesis, spectroscopy, electrochemistry, and singlet state properties. Inorg. Chem. 1999, 38, 4971–4980. [Google Scholar] [CrossRef]
- Burrell, A.K.; Officer, D.L.; Plieger, P.G.; Reid, D.C.W. Synthetic routes to multiporphyrin arrays. Chem. Rev. 2001, 101, 2751–2796. [Google Scholar] [CrossRef]
- Nakamura, Y.; Hwang, I.W.; Aratani, N.; Ahn, T.K.; Ko, D.M.; Takagi, A.; Kawai, T.; Matsumoto, T.; Kim, D.; Osuka, A. Directly meso-meso linked porphyrin rings: Synthesis, characterization, and efficient excitation energy hopping. J. Am. Chem. Soc. 2005, 127, 236–246. [Google Scholar] [CrossRef]
- Kodis, G.; Liddell, P.A.; De la Garza, L.; Clausen, P.C.; Lindsey, J.S.; Moore, A.L.; Moore, T.A.; Gust, D. Efficient energy transfer and electron transfer in an artificial photosynthetic antenna-reaction center complex. J. Phys. Chem. A 2002, 106, 2036–2048. [Google Scholar] [CrossRef]
- Terazono, Y.; Kodis, G.; Bhushan, K.; Zaks, J.; Madden, C.; Moore, A.L.; Moore, T.A.; Fleming, G.R.; Gust, D. Mimicking the role of the antenna in photosynthetic photoprotection. J. Am. Chem. Soc. 2011, 133, 2916–2922. [Google Scholar] [CrossRef]
- Aratani, N.; Osuka, A.; Kim, Y.H.; Jeong, D.H.; Kim, D. Extremely long, discrete meso-meso-coupled porphyrin arrays. Angew. Chem. Int. Ed. 2000, 39, 1458–1462. [Google Scholar] [CrossRef]
- Gust, D.; Moore, T.A.; Moore, A.L. Solar fuels via artificial photosynthesis. Acc. Chem. Res. 2009, 42, 1890–1898. [Google Scholar] [CrossRef]
- Holten, D.; Bocian, D.F.; Lindsey, J.S. Probing electronic communication in covalently linked multiporphyrin arrays. A guide to the rational design of molecular photonic devices. Acc. Chem. Res. 2002, 35, 57–69. [Google Scholar] [CrossRef]
- Uetomo, A.; Kozaki, M.; Suzuki, S.; Yamanaka, K.I.; Ito, O.; Okada, K. Efficient light-harvesting antenna with a multi-porphyrin cascade. J. Am. Chem. Soc. 2011, 133, 13276–13279. [Google Scholar] [CrossRef]
- Karam, T.E.; Siraj, N.; Ranasinghe, J.C.; Kolic, P.E.; Regmi, B.P.; Warner, I.M.; Haber, L.H. Efficient Photoinduced Energy Transfer in Porphyrin-Based Nanomaterials. J. Phys. Chem. C 2020. [Google Scholar] [CrossRef]
- Seo, C.; Kim, M.; Lee, J.; Lee, C.Y.; Kim, J. Spectroscopic evidence of energy transfer in bodipy-incorporated nano-porphyrinic metal-organic frameworks. Nanomaterials 2020, 10, 1925. [Google Scholar] [CrossRef] [PubMed]
- Obondi, C.O.; Lim, G.N.; Martinez, P.; Swamy, V.; D’Souza, F. Controlling electron and energy transfer paths by selective excitation in a zinc porphyrin-BODIPY-C60 multi-modular triad. Nanoscale 2017, 9, 18054–18065. [Google Scholar] [CrossRef]
- Qin, W.; Baruah, M.; Stefan, A.; Van Der Auweraer, M.; Boens, N. Photophysical properties of BODIPY-derived hydroxyaryl fluorescent pH probes in solution. ChemPhysChem 2005, 6, 2343–2351. [Google Scholar] [CrossRef]
- López Arbeloa, F.; Bañuelos, J.; Martínez, V.; Arbeloa, T.; López Arbeloa, I. Structural, photophysical and lasing properties of pyrromethene dyes. Int. Rev. Phys. Chem. 2005, 24, 339–374. [Google Scholar] [CrossRef]
- Lazarides, T.; Charalambidis, G.; Vuillamy, A.; Réglier, M.; Klontzas, E.; Froudakis, G.; Kuhri, S.; Guldi, D.M.; Coutsolelos, A.G. Promising fast energy transfer system via an easy synthesis: Bodipy-porphyrin dyads connected via a cyanuric chloride bridge, their synthesis, and electrochemical and photophysical investigations. Inorg. Chem. 2011, 50, 8926–8936. [Google Scholar] [CrossRef]
- Hu, Q.Q.; Zhu, Y.Z.; Zhang, S.C.; Tong, Y.Z.; Zheng, J.Y. Meso-2′-Linked porphyrin-BODIPY hybrids: Synthesis and efficient excitation energy transfer. Dalt. Trans. 2015, 44, 15523–15530. [Google Scholar] [CrossRef]
- Whited, M.T.; Djurovich, P.I.; Roberts, S.T.; Durrell, A.C.; Schlenker, C.W.; Bradforth, S.E.; Thompson, M.E. Singlet and triplet excitation management in a bichromophoric near-infrared-phosphorescent BODIPY-benzoporphyrin platinum complex. J. Am. Chem. Soc. 2011, 133, 88–96. [Google Scholar] [CrossRef][Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tutel, Y.; Sevinç, G.; Küçüköz, B.; Akhuseyin Yildiz, E.; Karatay, A.; Dumanoğulları, F.M.; Yılmaz, H.; Hayvali, M.; Elmali, A. Ultrafast Electron/Energy Transfer and Intersystem Crossing Mechanisms in BODIPY-Porphyrin Compounds. Processes 2021, 9, 312. https://doi.org/10.3390/pr9020312
Tutel Y, Sevinç G, Küçüköz B, Akhuseyin Yildiz E, Karatay A, Dumanoğulları FM, Yılmaz H, Hayvali M, Elmali A. Ultrafast Electron/Energy Transfer and Intersystem Crossing Mechanisms in BODIPY-Porphyrin Compounds. Processes. 2021; 9(2):312. https://doi.org/10.3390/pr9020312
Chicago/Turabian StyleTutel, Yusuf, Gökhan Sevinç, Betül Küçüköz, Elif Akhuseyin Yildiz, Ahmet Karatay, Fatih Mehmet Dumanoğulları, Halil Yılmaz, Mustafa Hayvali, and Ayhan Elmali. 2021. "Ultrafast Electron/Energy Transfer and Intersystem Crossing Mechanisms in BODIPY-Porphyrin Compounds" Processes 9, no. 2: 312. https://doi.org/10.3390/pr9020312
APA StyleTutel, Y., Sevinç, G., Küçüköz, B., Akhuseyin Yildiz, E., Karatay, A., Dumanoğulları, F. M., Yılmaz, H., Hayvali, M., & Elmali, A. (2021). Ultrafast Electron/Energy Transfer and Intersystem Crossing Mechanisms in BODIPY-Porphyrin Compounds. Processes, 9(2), 312. https://doi.org/10.3390/pr9020312






