Screening of Ion Exchange Resins for Hazardous Ni(II) Removal from Aqueous Solutions: Kinetic and Equilibrium Batch Adsorption Method
Abstract
1. Introduction
2. Materials and Methods
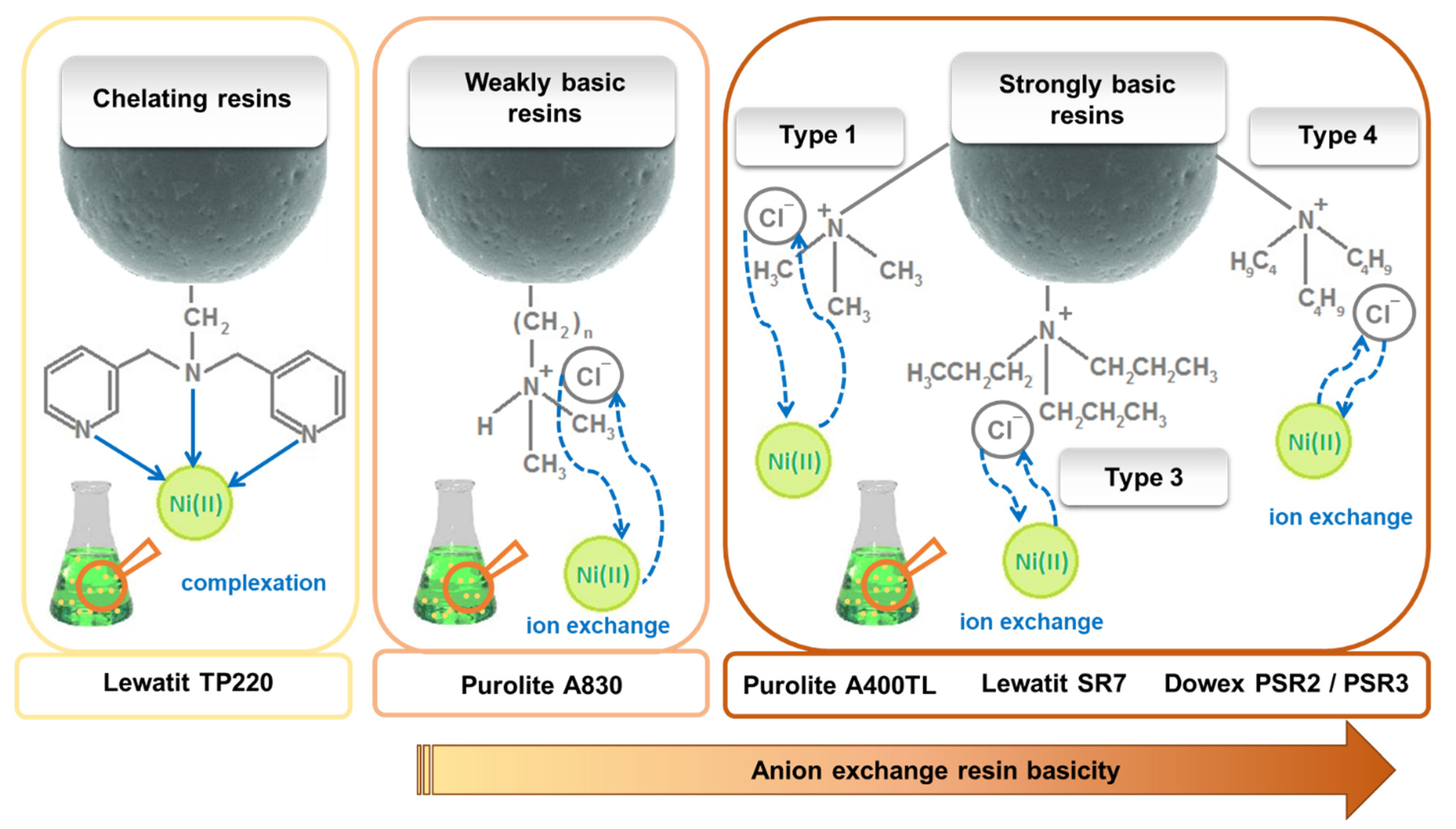
2.1. Materials
2.2. Batch Adsorption Studies
2.2.1. Kinetic Studies
2.2.2. Equilibrium Studies
2.2.3. Error Analysis
2.2.4. FTIR-ATR Analysis
2.2.5. Batch Desorption Experiments
3. Results and Discussion
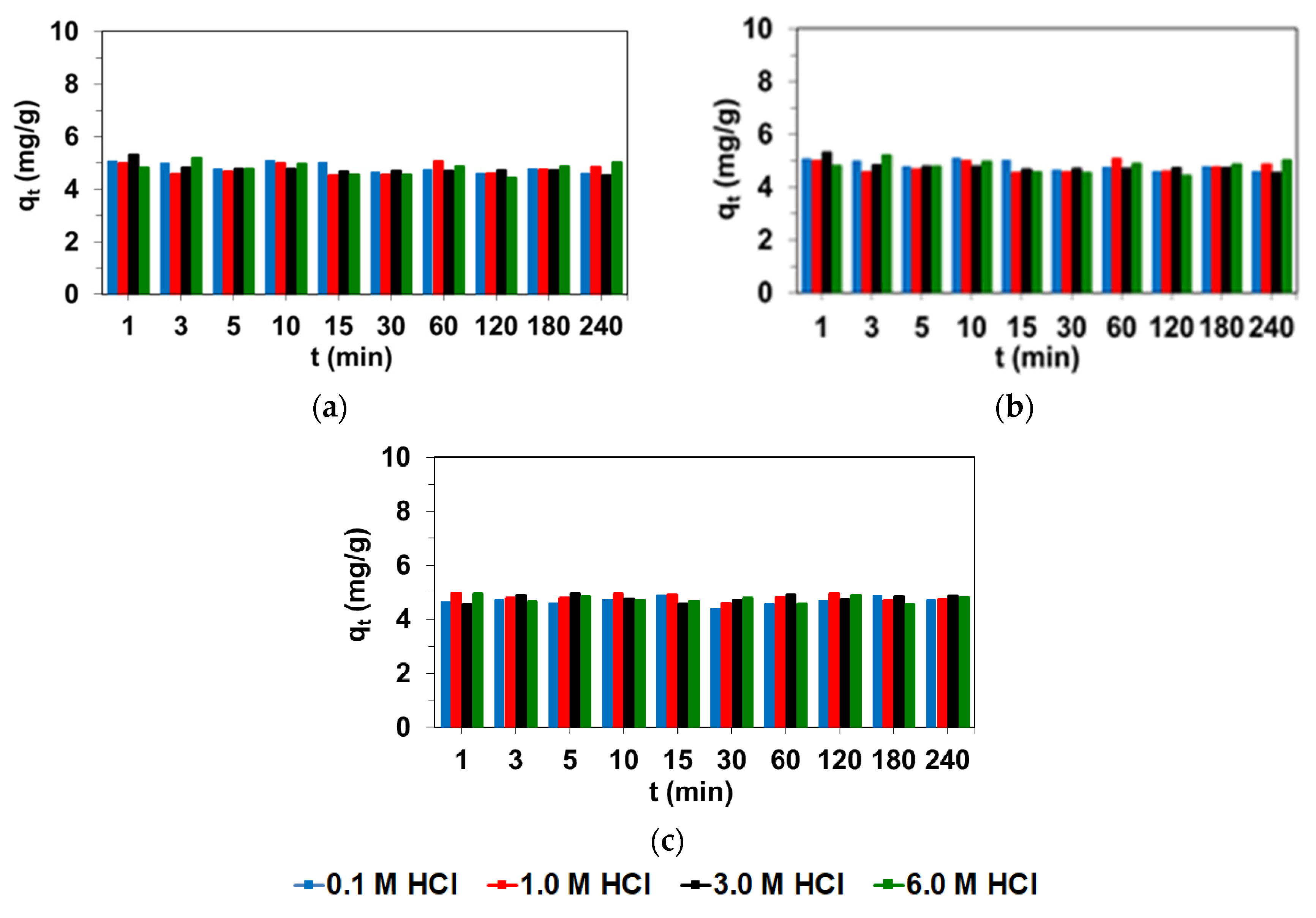
3.1. Effect of Phase Contact Time and Acids Concentration on Ni(II) Adsorption
3.2. Equilibrium Studies
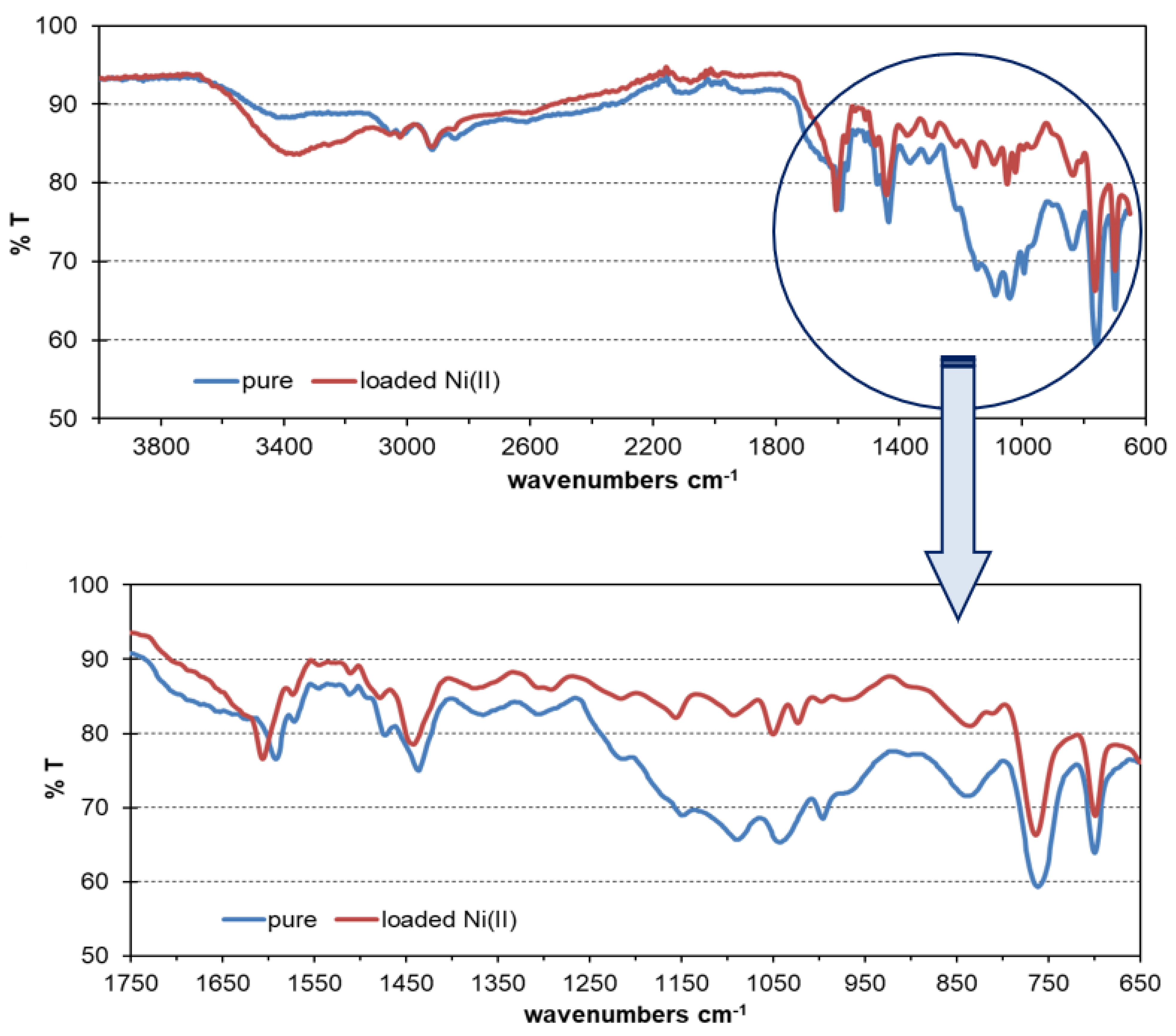
3.3. FTIR-ATR Analysis of Pure and Loaded TP220 by Ni(II)
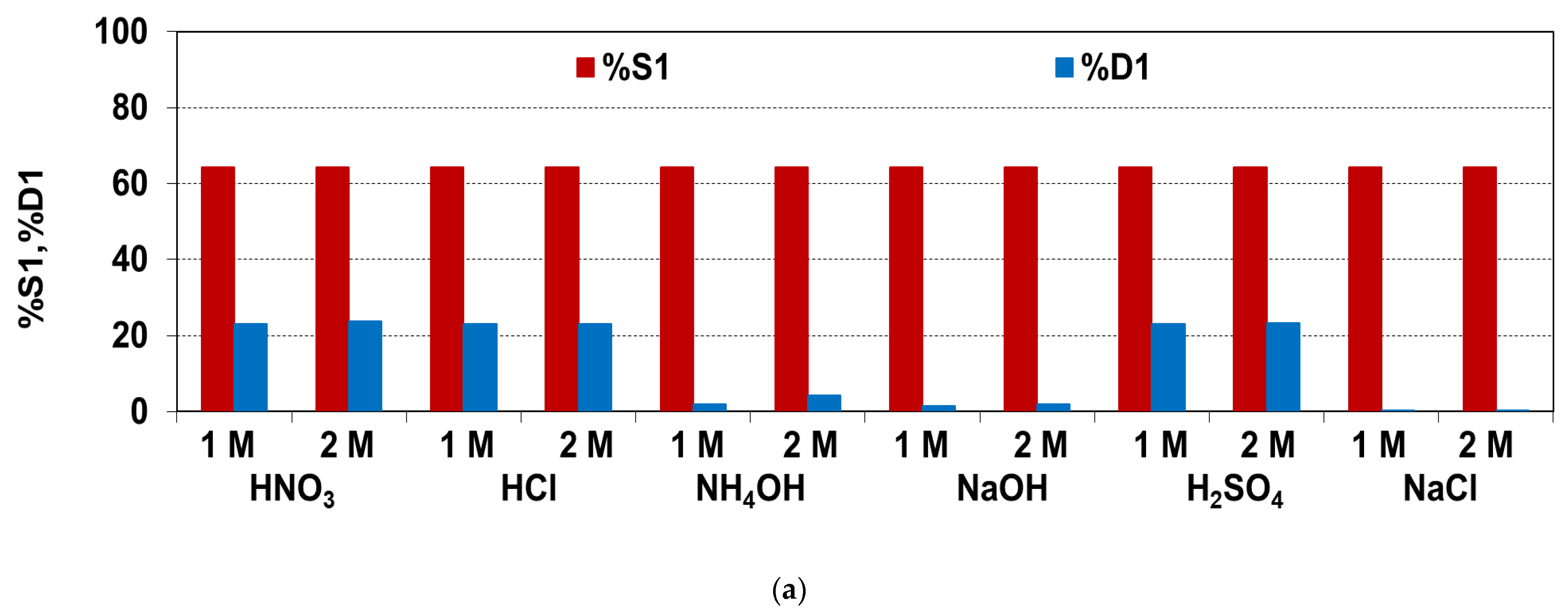
3.4. Desorption of Ni(II)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MCL (mg/L) | maximum contaminant level |
| HYDRA | Hydrochemical Equilibrium Constant Database |
| MEDUSA | Make Equilibrium Diagrams Using Sophisticated Algorithms |
| qt (mg/g) | the amount of Ni(II) sorbed by the adsorbents at time t |
| qe (mg/g) | the amount of Ni(II) sorbed at the equilibrium |
| C0 (mg/L) | the Ni(II) concentration in the solution before sorption |
| Ct (mg/L) | the Ni(II) concentration in the solution after sorption |
| Ce (mg/L) | the Ni(II) concentrations in the solution after sorption at equilibrium |
| t (min) | the phase contact time |
| V (L) | the volume of the Ni(II) solution |
| m (g) | the mass of the adsorbent |
| PFO | the pseudo-first-order kinetic model |
| k1 (1/min) | the rate constant of sorption determined from PFO equation |
| PSO | the pseudo-second-order kinetic model |
| k2 (g/mg min) | the rate constant of sorption determined from PSO equation |
| IPD | the intraparticle diffusion kinetic model |
| ki (mg/g min0.5) | the intraparticle diffusion rate constant |
| kF (mg1−1/n L1/n/g) | the Freundlich constant related to the adsorption capability |
| n | the Freundlich constant related to adsorption intensity |
| kL (L/mg) | the constant parameter of adsorption equilibrium |
| Q0 (mg/g) | the monolayer adsorption capacity |
| bT (J g/mol mg) | Temkin constant related to the heat of adsorption |
| A (L/mg) | the Temkin isotherm equilibrium binding constant |
| qm (mg/g) | the maximum adsorption capacity |
| kDR (mol2 J2) | the constant related to the adsorption energy |
| ε (J/mol) | the adsorption potential |
| R (J/mol K) | the gas constant |
| T (K) | the temperature |
| MPSD | Marquardt’s percent standard deviation |
| R2 | the determination coefficient |
| the adjusted R-squared | |
| qe exp (mg/g) | the experimental amount of Ni(II) sorbed at equilibrium |
| qe cal (mg/g) | the amount of Ni(II) sorbed calculated from the non-linear models |
| qe mean (mg/g) | the measured by the means of qe exp values |
| n | the number of points in the data sample |
| k | the number of independent regressors |
| FTIR-ATR | the Fourier-transform infrared spectroscopy with the attenuated total reflection |
| D (%) | the percentage values of Ni(II) desorbed from adsorbent |
| mdes (mg) | the mass of Ni(II) desorbed |
| mads (mg) | the mass of Ni(II) adsorbed. |
References
- Iyaka, Y.A. Nickel in soils: A review of its distribution and impacts. Sci. Res. Essays 2011, 6, 6774–6777. [Google Scholar] [CrossRef]
- Vakili, M.; Rafatullah, M.; Yuan, J.; Zwain, H.M.; Mojiri, A.; Gholami, Z.; Gholami, F.; Wang, W.; Giwa, A.S.; Yu, Y.; et al. Nickel ion removal from aqueous solutions through the adsorption process: A review. Rev. Chem. Eng. 2020. [Google Scholar] [CrossRef]
- Harasim, P.; Filipek, T. Nickel in the environment. J. Elem. 2015, 20, 525–534. [Google Scholar] [CrossRef]
- Duda-Chodak, A.; Błaszczyk, U. The impact of nickel on human health. J. Elementol. 2008, 13, 685–696. [Google Scholar]
- Nieminen, T.M.; Ukonmaanaho, L.; Rausch, N.; Shotyk, W. Biogeochemistry of Nickel and Its Release into the Environment. Nickel Surpris. Impact Nat. 2007, 2, 1–29. [Google Scholar] [CrossRef]
- Pacyna, J.M.; Pacyna, E.G. An assessment of global and regional emissions of trace metals to the atmosphere from anthropogenic sources worldwide. Environ. Rev. 2001, 9, 269–298. [Google Scholar] [CrossRef]
- Gambuś, F.; Wieczorek, J. Pollution of fertilizers with heavy metals. Ecol. Chem. Eng. A 2012, 19, 353–360. [Google Scholar] [CrossRef]
- Łukowski, A.; Wiater, J. The influence of mineral fertilization on heavy metal fraction contents in soil. Part II. Copper and nickel. Pol. J. Environ. Stud. 2009, 18, 645–650. [Google Scholar]
- Coman, V.; Robotin, B.; Ilea, P. Nickel recovery/removal from industrial wastes: A review. Resour. Conserv. Recycl. 2013, 73, 229–238. [Google Scholar] [CrossRef]
- Barakat, M. New trends in removing heavy metals from industrial wastewater. Arab. J. Chem. 2011, 4, 361–377. [Google Scholar] [CrossRef]
- Renu; Agrawal, M.; Singh, K. Heavy metal removal from wastewater using various adsorbents: A review. J. Water Reuse Desalin. 2016, 7, 387–419. [Google Scholar] [CrossRef]
- Tripathi, A.; Ranjan, M.R. Heavy Metal Removal from Wastewater Using Low Cost Adsorbents. J. Bioremediat. Biodegrad. 2015, 6, 315–320. [Google Scholar] [CrossRef]
- Borba, C.; Guirardello, R.; Silva, E.; Veit, M.; Tavares, C. Removal of nickel(II) ions from aqueous solution by biosorption in a fixed bed column: Experimental and theoretical breakthrough curves. Biochem. Eng. J. 2006, 30, 184–191. [Google Scholar] [CrossRef]
- Genchi, G.; Carocci, A.; Lauria, G.; Sinicropi, M.S.; Catalano, A. Nickel: Human Health and Environmental Toxicology. Int. J. Environ. Res. Public Health 2020, 17, 679. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Scientific Opinion on the risks to public health related to the presence of nickel in food and drinking water. EFSA J. 2015, 13, 4002. [Google Scholar] [CrossRef]
- Khulbe, K.C.; Matsuura, T. Removal of heavy metals and pollutants by membrane adsorption techniques. Appl. Water Sci. 2018, 8, 19. [Google Scholar] [CrossRef]
- Liu, L.; Li, W.; Song, W.; Guo, M. Remediation techniques for heavy metal-contaminated soils: Principles and applicability. Sci. Total Environ. 2018, 633, 206–219. [Google Scholar] [CrossRef]
- Kumar, D.; Khan, E.A. 12—Remediation and detection techniques for heavy metals in the environment. In Heavy Metals in the Environment: Impact, Assessment, and Remediation; Candice Janco: Cambridge, MA, USA, 2020; pp. 205–222. [Google Scholar]
- Geng, H.; Xu, Y.; Zheng, L.; Gong, H.; Dai, L.; Dai, X. An overview of removing heavy metals from sewage sludge: Achievements and perspectives. Environ. Pollut. 2020, 266, 115375. [Google Scholar] [CrossRef]
- Azimi, A.; Azari, A.; Rezakazemi, M.; Ansarpour, M. Removal of Heavy Metals from Industrial Wastewaters: A Review. ChemBioEng Rev. 2017, 4, 37–59. [Google Scholar] [CrossRef]
- Beltrán-Heredia, J.; Martín, J.S. Removing heavy metals from polluted surface water with a tannin-based flocculant agent. J. Hazard. Mater. 2009, 165, 1215–1218. [Google Scholar] [CrossRef]
- Al-Shannag, M.; Al-Qodah, Z.; Bani-Melhem, K.; Qtaishat, M.R.; Alkasrawi, M. Heavy metal ions removal from metal plating wastewater using electrocoagulation: Kinetic study and process performance. Chem. Eng. J. 2015, 260, 749–756. [Google Scholar] [CrossRef]
- Lakhdhar, I.; Belosinschi, D.; Mangin, P.; Chabot, B. Development of a bio-based sorbent media for the removal of nickel ions from aqueous solutions. J. Environ. Chem. Eng. 2016, 4, 3159–3169. [Google Scholar] [CrossRef]
- Mohsen-Nia, M.; Montazeri, P.; Modarress, H. Removal of Cu2+ and Ni2+ from wastewater with a chelating agent and reverse osmosis processes. Desalination 2007, 217, 276–281. [Google Scholar] [CrossRef]
- Samper, E.; Rodríguez, M.; De La Rubia, M.; Rico, D.P. Removal of metal ions at low concentration by micellar-enhanced ultrafiltration (MEUF) using sodium dodecyl sulfate (SDS) and linear alkylbenzene sulfonate (LAS). Sep. Purif. Technol. 2009, 65, 337–342. [Google Scholar] [CrossRef]
- Moghbeli, M.; Khajeh, A.; Alikhani, M. Nanosilica reinforced ion-exchange polyHIPE type membrane for removal of nickel ions: Preparation, characterization and adsorption studies. Chem. Eng. J. 2017, 309, 552–562. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Radhakrishnan, P.G. Uptake and desorption of nickel(II) using polymerised tamarind fruit shell with acidic functional groups in aqueous environments. Chem. Ecol. 2010, 26, 93–109. [Google Scholar] [CrossRef]
- Fil, B.A.; Boncukcuoğlu, R.; Yilmaz, A.E.; Bayar, S. Adsorption of Ni(II) on ion exchange resin: Kinetics, equilibrium and thermodynamic studies. Korean J. Chem. Eng 2020, 29, 1232–1238. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- Sharma, S.; Bhattacharya, A. Drinking water contamination and treatment techniques. Appl. Water Sci. 2017, 7, 1043–1067. [Google Scholar] [CrossRef]
- Kanamarlapudi, S.L.R.K.; Chintalpudi, V.K.; Muddada, S. Application of biosorption for removal of heavy metals from wastewater. InTech 2018, 4, 69–116. [Google Scholar]
- Couto, C.F.; Lange, L.C.; Amaral, M.C.S. A critical review on membrane separation processes applied to remove pharmaceutically active compounds from water and wastewater. J. Water Process. Eng. 2018, 26, 156–175. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Rubio, J.; Souza, M.L.; Smith, R.W. Overview of flotation as a wastewater treatment technique. Miner. Eng. 2002, 15, 139–155. [Google Scholar] [CrossRef]
- Ahmad Panahi, H.; Samadi Zadeh, M.; Tavangari, S.; Moniri, E.; Ghassemi, J. Nickel adsorption from environmen-tal samples by ion imprinted aniline-formaldehyde polymer. Iranian J. Chem. Eng. 2012, 31, 35–44. [Google Scholar]
- El-Sadaawy, M.; Abdelwahab, O. Adsorptive removal of nickel from aqueous solutions by activated carbons from doum seed (Hyphaenethebaica) coat. Alex. Eng. J. 2014, 53, 399–408. [Google Scholar] [CrossRef]
- Do, Q.C.; Choi, S.; Kim, H.; Kang, S. Adsorption of Lead and Nickel on to Expanded Graphite Decorated with Manganese Oxide Nanoparticles. Appl. Sci. 2019, 9, 5375. [Google Scholar] [CrossRef]
- Silva, R.M.P.; Manso, J.P.H.; Rodrigues, J.R.; Lagoa, R. A comparative study of alginate beads and an ion-exchange resin for the removal of heavy metals from a metal plating effluent. J. Environ. Sci. Health Part A 2008, 43, 1311–1317. [Google Scholar] [CrossRef]
- Shen, Z.; Zhang, Y.; McMillan, O.; Jin, F.; Al-Tabbaa, A. Characteristics and mechanisms of nickel adsorption on biochars produced from wheat straw pellets and rice husk. Environ. Sci. Pollut. Res. 2017, 24, 12809–12819. [Google Scholar] [CrossRef]
- Kołodyńska, D. Polyacrylate anion exchangers in sorption of heavy metal ions with the biodegradable complexing agent. Chem. Eng. J. 2009, 150, 280–288. [Google Scholar] [CrossRef]
- Anah, L.; Astrini, N. Isotherm adsorption studies of Ni(II) ion removal from aqueous solutions by modified carboxymethyl cellulose hydrogel. IOP Conf. Ser. Earth Environ. Sci. 2018, 160, 012017. [Google Scholar] [CrossRef]
- Wołowicz, A.; Hubicki, Z. Polyactrylate ion exchangers in sorption of noble and base metal ions from single and tertiary component solutions. Solvent Extr. Ion Exch. 2014, 32, 189–205. [Google Scholar] [CrossRef]
- Wołowicz, A.; Hubicki, Z. The use of the chelating resin of a new generation Lewatit MonoPlus TP-220 with the bis-picolylamine functional groups in the removal of selected metal ions from acidic solutions. Chem. Eng. J. 2012, 197, 493–508. [Google Scholar] [CrossRef]
- Wołowicz, A.; Hubicki, Z. Sorption Behavior of Dowex PSR-2 and Dowex PSR-3 Resins of Different Structures for Metal(II) Removal. Solvent Extr. Ion Exch. 2016, 34, 375–397. [Google Scholar] [CrossRef]
- Wołowicz, A.; Hubicki, Z. Carbon-based adsorber resin Lewatit AF 5 applicability in metal ion recovery. Microporous Mesoporous Mater. 2016, 224, 400–414. [Google Scholar] [CrossRef]
- Wołowicz, A. Zinc(II) removal from model chloride and chloride–nitrate(V) solutions using various sorbents. Physicochem. Probl. Miner. Process. 2019, 55, 1517–1534. [Google Scholar]
- Wołowicz, A.; Hubicki, Z. Enhanced removal of copper(II) from acidic streams using functional resins: Batch and column studies. J. Mater. Sci. 2020, 55, 13687–13715. [Google Scholar] [CrossRef]
- How Much Does It Cost to Buy, Maintain, and Dispose of Ion Exchange Resins? Available online: Samcotech.com/how-much-does-it-cost-to-buy-maintain-and-dispose-of-ion-exchange-resins/ (accessed on 22 January 2021).
- Zong, L.; Liu, F.; Chen, D.; Zhang, X.; Ling, C.; Li, A. A novel pyridine based polymer for highly efficient separation of nickel from high-acidity and high-concentration cobalt solutions. Chem. Eng. J. 2018, 334, 995–1005. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process. Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption kinetic models: Physical meanings, applications, and solving methods. J. Hazard. Mater. 2020, 390, 122156. [Google Scholar] [CrossRef]
- Foo, K.; Hameed, B. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Marković, D.D.; Lekić, B.; Rajaković-Ognjanović, V.N.; Onjia, A.; Rajaković, L.V. A New Approach in Regression Analysis for Modeling Adsorption Isotherms. Sci. World J. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Sivarajasekar, N.; Baskar, R. Adsorption of Basic Magenta II onto H2SO4 activated immature Gossypium hirsutum seeds: Kinetics, isotherms, mass transfer, thermodynamics and process design. Arab. J. Chem. 2019, 12, 1322–1337. [Google Scholar] [CrossRef]
- Uzun, I.; Guzel, F. Adsorption of some heavy metal ions from aqueous solution by activated carbon and comparison of percent adsorption results of activated carbon with those of some other adsorbents. Turk. J. Chem. 2000, 24, 291–297. [Google Scholar]
- Satapathy, D.; Natarajan, G.S. Potassium bromate modification of the granular activated carbon and its effect on nickel adsorption. Adsorption 2006, 12, 147–154. [Google Scholar] [CrossRef]
- Wei, Y.-Z.; Feng, Q.; Arai, T.; Kumagai, M. Adsorption and Separation Behaviour of Cobalt, Nickel, and Copper in Nitrite Medium by Anion Exchanger. Solvent Extr. Ion Exch. 2002, 20, 561–573. [Google Scholar] [CrossRef]
- Keränen, A.; Leiviskä, T.; Salakka, A.; Tanskanen, J. Removal of nickel and vanadium from ammoniacal industrial wastewater by ion exchange and adsorption on activated carbon. Desalin. Water Treat. 2015, 53, 2645–2654. [Google Scholar] [CrossRef]
- Xiuling, S.; HuiPu, D.; Shijun, L.; Hui, Q. Adsorption Properties of Ni(II) by D301R Anion Exchange Resin. J. Chem. 2014, 2014, 1–5. [Google Scholar] [CrossRef][Green Version]
- Deepatana, A.; Valix, M. Recovery of nickel and cobalt from organic acid complexes: Adsorption mechanisms of metal-organic complexes onto aminophosphonate chelating resin. J. Hazard. Mater. 2006, 137, 925–933. [Google Scholar] [CrossRef]
- Kołodyńska, D.; Hubicka, H. Polyacrylate anion exchangers in sorption of heavy metal ions with non-biodegradable complexing agents. Chem. Eng. J. 2009, 150, 308–315. [Google Scholar] [CrossRef]
- Douven, S.; Paez, C.A.; Gommes, C.J. The range of validity of sorption kinetic models. J. Colloid Interface Sci. 2015, 448, 437–450. [Google Scholar] [CrossRef]
- Płaziński, W.; Rudziński, W. Kinetyka adsorpcji na granicy faz roztwór/ciało stałe. Znaczenie równań pseudo-first order oraz pseudo-second order. Wiadomości Chem. 2011, 65, 11–12. [Google Scholar]
- Hu, Q.; Zhang, Z. Application of Dubinin–Radushkevich isotherm model at the solid/solution interface: A theoretical analysis. J. Mol. Liq. 2019, 277, 646–648. [Google Scholar] [CrossRef]
- Morris, D.; Reed, G.; Short, E.; Slater, D.; Waters, D. Nickel (II) chloride complexes in aqueous solution. J. Inorg. Nucl. Chem. 1965, 27, 377–382. [Google Scholar] [CrossRef]
- Mohanty, U.S.; Rintala, L.; Halli, P.; Avarmaa, K.; Reuter, M.A. Hydrometallurgical Approach for Leaching of Metals from Copper Rich Side Stream Originating from Base Metal Production. Metal 2018, 8, 40. [Google Scholar] [CrossRef]
- Lee, M.S.; Nam, S.H. Chemical Equilibria of Nickel Chloride in HCl Solution at 25 °C. Bull. Korean Chem. Soc. 2009, 30, 2203–2207. [Google Scholar] [CrossRef]
- Kołodyńska, D.; Hałas, P.; Michalski, R. Development of ion exchangers for the removal of health-hazardous perchlorate ions from aqueous systems. Appl. Geochem. 2019, 101, 75–87. [Google Scholar] [CrossRef]
- Zagorodni, A.A.; Kotova, D.L.; Selemenev, V.F. Infrared spectroscopy of ion exchange resins: Chemical deterioration of the resins. React. Funct. Polym. 2002, 53, 157–171. [Google Scholar] [CrossRef]
- Lazar, L.; Bulgariu, L.; Bandrabur, B.; Tataru-Farmus, R.-E.; Drobotă, M.; Gutt, G. FTIR Analisys of ion Exchange Resins with Application in Permanent Hard Water Softening. Environ. Eng. Manag. J. 2014, 13, 2145–2152. [Google Scholar] [CrossRef]
- Ghosh, S.; Dhole, K.; Tripathy, M.K.; Kumar, R.; Sharma, R.S. FTIR spectroscopy in the characterization of the mixture of nuclear grade cation and anion exchange resins. J. Radioanal. Nucl. Chem. 2015, 304, 917–923. [Google Scholar] [CrossRef]
- Traboulsi, A.; Dupuy, N.; Rebufa, C.; Sergent, M.; Labed, V. Investigation of gamma radiation effect on the anion exchange resin Amberlite IRA-400 in hydroxide form by Fourier transformed infrared and 13C nuclear magnetic resonance spectroscopies. Anal. Chim. Acta 2012, 717, 110–121. [Google Scholar] [CrossRef]

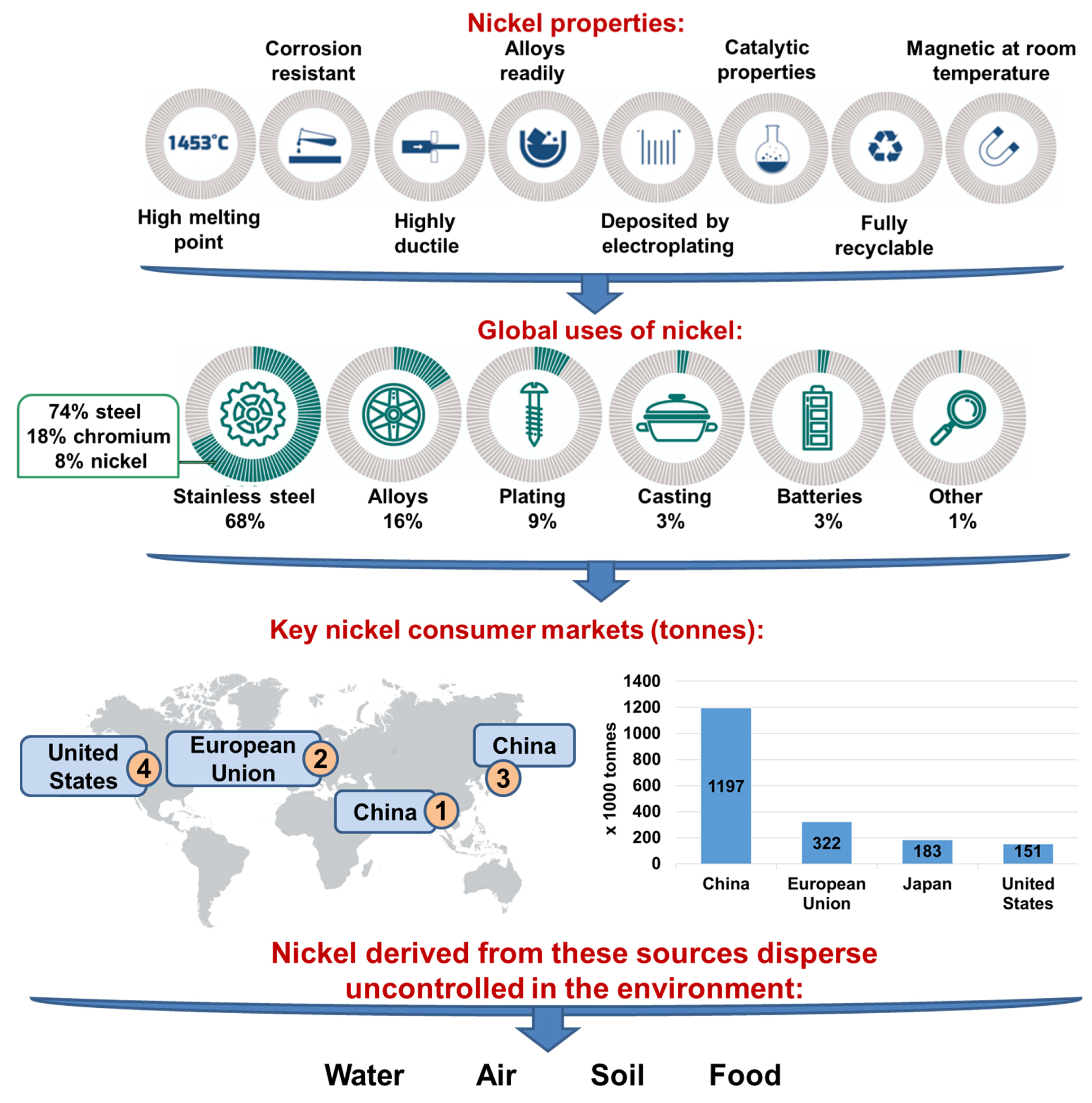



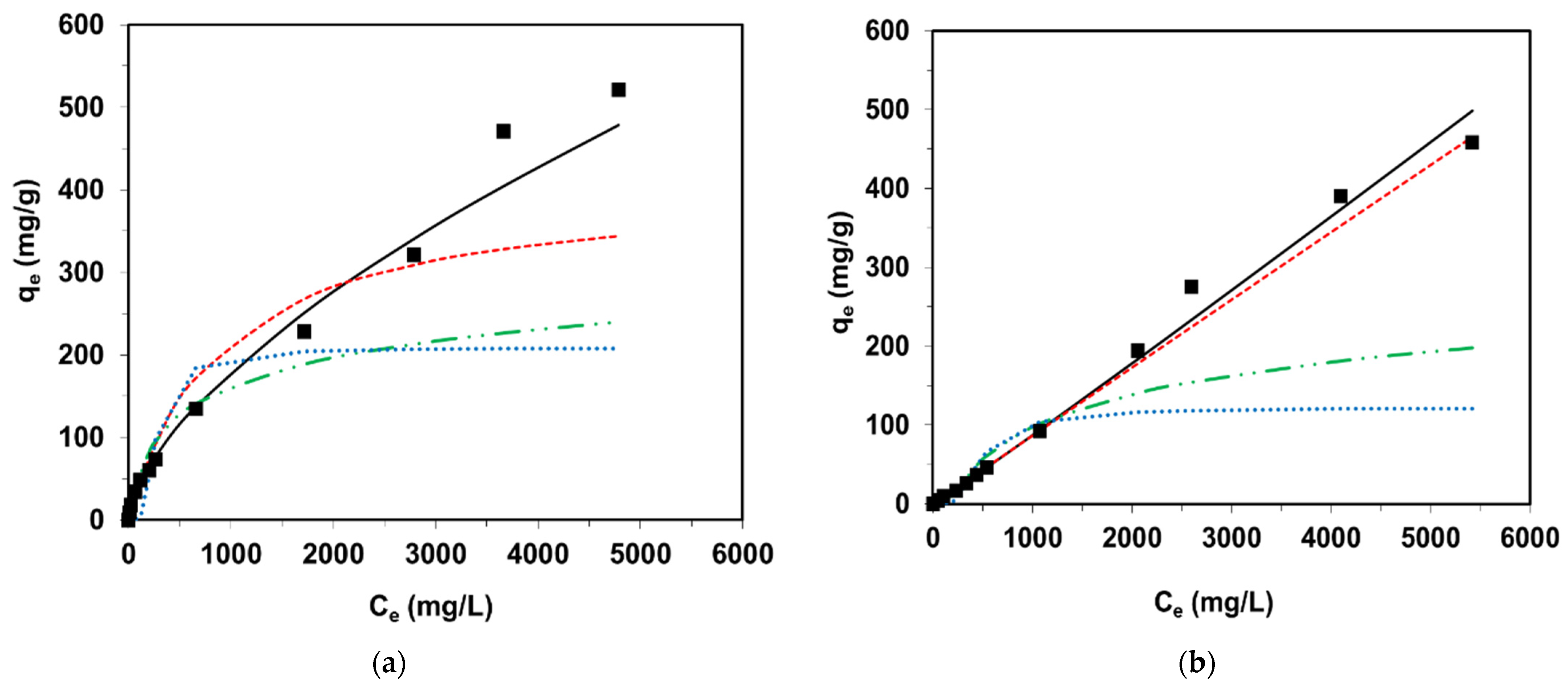
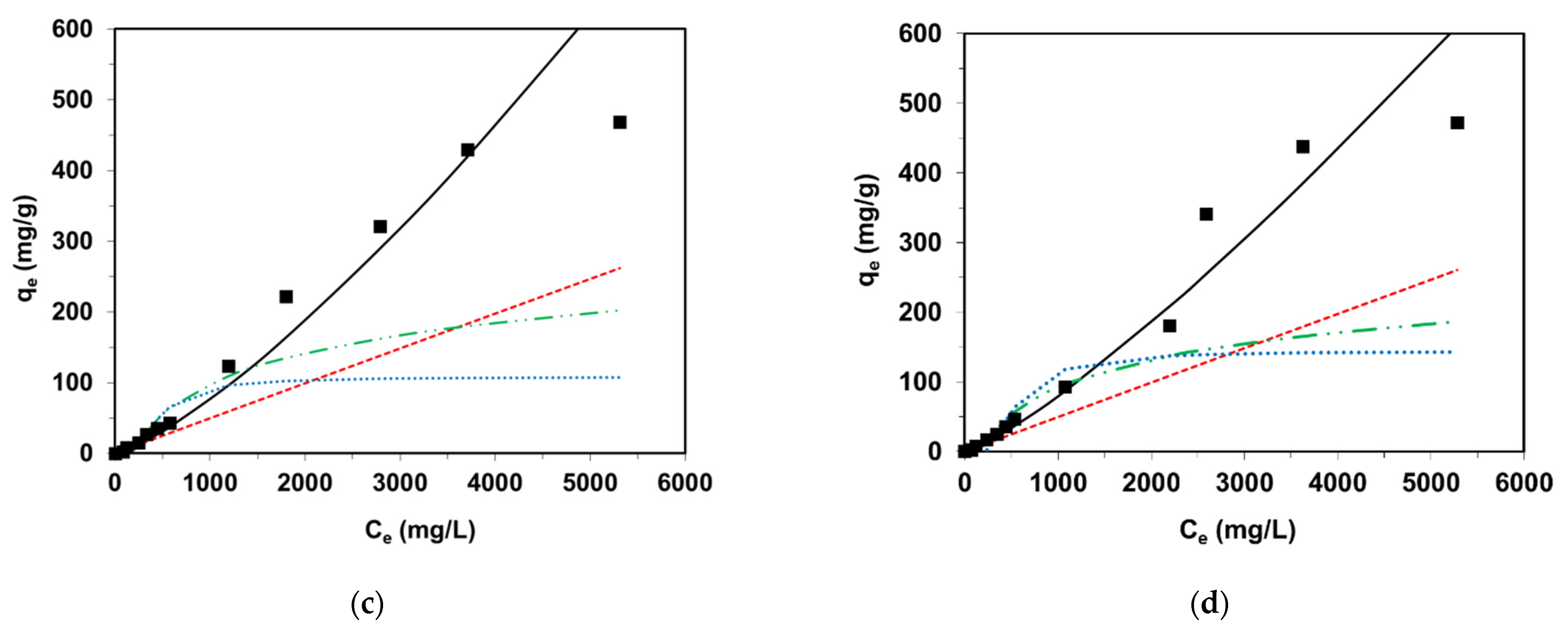
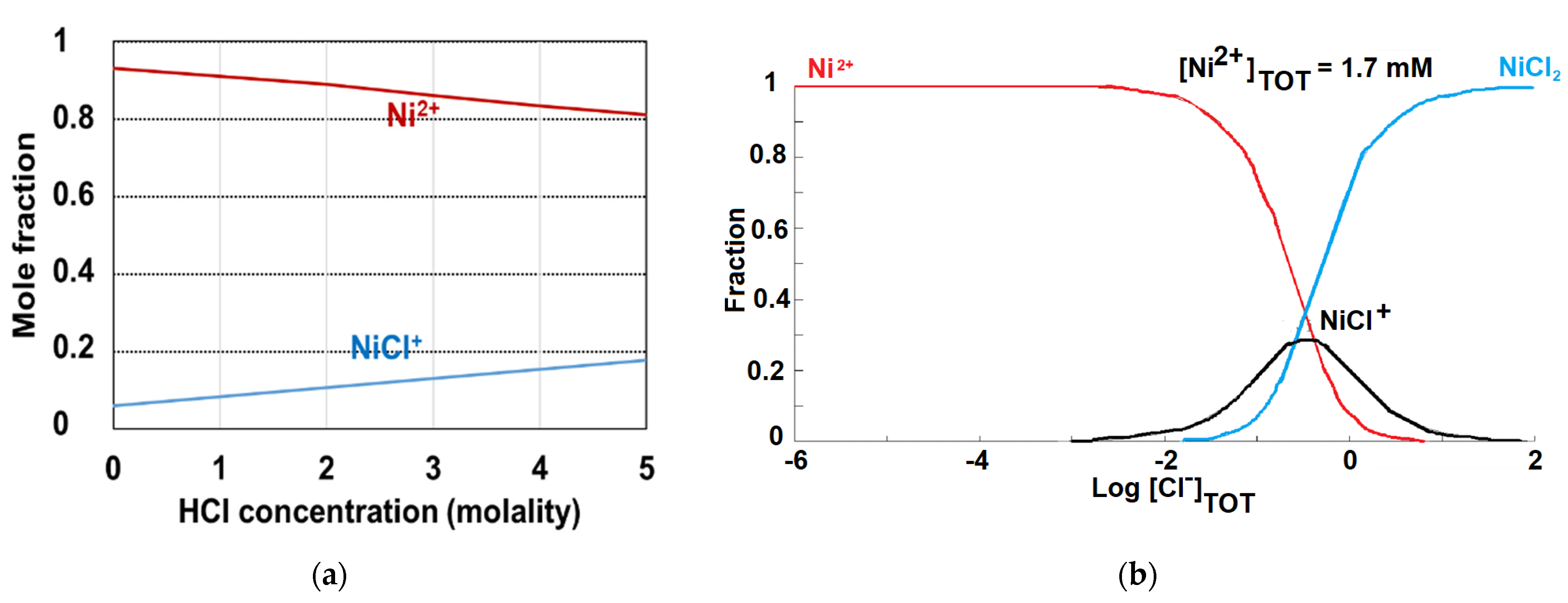

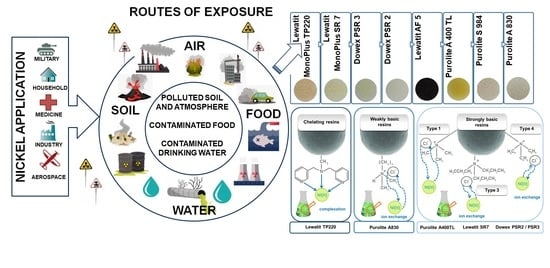
| Ni Occurrence | Concentration of Ni | References | |
|---|---|---|---|
| water | Baltic water | 0.09–1.08 μg/L | [2] |
| river water | 0.7 μg/L | ||
| bottled mineral waters | 0.71–3.20 μg/L | ||
| drinking water from Stalowa Wola (an area affected by industrial emissions) | 17 μg/L | ||
| uncontaminated water | 300 ng/L | [3] | |
| air | ambient air | 6–20 ng/L | |
| air (anthropogenic sources) | 150 ng/L | ||
| soil | farm soils | 3–1000 mg/L | [2] |
| soil | 0.2–450 mg/kg | ||
| soil near metal refineries dried sludge | 24,000–53,000 mg/kg | ||
| average content of nickel in Poland | 6.5 mg/kg | ||
| average content of nickel in the world | 13–37 mg/kg | ||
| soil affected by industrial emissions from Stalowa Wola | 17.20 mg/kg | ||
| soil affected by the Bolesław Mining and Metallurgical Plant | 19.62 mg/kg | [6] | |
| fertilizer | fertilizer based on dolomite | 7.6–396.0 mg/kg | |
| 11 types of fertilizer 1 | 0.4–295.1 mg/kg | [7,8] | |
| Name | Type | Matrix | Structure | Functional Groups | Mean Bead Size (mm) | Total Capacity (val/L) | Water Retention (%) |
|---|---|---|---|---|---|---|---|
| S984 | Chelating ion exchanger | Cross-linked polyacrylic | Macroporous | Polyamine | – | 2.7 | 44–55 |
| TP220 | Chelating/Weakly basic anion exchanger | Cross-linked polystyrene | Macroporous | Bis-picolylamine, bis(2-pyridyl-methyl)amine | 0.62 (±0.05) | 2.2 | 48–60 |
| A830 | Weakly basic anion exchanger | Cross-linked polyacrylic | Macroporous | Complex amine | 0.3–1.2 | 2.75 | 47–53 |
| SR7 | Strongly basic anion exchanger | Cross-linked polystyrene | Macroporous | Quaternary ammonium, type 3 | 0.57–0.67 | 0.6 | 59–64 |
| A400 TL | Strongly basic anion exchanger | Cross-linked polyacrylic | Microporous | Quaternary ammonium, type 1 | 0.425–0.85 | 1.3 | 48–54 |
| PSR2 | Strongly basic anion exchanger | Cross-linked polystyrene | Microporous | Quaternary ammonium, type, tri-n-butyl amine | 0.3–1.2 | 0.65 | 40–48 |
| PSR3 | Strongly basic anion exchanger | Cross-linked polystyrene | Macroporous | Quaternary ammonium, type, tri-n-butyl amine | 0.3–1.2 | 0.6 | 50–65 |
| AF5 | Adsorbent without functional group | Carbonaceous | Microporous | – | 0.4–0.8 | – | 48–60 |
| Adsorbent | HCl (M) | Ref. | HCl (M)/HNO3 (M) | Ref. | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.1 | 1.0 | 3.0 | 6.0 | 0.1/0.9 | 0.2/0.8 | 0.5/0.5 | 0.8/0.2 | 0.9/0.1 | |||
| S984 | 4.95 | 4.83 | 5.52 | 4.74 | [42] | 5.43 | 4.93 | 4.75 | 5.27 | 6.41 | [42] |
| TP220 | 6.24 | 4.93 | 4.68 | 4.89 | [43] | 4.88 | 4.93 | 4.91 | 5.00 | 4.95 | [43] |
| A830 | 4.60 | 4.85 | 4.53 | 5.02 | * | 4.59 | 4.49 | 4.36 | 4.40 | 4.53 | * |
| SR7 | 4.56 | 4.74 | 4.87 | 4.82 | * | 4.55 | 4.44 | 4.25 | 4.34 | 4.32 | * |
| A400TL | 4.72 | 4.79 | 4.91 | 4.76 | * | 4.17 | 4.40 | 4.30 | 3.92 | 4.02 | * |
| PSR2 | 3.70 | 3.29 | 4.36 | 4.14 | [44] | 4.78 | 4.75 | 4.76 | 4.69 | 4.78 | * |
| PSR3 | 4.73 | 4.82 | 4.53 | 3.92 | [44] | 4.69 | 4.78 | 4.57 | 4.60 | 4.64 | * |
| AF5 | 4.89 | 4.76 | 4.73 | 4.72 | [45] | 4.83 | 4.90 | 4.84 | 5.00 | 4.88 | [45] |
| Parameters | Pseudo-First Order | Pseudo-Second Order | Intraparticle Diffusion Model |
|---|---|---|---|
| qe exp = 6.24 mg/g | |||
| qe cal (mg/g) | 5.20 | 5.31 | 5.52 |
| k1 (1/min) | 2.75 | - | - |
| k2 (g/mg min) | - | 1.22 | |
| ki (mg/g min0.5) | - | - | 0.05 |
| MPSD | 0.0748 | 0.0631 | - |
| R2 | 0.9131 | 0.9280 | 0.6869 |
| R2adj | 0.8883 | 0.9074 | 0.5974 |
| Model | Parameters | Adsorbents | |||||||
|---|---|---|---|---|---|---|---|---|---|
| S984 | TP220 | A830 | SR7 | A400TL | PSR2 | PSR3 | AF5 | ||
| Freundlich | 0.0479 | 2.317 | 0.070 | 0.0096 | 0.0260 | 0.0363 | 0.0376 | 0.0169 | |
| 1/n | 1.081 | 0.629 | 1.031 | 1.299 | 1.165 | 1.118 | 1.108 | 1.224 | |
| MPSD | 0.272 | 0.079 | 0.097 | 0.905 | 0.248 | 0.368 | 0.212 | 0.582 | |
| R2 | 0.982 | 0.986 | 0.987 | 0.927 | 0.969 | 0.881 | 0.962 | 0.929 | |
| 0.977 | 0.983 | 0.984 | 0.909 | 0.961 | 0.852 | 0.952 | 0.911 | ||
| Langmuir | kL | 2.07 × 10−6 | 0.0011 | 2.27 × 10−6 | 1.30 × 10−6 | 1.72 × 10−6 | 1.30 × 10−6 | 1.91 × 10−6 | 1.30 × 10−6 |
| Q0 | 38,371.1 | 408.98 | 38,322.8 | 38,322.8 | 40,472.9 | 38,682.4 | 38,604.4 | 38,322.7 | |
| MPSD | 0.474 | 1.002 | 0.123 | 3.119 | 0.944 | 0.737 | 0.538 | 2.153 | |
| R2 | 0.986 | 0.901 | 0.989 | 0.965 | 0.978 | 0.892 | 0.970 | 0.947 | |
| 0.983 | 0.877 | 0.986 | 0.956 | 0.972 | 0.865 | 0.962 | 0.934 | ||
| Temkin | bT | 48.05 | 49.74 | 41.78 | 39.72 | 48.29 | 46.19 | 45.56 | 43.75 |
| A | 0.0061 | 0.0256 | 0.0052 | 0.0048 | 0.0055 | 0.0054 | 0.0053 | 0.0052 | |
| MPSD | 1.921 | 2.089 | 1.797 | 1.672 | 1.942 | 1.894 | 1.837 | 1.756 | |
| R2 | 0.900 | 0.858 | 0.906 | 0.926 | 0.882 | 0.850 | 0.908 | 0.877 | |
| 0.875 | 0.823 | 0.883 | 0.907 | 0.852 | 0.813 | 0.884 | 0.846 | ||
| Dubinin–Radushkevich | qm | 183.86 | 208.12 | 121.59 | 107.8 | 90.41 | 141.33 | 165.63 | 144.56 |
| kDR | 0.0048 | 0.0086 | 0.0282 | 0.0263 | 0.0216 | 0.0374 | 0.0458 | 0.038 | |
| E | 3.237 | 7.605 | 4.178 | 4.359 | 4.811 | 3.655 | 3.304 | 3.624 | |
| MPSD | 4.391 | 4.889 | 4.266 | 4.602 | 4.708 | 4.533 | 4.297 | 4.389 | |
| R2 | 0.773 | 0.706 | 0.680 | 0.689 | 0.606 | 0.698 | 0.767 | 0.711 | |
| 0.717 | 0.632 | 0.600 | 0.612 | 0.508 | 0.623 | 0.709 | 0.639 | ||
| Sorbent | Isotherm Model | Equilibrium Parameters | Ref. |
|---|---|---|---|
| Activated carbons from the doum seed | Freundlich | kF = 0.36–0.98 L/g, T = room temperature, pH = 7, a.d. = 5 g/L | [36] |
| Expanded graphite decorated with manganese oxide nanoparticles | Langmuir | qe = 0.0065 mg/g, T = 25 °C, a.d. = 0.5 g/L, | [37] |
| Lewatit TP207 (chelating iminodiacetic acid groups in PS-DVB matrix) | - | qe = 1.23 mg/g, T = 25 °C, a.d. = 0.27 g/L, pH = 6 | [38] |
| Biochars produced from the wheat straw pellets (WSP550, WSP700) and rice husk (RH550, RH700) at 550 and 700 °C | Freundlich | WSP700: qe = 25.1 mg/g WSP550: qe = 12.6 mg/g RH700: qe = 10.15 mg/g RH550: qe = 6.87 mg/g T = 20 °C, a.d. = 0.1 g/20 mL, pH = 5 | [39] |
| Amberlite IRA 458 (quaternary ammonium groups in the PA-DVB matrix) Amberlite IRA 958 (quaternary ammonium groups in the PA-DVB matrix) Amberlite IRA 67 (tertiary amine groups in the PA-DVB matrix) | Langmuir | qe = 16.72 mg/g qe = 13.22 mg/g qe = 10.03 mg/g T = room temperature, a.d. = 0.5 g/50 mL, pH = 4–8 | [40] |
| Modified carboxymethyl cellulose hydrogel | Freundlich | kF = 4.614 L/g, T = 30 °C, a.d. = 100 mg/L, pH = 5 | [41] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wołowicz, A.; Wawrzkiewicz, M. Screening of Ion Exchange Resins for Hazardous Ni(II) Removal from Aqueous Solutions: Kinetic and Equilibrium Batch Adsorption Method. Processes 2021, 9, 285. https://doi.org/10.3390/pr9020285
Wołowicz A, Wawrzkiewicz M. Screening of Ion Exchange Resins for Hazardous Ni(II) Removal from Aqueous Solutions: Kinetic and Equilibrium Batch Adsorption Method. Processes. 2021; 9(2):285. https://doi.org/10.3390/pr9020285
Chicago/Turabian StyleWołowicz, Anna, and Monika Wawrzkiewicz. 2021. "Screening of Ion Exchange Resins for Hazardous Ni(II) Removal from Aqueous Solutions: Kinetic and Equilibrium Batch Adsorption Method" Processes 9, no. 2: 285. https://doi.org/10.3390/pr9020285
APA StyleWołowicz, A., & Wawrzkiewicz, M. (2021). Screening of Ion Exchange Resins for Hazardous Ni(II) Removal from Aqueous Solutions: Kinetic and Equilibrium Batch Adsorption Method. Processes, 9(2), 285. https://doi.org/10.3390/pr9020285