Black Soldier Fly Larval Valorization Benefitting from Ex-Situ Fungal Fermentation in Reducing Coconut Endosperm Waste
Abstract
1. Introduction
2. Materials and Methods
2.1. Activation of Rhizopus Oligosporus and Spore Suspension Preparation
2.2. Growing Black Soldier Fly Larvae (BSFL)
2.3. Measured Parameters from BSFL Growth
2.4. Statistical Analysis
3. Results and Discussion
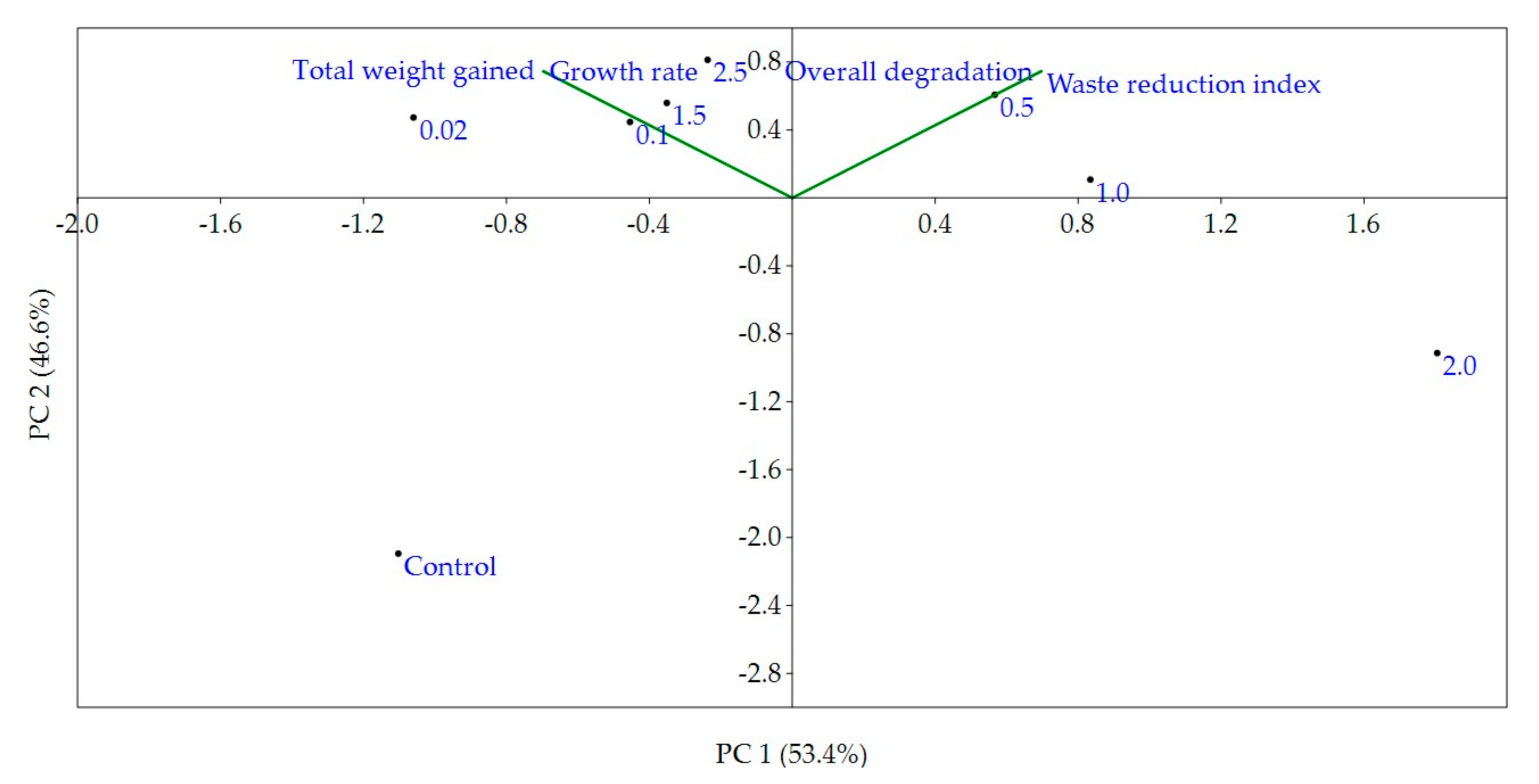
3.1. Larval Growths
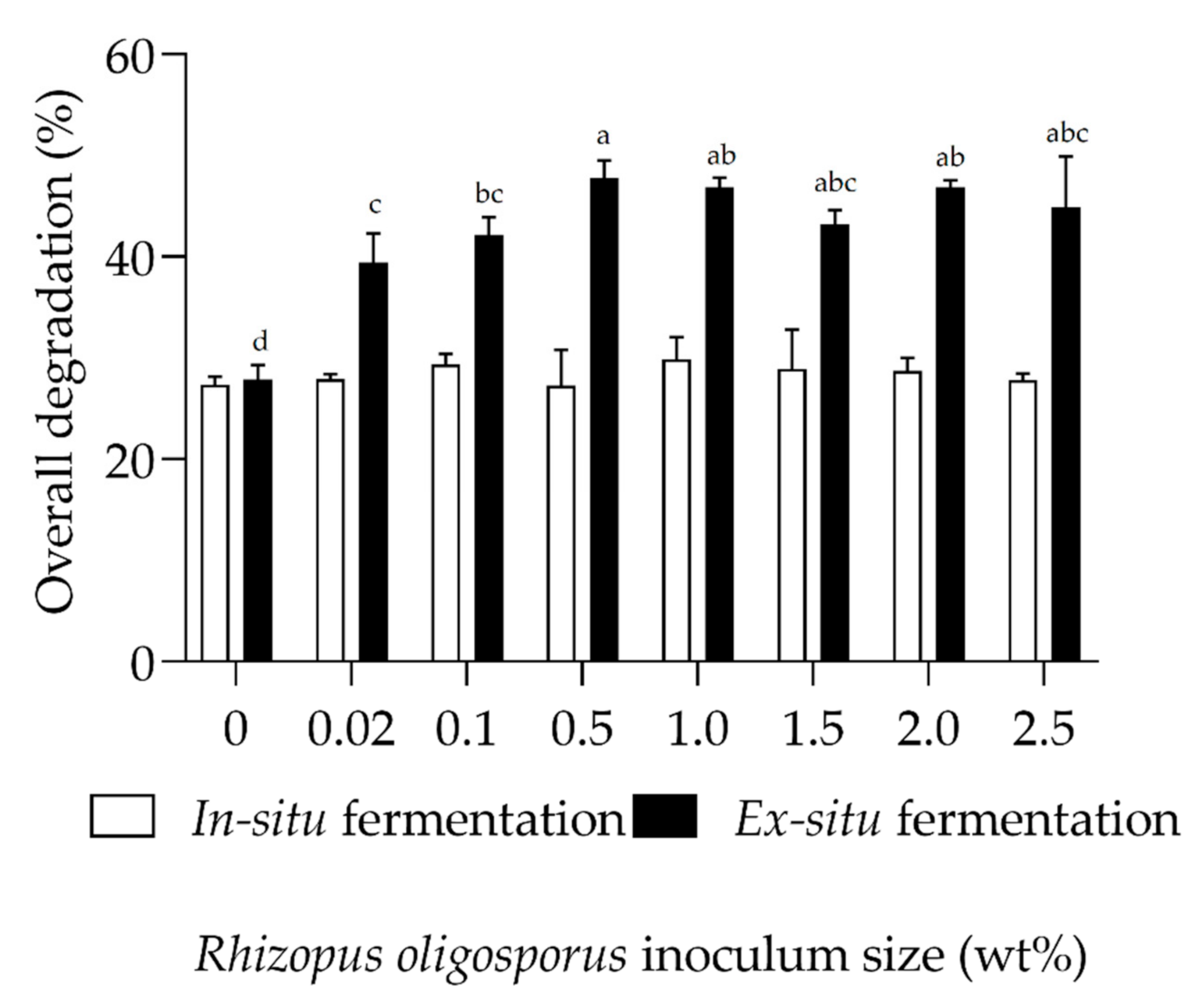
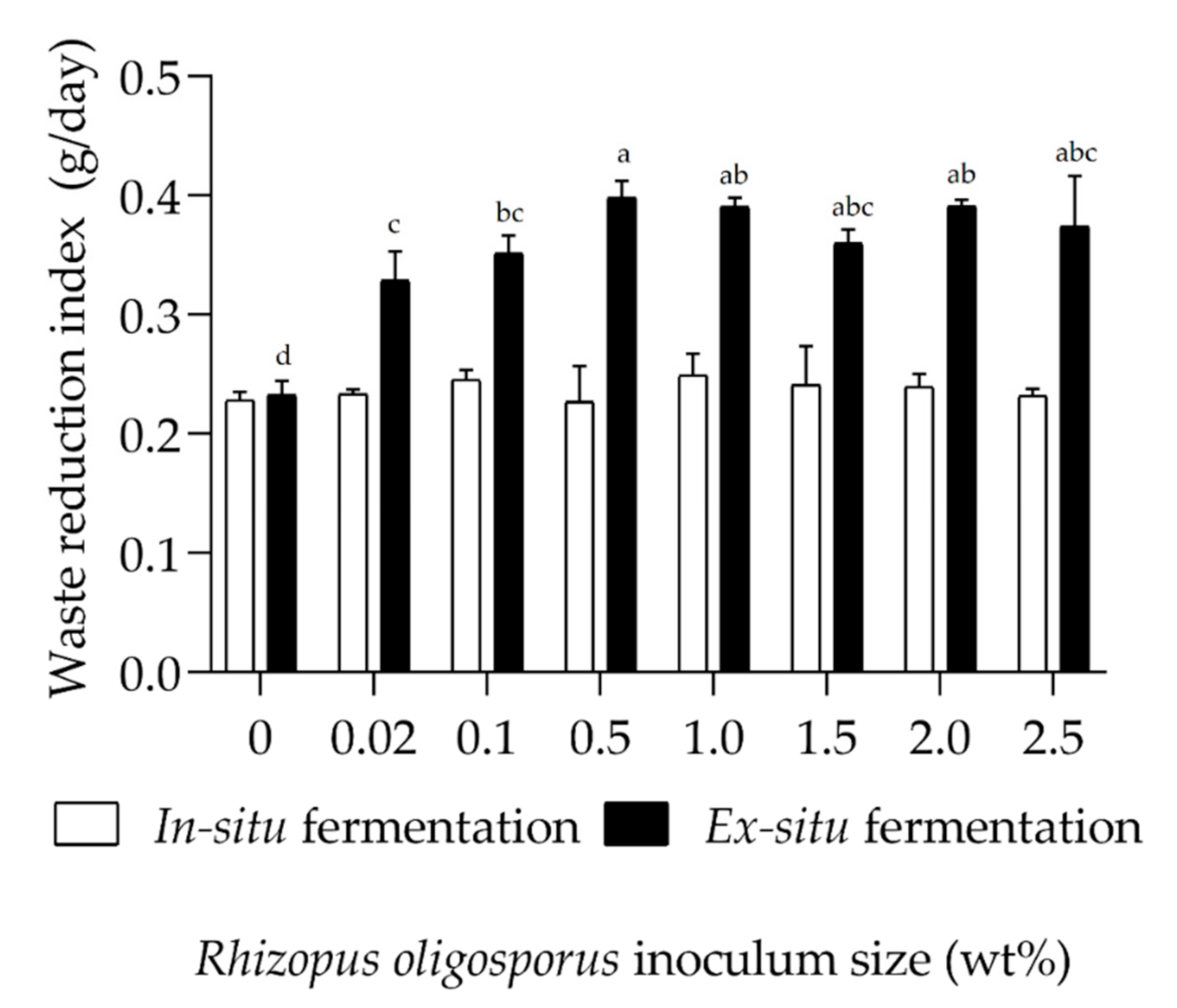
3.2. Reduction of Fermented Organic Wastes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bulak, P.; Polakowski, C.; Nowak, K.; Waśko, A.; Wiącek, D.; Bieganowski, A. Hermetia illucens as a new and promising species for use in entomoremediation. Sci. Total Environ. 2018, 633, 912–919. [Google Scholar] [CrossRef]
- Ewuim, S.C. Entomoremediation-a novel in-situ bioremediation approach. Anim. Res. Int. 2013, 10, 1681–1684. [Google Scholar]
- Zhou, F.; Tomberlin, J.K.; Zheng, L.; Yu, Z.; Zhang, J. Developmental and waste reduction plasticity of three black soldier fly strains (Diptera: Stratiomyidae) raised on different livestock manures. J. Med. Entomol. 2013, 50, 1224–1230. [Google Scholar] [CrossRef]
- Diener, S.; Solano, N.M.S.; Gutiérrez, F.R.; Zurbrügg, C.; Tockner, K. Biological treatment of municipal organic waste using black soldier fly larvae. Waste Biomass Valoriz. 2011, 2, 357–363. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Tomberlin, J.K.; Vanlaerhoven, S. Ability of black soldier fly (Diptera: Stratiomyidae) larvae to recycle food waste. Environ. Entomol. 2015, 44, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Lalander, C.H.; Fidjeland, J.; Diener, S.; Eriksson, S.; Vinnerås, B. High waste-to-biomass conversion and efficient salmonella spp. Reduction using black soldier fly for waste recycling. Agron. Sustain. Dev. 2015, 35, 261–271. [Google Scholar] [CrossRef]
- Schiavone, A.; Cullere, M.; De Marco, M.; Meneguz, M.; Biasato, I.; Bergagna, S.; Dezzutto, D.; Gai, F.; Dabbou, S.; Gasco, L. Partial or total replacement of soybean oil by black soldier fly larvae (Hermetia illucens L.) fat in broiler diets: Effect on growth performances, feed-choice, blood traits, carcass characteristics and meat quality. Ital. J. Anim. Sci. 2017, 16, 93–100. [Google Scholar] [CrossRef]
- Rana, K.S.; Salam, M.; Hashem, S.; Islam, M.A. Development of black soldier fly larvae production technique as an alternate fish feed. Int. J. Res. Fish Aquac. 2015, 5, 41–47. [Google Scholar]
- Cullere, M.; Tasoniero, G.; Giaccone, V.; Miotti-Scapin, R.; Claeys, E.; De Smet, S.; Dalle Zotte, A. Black soldier fly as dietary protein source for broiler quails: Apparent digestibility, excreta microbial load, feed choice, performance, carcass and meat traits. Animal 2016, 10, 1923–1930. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-S.; Shelomi, M. Review of black soldier fly (Hermetia illucens) as animal feed and human food. Foods 2017, 6, 91. [Google Scholar] [CrossRef]
- Elsayed, M.; Ran, Y.; Ai, P.; Azab, M.; Mansour, A.; Jin, K.; Zhang, Y.; Abomohra, A.E.-F. Innovative integrated approach of biofuel production from agricultural wastes by anaerobic digestion and black soldier fly larvae. J. Clean. Prod. 2020, 121495. [Google Scholar] [CrossRef]
- Li, W.; Li, Q.; Zheng, L.; Wang, Y.; Zhang, J.; Yu, Z.; Zhang, Y. Potential biodiesel and biogas production from corncob by anaerobic fermentation and black soldier fly. Bioresour. Technol. 2015, 194, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Chen, H. Modern Solid State Fermentation; Springer: Dordrecht, The Netherlands, 2013. [Google Scholar]
- Gao, Z.; Wang, W.; Lu, X.; Zhu, F.; Liu, W.; Wang, X.; Lei, C. Bioconversion performance and life table of black soldier fly (Hermetia illucens) on fermented maize straw. J. Clean. Prod. 2019, 230, 974–980. [Google Scholar] [CrossRef]
- Wong, C.Y.; Lim, J.W.; Chong, F.K.; Lam, M.K.; Uemura, Y.; Tan, W.N.; Bashir, M.J.K.; Lam, S.M.; Sin, J.C.; Lam, S.S. Valorization of exo-microbial fermented coconut endosperm waste by black soldier fly larvae for simultaneous biodiesel and protein productions. Environ. Res. 2020, 185, 109458. [Google Scholar] [CrossRef]
- Mazza, L.; Xiao, X.; Ur Rehman, K.; Cai, M.; Zhang, D.; Fasulo, S.; Tomberlin, J.K.; Zheng, L.; Soomro, A.A.; Yu, Z. Management of chicken manure using black soldier fly (Diptera: Stratiomyidae) larvae assisted by companion bacteria. Waste Manag. 2020, 102, 312–318. [Google Scholar] [CrossRef]
- Handoyo, T.; Morita, N. Structural and functional properties of fermented soybean (tempeh) by using Rhizopus oligosporus. Int. J. Food Prop. 2006, 9, 347–355. [Google Scholar] [CrossRef]
- Nelofer, R.; Nadeem, M.; Irfan, M.; Syed, Q. Nutritional enhancement of barley in solid state fermentation by Rhizopus oligosporus ml-10. Nutr. Food Sci. Int. J. 2018, 6, 555700. [Google Scholar] [CrossRef]
- Diclaro, J.; Kaufman, P.E. Black soldier fly hermetia illucens linnaeus (Insecta: Diptera: Stratiomyidae). EENY 2009, 461, 1–3. [Google Scholar]
- Kim, W.-T.; Bae, S.-W.; Park, H.-C.; Park, K.-H.; Lee, S.-B.; Choi, Y.-C.; Han, S.-M.; Koh, Y.-H. The larval age and mouth morphology of the black soldier fly, Hermetia illucens (Diptera: Stratiomyidae). Int. J. Ind. Entomol. 2010, 21, 185–187. [Google Scholar]
- Li, Q.; Zheng, L.; Qiu, N.; Cai, H.; Tomberlin, J.K.; Yu, Z. Bioconversion of daiy manure by black soldier fly (Diptera: Stratiomyidae) for biodiesel and sugar production. Waste Manag. 2011, 31, 1316–1320. [Google Scholar] [CrossRef]
- Mohd-Noor, S.N.; Wong, C.Y.; Lim, J.W.; Uemura, Y.; Lam, M.K.; Ramli, A.; Bashir, M.J.; Tham, L. Optimization of self-fermented period of waste coconut endosperm destined to feed black soldier fly larvae in enhancing the lipid and protein yields. Renew. Energy 2017, 111, 646–654. [Google Scholar] [CrossRef]
- Wong, C.Y.; Mohd Aris, M.N.; Daud, H.; Lam, M.K.; Yong, C.S.; Abu Hasan, H.; Chong, S.; Show, P.L.; Hajoeningtijas, O.D.; Ho, Y.C.; et al. In-Situ Yeast Fermentation to Enhance Bioconversion of Coconut Endosperm Waste into Larval Biomass of Hermetia illucens: Statistical Augmentation of Larval Lipid Content. Sustainability 2020, 12, 1558. [Google Scholar] [CrossRef]
| R. oligosporus Inoculum Size (wt %) | Total Weight Gained (g) | Growth Rate (g/day) |
|---|---|---|
| 0 (Control) | 0.71 ± 0.06 | 0.059 ± 0.005 |
| 0.02 | 0.77 ± 0.05 | 0.065 ± 0.004 |
| 0.1 | 0.76 ± 0.01 | 0.063 ± 0.001 |
| 0.5 | 0.73 ± 0.03 | 0.061 ± 0.002 |
| 1.0 | 0.71 ± 0.04 | 0.059 ± 0.003 |
| 1.5 | 0.76 ± 0.04 | 0.063 ± 0.004 |
| 2.0 | 0.65 ± 0.01 | 0.055 ± 0.001 |
| 2.5 | 0.75 ± 0.02 | 0.063 ± 0.002 |
| R. oligosporus Inoculum Size (wt %) | Total Weight Gained (g) | Growth Rate (g/day) |
|---|---|---|
| 0 (Control) | 0.70 ± 0.06 | 0.058 ± 0.005 |
| 0.02 | 1.08 ± 0.10 | 0.090 ± 0.009 |
| 0.1 | 1.02 ± 0.11 | 0.085 ± 0.009 |
| 0.5 | 1.13 ± 0.11 | 0.094 ± 0.009 |
| 1.0 | 1.04 ± 0.03 | 0.087 ± 0.003 |
| 1.5 | 0.95 ± 0.04 | 0.079 ± 0.003 |
| 2.0 | 1.06 ± 0.07 | 0.089 ± 0.006 |
| 2.5 | 0.97 ± 0.25 | 0.081 ± 0.021 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, C.Y.; Kiatkittipong, K.; Kiatkittipong, W.; Ntwampe, S.K.O.; Lam, M.K.; Goh, P.S.; Cheng, C.K.; Bashir, M.J.K.; Lim, J.W. Black Soldier Fly Larval Valorization Benefitting from Ex-Situ Fungal Fermentation in Reducing Coconut Endosperm Waste. Processes 2021, 9, 275. https://doi.org/10.3390/pr9020275
Wong CY, Kiatkittipong K, Kiatkittipong W, Ntwampe SKO, Lam MK, Goh PS, Cheng CK, Bashir MJK, Lim JW. Black Soldier Fly Larval Valorization Benefitting from Ex-Situ Fungal Fermentation in Reducing Coconut Endosperm Waste. Processes. 2021; 9(2):275. https://doi.org/10.3390/pr9020275
Chicago/Turabian StyleWong, Chung Yiin, Kunlanan Kiatkittipong, Worapon Kiatkittipong, Seteno K. O. Ntwampe, Man Kee Lam, Pei Sean Goh, Chin Kui Cheng, Mohammed J. K. Bashir, and Jun Wei Lim. 2021. "Black Soldier Fly Larval Valorization Benefitting from Ex-Situ Fungal Fermentation in Reducing Coconut Endosperm Waste" Processes 9, no. 2: 275. https://doi.org/10.3390/pr9020275
APA StyleWong, C. Y., Kiatkittipong, K., Kiatkittipong, W., Ntwampe, S. K. O., Lam, M. K., Goh, P. S., Cheng, C. K., Bashir, M. J. K., & Lim, J. W. (2021). Black Soldier Fly Larval Valorization Benefitting from Ex-Situ Fungal Fermentation in Reducing Coconut Endosperm Waste. Processes, 9(2), 275. https://doi.org/10.3390/pr9020275












