Abstract
In the current research, the supercritical carbon dioxide (SCCO2) procedure was used to extract volatile oils from perilla leaves. The yields of the volatile oils and the four main constituents, limonene, perillaldehyde, β-caryophyllene, and (Z,E)-α-farnesene obtained by the SCCO2 procedure were 1.31-, 1.12-, 1.04-, 1.05-, and 1.07-fold higher than those obtained by the hydrodistillation technique, respectively. Furthermore, the duration and temperature of extraction were 40 min and 45 °C lower, respectively, in the former procedure compared to the latter technique. These advantages reveal that SCCO2 not only obtains high-quality extracts, but also meets the requirements of green environmental protection. The theoretical solubilities of the volatile oils acquired by the SCCO2 dynamic extraction at various temperatures and pressures were 1.385 × 10−3–8.971 × 10−3 (g oil/g CO2). Moreover, the three density-based models were well correlated with these theoretical solubility data, with a high coefficient of determination and low average absolute relative deviation.
1. Introduction
Perilla frutescens (L.) Britton is a famous edible and medicinal plant that belongs to the Lamiaceae family [1,2]. Due to its special flavor, perilla leaves have a special flavor, they are often eaten directly as fresh vegetables or used as food additive material or in sushi. Furthermore, perilla leaves are a widely used traditional medicinal material because of their rich and diverse pharmacological properties, such as anticancer, antioxidative, neuroprotective, anti-inflammatory, and antimicrobial activities [2,3,4]. Essential oil has been reported to be an important bioactive ingredient in perilla leaves [5]. Perilla essential oil is not only used as a food and nutritional additive, but also has been extensively researched for its anticancer, antioxidative, neuroprotective, anti-inflammatory, and antimicrobial effects [3,6]. The chemical composition of perilla essential oil varies according to the variety, geographical factors, climatic conditions, and planting and harvest times. However, for red-leafed varieties of P. frutescens, the main volatile component has been found to be perillaldehyde [3,6]. Gas chromatography was used to study essential oils obtained from perilla leaves, providing qualitative and quantitative analyses with sufficient accuracy for the aforementioned components [5].
Hydrodistillation (HD) and steam distillation (STD) have been widely used to separate essential oils from P. frutescens because of the use of water as the solvent and simple operation [7,8,9]. However, these traditional procedures require the use of high temperatures; they often cause the degradation of heat-sensitive ingredients, thus affecting the quality of essential oils. Moreover, the procedures often cause the mixing of water-soluble components and essential oils, and further separation procedures cause the loss of essential oils. However, the long extraction time is the most frequently criticized drawback of these traditional procedures.
Because of the unique properties of supercritical carbon dioxide (SCCO2), such as a low critical point (7.4 MPa, 31.1 °C), chemical inertness, and high penetration and diffusion capabilities, SCCO2 extraction can perfectly solve the shortcomings of traditional procedures [10]. This is mainly due to the low critical point of CO2, it does not need a very high temperature to achieve good extraction results, so it does not cause degradation of heat-sensitive components. Additionally, SCCO2 does not react with the constituents of the extract, so the SCCO2 procedure obtains a highly stable extract. Furthermore, after the extraction process is decompressed, CO2 and the extract can be completely separated, so there is no concern about solvent residue in the extract. Based on the abovementioned advantages, SCCO2 extraction can obtain excellent quality extracts that are very suitable for the food, functional nutrition, cosmetics, and pharmaceutical industries. In addition, because of the high penetration and diffusibility of SCCO2, it effectively promotes the penetration of the solvent through the plant substrate, thereby accelerating the desorption and release from the substrate of the analyte, which then diffuses into the solvent, thus shortening the extraction time [11,12,13]. Therefore, SCCO2 extraction meets the requirements of economic efficiency in the industrialization process.
Based on the foregoing description, it is clear that SCCO2 is an excellent extraction solvent for nonpolar to low-polar components. Therefore, SCCO2 extraction has been widely used to extract volatile components from different substrates [14]. However, literature reports on the extraction of perilla oil with SCCO2 are scarce. In addition, the solubility of the analyte in SCCO2 is one of the important data for judging whether the SCCO2 extraction procedure is feasible and even good or bad in progress. Furthermore, the solubility data of the analyte are an indispensable parameter for subsequent pharmacokinetic experiments.
Although the diverse uses and pharmacological activities of perilla oil have been widely reported, data on its solubility in SCCO2 remain very limited. Therefore, this study primarily uses the SCCO2 dynamic extraction of perilla oil and carefully explores the effects of the CO2 flow rate, temperature, and pressure on the yield to obtain the highest yield and best quality of the oil. Furthermore, three density-based correlation models with excellent accuracy are used to correlate the solubility of perilla oil obtained through experiments to expand the solubility data for subsequent pharmacokinetic experiments.
2. Materials and Methods
2.1. Raw Materials and Reagents
Dried P. frutescens leaves (the red-leaf form) were purchased from different local herbal shops (Kaohsiung, Taiwan) and then authenticated, triturated, sieved (W.S. Tyler, Mentor, OH, USA, 0.36 mm), and deposited [15]. Subsequently, the moisture content of the herb sample measured by volumetric Karl Fischer titration was 8.71% [16]. Helium (99.99%) and carbon dioxide (99.99%) were purchased from the Yun-Shan Gas Co. Ltd. (Tainan, Taiwan). Perillaldehyde, β-caryophyllene, (Z,E)-α-farnesene and limonene were obtained from Sigma Chemical Co. (St. Louis, MO, USA); n-hexane was bought from Merck Co. (Darmstadt, Germany).
2.2. Supercritical Carbon Dioxide (SCCO2) Extraction
A semibatch flow extraction procedure (Isco 260D, Lincoln, NE, USA) was used for SCCO2 extraction [17]. The extraction procedure was carried out by adding 20 g of plant sample (0.36 mm) into a stainless steel vessel (SS304, i.d. of 22 mm and length of 300 mm), which was later immersed in a large stainless steel water bath (SS304, length: 300 mm, width: 200 mm, height: 500 mm). After reaching the desired temperature and pressure, a static extraction stage (for 30 min) followed by a dynamic extraction stage (5–210 min) was carried out [18]. The oils were collected in two consecutive separators. The first separation tank is operated at 4 °C and 9 MPa, while the second separation tank is operated at 4 °C and 0.15 MPa.
2.3. Essential Oil Extraction by Hydrodistillation (HD)
In brief, 100 g of an herbal sample (0.36 mm) was placed in a 1.5 L distillation flask (3 holes), a certain amount of distilled water was added, and the device was connected with a Clevenger condenser. Subsequently, the distillation flask was immersed in a temperature-controlled water bath (Haake F3-K, Haake, Karlsruhe, Germany). After the desired temperature (100 °C) was obtained, a 4-h static stage extraction was performed for the HD procedure. The essential oil was further separated by the action of gravity. Anhydrous sodium sulfate was used to dry the obtained oils [19,20].
2.4. Gas Chromatographic (GC) Analysis
GC analyses of the essential oils were performed using a GC/flame ionization detector (FID) system (Shimadzu, model CG-14A, Kyoto, Japan) equipped with a DB-5 silica capillary column (30 m × 0.25 mm × 0.25 μm, J&W Scientific, Folsom, CA, USA) [21]. The quantities of the four main constituents, limonene, perillaldehyde, β-caryophyllene, and (Z,E)-α-farnesene were evaluated by comparing their peak areas to those of the calibration curves of the standards of constituents.
2.5. Statistical Analysis
All yields are estimated on the basis of plant dry weight, which is expressed using the average and standard deviation (SD) of three experimental results. The SD was calculated as follows:
where ye,i is the experimental value, ym is the mean value, and n is the number of experimental runs [22].
To evaluate correlations in the applied models, the two objective functions average absolute relative deviation (AARD(%)) and coefficient of determination (R2) were determined using the predicted and experimental values. These parameters were calculated using Equations (2) and (3).
where yp,i is the predicted value corresponding to point i.
3. Results and Discussion
3.1. Essential Oils Obtained by HD
Hydrodistillation (HD) and steam distillation (STD) are well-known standard procedures for extracting essential oils from natural sources. Therefore, to evaluate the performance of the SCCO2 extraction, this study first used the HD procedure for 4 h to extract the essential oil from P. frutescens leaves. The obtained essential oil has a pale yellow color and a strong fragrance. The yields of the essential oil and its four main components are shown in Table 1. The yields of essential oils and individual components were expressed in terms of the mass percent of the essential oil per gram of dry plant (%, g of oil/g of dry plant) and the mass percent of each component per gram of essential oil (%, g of component/g of oil), respectively.

Table 1.
Comparison of the extraction conditions and yields obtained by hydrodistillation (HD) and supercritical carbon dioxide (SCCO2) extraction.
3.2. Influence of the CO2 Flow Rate
The extraction kinetic curve of the natural solid substratum using the SCCO2 dynamic procedure is usually called the overall extraction curve (OEC), which is often expressed as a graph of extraction yield versus extraction time or the mass of spent CO2 for the extracting conditions (T, P, CO2 flow rate, and cosolvent) [23]. The OEC not only predicts the extraction capacity of the procedure but also indicates the composition and physicochemical and biological characteristics of the extract. Furthermore, based on the experimental data of the OEC, a mathematical model of the extraction procedure can be derived to establish the parameters required by the extraction procedure, which can then be used as the basis for establishing optimized industrial procedures. Experimental OEC data fitted to a spline using two straight lines can usually be clearly divided into three stages: the constant extraction rate (CER), decreasing extraction rate and diffusion period [24,25,26]. Consequently, according to the kinetic parameters obtained in the CER period, such as the duration, mass transfer rate, and yield, the analyte concentration in the solvent phase at the vessel outlet (YCER) is further calculated. At a certain temperature and pressure and the most appropriate CO2 flow rate, the YCER value will approach the maximum value (YCER *), which can be considered the theoretical solubility of the analyte in SCCO2. The proper CO2 flow rate have to be sufficiently low to guarantee that the SCCO2 is saturated with solute at the outlet of the vessel, but it cannot restrict the mass transfer of the extraction process. Therefore, the CO2 flow rate is one of the most crucial factors for determining the solubility of the solute in the supercritical solvent phase via the SCCO2 dynamic extraction method.
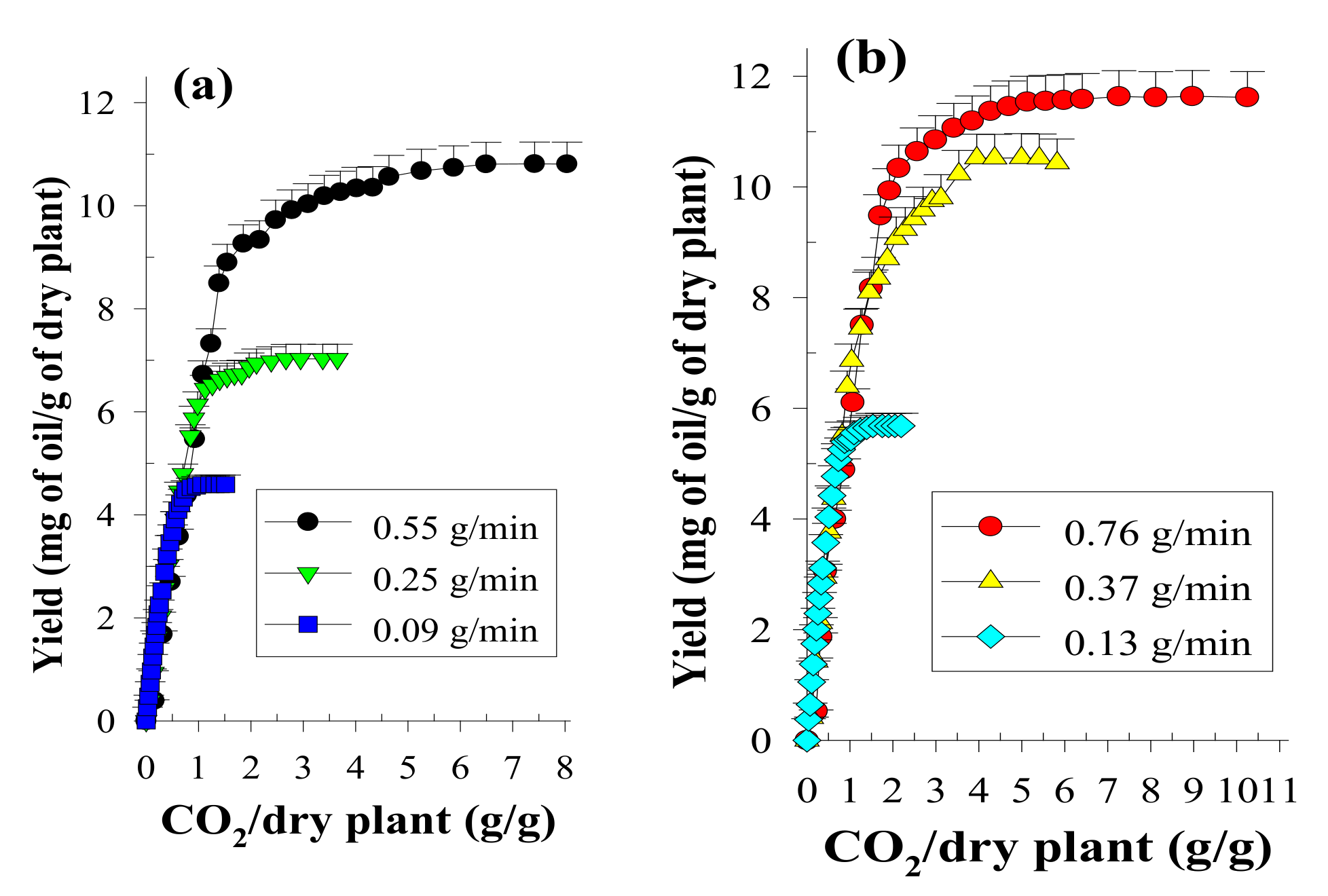
To evaluate the influence of CO2 flow rate on the oil yield, SCCO2 extraction was performed at a static stage of 30 min followed by a dynamic stage of 5–270 min at a mean particle size of 0.36 mm [27]. To establish the OEC data, the extracts were collected at equal intervals of 5–10 min, and qualitative and quantitative analyses were further performed using GC analysis. The OECs of volatile oils using SCCO2 dynamic extraction at a serial flow rates were expressed as the oils/dry plant mass ratio (g/g, %) as a function of the CO2/dry plant mass ratio (Figure 1). Then, the OECs obtained at different CO2 flow rates were fitted using two straight lines, and the YCER value obtained at each flow rate in the CER period (first straight line) was therefore generated, as shown in Figure 2.
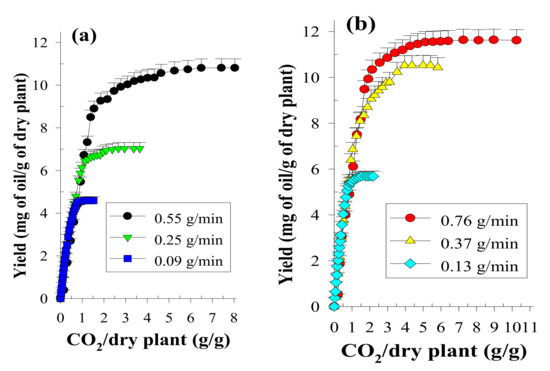
Figure 1.
Overall extraction curves at 32 °C, 33.0 MPa and various CO2 flow rates (a,b) for the SCCO2 extraction of volatile oils from P. frutescens leaves.
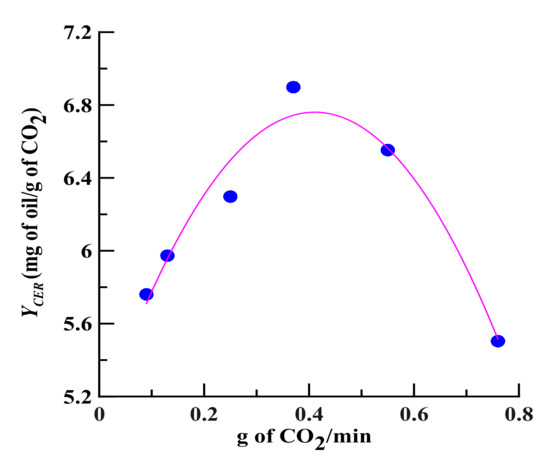
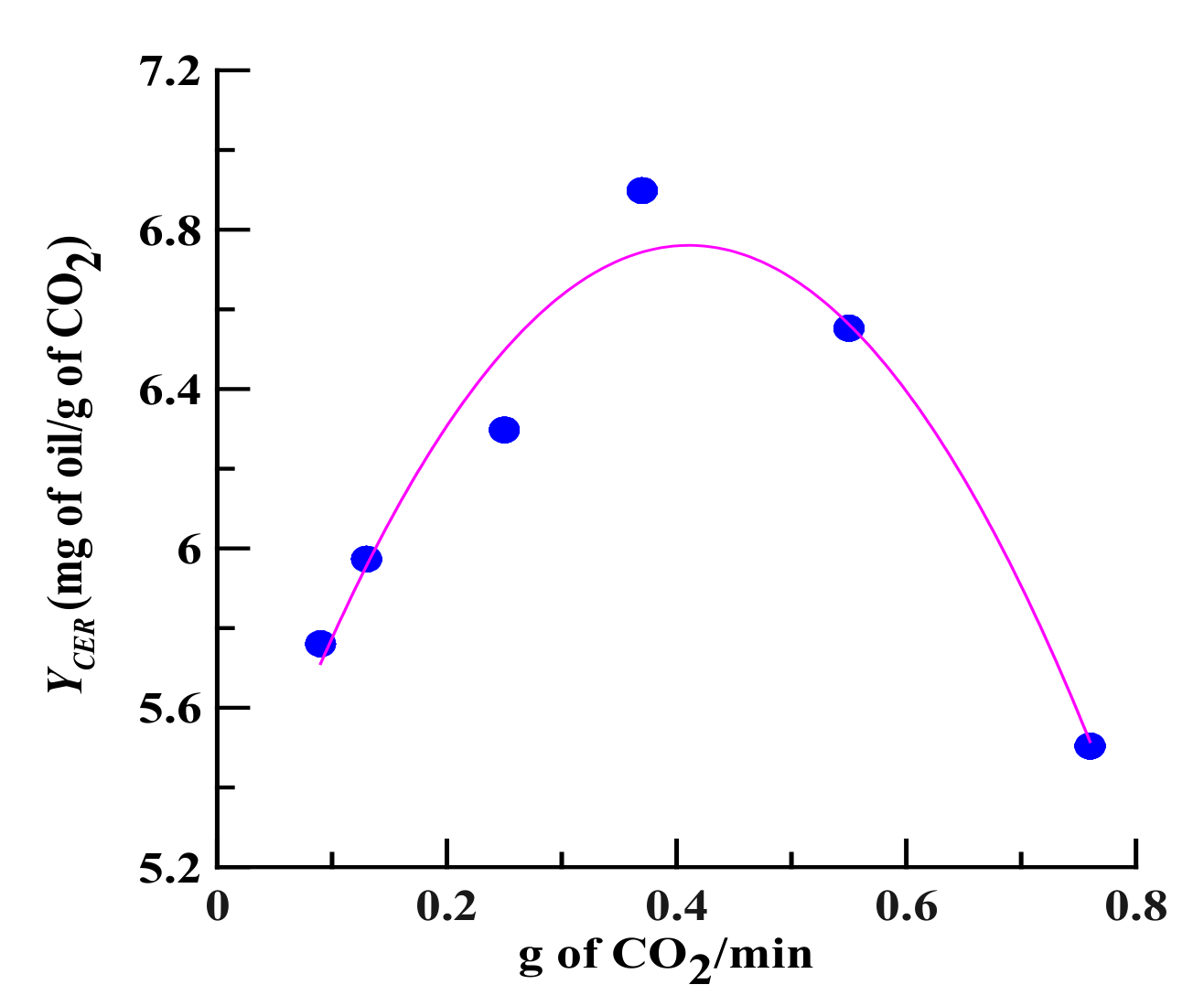
Figure 2.
The effect of CO2 flow rate on YCER (mg of oil/g of CO2) value at 32 °C. The slope of the constant extraction rate (CER) period (a straight line) is defined as the YCER value, which is obtained by dividing the yield by the solvent/plant weight ratio. If the solvent is saturated with the solute, YCER should be maximized; this value is called the theoretical solubility (YCER*).
Increasing the CO2 flow rate (0.09 to 0.37 g/min) increased the contact area and collision opportunity frequency between the solvent and the plant sample, which in turn increased the value of YCER (Figure 2). Additionally, the greater the CO2 flow rate is, the steeper the concentration gradient difference between the analyte and the SCCO2 is, which promotes the diffusion and mass transfer of the analyte and thereby enhances the value of YCER. However, if the CO2 flow rate is too high (0.37 to 0.76 g/min), because of the declined extent of saturation of the supercritical solvent at higher flow rates, the value of YCER begins to decrease. Using SCCO2 extraction, a flow rate of 0.37 g/min generated the maximum value of YCER (YCER*) and was therefore determined as an appropriate CO2 flow rate for evaluating the theoretical solubility of volatile oil in SCCO2.
3.3. Influences of Temperature and Pressure
Pressure and temperature are the most important factors for the performance and selectivity of SCCO2 extraction, and this importance has been verified for different natural species [28]. To estimate the effects of both factors on the oil yields, SCCO2 extraction was conducted at pressures ranging from 10.5 to 33.0 MPa and temperatures ranging from 32 °C to 55 °C, while other conditions were kept fixed as mentioned above.
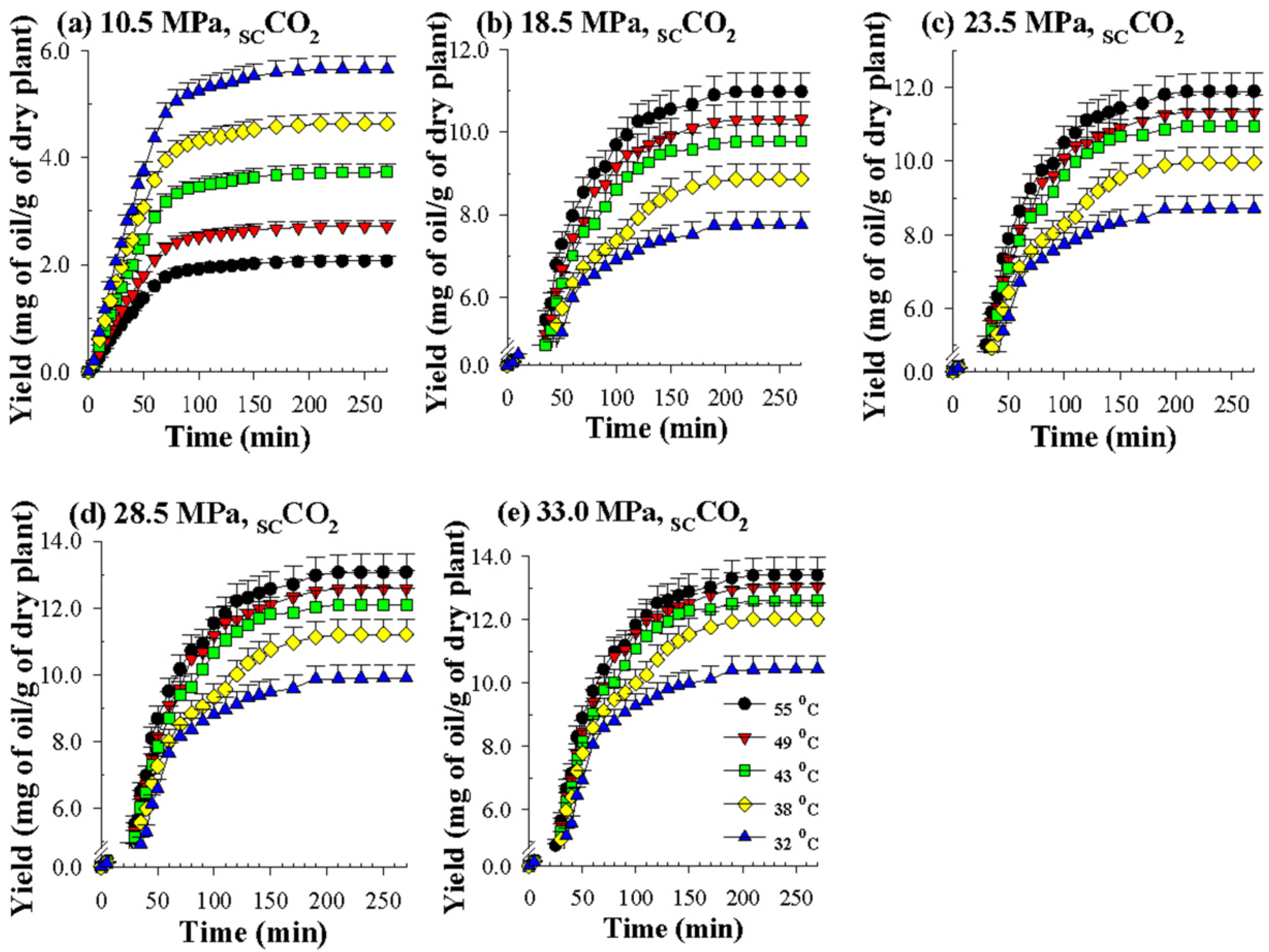
Figure 3 indicates that when the pressure increases from 10.5 MPa to 33.0 MPa, the yield of volatile oil increases substantially. This result is due to the increase in the density of the supercritical CO2 with the increase in pressure, so the solubility of the oil in the supercritical solvent increases, enhancing the yield of oil [29,30]. However, at a pressure of 33.0 MPa, the increase in the oil yield was smaller, which may be due to the decrease in mass transfer and diffusion parameters as the pressure increased.
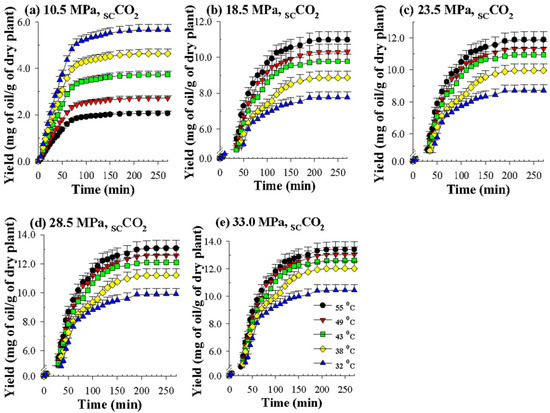
Figure 3.
Overall extraction curves for SCCO2 dynamic extraction of volatile oils from P. frutescens leaves at various temperatures and pressures (a–e) and a CO2 flow rate of 0.37 g/min.
As shown in Figure 3, the effect of temperature obviously has two opposing trends on the yields obtained by SCCO2 extraction at different pressures. Under the lower pressure of 10.5 MPa (Figure 3a), because it is closer to the critical pressure of CO2 (7.39 MPa), when the extraction temperature increases from 32 °C to 55 °C, the density of supercritical CO2 drops substantially, so the SCCO2 extraction capacity is reduced. However, under the four higher pressures (18.5, 23.5, 28.5, and 33.0 MPa) (Figure 3b–e), the extraction temperature obviously increases (32 °C to 55 °C), which is conducive to an increase in the extraction yield.
Although the increase in temperature causes the density of supercritical CO2 to decrease, the vapor pressure, diffusion coefficient, and mass transfer coefficient of the solute and solvent all increase with the temperature. The driving forces of these two different trends compete with each other; that is, the rise in the latter driving force offsets the fall in the former driving force, which benefits the improvement in the extraction capacity of SCCO2. The competing effects of the two apparently contradictory driving forces caused by the extraction temperature result in a crossover trend when SCCO2 dynamically extracts the perilla oil [31]. Similar to this phenomenon, it has also been observed that SCCO2 dynamically extracts different analytes, such as rice bran oil [31], crude oil [32] and coenzyme Q10 [33].
3.4. Characterization of Oils Obtained by SCCO2 Extraction and HD
As indicated in Table 1, the yields of oil and the main volatile components obtained by SCCO2 extraction for 200 min at 33.0 MPa and 55 °C were obviously higher than those of the HD procedure. The yield of volatile oils was 23.9% higher when acquired by SCCO2 extraction instead of HD. In addition, the contents of perillaldehyde, limonene, caryophyllene and (Z,E)-α-farnesene were approximately 1.02-, 1.12-, 1.05-, and 1.07-fold higher, respectively, when acquired by the SCCO2 procedure instead of HD extraction. In addition, the extracts obtained by SCCO2 extraction are stronger and deeper than those obtained by HD in terms of aroma and color. Therefore, it is inferred that SCCO2 extraction can obtain higher quality perilla oil than the HD procedure. Furthermore, the SCCO2 procedure reduced the time required (1.2-fold) and the operating temperature (45 °C). Therefore, these advantages of the SCCO2 procedure enable it to meet the green requirements.
3.5. Theoretical Solubility of Volatile Oils and Models
The OECs in Figure 3 obtained by SCCO2 dynamic extraction at different pressures and temperatures were further fitted to a spline using two straight lines. The slope of the first straight line (CER period) was used to calculate the theoretical solubility of volatile oil in supercritical CO2, and the results are shown in Table 2. At all five extraction temperatures, the theoretical solubility increased substantially with pressure because of the increase in the supercritical CO2 density. This result is similar to those of most studies using SCCO2 dynamic extraction [33,34].

Table 2.
Solubility data for the P. frutescens oils obtained by dynamic SCCO2 extraction.
However, when examining the influence of temperature on the theoretical solubility of oils in supercritical CO2, the phenomenon of retrograde solubility in the supercritical solvent was inspected at 10.5 MPa (Table 2), where an increase in temperature leads to a decrease in the theoretical solubility under a pressure below that of the crossover zone, and at a pressure higher than that of this zone, the theoretical solubility of oils increases with temperature (32 °C to 55 °C). When SCCO2 is used to extract different analytes dynamically, retrograde solubility behavior is often observed [33,35].
The Chrastil [36] model is the most commonly used model for correlating solubility data and describes the relationship between the solubility of the volatile oil (Y*oil) and the density of supercritical CO2 (ρ) as follows:
where T is the extraction temperature (K); a0, a1 and a2 are the model parameters; and a2 is further denoted as
where ∆HT is the total heat of the reaction or solution, and R is the universal gas constant (8.314 J/mol K).
Additionally, two models frequently utilized to correlate experimental data were proposed by Bartle [37] and Kumar and Johnston (K–J) [38]. The common features of these two models are simplicity and high accuracy. In the Bartle model, the correlation between the experimental data and the density of supercritical CO2 is demonstrated in a semi-logarithmic relationship.
where b0, b1 and b2 are the model parameters; and b2 is further denoted as
Furthermore, in the Bartle model, in addition to the solubility data, density and temperature, the pressure parameter is added and is expressed in the following formula.
where c0, c1 and c2 are the model parameters; Pref is 0.1 MPa; ρref is a reference density with a value of 700 g/L; and c2 is further expressed as
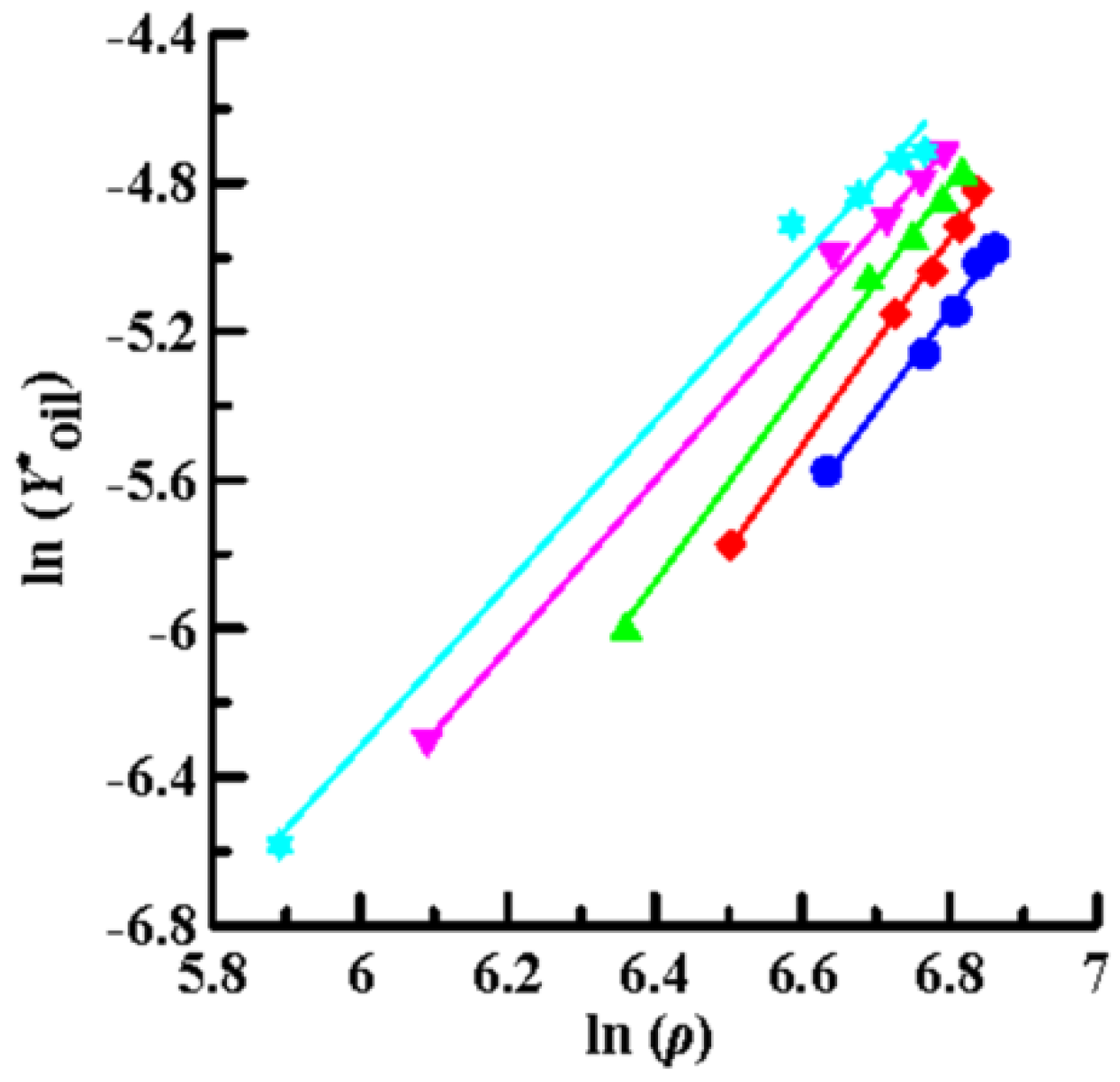
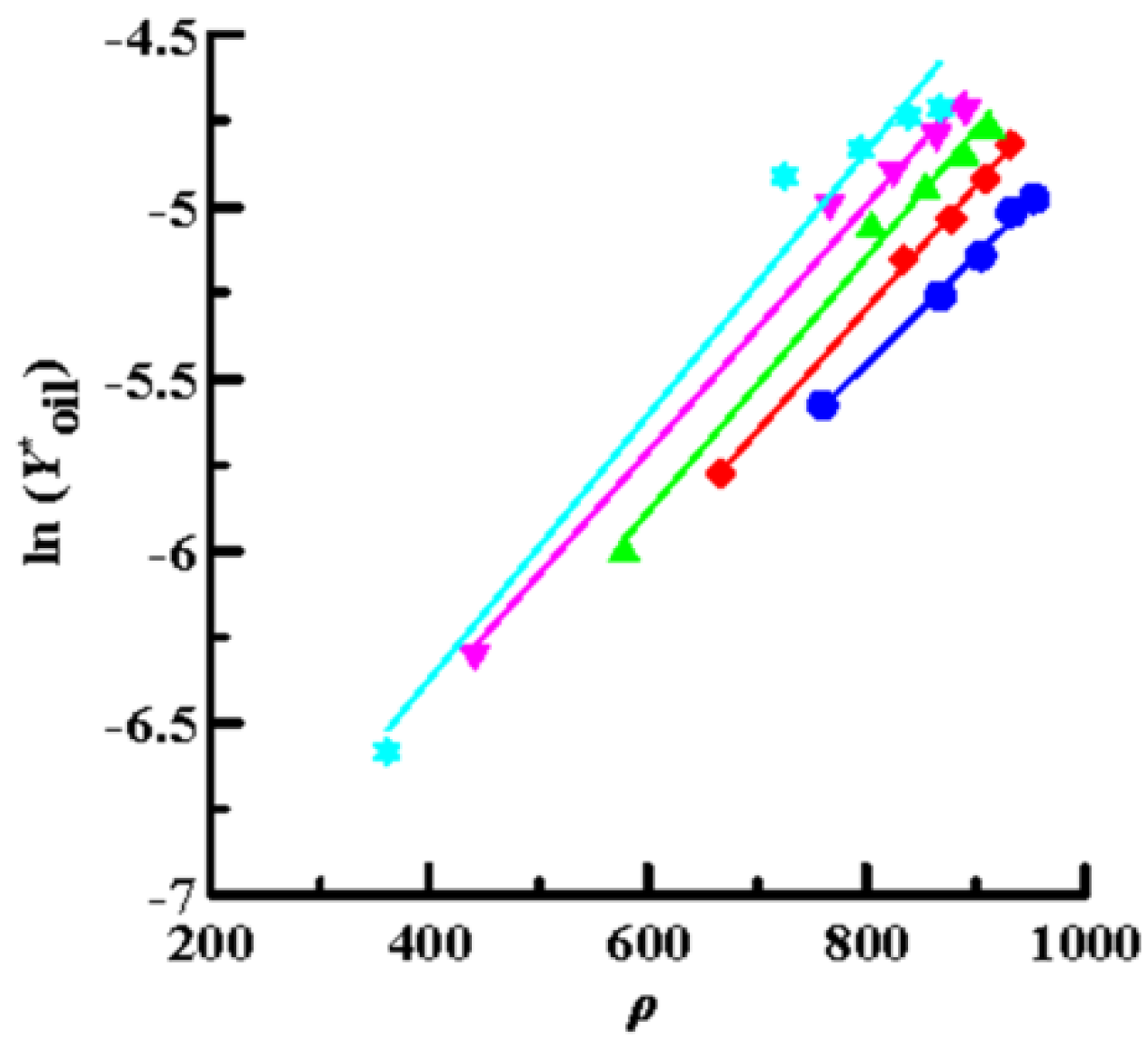
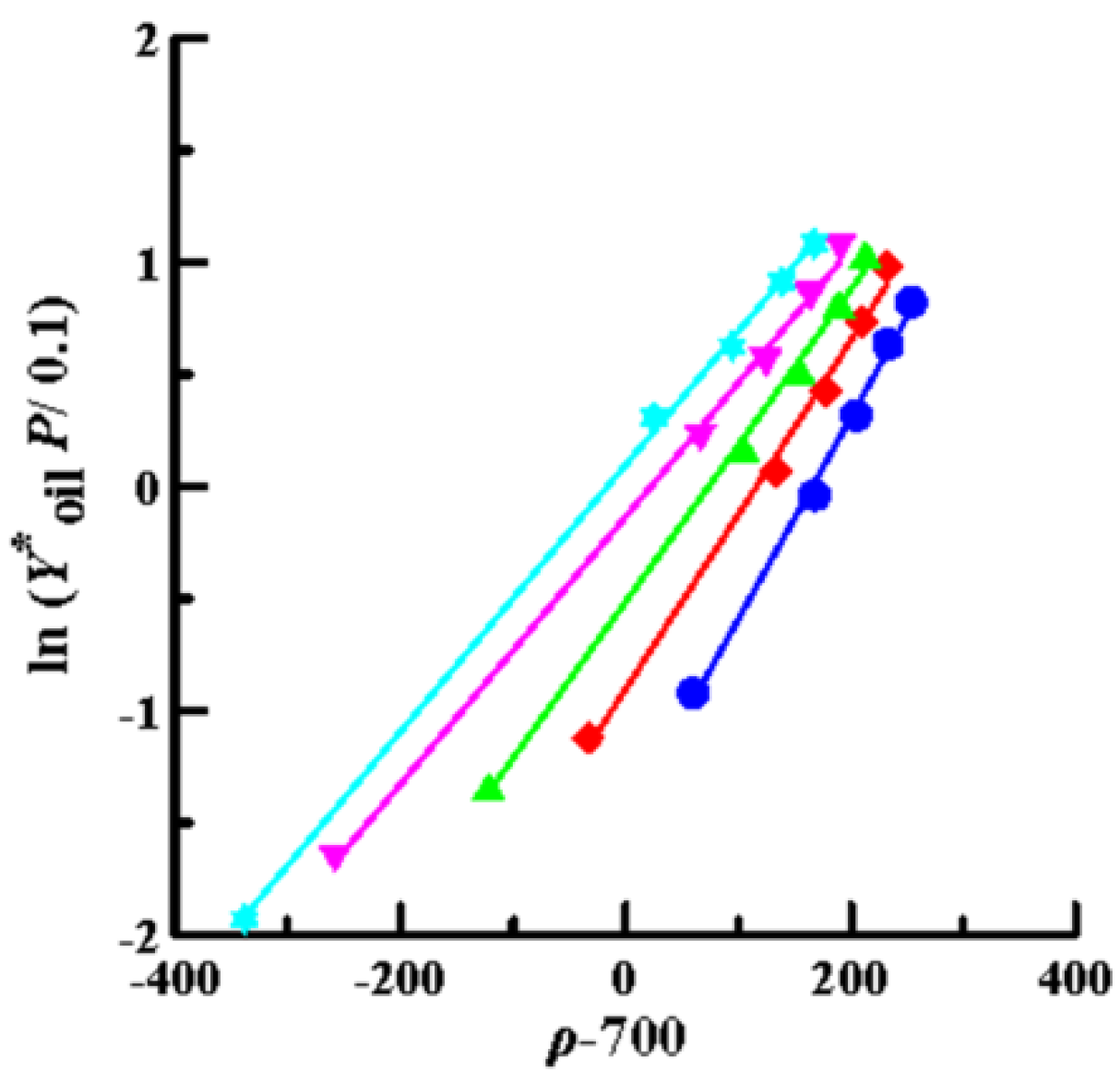
Then, the solubility data obtained by SCCO2 dynamic extraction at different temperatures and pressures (Table 2) was linearly regressed using Equations (4) (Chrastil model), (6) (K–J model) and (8) (Bartle model), as shown in Figure 4, Figure 5 and Figure 6, respectively. The coefficient of determination (R2) was high, in the range of 0.992–0.999, which means that the three models mentioned above were sufficiently accurate to illustrate the solubility of the oils in supercritical CO2. Furthermore, Equations (4), (6), and (8) were used to perform multiple linear regression on the solubility data of the volatile oil, according to which all parameters of the three models were obtained (Table 3). Table 3 shows that all AARD(%) were less than 3.3% (1.39–3.25%), which again means that these three models reliably and accurately describe the solubility of volatile oil in supercritical solvents. In addition, using the three parameter values of a2, b2, and c2, the heat of the solution (∆HT), vaporization (∆Hvap), and solvation (∆Hsolv) for the supercritical binary systems were further calculated using Equations (5), (7), and (9), respectively (Table 3). The ∆HT values obtained from the Chrastil (∆HT,C) and K–J (∆HT,K-J) models were 22.87 and 25.23 kJ/mol, respectively, and the value of ∆Hvap obtained using the Bartle model was 39.08 kJ/mol. It shows that vaporization is an endothermic process for binary systems. Therefore, the value of ∆Hsolv was further calculated using the following equation [39]:
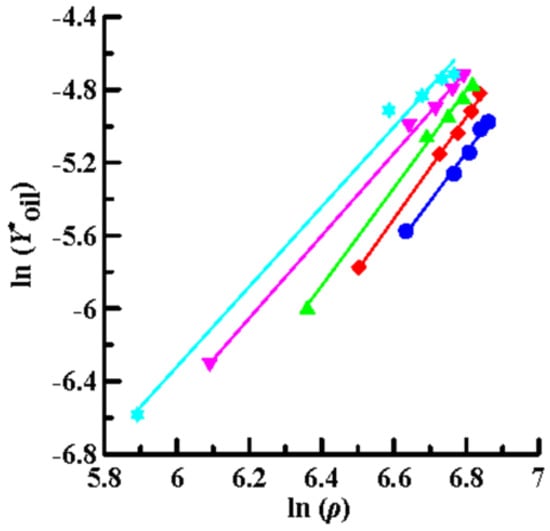
Figure 4.
Plots of ln (Y*oil) versus ln (ρ) using the Chrastil model for oil extraction from P. frutescens leaves at various temperatures and pressures of 10.5–33.0 MPa. (●:32 °C; ◆: 38 °C; ▲: 43 °C; ▼: 49 °C; ★: 55 °C).
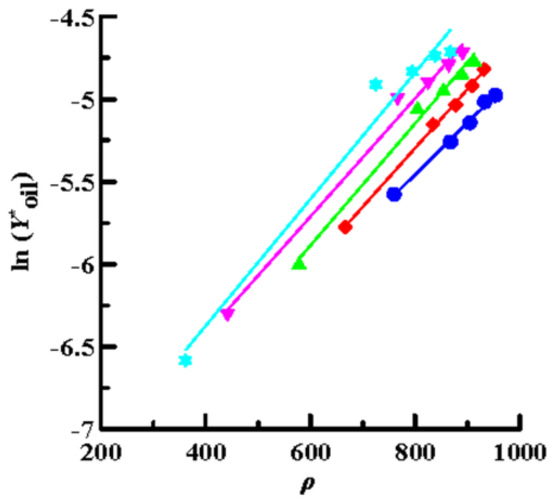
Figure 5.
Plots of ln (Y*oil) versus ρ using the K–J model for oil extraction from P. frutescens leaves at various temperatures and pressures of 10.5–33.0 MPa. (●:32 °C; ◆: 38 °C; ▲: 43 °C; ▼: 49 °C; ★: 55 °C).
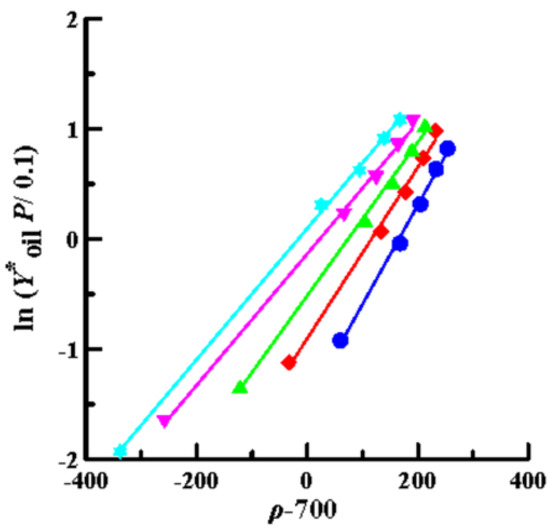
Figure 6.
Plots of ln (Y*oil P/0.1) versus (ρ–700) using the Bartle model for oil extraction from P. frutescens leaves at various temperatures and pressures of 10.5–33.0 MPa. (●:32 °C; ◆: 38 °C; ▲: 43 °C; ▼: 49 °C; ★: 55 °C).

Table 3.
The fitted solubility parameters of three density-based models and the approximate heat of vaporization, total heat of solution and heat of solvation for P. frutescens oils obtained by dynamic SCCO2 extraction.
The thermodynamic parameters in Table 3 indicate that the value of ∆Hsolv is −15.03 kJ/mol, which demonstrates that solvation is an exothermic process for the oil-supercritical CO2 binary systems. The value of ∆Hsolv is negative because ∆Hvap was higher than ∆HT in the oil-supercritical binary systems.
4. Conclusions
SCCO2 extraction and hydrodistillation (HD) extraction were used to separate the oils and the four main constituents in oils from Perilla frutescens leaves. SCCO2 extraction obtains higher yields of the oil and its four main components than the latter procedure, and uses milder operating conditions (duration time and temperature). Additionally, the theoretical solubilities of the oils obtained by the SCCO2 dynamic extraction at various temperatures and pressures were systematically evaluated, and retrograde solubility behavior was found in the oil-supercritical CO2 binary systems. At a minimum experimental pressure (10.5 MPa), the density of CO2 dominates the extraction capacity of SCCO2; therefore, this extraction procedure is suitable for lower temperatures. However, at higher pressures (18.5, 23.5, 28.5, and 33.0 MPa) the vapor pressure, mass transfer, and diffusion coefficients prevailed in the SCCO2 extraction; therefore, a higher temperature benefits the extraction process. Furthermore, the theoretical solubility was excellently correlated with three density-based models. Consequently, the heat of the solution, vaporization, and solvation for the oil-supercritical CO2 binary systems were perfectly estimated. Because the heat of the solution and vaporization were both positive values, the entire SCCO2 extraction was an endothermic process. However, because the heat of solvation was negative, this step is an exothermic process.
Author Contributions
Conceptualization, M.-C.W.; data curation, D.-H.W. and Y.-C.Y.; formal analysis, D.-H.W. and Y.-C.Y.; investigation, M.-C.W., D.-H.W., and Y.-C.Y.; methodology, D.-H.W. and Y.-C.Y.; software, M.-C.W. and C.-S.W.; supervision, M.-C.W. and C.-S.W.; validation, M.-C.W., D.-H.W., and Y.-C.Y.; visualization, C.-S.W.; writing—original draft, Y.-C.Y.; writing—review and editing, M.-C.W., C.-S.W., and Y.-C.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Science Council of Taiwan (105-2914-I-037-012-A1, 106-2914-I-041-001-A1, and 107-2914-I-041-002-A1) Kaohsiung Medical University (Kaohsiung, Taiwan) (KMU-M108028, KMU-M106034, KMU-M104020, S102035, S102036, ORD, CRRD, etc.), and Chia Nan University of Pharmacy and Science (Tainan, Taiwan) (151200-CN10527).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
The authors thank the National Science Council of Taiwan (105-2914-I-037-012-A1, 106-2914-I-041-001-A1, and 107-2914-I-041-002-A1), Kaohsiung Medical University (Kaohsiung, Taiwan) (KMU-M108028, KMU-M106034, KMU-M104020, S102035, S102036, ORD, CRRD, etc.), and Chia Nan University of Pharmacy and Science (Tainan, Taiwan) (151200-CN10527) for the financial support. We greatly thank KMU colleagues and students (Li-Mei An, Min-Yuan, Hung, Yen-Jung Lee, Shui-Chin Lu, Wen-Jeng Su, Hau-Lun Hung, Ting-Wei Chiu, Yi-Tsung Cheng, Liang-Yi Chou, Pin-Chieh Huang, etc.) for technical and editorial assistance. We greatly thank the technical and financial supports of Kaiser Pharmaceutical Limited Company (KPC, Tainan, Taiwan) and Chuang Song Zong Pharmaceutical Co., Ltd. (Kaohsiung, Taiwan) (S102035 and S102036). Finally, the authors gratefully acknowledge the editors and referees for their constructive comments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tabanca, N.; Demirci, B.; Ali, A.; Ali, Z.; Blythe, E.K.; Khan, I.A. Essential oils of green and red Perilla frutescens as potential sources of compounds for mosquito management. Ind. Crops Prod. 2015, 65, 36–44. [Google Scholar] [CrossRef]
- Torri, L.; Bondioli, P.; Folegatti, L.; Rovellini, P.; Piochi, M.; Morini, G. Development of perilla seed oil and extra virgin olive oil blends for nutritional, oxidative stability and consumer acceptance improvements. Food Chem. 2019, 286, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Zeng, X.B.; Zhang, S.; Wang, Y.Z.; Zhang, P.; Lu, A.J.; Peng, X. Regional variation in components and antioxidant and antifungal activities of Perilla frutescens essential oils in China. Ind. Crops Prod. 2014, 59, 69–79. [Google Scholar] [CrossRef]
- Park, S.H.; Paek, J.H.; Shin, D.; Lee, J.Y.; Lim, S.S.; Kang, Y.H. Purple perilla extracts with α-asarone enhance cholesterol efflux from oxidized LDL-exposed macrophages. Int. J. Mol. Med. 2015, 35, 957–965. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.C.; Wang, C.S.; Wei, M.C. Development and validation of an ultrasound-assisted supercritical carbondioxide procedure for the production of essential oils from Perilla frutescens. LWT Food Sci. Technol. 2020, 128, 109503. [Google Scholar] [CrossRef]
- Chang, X.L.; Zhao, Z.L.; Li, X.L.; Xu, H.; Sun, Y.; Wang, W.H. Extraction and advanced adsorbents for the separation of perillaldehyde from Perilla frutescens (L.) Britton var. crispa f. viridis leaves. Food Sci. Technol. Res. 2014, 20, 189–199. [Google Scholar] [CrossRef][Green Version]
- You, C.X.; Wang, Y.; Zhang, W.J.; Yang, K.; Wu, Y.; Geng, Z.F.; Chen, H.P.; Jiang, H.Y.; Du, S.S.; Deng, Z.W.; et al. Chemical constituents and biological activities of the purple Perilla essential oil against Lasioderma serricorne. Ind. Crops Prod. 2014, 61, 331–337. [Google Scholar] [CrossRef]
- Ahmed, H.M.; Tavaszi-Sarosi, S. Identification and quantification of essential oil content and composition, total polyphenols and antioxidant capacity of Perilla frutescens (L.) Britt. Food Chem. 2019, 275, 730–738. [Google Scholar] [CrossRef]
- Chen, F.; Liu, S.; Zhao, Z.; Gao, W.; Ma, Y.; Wang, X.; Yan, S.; Luo, D. Ultrasound pre-treatment combined with microwave-assisted hydrodistillation of essential oils from Perilla frutescens (L.) Britt. leaves and its chemical composition and biological activity. Ind. Crops Prod. 2020, 143, 111908. [Google Scholar] [CrossRef]
- Yang, Y.C.; Wang, C.S.; Wei, M.C. Insights into the major phenolic acids in Perilla frutescens obtained by a sustainable procedure. J. Ind. Eng. Chem. 2021. In Press. [Google Scholar] [CrossRef]
- Szydłowska-Czerniak, A.; Tułodziecka, A.; Karlovits, G.; Szłyk, E. Optimisation of ultrasound-assisted extraction of natural antioxidants from mustard seed cultivars. J. Sci. Food Agric. 2015, 95, 1445–1453. [Google Scholar] [CrossRef] [PubMed]
- El Marsni, Z.; Torres, A.; Varela, R.M.; Molinillo, J.M.; Casas, L.; Mantell, C.; Martinez de la Ossa, E.J.; Macias, F. Isolation of bioactive compounds from sunflower leaves (Helianthus annuus L.) extracted with supercritical carbon dioxide. J. Agric. Food Chem. 2015, 63, 6410–6421. [Google Scholar] [CrossRef] [PubMed]
- Sanjaya, R.E.; Tedjo, Y.Y.; Kurniawan, A.; Ju, Y.H.; Ayucitra, A.; Ismadj, S. Investigation on supercritical CO2 extraction of phenolic-phytochemicals from an epiphytic plant tuber (Myrmecodia pendans). J. CO2 Util. 2014, 6, 26–33. [Google Scholar] [CrossRef]
- Yousefi, M.; Rahimi-Nasrabadi, M.; Pourmortazavi, S.M.; Wysokowski, M.; Jesionowski, T.; Ehrlich, H.; Mirsadeghi, S. Supercritical fluid extraction of essential oils. Trends Anal. Chem. 2019, 118, 182–193. [Google Scholar] [CrossRef]
- Yang, Y.C.; Wei, M.C.; Lian, F.Y.; Huang, T.C. Simultaneous extraction and quantitation of oleanolic acid and ursolic acid from Scutellaria barbata D. Don by ultrasound-assisted extraction and high-performance liquid chromatography. Chem. Eng. Comm. 2014, 201, 482–500. [Google Scholar] [CrossRef]
- Yang, Y.C.; Wei, M.C.; Hong, S.J. Ultrasound-assisted extraction and quantitation of oils from Syzygium aromaticum flower bud (clove) with supercritical carbon dioxide. J. Chromatogr. A 2014, 1323, 18–27. [Google Scholar] [CrossRef]
- Yang, Y.C.; Wang, C.S.; Wei, M.C. Kinetics and mass transfer considerations for an ultrasound-assisted supercritical CO2 procedure to produce extracts enriched in flavonoids from Scutellaria barbata. J. CO2 Util. 2019, 32, 219–231. [Google Scholar] [CrossRef]
- Yang, Y.C.; Wei, M.C. A combined procedure of ultrasound-assisted and supercritical carbon dioxide for extraction and quantitation oleanolic and ursolic acids from Hedyotis corymbosa. Ind. Crops Prod. 2016, 79, 7–17. [Google Scholar] [CrossRef]
- Huang, B.K.; Lei, Y.L.; Tang, Y.H.; Zhang, J.C.; Qin, L.P.; Liu, J.A. Comparison of HS-SPME with hydrodistillation and SFE for the analysis of the volatile compounds of Zisu and Baisu, two varietal species of Perilla frutescens of Chinese origin. Food Chem. 2011, 125, 268–275. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, H.; Zhang, J. Comparison of MAHD with UAE and hydrodistillation for the analysis of volatile oil from four parts of Perilla frutescens cultivated in southern China. Anal. Lett. 2012, 45, 1894–1909. [Google Scholar] [CrossRef]
- Wei, M.C.; Xiao, J.; Yang, Y.C. Extraction of α-humulene-enriched oil from clove by ultrasound-assisted supercritical carbon dioxide extraction and studies of its fictitious solubility. Food Chem. 2016, 210, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.C.; Wang, C.S.; Wei, M.C. Separation and quantification of bioactive flavonoids from Scutellaria barbata using a green procedure. Food Bioprod. Process. 2019, 118, 77–90. [Google Scholar] [CrossRef]
- Wei, M.C.; Yang, Y.C. Kinetic studies for ultrasound-assisted supercritical carbon dioxide extraction of triterpenic acids from healthy tea ingredient Hedyotis diffusa and Hedyotis corymbosa. Sep. Purif. Technol. 2015, 142, 316–325. [Google Scholar] [CrossRef]
- Yang, Y.C.; Wang, C.S.; Wei, M.C. A green approach for the extraction and characterization of oridonin and ursolic and oleanolic acids from Rabdosia rubescens and its kinetics behavior. Food Chem. 2020, 319, 126582. [Google Scholar] [CrossRef]
- Ferreira, S.R.S.; Meireles, M.A.A. Modeling the supercritical fluid extraction of black pepper (Piper nigrum L.) essential oil. J. Food Eng. 2002, 54, 263–269. [Google Scholar] [CrossRef]
- Santana, Á.L.; Queirós, L.D.; Martínez, J.; Macedo, G.A. Pressurized liquid- and supercritical fluid extraction of crude and waste seeds of guarana (Paullinia cupana): Obtaining of bioactive compounds and mathematical modeling. Food Bioprod. Process. 2019, 117, 194–202. [Google Scholar] [CrossRef]
- Wei, M.C.; Hong, S.J.; Yang, Y.C. Isolation of triterpenic acid-rich extracts from Hedyotis corymbosa using ultrasound-assisted supercritical carbon dioxide extraction and determination of their fictitious solubilities. J. Ind. Eng. Chem. 2017, 48, 202–211. [Google Scholar] [CrossRef]
- Yang, Y.C.; Wei, M.C. Development and characterization of a green procedure for apigenin extraction from Scutellaria barbata D. Don. Food Chem. 2018, 252, 381–389. [Google Scholar] [CrossRef]
- Said, P.P.; Pradhan, R.C.; Rai, B.N. A green separation of Lagenaria siceraria seed oil. Ind. Crops Prod. 2014, 52, 796–800. [Google Scholar] [CrossRef]
- Wei, M.C.; Yang, Y.C.; Hong, S.J. Determination of oleanolic and ursolic acids in Hedyotis diffusa using hyphenated ultrasound-assisted supercritical carbon dioxide extraction and chromatography. Evid. Based Complement. Alternat. Med. 2015, 2015, 450547. [Google Scholar] [CrossRef]
- Tomita, K.; Machmudah, S.; Fukuzato, R.; Kanda, H.; Quitain, A.T.; Sasaki, M.; Goto, M. Extraction of rice bran oil by supercritical carbon dioxide and solubility consideration. Sep. Purif. Technol. 2014, 12, 5319–5325. [Google Scholar] [CrossRef]
- Rudyk, S.; Spirov, P.; Tyrovolas, A. Effect of temperature on crude oil extraction by SC-CO2 at 40–70 °C and 40–60 MPa. J. CO2 Util. 2018, 24, 471–478. [Google Scholar] [CrossRef]
- Couto, R.; Seifried, B.; Moquin, P.; Temellia, F. Coenzyme Q10 solubility in supercritical CO2 using a dynamic system. J. CO2 Util. 2018, 24, 315–320. [Google Scholar] [CrossRef]
- Yang, Y.C.; Wei, M.C. Ethanol solution-modified supercritical carbon dioxide extraction of triterpenic acids from Hedyotis corymbosa with ultrasound assistance and determination of their solubilities. Sep. Purif. Technol. 2015, 150, 204–214. [Google Scholar] [CrossRef]
- Wei, M.C.; Lin, P.H.; Hong, S.J.; Chen, J.M.; Yang, Y.C. Development of a green alternative procedure for simultaneous separation and quantification of clove oil and its major bioactive constituents. ACS Sustain. Chem. Eng. 2016, 4, 6491–6499. [Google Scholar] [CrossRef]
- Chrastil, J. Solubility of solids and liquids in supercritical gases. J. Phys. Chem. 1982, 86, 3016–3021. [Google Scholar] [CrossRef]
- Bartle, K.D.; Clifford, A.; Jafar, S.A.; Shilton, G.F. Solubilities of solids and liquids of low volatility in supercritical carbon dioxide. J. Phys. Chem. Ref. Data 1991, 20, 713–756. [Google Scholar] [CrossRef]
- Kumar, S.K.; Johnston, K.P. Modeling the solubility of solids in supercritical fluids with density as the independent variable. J. Supercrit. Fluids 1988, 1, 15–22. [Google Scholar] [CrossRef]
- Yang, Y.C.; Lin, P.H.; Wei, M.C. Production of oridonin-rich extracts from Rabdosia rubescens using hyphenated ultrasound-assisted supercritical carbon dioxide extraction. J. Sci. Food Agric. 2017, 97, 3323–3332. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).