Sequential Abatement of FeII and CrVI Water Pollution by Use of Walnut Shell-Based Adsorbents
Abstract
1. Introduction
2. Results and Discussion
2.1. Adsorbent Characterization
2.1.1. Fourier-Transform Infrared Spectroscopy (FTIR) Analysis
2.1.2. Scanning Electron Microscopy (SEM) Analysis
2.1.3. Energy Dispersive X-ray Spectroscopy Analysis (EDX) Analysis
2.2. AMD Treatability Experiments
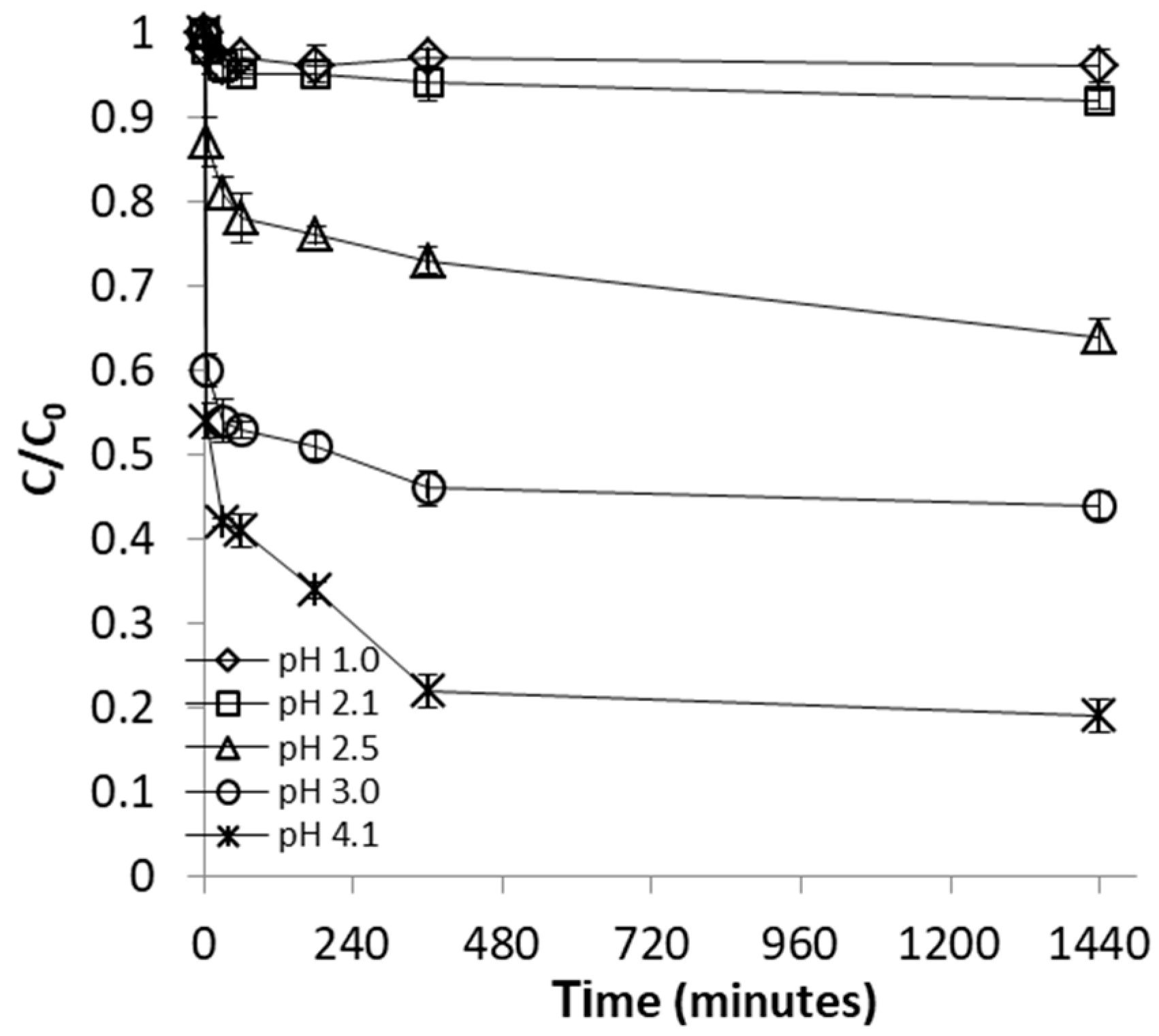
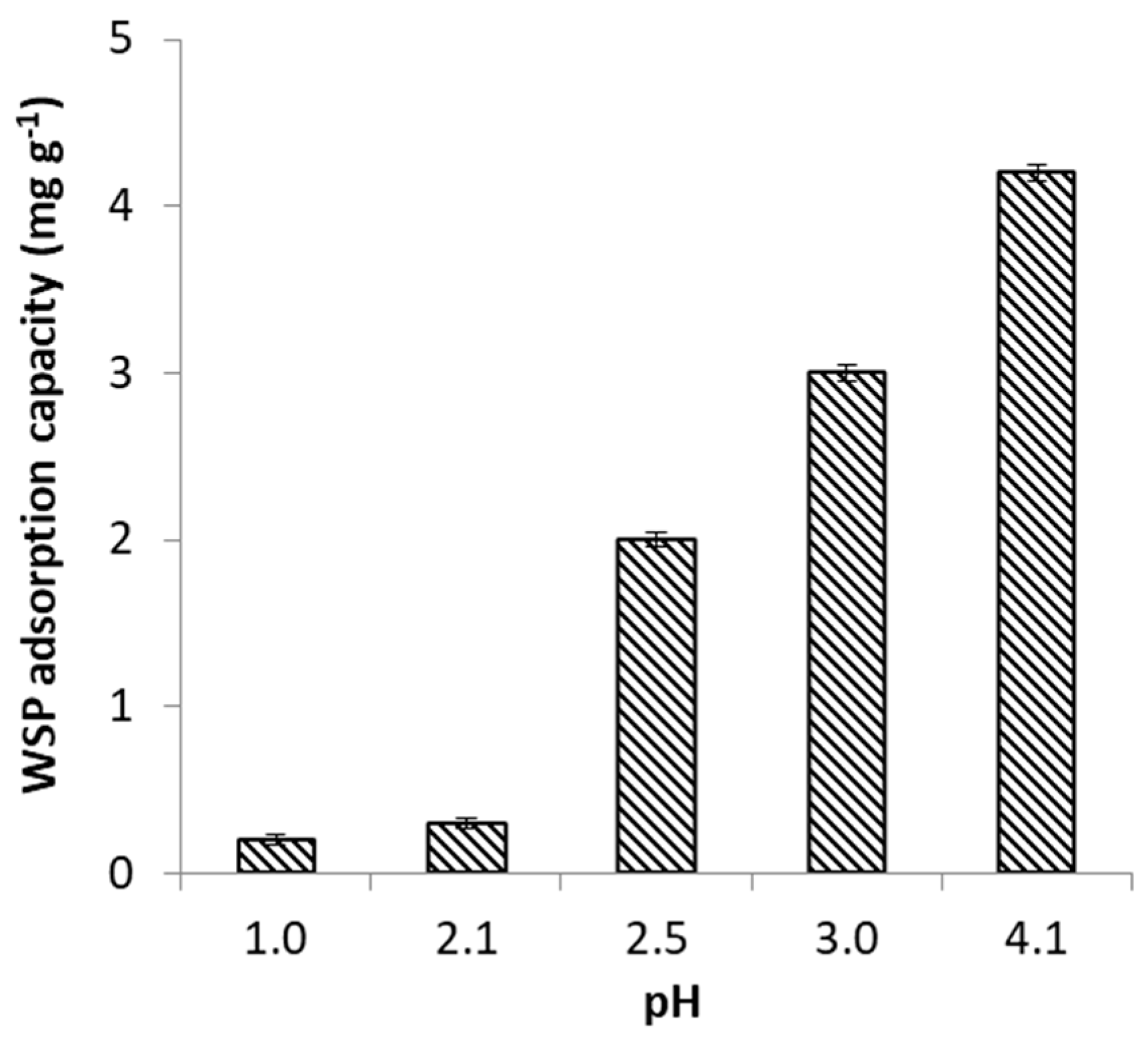
2.2.1. Effect of pH
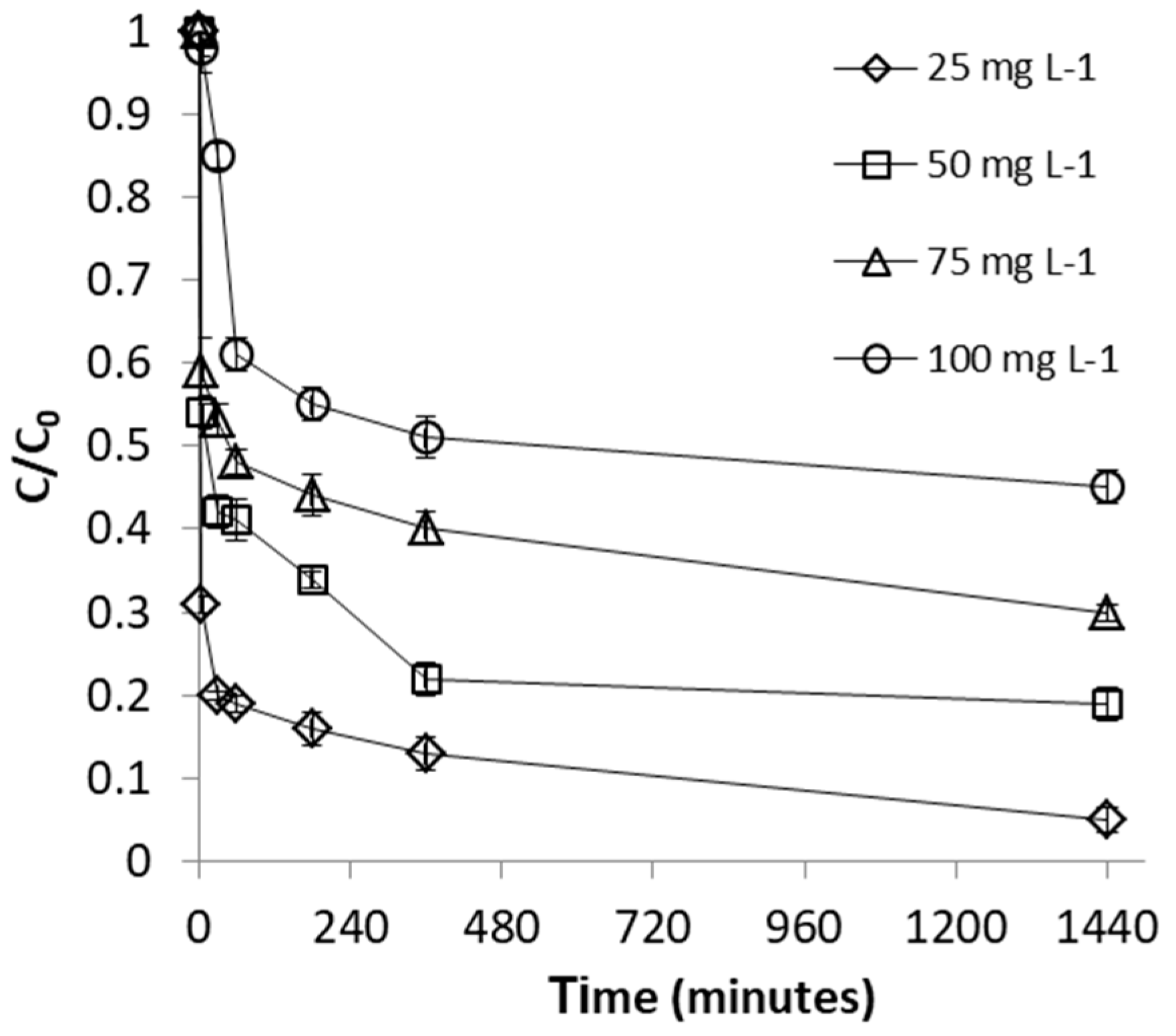
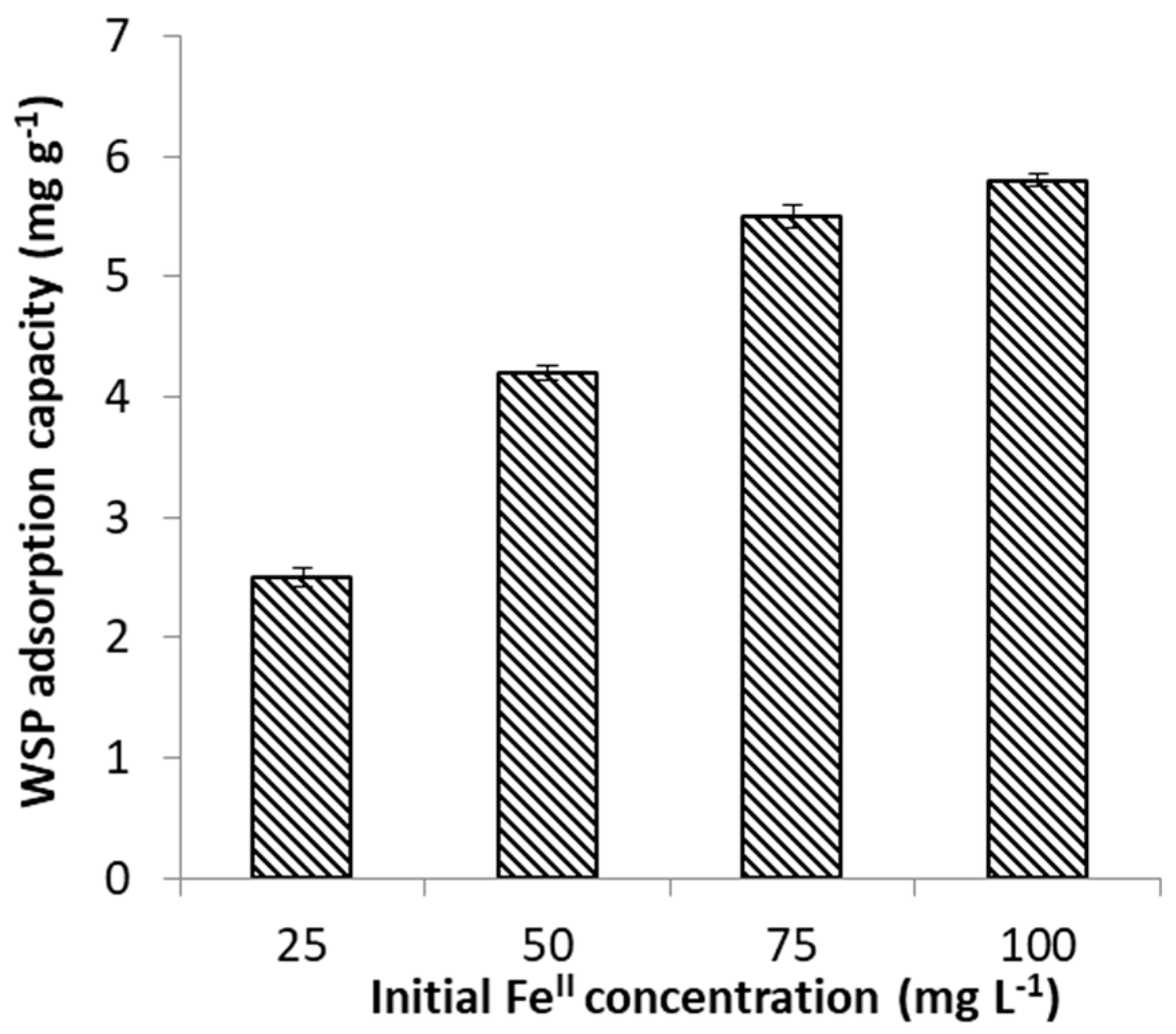
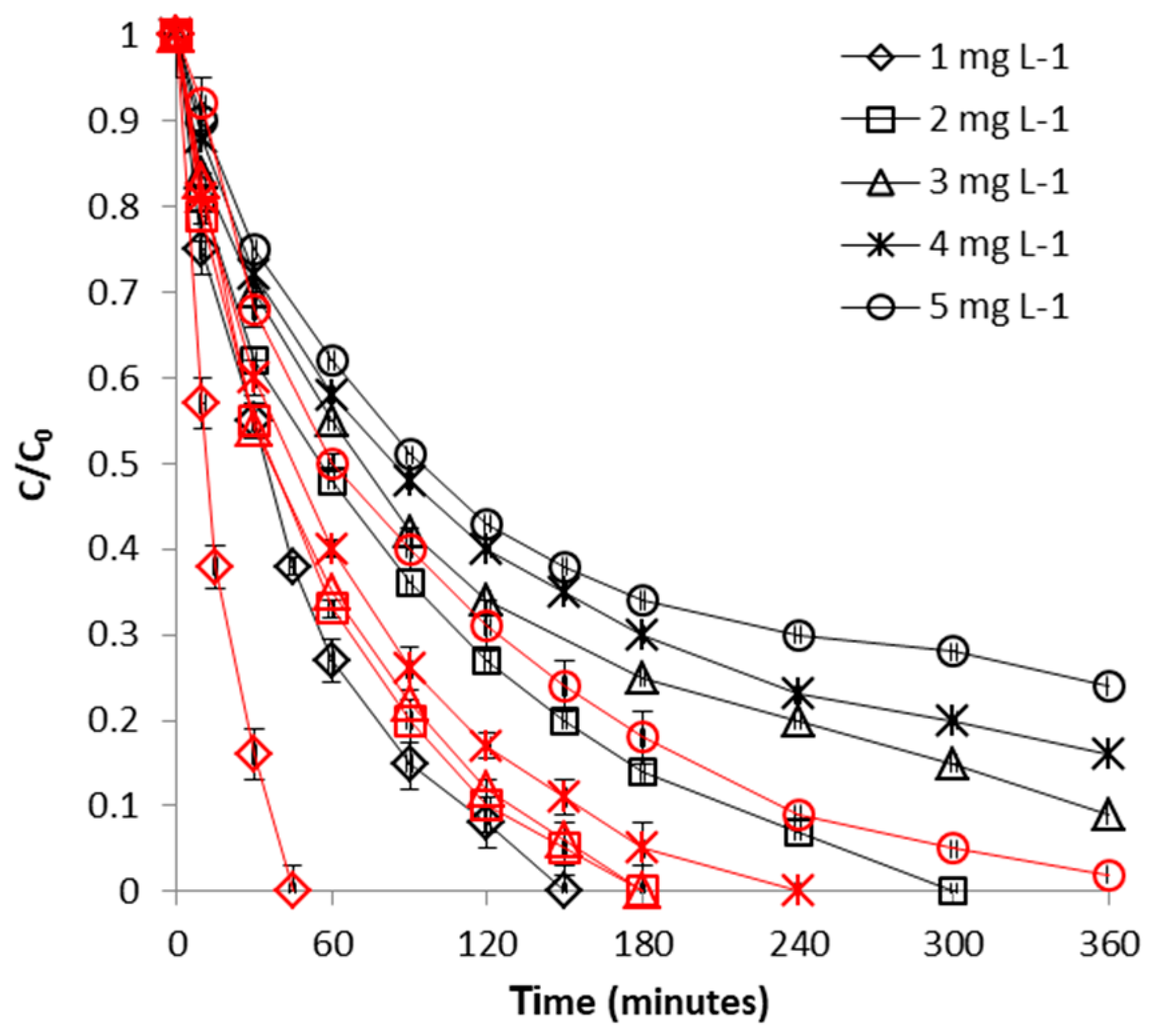
2.2.2. Effect of FeII Initial Concentration
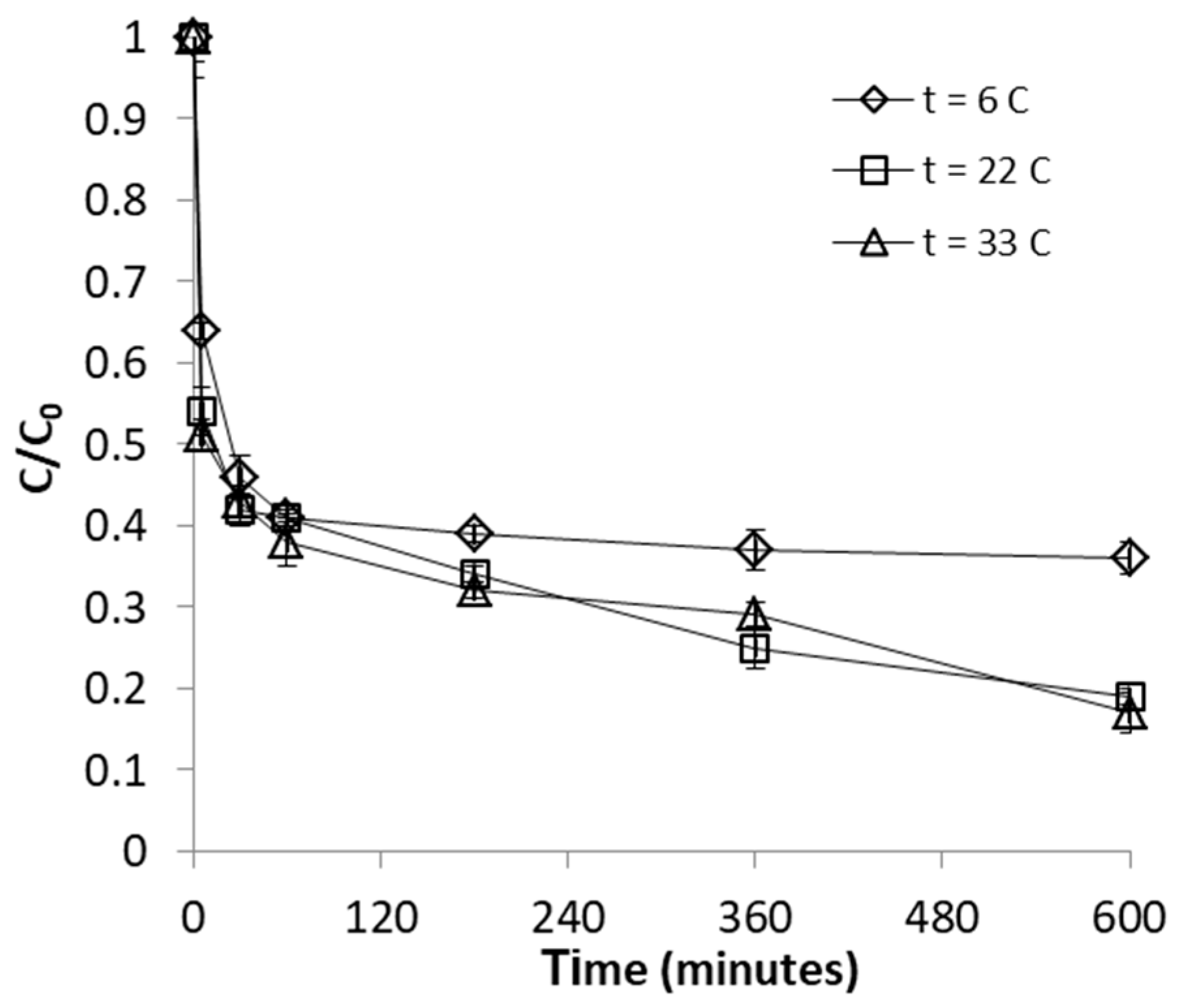
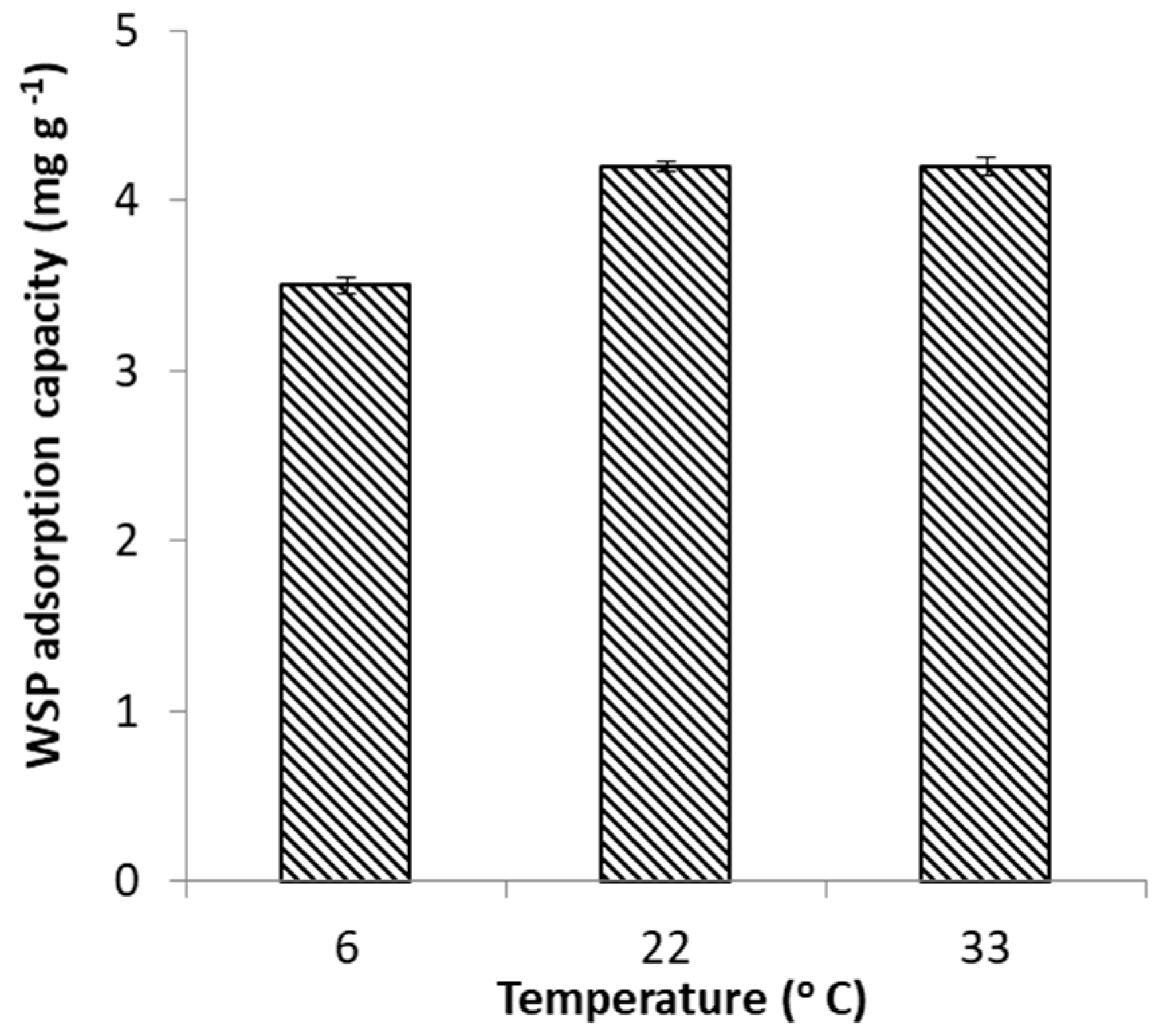
2.2.3. Effect of Temperature
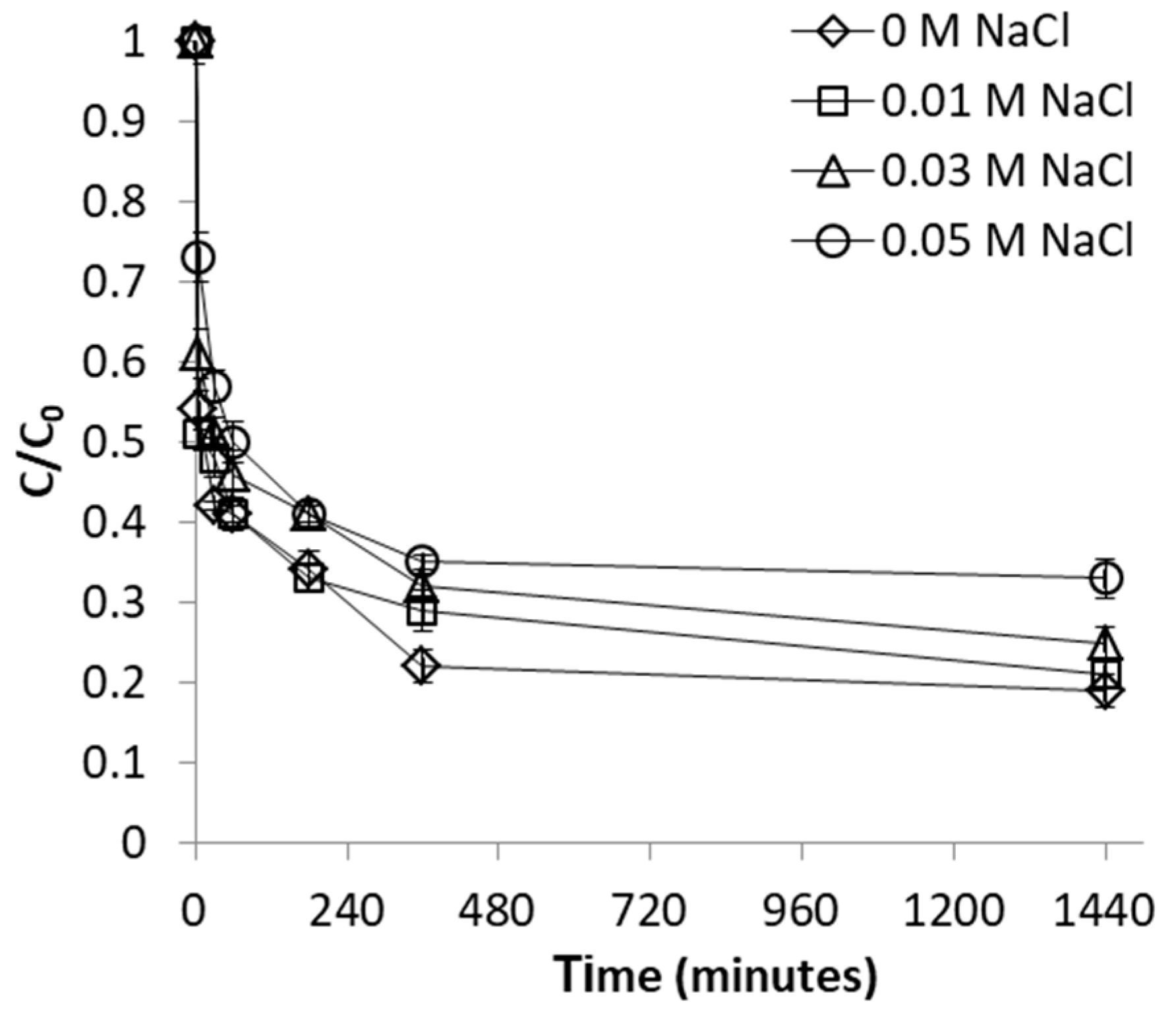
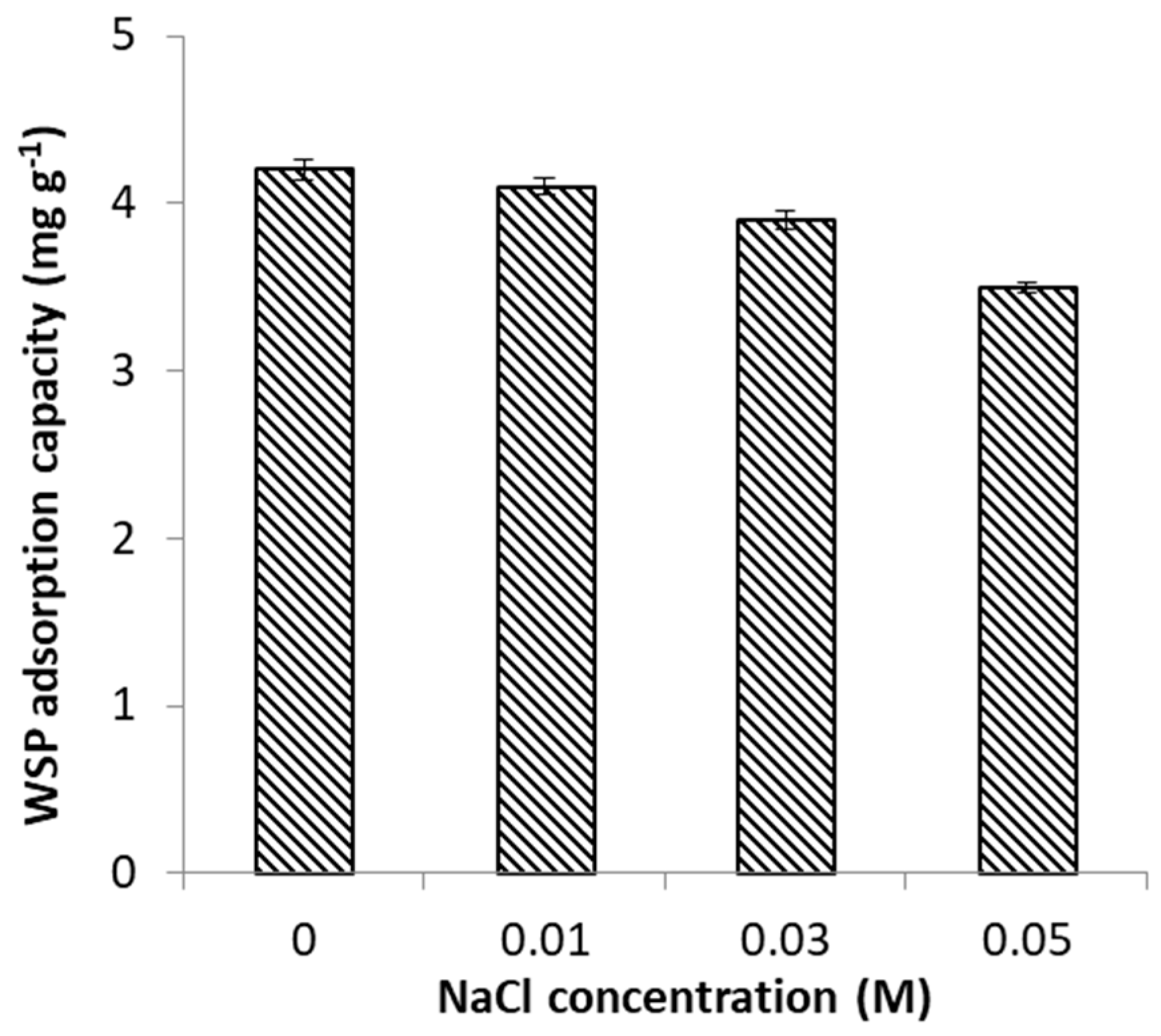
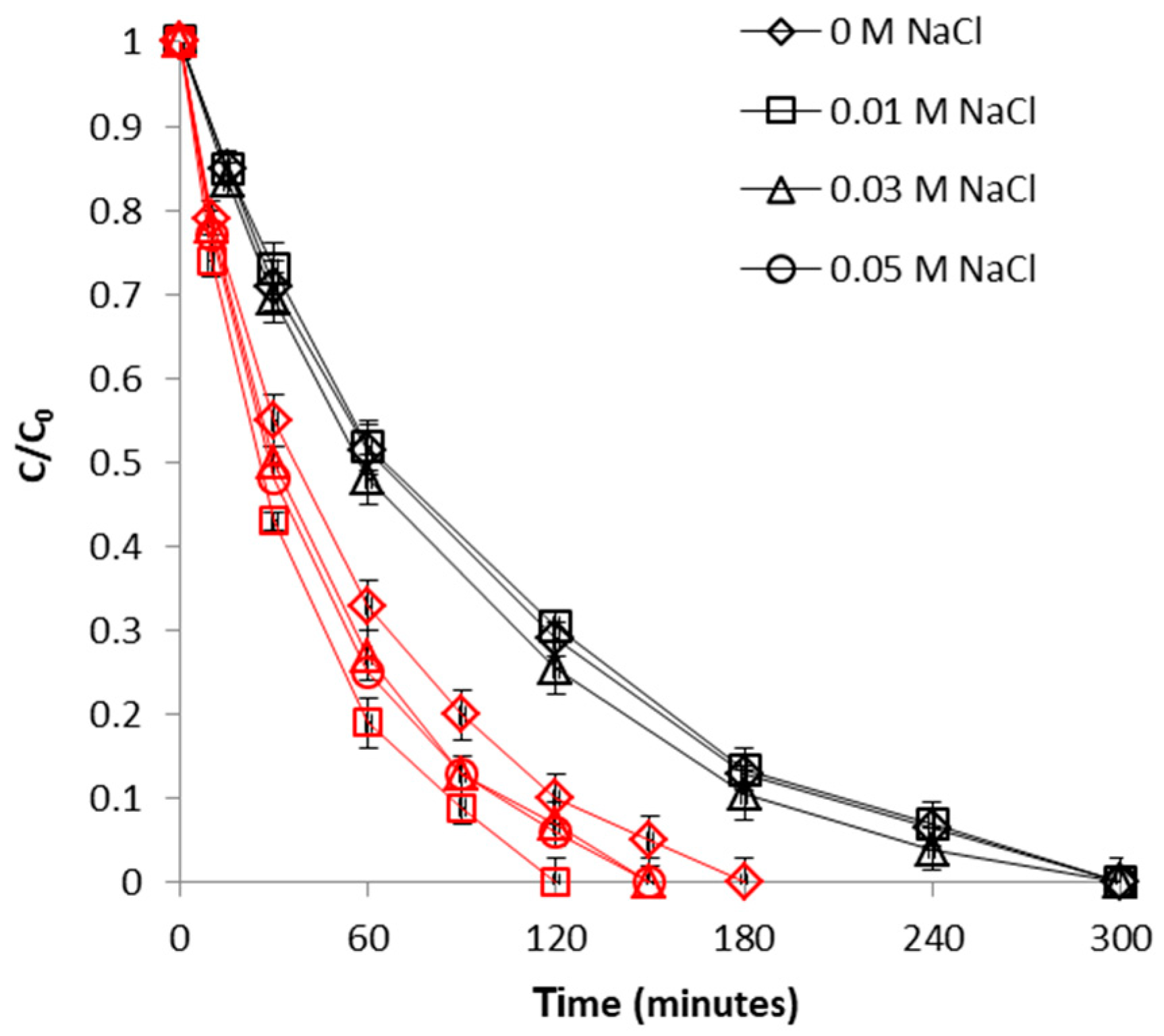
2.2.4. Effect of Ionic Strength
2.3. CrVI Treatability Experiments
2.3.1. Effect of pH
2.3.2. Effect of CrVI Initial Concentration
2.3.3. Effect of Temperature
2.3.4. Effect of Ionic Strength
2.4. Kinetic Modeling
2.4.1. Identification of the Kinetic Order
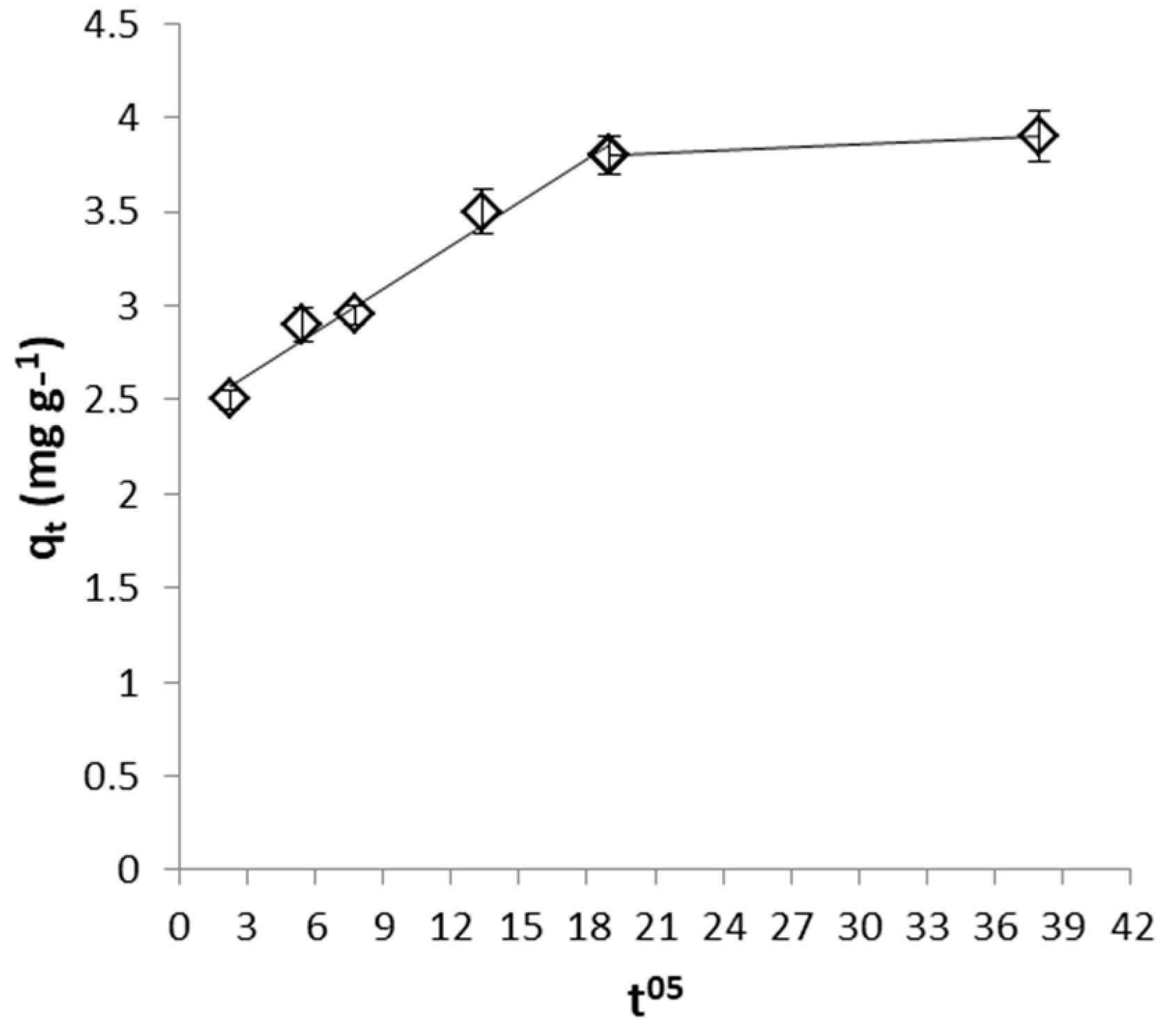
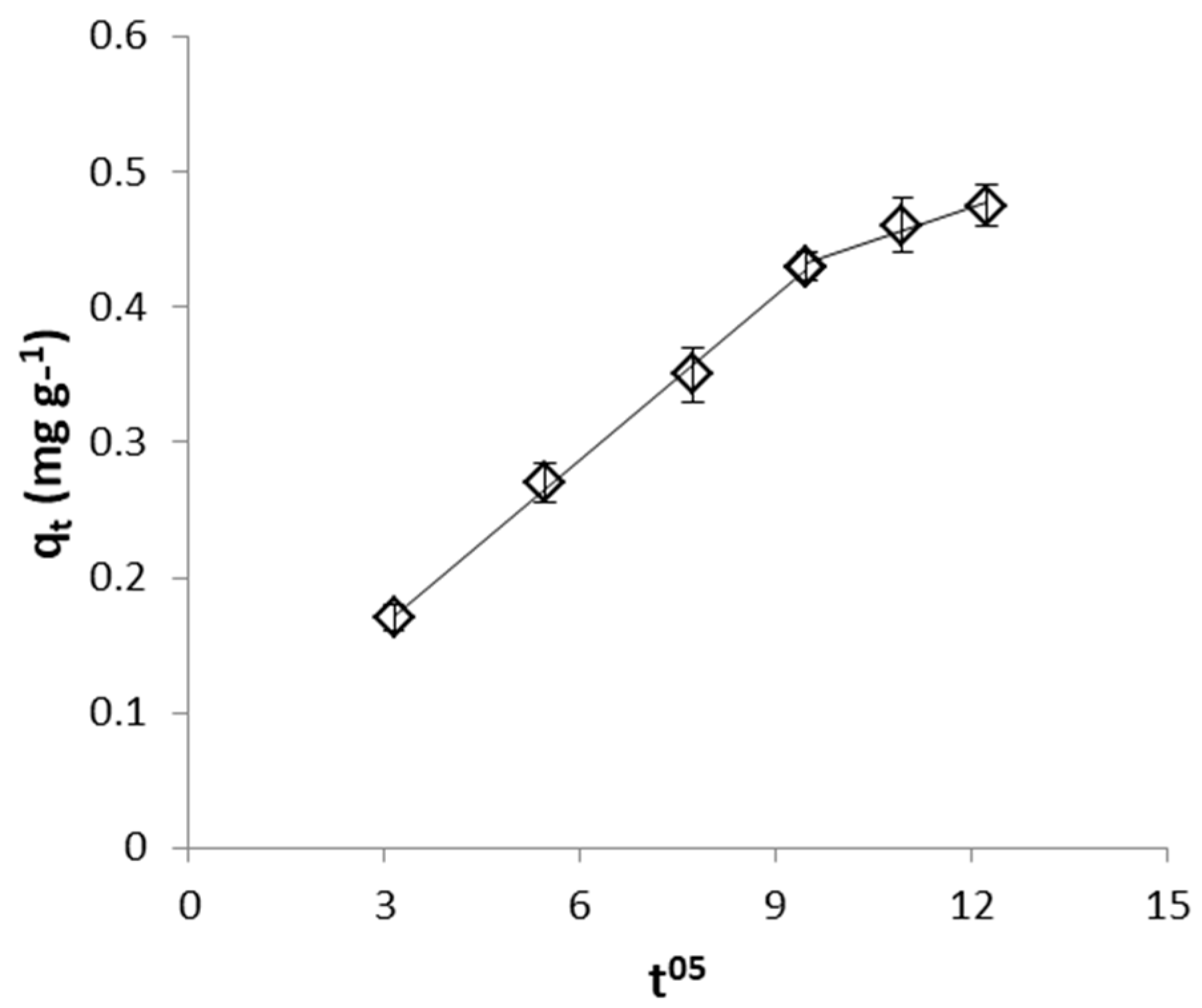
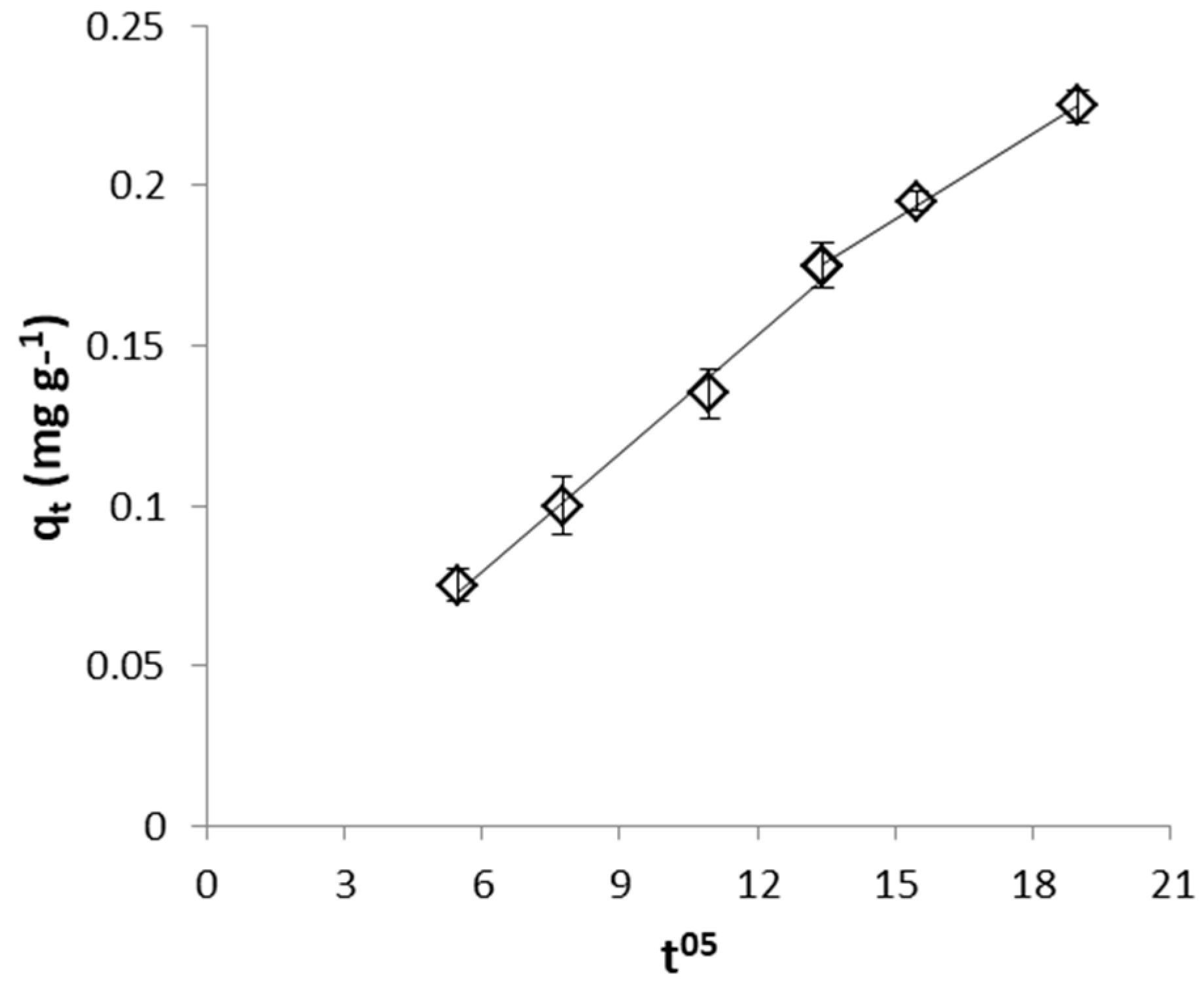
2.4.2. Identification of the Rate Limiting Step
2.5. Mechanism of Metal Removal
3. Materials and Methods
3.1. Preparation of the Adsorbent for AMD Treatability Experiments
3.2. Preparation of the Reactive Material for CrVI Treatability Experiments
3.3. AMD Treatability Experiments
3.4. CrVI Treatability Experiments
3.5. Analytical Procedure
3.6. Kinetic Modeling of Experimental Data
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vardhan, K.H.; Kumar, P.S.; Panda, R.C. A review on heavy metal pollution, toxicity and remedial measures: Current trends and future perspectives. J. Mol. Liq. 2019, 290, 111197. [Google Scholar] [CrossRef]
- Bozic, D.; Stankovic, V.; Gorgievski, M.; Bogdanovic, G.; Kovacevic, R. Adsorption of heavy metal ions by sawdust of deciduous trees. J. Hazard. Mater. 2009, 171, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Barakat, M.A. New trends in removing heavy metals from industrial wastewater. Arab. J. Chem. 2011, 4, 361–377. [Google Scholar] [CrossRef]
- Gautam, R.K.; Mudhoo, A.; Lofrano, G.; Chattopadhyaya, M.C. Biomass-derived biosorbents for metal ions sequestration: Adsorbent modification and activation methods and adsorbent regeneration. J. Environ. Chem. Eng. 2014, 2, 239–259. [Google Scholar] [CrossRef]
- Akhbarizadeh, R.; Shayestefar, M.R.; Darezereshki, E. Competitive removal of metals from wastewater by maghemite nanoparticles: A comparison between simulated wastewater and AMD. Mine Water Environ. 2014, 33, 89–96. [Google Scholar] [CrossRef]
- Gheju, M.; Pode, R.; Manea, F. Comparative heavy metal chemical extraction from anaerobically digested biosolids. Hydrometallurgy 2011, 108, 115–121. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Bolisetty, S.; Peydayesh, M.; Mezzenga, R. Sustainable technologies for water purification from heavy metals: Review and analysis. Chem. Soc. Rev. 2019, 48, 463–487. [Google Scholar] [CrossRef]
- Hashim, M.A.; Mukhopadhyay, S.; Sahu, J.N.; Sengupta, B. Remediation technologies for heavy metal contaminated groundwater. J. Environ. Manag. 2011, 92, 2355–2388. [Google Scholar] [CrossRef]
- Selvi, A.; Rajasekar, A.; Theerthagiri, J.; Ananthaselvam, A.; Sathishkumar, K.; Madhavan, J.; Rahman, P.K.S.M. Integrated remediation processes toward heavy metal removal/recovery from various environments–A review. Front. Environ. Sci. 2019, 7, 66. [Google Scholar] [CrossRef]
- Hu, M.; Zhang, S.; Pan, B.; Zhang, W.; Lv, L.; Zhang, Q. Heavy metal removal from water/wastewater by nanosized metal oxides: A review. J. Hazard. Mater. 2012, 211–212, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Kurniawan, T.A.; Chan, G.Y.S.; Lo, W.H.; Babel, S. Comparisons of low-cost adsorbents for treating wastewaters laden with heavy metals. Sci. Total Environ. 2006, 366, 409–426. [Google Scholar] [CrossRef] [PubMed]
- Heidarinejad, Z.; Dehghani, M.H.; Heidari, M.; Javedan, G.; Ali, I.; Sillanpää, M. Methods for preparation and activation of activated carbon: A review. Environ. Chem. Lett. 2019, 18, 393–415. [Google Scholar] [CrossRef]
- Dias, J.M.; Alvim-Ferraz, M.C.M.; Almeida, M.F.; Rivera-Utrilla, J.; Sanchez-Polo, M. Waste materials for activated carbon preparation and its use in aqueous-phase treatment: A review. J. Environ. Manag. 2007, 85, 833–846. [Google Scholar] [CrossRef]
- Sirry, S.M.; Aldakhil, F.; Alharbi, O.M.L.; Ali, I. Chemically treated date stones for uranium (VI) uptake and extraction in aqueous solutions. J. Mol. Liq. 2019, 273, 192–202. [Google Scholar] [CrossRef]
- Mosoarca, G.; Vancea, C.; Popa, S.; Gheju, M.; Boran, S. Syringa vulgaris leaves powder a novel low-cost adsorbent for methylene blue removal: Isotherms, kinetics, thermodynamic and optimization by Taguchi method. Sci. Rep. 2020, 10, 17676. [Google Scholar] [CrossRef]
- Abdel-Ghani, N.T.; El-Chaghaby, G.A. Biosorption for metal ions removal from aqueous solutions: A review of recent studies. Int. J. Lat. Res. Sci. Technol. 2014, 3, 24–42. [Google Scholar]
- Mohan, D.; Pittman, C.U., Jr. Activated carbons and low cost adsorbents for remediation of tri- and hexavalent chromium from water. J. Hazard. Mater. 2006, B137, 762–811. [Google Scholar] [CrossRef]
- Joseph, L.; Jun, B.M.; Flora, J.R.V.; Park, C.M.; Yoon, Y. Removal of heavy metals from water sources in the developing world using low-cost materials: A review. Chemosphere 2019, 229, 142–159. [Google Scholar] [CrossRef]
- Ahmad, T.; Ahmad, K.; Alam, M. Sustainable management of water treatment sludge through 3‘R’ concept. J. Clean. Prod. 2016, 124, 1–13. [Google Scholar] [CrossRef]
- Ippolito, J.A.; Barbarick, K.A.; Elliott, H.A. Drinking water treatment residuals: A review of recent uses. J. Environ. Qual. 2011, 40, 1–12. [Google Scholar] [CrossRef]
- Turner, T.; Wheeler, R.; Stone, A.; Oliver, I. Potential alternative reuse pathways forwater treatment residuals: Remaining barriers and questions–A Review. Water Air Soil Pollut. 2019, 230, 227. [Google Scholar] [CrossRef]
- Babatunde, A.; Zhao, Y. Constructive approaches toward water treatment works sludge management: An international review of beneficial reuses. Crit. Rev. Environ. Sci. Technol. 2007, 37, 129–164. [Google Scholar] [CrossRef]
- Wolowiec, M.; Komorowska-Kaufman, M.; Pruss, A.; Rzepam, G.; Bajda, T. Removal of heavy metals and metalloids from water using drinking water treatment residuals as adsorbents: A review. Minerals 2019, 9, 487. [Google Scholar] [CrossRef]
- Gheju, M.; Balcu, I. Mitigation of Cr(VI) aqueous pollution by reuse of iron-contaminated water treatment residues. ChemEngineering 2017, 1, 9. [Google Scholar] [CrossRef]
- Sagar, N.A.; Pareek, S.; Sharma, S.; Yahia, E.M.; Lobo, M.G. Fruit and vegetable waste: Bioactive compounds, their extraction, and possible utilization. Compr. Rev. Food Sci. Food Saf. 2018, 17, 512–531. [Google Scholar] [CrossRef]
- Gowe, C. Review on potential use of fruit and vegetables by-products as a valuable source of natural food additives. Food Sci. Qual. Manag. 2015, 45, 47–61. [Google Scholar]
- USDA. Tree nuts: World Market and Trade; United States Department of Agriculture Foreign Agricultural Service: Washington, DC, USA, 2017.
- Ghasemi, M.; Ghoreyshi, A.A.; Younesi, H.; Khoshhal, S. Synthesis of a high characteristics activated carbon from walnut shell for the removal of Cr(VI) and Fe(II) from aqueous solution: Single and binary solutes adsorption. Iran. J. Chem. Eng. 2015, 12, 28–51. [Google Scholar]
- Adebayo, G.B.; Mohammed, A.A.; Sokoya, S.O. Biosorption of Fe(II) and Cd(II) ions from aqueous solution using a low cost adsorbent from orange peels. J. Appl. Sci. Environ. Manag. 2016, 20, 702–714. [Google Scholar] [CrossRef][Green Version]
- Noubactep, C. Metallic iron for environmental remediation: A review of reviews. Water Res. 2015, 85, 114–123. [Google Scholar] [CrossRef]
- Bansal, M.; Singh, D.; Garg, V.K. A comparative study for the removal of hexavalent chromium from aqueous solution by agriculture wastes’ carbons. J. Hazard. Mater. 2009, 171, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Savy, D.; Piccolo, A. Physical-chemical characteristics of lignins separated from biomasses for second-generation ethanol. Biomass Bioenergy 2014, 62, 58–67. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of infrared spectra, a practical approach. In Encyclopedia of Analytical Chemistry; Meyers, R.A., Ed.; John Wiley & Sons: Chichester, UK, 2000; pp. 10815–10837. [Google Scholar]
- Domínguez-Robles, J.; Sánchez, R.; Espinosa, E.; Savy, D.; Mazzei, P.; Piccolo, A.; Rodríguez, A. Isolation and characterization of Gramineae and Fabaceae soda lignins. Int. J. Mol. Sci. 2017, 18, 327. [Google Scholar] [CrossRef] [PubMed]
- Aravindhan, R.; Madhan, B.; Rao, J.R.; Nair, B.U.; Ramasam, T. Bioaccumulation of chromium from tannery wastewater: An approach for chrome recovery and reuse. Environ. Sci. Technol. 2004, 38, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Bykov, I. Characterization of Natural and Technical Lignins Using FTIR Spectroscopy. Master’s Thesis, Lulea University of Technology, Lulea, Sweden, 2008. [Google Scholar]
- Bhattacharyya, K.G.; Sen Gupta, S. Adsorption of chromium(VI) from water by clays. Ind. Eng. Chem. Res. 2006, 45, 7232–7240. [Google Scholar] [CrossRef]
- Espana, J.S.; Pamo, E.L.; Pastor, E.S. The oxidation of ferrous iron in acidic mine effluents from the Iberian Pyrite Belt (Odiel Basin, Huelva, Spain): Field and laboratory rates. J. Geochem. Explor. 2007, 92, 120–132. [Google Scholar] [CrossRef]
- Espana, J.S. Acid mine drainage in the Iberian pyrite belt: An overview with special emphasis on generation mechanisms, aqueous composition and associated mineral phases. Macla 2008, 10, 34–43. [Google Scholar]
- Yu, L.J.; Shukla, S.S.; Doris, K.L.; Shukla, A.; Margrave, J.L. Adsorption of chromium from aqueous solutions by maple sawdust. J. Hazard. Mater 2003, B100, 53–63. [Google Scholar] [CrossRef]
- Baral, S.S.; Das, S.N.; Rath, P. Hexavalent chromium removal from aqueous solution by adsorption on treated sawdust. Biochem. Eng. J. 2006, 31, 216–222. [Google Scholar] [CrossRef]
- Das, D.D.; Mahapatra, R.; Pradhan, J.; Das, S.N.; Thakur, R.S. Removal of Cr(VI) from aqueous solution using activated cow dung carbon. J. Coll. Interf. Sci. 2000, 232, 235–240. [Google Scholar] [CrossRef]
- McBride, M.B. A critique of diffuse double layer models applied to colloid and surface chemistry. Clays Clay Miner. 1997, 45, 598–608. [Google Scholar] [CrossRef]
- Gheju, M. Decontamination of hexavalent chromium-polluted waters: Significance of metallic iron technology. In Enhancing Cleanup of Environmental Pollutants. Volume 2. Non biological Approaches; Anjum, N., Gill, S., Tuteja, N., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 209–254. [Google Scholar]
- Albadarin, A.B.; Mangwandi, C.; Walker, G.M.; Allen, S.J.; Ahmad, M.N.M.; Khraisheh, M. Influence of solution chemistry on Cr(VI) reduction and complexation onto date-pits/tea-waste biomaterials. J. Environ. Manag. 2013, 114, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Acharya, J.; Sahu, J.N.; Sahoo, B.K.; Mohanty, C.R.; Meikap, B.C. Removal of chromium(VI) from wastewater by activated carbon developed from Tamarind wood activated with zinc chloride. Chem. Eng.J. 2009, 150, 25–39. [Google Scholar] [CrossRef]
- Chen, C.; Zhao, P.; Li, Z.; Tong, Z. Adsorption behavior of chromium(VI) on activated carbon from eucalyptus sawdust prepared by microwave-assisted activation with ZnCl2. Desal. Water Treat. 2016, 57, 12572–12584. [Google Scholar] [CrossRef]
- Amuda, O.S.; Adelowo, F.E.; Ologunde, M.O. Kinetics and equilibrium studies of adsorption of chromium(VI) ion from industrial wastewater using Chrysophyllum albidum (Sapotaceae) seed shells. Colloids Surf. B Biointerfaces 2009, 68, 184–192. [Google Scholar] [CrossRef]
- Flury, B.; Eggenberger, U.; Mader, U. First results of operating and monitoring an innovative design of a permeable reactive barrier for the remediaton of chromate contaminated groundwater. J. Appl. Geochem. 2009, 24, 687–697. [Google Scholar] [CrossRef]
- Gao, R.M. Simultaneous determination of hexavalent and total chromium in water and plating baths by spectrophotometry. Talanta 1993, 40, 637–640. [Google Scholar] [CrossRef]
- Ye, J.; Yin, H.; Mai, B.; Peng, H.; Qin, H.; He, B.; Zhang, N. Biosorption of chromium from aqueous solution and electroplating wastewater using mixture of Candida lipolytica and dewatered sewage sludge. Biores. Technol. 2010, 101, 3893–3902. [Google Scholar] [CrossRef]
- Altun, T.; Pehlivan, E. Removal of Cr(VI) from aqueous solutions by modified walnut shells. Food Chem. 2012, 132, 693–700. [Google Scholar] [CrossRef]
- Fiol, N.; Villaescusa, I.; Martinez, M.; Miralles, N.; Poch, J.; Serarols, J. Biosorption of Cr(VI) using low cost sorbents. Environ. Chem. Lett. 2003, 1, 135–139. [Google Scholar] [CrossRef]
- Jing, X.; Cao, Y.; Zhang, X.; Wang, D.; Wu, X.; Xu, H. Biosorption of Cr(VI) from simulated wastewater using a cationic surfactant modified spent mushroom. Desalination 2011, 269, 120–127. [Google Scholar] [CrossRef]
- Tepong-Tsindé, R.; Phukan, M.; Nassi, A.; Noubactep, C.; Ruppert, H. Validating the Efficiency of the MB Discoloration Method for the Characterization of Fe0/H2O systems using accelerated corrosion by chloride ions. Chem. Eng. J. 2015, 279, 353–362. [Google Scholar] [CrossRef]
- Gheju, M.; Balcu, I.; Enache, A.; Flueras, A. A kinetic approach on hexavalent chromium removal with metallic iron. J. Environ. Manag. 2017, 203, 937–941. [Google Scholar] [CrossRef] [PubMed]
- Mohan, D.; Singh, K.P.; Singh, V.K. Removal of hexavalent chromium from aqueous solution using low-cost activated carbons derived from agricultural waste materials and activated carbon fabric cloth. Ind. Eng. Chem. Res. 2005, 44, 1027–1042. [Google Scholar] [CrossRef]
- Wang, H.; Tian, Z.; Jiang, L.; Luo, W.; Wei, Z.; Li, S.; Cui, J.; Wei, W. Highly efficient adsorption of Cr(VI) from aqueous solution by Fe3+ impregnated biochar. J. Disp. Sci. Technol. 2017, 38, 815–825. [Google Scholar] [CrossRef]
- Mohanty, K.; Jha, M.; Meikap, B.C.; Biswas, M.N. Removal ofchromium (VI) from dilute aqueous solutions by activated carbon developed from Terminalia arjuna nuts activated with zinc chloride. Chem. Eng. Sci. 2005, 60, 3049–3059. [Google Scholar] [CrossRef]
- Malkoc, E.; Nuhoglu, Y. Potential of tea factory waste for chromium(VI) removal from aqueous solutions: Thermodynamic and kinetic studies. Sep. Purif. Technol. 2007, 54, 291–298. [Google Scholar] [CrossRef]
- Maitlo, H.A.; Kim, K.H.; Kumar, V.; Kim, S.; Park, J.W. Nanomaterials-based treatment options for chromium in aqueous environments. Environ. Int. 2019, 130, 104748. [Google Scholar] [CrossRef]
- Rangabhashiyam, S.; Selvaraju, N. Adsorptive remediation of hexavalent chromium from synthetic wastewater by a natural and ZnCl2 activated Sterculia guttata shell. J. Molec. Liq. 2015, 207, 39–49. [Google Scholar] [CrossRef]
- El Nemr, A. Potential of pomegranate husk carbon for Cr(VI) removal from wastewater: Kinetic and isotherm studies. J. Hazard. Mater. 2009, 161, 132–141. [Google Scholar] [CrossRef]
- Zhu, Q.; Moggridge, G.D.; D’Agostino, C. Adsorption of pyridine from aqueous solutions by polymeric adsorbents MN 200 and MN 500. Part 2: Kinetics and diffusion analysis. Chem. Eng. J. 2016, 306, 1223–1233. [Google Scholar] [CrossRef]
- Golban, A.; Lupa, L.; Cocheci, L.; Pode, R. Synthesis of MgFe layered double hydroxide from iron-containing acidic residual solution and its adsorption performance. Crystals 2019, 9, 514. [Google Scholar] [CrossRef]
- Khambhaty, Y.; Mody, K.; Basha, S.; Jha, B. Kinetics, equilibrium and thermodynamic studies on biosorption of hexavalent chromium by dead fungal biomass of marine Aspergillus niger. Chem. Eng. J. 2009, 145, 489–495. [Google Scholar] [CrossRef]
- El Nemr, A.; Khaled, A.; Abdelwahab, O.; El-Sikaily, A. Treatment of wastewater containing toxic chromium using new activated carbon developed from date palm seed. J. Hazard. Mater. 2009, 152, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, K.; Jha, M.; Meikap, B.C.; Biswas, M.N. Biosorption of Cr(VI) from aqueous solutions by Eichhornia crassipes. Chem. Eng. J. 2006, 117, 71–77. [Google Scholar] [CrossRef]
- Park, D.; Lim, S.R.; Yun, Y.S.; Park, J.M. Reliable evidences that the removal mechanism of hexavalent chromium by natural biomaterials is adsorption-coupled reduction. Chemosphere 2007, 70, 298–305. [Google Scholar] [CrossRef]
- Lin, Y.C.; Wang, S.L. Cr K-edge X-ray absorption and FTIR spectroscopic study on the reaction mechanisms of Cr(III) and Cr(VI) with lignin. Desal. Water Treat. 2016, 57, 21598–21609. [Google Scholar] [CrossRef]
- Uzum, C.; Shahwan, T.; Eroglu, A.E.; Lieberwirth, I.; Scott, T.B.; Hallam, K.R. Application of zero-valent iron nanoparticles for the removal of aqueous Co2+ ions under various experimental conditions. Chem. Eng. J. 2008, 144, 213–220. [Google Scholar] [CrossRef]
- Ali, S.W.; Mirza, M.L.; Bhatti, T.M. Removal of Cr(VI) using iron nanoparticles supported on porous cation-exchange resin. Hydrometallurgy 2015, 157, 82–89. [Google Scholar] [CrossRef]
- Rakotonimaro, T.V.; Neculita, C.M.; Bussiere, B.; Zagury, G.J. Comparative column testing of three reactive mixtures for the bio-chemical treatment of iron-rich acid mine drainage. Miner. Eng. 2017, 111, 79–89. [Google Scholar] [CrossRef]
- APHA, AWWA, WEF. Standard Methods for the Examination of Water and Wastewater, 19th ed.; United Book Press: Baltimore, MD, USA, 1995. [Google Scholar]
- Zach-Maor, A.; Semiat, R.; Shemer, H. Synthesis, performance, and modeling of immobilized nano-sized magnetite layer for phosphate removal. J. Colloid Interface Sci. 2011, 357, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Cocheci, L.; Pode, R.; Popovici, E.; Dvininov, E.; Iovi, A. Sorption removal of chromate in single batch systems by uncalcined and calcined Mg/Zn-Al-type hydrotalcites. Environ. Eng. Manag. J. 2009, 8, 865–870. [Google Scholar] [CrossRef]
- Ho, Y.S. Review of second-order models for adsorption systems. J. Hazard. Mater. 2006, B136, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Albadarin, A.B.; Mangwandi, C.; Al-Muhtase, A.H.; Walker, G.M.; Allen, S.J.; Ahmad, M.N.M. Kinetic and thermodynamics of chromium ions adsorption onto low-cost dolomite adsorbent. Chem. Eng. J. 2012, 179, 193–202. [Google Scholar] [CrossRef]


| Test | Pseudo 1st Order | Pseudo 2nd Order | qeexp (mg g−1) | ||||
|---|---|---|---|---|---|---|---|
| k1 (min−1) | qe (mg g−1) | R2 | k2 (g mg−1 min−1) | qe (mg g−1) | R2 | ||
| FeII + WSP | 1.4 × 10−3 | 1.20 | 0.7936 | 18.8 × 10−3 | 3.93 | 0.9998 | 4.20 |
| CrVI + WSP | 6.9 × 10−3 | 0.35 | 0.9891 | 1.9 × 10−2 | 0.33 | 0.9874 | 0.40 |
| CrVI + WSP-Fe0 | 1.8 × 10−2 | 0.46 | 0.9948 | 5.9 × 10−2 | 0.56 | 0.9923 | 0.50 |
| kdiff (mg g−1 min−0.5) | C | |
|---|---|---|
| FeII removal by WSP | 5.3 × 10−3 | 2.4 |
| CrVI removal by WSP | 0.9 × 10−2 | 2 × 10−2 |
| CrVI removal by WSP-Fe0 | 1.6 × 10−2 | 3 × 10−2 |
| Investigation of the Influence of | ||||
|---|---|---|---|---|
| pH | FeII Concentration | Temperature | Ionic Strength | |
| pH | 1.0–4.1 | 4.1 | 4.1 | 4.1 |
| FeII concentration (mg L−1) | 50 | 25–100 | 50 | 50 |
| Temperature (°C) | 22 | 22 | 6–33 | 22 |
| Ionic strength (mole L−1 NaCl) | 0 | 0 | 0 | 0–0.05 |
| Investigation of the Influence of | ||||
|---|---|---|---|---|
| pH | CrVI Concentration | Temperature | Ionic Strength | |
| pH | 1.0–5.9 | 3.0 | 3.0 | 3.0 |
| CrVI concentration (mg L−1) | 2 | 1–5 | 2 | 2 |
| Temperature (°C) | 22 | 22 | 6–33 | 22 |
| Ionic strength (mole L−1 NaCl) | 0 | 0 | 0 | 0–0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gheju, M.; Balcu, I. Sequential Abatement of FeII and CrVI Water Pollution by Use of Walnut Shell-Based Adsorbents. Processes 2021, 9, 218. https://doi.org/10.3390/pr9020218
Gheju M, Balcu I. Sequential Abatement of FeII and CrVI Water Pollution by Use of Walnut Shell-Based Adsorbents. Processes. 2021; 9(2):218. https://doi.org/10.3390/pr9020218
Chicago/Turabian StyleGheju, Marius, and Ionel Balcu. 2021. "Sequential Abatement of FeII and CrVI Water Pollution by Use of Walnut Shell-Based Adsorbents" Processes 9, no. 2: 218. https://doi.org/10.3390/pr9020218
APA StyleGheju, M., & Balcu, I. (2021). Sequential Abatement of FeII and CrVI Water Pollution by Use of Walnut Shell-Based Adsorbents. Processes, 9(2), 218. https://doi.org/10.3390/pr9020218






