Abstract
Marine sources are gaining popularity and attention as novel materials for manufacturing biopolymers such as proteins and polysaccharides. Due to their biocompatibility, biodegradability, and non-toxicity features, these biopolymers have been claimed to be beneficial in the development of food packaging materials. Several studies have thoroughly researched the extraction, isolation, and latent use of marine biopolymers in the fabrication of environmentally acceptable packaging. Thus, a review was designed to provide an overview of (a) the chemical composition, unique properties, and extraction methods of marine biopolymers; (b) the application of marine biopolymers in film and coating development for improved shelf-life of packaged foods; (c) production flaws and proposed solutions for better isolation of marine biopolymers; (d) methods of preparation of edible films and coatings from marine biopolymers; and (e) safety aspects. According to our review, these biopolymers would make a significant component of a biodegradable food packaging system, reducing the amount of plastic packaging used and resulting in considerable environmental and economic benefits.
1. Introduction
The most important component of food packaging is that it aids in the preservation of food quality and ensures microbial safety [1,2]. Furthermore, packaging protects food products from physical, chemical, and microbiological harm by enabling the handling, transportation, and storage of various food products, resulting in improved quality preservation and shelf-life [3,4]. However, petroleum-based polymeric materials, sometimes known as plastic, are the most employed in food packaging [5]. Plastic packaging has limitations in terms of biodegradability, recyclability, biocompatibility, and reusability [5]. These restrictions on plastic packaging produce a massive amount of garbage, causing serious environmental difficulties such as soil contamination, marine pollution, air pollution, and animal deaths around the world [5,6]. Discarded plastics have now contaminated the entire human habitat. Aquatic life is also likely to be the most endangered due to the widespread pollution of the ocean by plastics [6]. Nonetheless, the plastic packaging industry is thriving due to its unique characteristics, such as its capacity to be used as a global marketing tool, low production costs, flexibility in usage, versatility in application, and critical food product safety [7,8]. The food packaging sector generated US$ 839 billion in revenue in 2015, with a 3.5 percent annual growth rate between 2015 and 2020, implying a value of about 1000 billion in 2020 [9,10]. Furthermore, global plastics output grew by roughly 10 million metric tons per year, from 322 million metric tons in 2015 to 367 million metric tons in 2020 [11]. This rise suggests that plastic production progresses while having a corresponding impact on the environment [5]. As a result, the concept of biodegradable packaging has been gaining traction for years, pushing researchers, stakeholders, concerned organizations, and social groups to seek eco-friendly materials to build biodegradable packages that have little or no environmental impact. Biodegradable plastic’s market value is soaring over the world. The global market for biodegradable plastic packaging reached about 4.7 billion dollars in 2019. By 2025, it is expected that the value would have risen to roughly US$ 12.1 billion [12]. The market value of biodegradable plastics is also increasing in the United States. The market value of biodegradable plastics in the United States reached US$ 822 million in 2019, with a projected increase of 116% (1774 million dollars) by 2025 [13]. Figure 1 shows the projected rise of the biodegradable plastic market value globally and in the United States.
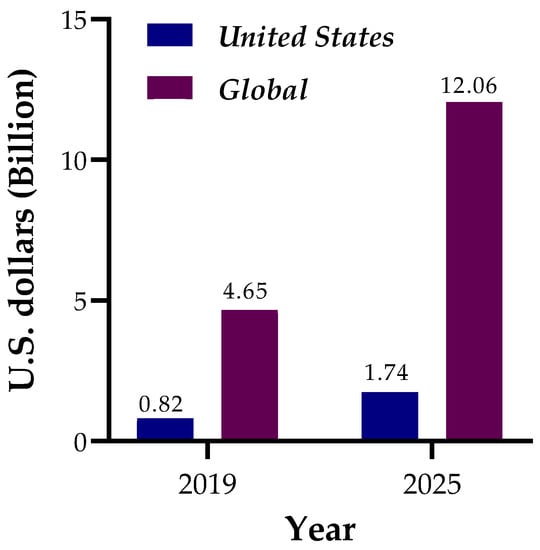
Figure 1.
Market growth of biodegradable plastic packaging worldwide and in the United States. STATISTA analysis of the biodegradable plastic market value reports for 2021 [12,13] was used to collect the data.
Recently, the literature surrounding the theme of “biodegradable packaging” has increased considerably due to its little or no impact on the environment [9,14]. The actual intention of biodegradable packaging is to develop and promote bioplastics–plastics with eco-friendly properties [15,16]. More recently, there has been a growing interest in producing films and coatings using biopolymers from renewable sources for food packaging applications [17]. Numerous ongoing research studies are attempting to meet consumer demands and nature-friendly legislation, focusing on creating novel and sustainable food packaging opportunities [18]. In contrast to conventional plastics, bioplastics (developed from polymers, such as gelatin, collagen, chitin, chitosan, etc.) are biocompatible, renewable, and sometimes cheaper. They can be obtained with little effort from agricultural and marine sources [19,20]. Among various resources, polysaccharides and protein from marine sources are attracting considerable attention in food packaging development, manufacturing solid films and coatings for robust packaging. Polysaccharides from marine sources can be used to develop environmentally friendly packages to extend food quality and enhance microbial safety [21,22]. For instance, crustacean and mollusk by-products were previously reported to be a good source of chitin, a handy resource for eco-friendly bioplastic development for food packaging [22,23,24]. Alginates are indigestible, naturally occurring polysaccharides useful in filming and coating. They can be obtained from the cell walls of brown algae [25]. Alginates have been successfully employed in preserving potato strips and fresh-cut apples [26,27].
In addition, proteins (collagen, gelatin) from marine sources are also used to develop biodegradable packaging [27,28]. Fish by-products, another vital source of marine biopolymers, have been put under chemical extraction to produce collagen, which has excellent food packaging properties [28]. This study seeks to provide an overview of the marine polysaccharides and proteins used to develop packaging materials, focusing on their films and coatings perspectives. The chemical composition, unique properties, extraction methods, major drawbacks, proposed resolution, and safety aspects will also be discussed. Furthermore, this review will describe some of the more recent developments in marine-based packaging with essential insights into the future perspectives of marine resources for food packaging application.
2. Marine Biopolymers in Food Packaging
The significant development of marine biopolymers can be traced back to industrial processing where marine resources such as fish, crustaceans, and mollusks, etc. are processed to produce commercial products, leaving a considerable quantity of solid wastage (27–75%), depending on the type of product, towards final consumption [29]. For instance, studies reported that fish canning and lobster processing account for approximately 27% and 75% of wastage, respectively [29,30]. These by-products or wastages are the skins, bones, scales, tendons, and shells left after processing, which can be subjected for chemical and biological extractions to produce two crucial biopolymer groups—proteins and polysaccharides. Common marine proteins are divided into three major parts: muscle proteins, collagen, and gelatin, whereas common marine polysaccharides include chitin, chitosan, alginate, agar, and carrageenan. Afterward, these proteins and polysaccharides are employed in the development of biodegradable coatings and films with the required mechanical properties to protect the food inside from different spoilage factors. In the preparation of packaging materials from marine biopolymers, different solutions and solvents are then applied with different techniques, such as dipping, spraying, coating, wrapping, brushing, or panning [31,32,33]. After the final application in food packaging, the materials are usually discarded and subjected to decomposition for their biodegradable properties. The most important, and perhaps the most expected property of these packaging materials, is their ability to be decomposed in the environment. The decomposition of biodegradable polymers into the environment have been explained in a study and according to Figure 2; the initial step in the decomposition process usually starts with the involvement of ultraviolet (UV) radiation, mechanical force, and microorganisms, producing microplastic (fragmented small pieces) [34]. Then, the extracellular enzymes come into action, inaugurating the abiotic and biotic hydrolysis to break down the ester bond of the polymer, leading to the reduction of the molar mass and formulation of soluble intermediates. The soluble intermediates then go through the assimilation process by the action of intracellular enzymes. Intracellular enzymes use the degraded products as a source of carbon and energy to produce cell biomass and products such as carbon dioxide and water [34]. A complete projection of marine biopolymers, their chemical extraction, and how they can be biodegraded into the surrounding environment is presented in Figure 2. Some of the unique technological properties include water barrier ability, oxygen barrier ability, edibility, transparency, thickness, and elasticity, which are summarized in Figure 3 [35,36]. These properties aid in protecting the packaged food against oxidation and microbial contaminations [37]. Marine biopolymers have been reported to hold these unique properties and could be used in smart packaging, where the active monitoring of product condition and the interactive action between packages and internal ambiance of the package plays an important role in the extension of food shelf-life while maintaining the biochemical, sensorial, and microbial quality [38,39,40].
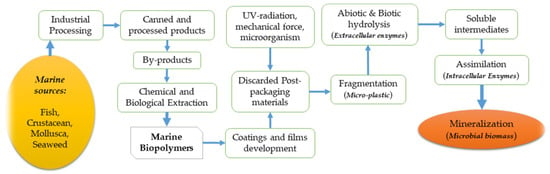
Figure 2.
Schematic representation of the complete cycle of marine biopolymers—from production through to the biodegrading of the environment.

Figure 3.
Technological properties of marine biopolymer-based packaging materials with specific functions.
In most cases, the films and coatings act as a medium to preclude the passage of water, oxygen, and carbon dioxide [41]. Even though the technological properties of marine biopolymer-based packaging have been reported, the efficacy is still lower and underdeveloped compared to synthetic and plastic polymer materials [42]. Consequently, several researchers are making significant attempts to build high barrier and thermal properties food packaging systems from cheap, available, and renewable biopolymers, especially marine sources [15,36,43]. To this aim, several propositions already have been made, and a recent upgrade has been brought with the application of active agents with potent antimicrobial and anti-oxidative properties that target to suppress the growth of microorganisms and to diminish the oxidation of lipids and pigments in food and the surroundings in a packaging arrangement [44,45].
3. Marine Proteins
3.1. Muscle Proteins
In recent years, utilization of protein biomaterials in the food industry has been increasing due to their excellent ability to form gels, nontoxic responses, and biodegradable nature [46,47]. The exploitation of marine stromal and myofibrillar proteins has received significant consideration for their ability to form edible films that are capable of acting as a barrier against gases, lipids, and organic volatiles [48,49,50]. Muscle proteins from marine resources are divided into three essential groups—myofibrillar, sarcoplasmic, and stromal proteins [50]. Myofibrillar proteins—contractile (myosin, actin) and regulatory (tropomyosin, troponin)—represent around 65–75% of the total muscle protein [51,52]. Myofibrillar proteins require a high ionic strength solution for their solubilization and extraction [49]. Sarcoplasmic proteins represent about 20–30% of the total muscle protein [51]. These water-soluble proteins are the enzymes involved in the biochemical process in muscle tissue [51]. Stromal proteins—collagen and elastin—represent a very low percentage of the total muscle proteins [52]. The most popular method of myofibrillar protein extraction is the application of a denaturing solution comprising thiourea, urea, beta-mercaptoethanol, and sodium dodecyl sulfate [53,54]. The denaturing method has shown an excellent way of extracting proteins and fragments with low molecular weight such as tropomyosin, actin, troponin-T, and myosin light chain 1 and 2 [53,54]. However, the toxicity and polluting nature of the reagents used in the denaturing method has been an issue, which also requires a safe disposal procedure. The nondenaturing extraction using KCl phosphate has also been found effective in extracting muscle proteins, especially high molecular-weight proteins such as myosin heavy chain, α-actinin, and desmin [55]. The use of the nondenaturing extraction method saves time and labor and also reduces the release of toxic and polluting agents as compared to the denaturing method [54,55]. The films and coatings developed from muscle proteins have been reported to have excellent advantages: a UV light barrier, good oxygen and carbon dioxide barrier, and transparency, thereby becoming an essential part of active packaging development.
3.2. Collagen and Gelatin
Collagen is one of the most abundant animal proteins found in all connective tissues, including bones, tendons, ligaments, skin, and cartilage [56]. Collagen is composed of polypeptides and characterized by a triple helix structure, resulting from three crosslinked α amino acid chains, consisting of two homologous α1 chains and one α2 chain [57,58]. Marine sources, mainly fish by-products, such as sea urchin, starfish, and sea cucumber, have been reported to be rich in collagen [59]. The extraction of collagen primarily involves two crucial steps: raw material pretreatment (alkaline and alcohol pretreatment to remove non-collagenous proteins and lipids) and demineralization (using ethylenediaminetetraacetic acid to remove calcium or other inorganic materials) [60,61,62]. There are several methods available for the extraction of collagen from marine sources: acid-soluble collagen, salt-soluble collagen, and enzyme-soluble collagen. The extraction method has an impact on the final yield and properties depending on the factors such as fish age and the type of the fish [63]. In acid extraction, acetic acid is used chiefly; however, recent studies successfully applied other organic acids (citric acid) and inorganic acids (hydrochloric acid and phosphoric acid) [64,65,66,67]. It is worth mentioning that the solubility of collagen in acidic media is restricted due to the interchain crosslinks and covalent bonds of aldehydes with lysine and hydroxylysine at the telopeptide region during the condensation reaction [68]. Regarding the NaCl extraction, lower yields are usually obtained, and for this reason, acid and enzyme-dependent extraction are mostly preferred. In enzyme extraction, a variety of enzymes have been used such as trypsin, pepsin digestive proteases, and bacterial collagenases [69,70]. These enzymes can remove the non-collagenous proteins to improve the quality of the collagen. Later, these non-collagenous proteins can be removed from the system using dialysis and salt precipitation [71,72]. The maximum activity of the enzyme and outcome of the enzyme extraction is dependent on the suitable enzyme, physiochemical conditions for the better enzymatic reaction, required pH, favorable temperature, adequate hydrolysis time, and optimum enzyme concentration [72]. After the extraction of collagen, hydrolysis can be applied to the collagen to produce gelatin [73]. Gelatin, a denatured protein, can be obtained from the partial hydrolysis of collagen followed by thermal treatment [73,74]. Depending on the parent collagen and extraction methods, the gelatin consists of different proteins and polypeptides with different molecular weights [75]. The molecular bonds between different strands are broken down during hydrolysis, which leaves a mixture of polypeptides (single and multi-stranded), each with extended left-handed helix conformations and containing 50–1000 amino acids [76]. Acid hydrolysis and alkaline hydrolysis are primarily used in the production of collagen, and the collagen is then hydrolyzed to obtain different types of gelatins—type A and type B [77]. Gelatin is a vital industrial biopolymer with versatile applications, ranging from food packaging to pharmaceutical fields. To improve the physical and chemical properties of gelatin, some chemical treatments, such as deamination, esterification, acylation, reaction with acids and bases, and crosslinking, can be helpful to enhance the rheological properties for better production of gelatin [78].
4. Marine Polysaccharides
4.1. Chitin and Chitosan
Chitin is a naturally occurring biopolymer that is produced by a large number of living organisms [79]. It is the second most abundant natural polysaccharide after cellulose. Chitin can be obtained from a variety of sources, the most common of which are shellfish wastes such as shrimp, crabs, and lobsters [80]. Chitin makes up around 14–27% and 13–15% of the dry weight of shrimp and crab processing waste, respectively [81]. Chitin can also be found in a few fungus species.
Chitin is a linear, highly crystalline homopolymer made up of −1, 4–linked N-acetyl glucosamine residues organized in antiparallel (α), parallel (β), or mixed (γ) strands, with the antiparallel (α) strand being the most common. Native chitin has a molecular weight of >1,000,000 Da [79].
Chitin is insoluble in water and most organic solvents. The hydrogen bonding in chitin is one of the reasons for its low solubility. To circumvent this solubility issue, chitin derivatives are commonly synthesized and employed. Gels, membranes, and colloids can all be made from chitin. Chitin is naturally crystalline, and the degree of crystallinity is proportional to the degree of deacetylation [82]. Chitin is generally converted to chitosan to widen its use. Chitosan is harmless to mammals, biocompatible, biodegradable, and nonallergenic [83]. Chitin deacetylation under alkaline conditions is required for the transformation of chitin to chitosan [84]. Chitosan’s functional qualities are influenced by structural characteristics such as average molecular weight and deacetylation degree [85]. Chitosan with lower viscosity is easier to handle and is often preferred. Chitosan’s viscosity is determined by its physical and chemical treatment. The viscosity decreases as the treatment duration and temperature increase.
Two types of approaches are utilized to isolate chitin: chemical and biological (microbial) methods [86]. Deproteinization and demineralization are two steps in the extraction of chitin [87]. Another step in the removal of color from shell debris is depigmentation treatment. To obtain chitosan, a deacetylation technique is used after chitin extraction. Deacetylation levels in chitin normally vary from 5% to 15%. The degree of deacetylation has a significant impact on chitosan’s chemical, physical, and biological characteristics [88]. The degree of deacetylation of chitosan varies depending on the harshness of the treatment, which affects the material’s final qualities [89]. In terms of chemical treatments, the deproteinization stage is carried out with an alkali solution (often NaOH), the demineralization step is carried out with an acidic solution (commonly HCl), and the depigmentation step is carried out by soaking the chitin in an alkali solution. Following that, an alkali solution (usually NaOH) is utilized to extract chitosan [90,91]. For deproteinization and demineralization, bacterial strains are used in biological treatments. For example, bacterial strains that produce proteases, such as Bacillus subtilis, Pseudomonas aeruginosa, and Serratia marcescens, have been tried for deproteinization, and Lactobacillus plantarum has been studied for the demineralization process to remove calcium from chitin [92]. Furthermore, Rhizopus japonicas have been exploited as a biological alternative for the conversion of chitin to chitosan due to its strong intracellular and extracellular deacetylation mechanisms [93,94]. However, no bacterial strains are used in the depigmentation process.
Chemical treatments are low-cost methods that can be used in large quantities [95]. Chemical treatments, on the other hand, require large concentrations and volumes of acid and alkali solutions at high temperatures, which is environmentally unfriendly and has a severe impact on the physicochemical properties of chitin.
Biological treatments, on the other hand, are more suited techniques from an environmental standpoint. Despite this, they take longer to produce, are more expensive, and have only been used on a small basis [96]. It is also possible to use a combination of chemical and biological approaches. Pachapur et al. [97] presented a combination approach that uses saltwater for all processes of chitin extraction, including enzymatic deproteinization (with Bacillus licheniformis) followed by a chemical demineralization process. The use of seawater reduces the amount of freshwater and chemicals used in the chitin extraction process. Furthermore, Lopes et al. [98] examined chitosan extraction by chemical and biological techniques on a pilot size based on environmental impact. Some of the reported advances to reduce the environmental impact of chemical treatments include the use of soft alkaline treatment and the possibility of recovery utilizing NaOH, water, and fish protein hydrolysates. Additionally, certain changes were proposed for biological processes to lessen the environmental impacts and costs connected with enzyme synthesis; for example, using crustacean biomass as peptones for bacterial growth could be a technique to reduce environmental loads.
A unique approach for chitosan extraction that uses microwave technology for all processes (demineralization, deproteinization, and deacetylation) was recently proposed [99,100]. This procedure uses the same preparation conditions as chemical treatments; however, it takes significantly less time. Furthermore, this unique technology cuts the deacetylation time from more than 6 h to less than 30 min while maintaining the same deacetylation degree. Furthermore, the structure, shape, and chemical makeup of chitosan were similar in both procedures. The molecular weight of a biomolecule is one of the primary characteristics that affect its structure–function relationship [101].
Chitosan is categorized into two types based on its molecular weight: high molecular weight (HMW) (> 300 kDa) and low molecular weight (LMW) (300 kDa). The molecular weight of chitosan varies depending on its origin, and it describes its physicochemical qualities such as viscosity, solubility, elasticity, tear strength, and biofunctionalities [102]. The average molecular weight of chitosan is 50 to 1000 kDa [92], 3.8 to 2000 kDa [87], or 50 to 2000 kDa [88], depending on the source and manufacturing method. Higher molecular weight chitosan produces highly viscous solutions, which are difficult to handle in industrial settings. The viscosity of a chitosan film was standardized using glycerol molecules of various molecular weights. Chitosan films with a high molecular weight exhibit non-Newtonian behavior [103]. Chitosan with a low molecular weight has a higher inhibitory effect on phytopathogens [104]. However, due to the high degree of acetylation, chitosan becomes less flexible and transparent, making it appropriate for anti-adhesive applications [105].
Chitosan’s properties are influenced by the origin of chitin. Seaweed chitin is thick, hard, and insoluble when removed. Because of its great flexibility, reactivity, and water solubility, chitosan produced from the deacetylation of marine chitin is excellent for packaging [106]. Chitosan obtained from marine sources is used in the food industry as a preservative, coating, as well as antibacterial and antioxidant agent after deproteinization and demineralization [107]. In comparison to chitosan derived from marine sources, which is suitable for medical use, chitosan isolated from insect cuticles has a very low mineral content [108]. Microorganism-derived chitin and chitosan have been found to aid in the production of consistent, pure products with specific properties. Antibacterial, biocompatibility, and adsorption characteristics are also seen in chitin and chitosan produced from fungi and yeast [109].
Chitosan has been approved by a few countries as a food additive and a wound-healing agent by the USFDA. However, its toxicity is increased by charge density. A few in vitro studies showed that chitosan with MW <5 kDa, 5–10 kDa, and >10 kDa shows little cytotoxicity for human lymphoblastic leukemia and human embryonic lung cells [110]. However, bacteria, fungi, and parasites are prone to the toxicity of chitosan and its derivatives; that is why their nanoparticles can be used in food packaging. Although, most in vivo studies show minimal toxicity for chitosan and its derivatives.
Biodegradation of chitin and chitosan depend on the MW, pH, degree of deacetylation and the method of preparation. In vitro studies showed that chitosan films degrade over 4 h if porcine pancreatic is used. While literature related to in vivo degradation of chitosan is scarce, studies have shown that liver and kidney of living organisms are able to degrade the chitosan. In general, the rate and extent of biodegradation of chitosan has a linear relationship with the degree of deacetylation [111].
4.2. Alginate
Alginate is a marine biopolymer found in all species of brown algae and some bacteria, where it provides flexibility and strength to the cell walls. Alginate is a long linear chain polymer made up of varying proportions and sequential arrangements of (14)-linked-ß-d-mannuronate (Block M) and (14)-linked-l-guluronate (Block G) units [110,111,112]. Alginate is made up of G residues that are repeated (GGGGGG), M residues (MMMMMM), and alternating M and G residues (GMGMGM). Although alginate has been used as a food since 600 B.C., the Krefting method [113,114] was used to extract the pure form of alginate from seaweed in 1896. When it was utilized as a stabilizer in ice cream in 1929, alginate became a commercial commodity.
Alginate is frequently linked with other cations, such as sodium and calcium, which combine to produce sodium and calcium alginate, respectively, and alter its characteristics. Alginate characteristics are determined by the kind of algae, more precisely, the species of algae, which include Ascophyllum nodosum, Laminaria hyperborean, Laminaria digitata, and Macrocystis pyrifera. Bacteria from the Pseudomonas and Azotobacter genera create polymeric compounds that resemble alginate [115,116].
Alginic acid is employed in a variety of sectors as a stabilizing, viscosifying, and binding agent [117,118]. It is also being used with other polymers such as chitosan and hyaluronic acid to serve a wider range of purposes [119,120]. The viscosity of alginate solutions increases as the pH decreases, peaking at pH 3.0–3.5 when the alginate molecule’s carboxylate groups get protonated and form hydrogen bonds. Increasing the molecular weight of the alginate improves the physical qualities of the resultant gels. An alginate solution made from a high molecular weight polymer, on the other hand, becomes quite viscous, which is generally undesired in treatment [121]. The films made from alginates and a plasticizer are generally robust and flexible, and they provide a good oxygen barrier. Alginates films can be soluble or insoluble; soluble sodium alginate films are formed by casting and drying, whereas insoluble alginates films are made by applying a layer of alginate solution, crosslinking with calcium salt, and drying.
Traditionally, alginates are extracted from brown seaweeds in five steps: (1) acidification; (2) alkaline ex-traction using sodium carbonate (Na2CO3); (3) solid/liquid separation; (4) precipitation; and (5) drying [120,121,122]. Algal biomass is physically and chemically pre-treated before alginates are extracted. To begin with, the biomass used for extraction must include at least 83% dried matter (17 percent moisture) and less than 3% sand [123]. Another crucial factor in alginate extraction is biomass size. The further processing of algae is made easier by reducing the size of the raw material [124]. Fertah et al. [125] extracted alginates from Laminaria digitate using ground biomass with particle sizes of 1–5 mm and concluded that biomass with smaller particle sizes provided more surface area, resulting in a better extraction yield. Santagata et al. [126] employed a low-frequency ultrasound to rupture the cell wall of seaweed (Sargassum), increasing the alginates extraction yield. To soften the seaweed tissue and avoid alginate coloring, in some studies the algae were soaked in formaldehyde/formalin for 1–12 h before extracting the alginates from brown seaweeds. When formaldehyde combines with algal phenolic compounds, the coloring compounds become insoluble [127]. The creation of alginate gel is a somewhat complicated procedure. The amount and length of guluronic acid blocks (G-blocks) in the polymeric chain, the capacity to bind divalent ions, the kind of gelling ions, and the gelling circumstances all have a significant effect on alginate’s hydrogel capabilities. When divalent cations (often Ca2+ ions) are added to the solution, alginate undergoes conformational changes such as alignment of the G-blocks and creation of the egg-box model as a result of calcium ions being bonded between two chains and producing divalent salt bridges [128,129]. A greater proportion of G-Blocks results in rigid, dense gels, whereas a greater proportion of M-Blocks results in flexible, porous gels [130]. As a result, gels containing a high proportion of polyguluronic alginate have a high diffusional resistance to high-molecular-weight molecules [131]. Additionally, Olivas and Barbosa-Cánovas [132] demonstrated that films with a higher fraction of G-Block (i.e., a lower M/G ratio) had superior moisture barrier qualities.
4.3. Agar
Agar is a high-molecular-weight polymer generated from the carbohydrate agarose, which serves as the supporting framework of certain algal species’ cell walls. Agarophytes are red algae that belong to the group Rhodophytes (red algae). The primary commercial genera of agarophytes include Gelidium, Pterocladiella, Gelidiella, and Gracilaria. Ahnfeltia, Acanthopeltis, Campylaephora, Ceramium, Gracilariopsis, and Phyllophora are among the species that contain agar [133].
Agar is a mixture of two polysaccharide components: agarose (gelling fraction) and agaropectin (non-gelling fraction). Agarose is a linear polysaccharide made up of repeating units of D-galactose and 3 6, anhydro-L-galactose linked by alternating a-(1.3) and ß-(1.4) glycosidic linkages, while agaropectin is branched and sulfated. Agarose makes up the majority of agar (50–90%) and is also responsible for agar’s gelling properties [134]. Different degrees of sulfation can be found in these polysaccharides. As a result, galactose, 3,6-anhydro-galactose, and inorganic sulfates linked to the carbohydrate are found in agar [135]. However, some variance exists based on factors such as algae species and environmental circumstances. Carrageenan, furcelleran (Danish Agar), and other agars have lower ash concentrations than agar. Agar’s ash content is typically kept between 2.5 and 4.0%, with a 5.0% ash concentration considered acceptable. Agar is manufactured in about 90% of the world for food use. Agar’s remarkable properties as a thickening, stabilizing, and the gelling agent makes it an indispensable ingredient in the production of processed foods. The traditional procedure for agar extraction includes (1) soaking dry algae in boiling water to detach the agar gum from the cell wall and dissolve it in water; (2) sieving the liquid extract; and (3) using freezing and thawing processes to separate the gum from the water [136,137]. Furthermore, an alkali treatment could be employed to improve the gelation ability of agar. L-galactose-6-sulfate is converted to 3,6-anhydro-L-galactose, which is responsible for gel strength [138]. Alkali treatment, on the other hand, may have deleterious effects on the films [139].
4.4. Carrageenans
Carrageenans are hydrophilic colloids that are isolated from red algae species and belong to a class of linear sulfated polysaccharide galactans (Rhodophyta). Carrageenans are the third most significant hydrocolloid in the food business, after gelatin and starch. Carrageenan was first extracted commercially in 1930 from the red seaweed species Chondrus crispus and Mastocarpus stellatus for the production of chocolate milk. Eucheuma denticulatum and Kappaphycus alvarezii are the most often used marketable carrageenans.
Carrageenan raw materials are primarily grown from wild-harvested genera such as Chondrus, Chondracanthus, Furcellaria, Gigartina, Iridaea, Mazzaella, Mastocarpus, Sarcothalia, and Tichocarpus. Carrageenans are produced in large quantities around the world, with 70% of them used in the food business [140]. Because of their gelling, thickening, and stabilizing qualities, they are widely employed in the food business. The repeating disaccharides unit of -d-galactose and -d-galactose or -d-3,6-anhydrogalactose in carrageenan is -d-galactose and -d-galactose or -d-3,6-anhydrogalactose. There are three types of carrageenans based on the degree of substitution (DS) that happens on free hydroxyl groups. Substitutions are usually caused by the addition of ester sulfate or the presence of 3,6-anhydride residues [140,141]. Each disaccharide in Kappa (κ)-carrageenan has one sulfate group, Iota (ι)-carrageenan has two, and Lambda (λ)-carrageenan has three. Although d-galactose and 3,6-anhydro-d-galactose are the main sugar residues and sulfate is the main substituent, other carbohydrate residues such as glucose, uronic acids, and xylose are commonly found in carrageenans [142]. As a result, the number and position of sulfate groups, the presence of 3,6-anhydro-d-galactose, and the conformation of the pyranose ring characterize the structure of distinct types of carrageenans [133,143]. Carrageenans undergo a conformational shift from random coils to organized double helix shapes under the right conditions, such as decreasing temperature or rising salt content. By crosslinking the double helixes, the branched polymer chains can then evolve into a macromolecular network and produce gels or aggregates. Carrageenans have the typical solubility of hydrophilic colloids, meaning they are soluble in water but not in most organic solvents. Although alcohols and ketones are water-miscible, they are not carrageenan solvents. They can, however, be tolerated in a solution containing more than 40% carrageenans. Carrageenans are available in two forms: semi-refined and refined. Carrageenans are not removed from the seaweed in semi-refined carrageenans production; instead, they are heated to roughly 75 °C with an alkaline potassium hydroxide solution. The hydroxide reacts with the sulfate esters at the precursors μ- and ν-carrageenan to produce κ- and ι-carrageenan, which improves the gel strength of the product, while potassium binds to the carrageenans and promotes gel formation by preventing the hydrocolloid chains from dissolving. The potassium-bound carrageenan-containing seaweed is washed, dried, and ground into a powder [144]. The technique of semi-refined carrageenan extraction is continued by heating (95–110 °C) the alkali-treated seaweed fronds to dissolve the gel matrix in the seaweed fronds to produce refined carrageenans. Alcohol precipitation or gel pressing are used to recover the carrageenans. The extraction of refined carrageenans is significantly more expensive than the processing of semi-refined carrageenans. Enzymatic extraction of carrageenans is used to avoid the use of chemicals and their detrimental effects on the environment.
5. Films and Coatings from Marine Biopolymers in Food Packaging
Food preservation technologies are now confronted with many difficulties in extending the shelf-life of different kinds of food such as fruits, vegetables, fish, meat, refrigerated products, etc. [29,145]. In this regard, the use of edible films and coatings made from biopolymers, especially food-grade biopolymers, has progressed dramatically in recent years [145]. Food–grade biopolymers, such as proteins and polysaccharides from plants, marine life, animals, or food-processing by-products, are enormously utilized to formulate edible packaging systems [145,146]. Remarkably, biopolymers from marine resources are gaining significant attraction in developing bio-based packaging materials for enhanced food packaging. In most cases, the marine-derived biopolymers are used to prepare films and coatings and then coated, wrapped, or sprayed over the food through packaging, focusing on enhancing the packaged food’s shelf-life [35,36]. The films and coating create a barrier environment that stands against the transmission of gases, oxygen, vapors, etc., thereby enhancing shelf-life and quality attributes of the packaged food [36]. However, the functionality of the films and coatings as a packaging material developed from marine biopolymers requires different modifications before selecting in a packaging application [147]. For instance, a material characterization (such as mechanical property) of the packaging material is required to determine its suitability in specific applications [147]. The packaging material must serve its purpose to keep the product undamaged and undeteriorated [29,90]. The suitability and strength of the packaging materials developed from marine biopolymers may vary; however, in some cases, they have been found with low technological and functional properties [29,36]. Likewise, the low technological and functional properties may affect food rheological, sensorial, and microbial attributes when packaged [148]. In this regard, the application of active agents, plant extracts, biopolymers blends, nanoparticles, essential oils, and organic acids, etc., in line with marine biopolymers have been found effective in enhancing the functional and technological properties of packaging materials, leading to significant improvement in final packaging quality [149,150,151,152,153]. These biopolymer–active agents modification provides active properties to the packaging that extend the shelf-life and quality attributes.
5.1. Marine Protein Films and Coatings
Kaewprachu et al. [154] reported that fish myofibrillar protein films prepared in combination with catechin-Kradon extract (Careya sphaerica Roxb.) could reduce the growth of psychrophilic bacteria and retain sensory attributes up to 8 days in bluefin tuna during refrigerated storage. The researchers determined the total volatile base nitrogen, TBARS (thiobarbituric acid reactive substance assay), and peroxide value that indicates the primary and secondary metabolites of lipid oxidation during refrigerated storage. The study’s findings concluded that myofibrillar protein films prepared from fish incorporated with catechin-Kradon extract could control microbial growth and keep lipid oxidation inhibition during 10 days at refrigeration temperature (4 ± 1 °C), thereby prolonging shelf-life [154].
Collagen, another important marine protein biopolymer, is mainly converted into gelatin to produce films and coatings suitable for food packaging applications. Marine collagen is most often used in edible meat casings, where it can shrink and stretch to mimic the contraction and expansion of meat batter throughout continuous processing [155]. Despite its use as a food additive to increase rheological characteristics, marine collagen is currently underused in food packaging. The low heat stability and poor mechanical properties of collagen might be a problem. As a result, most of it is transformed into gelatin, which has been shown to have strong technical, rheological, and functional qualities, especially when paired with active agents [36].
Gelatin, another important marine protein biopolymer, probably the best of other marine protein biopolymers, has already been declared effective in making films and coatings with active agents for food packaging application. Gelatin-based films and coatings have been applied in many food settings to improve the quality and shelf-life of packaged products. For instance, gelatin (0–6%) in combination with chitosan (0.5–1.5%) was applied to produce a film to preserve beef steak during 5 days of retail display [156]. The blend film was found effective in reducing weight loss, protecting lipid oxidation, and enhancing the color attributes of beef steak [156]. Another study developed a chitosan–gelatin film to preserve and improve the shelf-life of minced trout fillet during refrigerated storage over 11 days [157]. The findings concluded that fish spoilage was the lowest in the samples treated with film, with reduced bacterial growth [157]. In shrimp preservation, a gelatin and 2% orange leaf essential oil incorporated coating had a significant effect on the quality and shelf-life of the shrimp compared to the control group. The sensory evaluation found that a combination of gelatin film and orange leaf essential oil enhanced the shelf-life of the shrimp up to 14 days, whereas the control group only retained a shelf-life of 4 days [158]. Nowzari et al. [159] also reported the impact of gelatin–chitosan coatings and films on the quality of refrigerated rainbow trout. The chitosan–gelatin composite influenced the bacterial contamination of rainbow trout fillets, as evidenced by findings from TVB-N (total volatile basic nitrogen) and bacteriological studies [159]. A few findings related to the application of films and coatings made from marine biopolymers in food preservation, in line with other active agents, are presented in Table 1.
5.2. Marine Polysaccharide Films and Coatings
In the last few decades, the application of polysaccharide-based films and coatings in food packaging has increased dramatically. Noticeably, marine polysaccharides such as chitosan, alginate, agar, and carrageenan received significant attraction in the make-up of films and coatings in food packaging with the ability to protect from contamination and deterioration, thus improving the shelf-life and quality attributes [160].
Chitosan, one of the most ubiquitous marine biopolymers, has been applied in many food packaging applications as a film and coating material. Priyadarshi et al. [161] reported that a combination of chitosan and apricot kernel essential oil in the development of film significantly inhibited the fungal growth on packaged bread slices [161]. Besides, the modified films showed better water resistance and water vapor property. The result concluded that the developed film is filled with antimicrobial and antioxidant properties and can be an excellent approach to extend the shelf-life of bread for better preservation [161]. Alsaggaf et al. [162] reported that a chitosan–pomegranate peel extract could enhance the microbiological, chemical, and sensorial quality of Nile tilapia fillets during cold storage at 4 °C for 30 days. The coating application resulted in a strident decrease in the total microbial counts during storage. Furthermore, sensory assessments specified advanced preferences for the odor, texture, color, and overall quality of coated samples as compared to uncoated samples. Thus, the study recommends that a chitosan–pomegranate peel extract could be a natural approach to extend the shelf-life of Nile tilapia fillets during storage [162]. Halim et al. [163] also reported that a chitosan–tannic acid film could improve the physiochemical properties of cherry tomato and grapefruits during preservation. In another study, pork sausages in refrigerated storage were coated with a chitosan–clove oil blend, and the microbiological, physical, chemical, and sensory properties were monitored over 25 days during storage [164]. Although the psychrotrophic bacteria count, color value, peroxide value, and the thiobarbituric acid reactive substances were increasing, the changes were slowest in the samples with the chitosan–clove oil coating. Based on the sensory evaluation and microbiological quality, the chitosan–clove oil-treated samples retained a shelf-life of up to 20 days [164].
Alginate, another promising marine biopolymer, achieved significant attention due to its striking properties such as film-forming ability, non-toxicity, cheapness, and biocompatibility [165]. Albert et al. [166] reported that an alginate–salt film is able to protect microwaveable food during microwave operation. The alginate film successfully reduced the cooking time, lessened browning, and enhanced warming efficiency [166]. The application of a salty alginate edible film as a susceptor during microwave cooking seems to work well. The film layer that covers the substrate could warm up faster and distribute heat more evenly [166]. Alginate coating in combination with antimicrobials was effective in poached and deli turkey products preservation for 7 days storage at 22 °C [167]. The coating inhibited the growth of L. monocytogenes, with final counts of 4.3 log CFU (colony forming unit)/g (home-style poached turkey) and 6.5 log CFU/g (roasted deli turkey), respectively. In contrast, untreated equivalents had counts of 9.9 and 7.9 log CFU/g, respectively. As a result, this study shows that utilizing alginate-based antimicrobial coatings to improve the microbiological safety and quality of ready-to-eat poultry items during chilled storage is beneficial [167]. The effectiveness of a carrageenan–glycerol coating on the firmness and color components of papaya during storage was reported by Hamzah et al. [168]. Carrageenan and glycerol, at 0.78 percent (w/v) and 0.85 percent (w/v), respectively, were found to delay ripening and hence extend the shelf-life [168]. According to Seol et al. [169], a j-carrageenan-based film containing ovotransferrin and ethylene diamine tetra acetic acid (EDTA) had an antibacterial effect against E. coli, S. Typhimurium, S. aureus, and Candida albicans. Using a solvent casting process, antimicrobial films based on carrageenan were created by mixing grape seed extract in various quantities into the polymer. Because of the polyphenolic chemicals in the grape extract, the films were yellowish and showed strong antibacterial action against food-borne germs. These findings show that carrageenan films could be useful as antibacterial or active food packaging [170]. The essential oils of Zataria multiflora Boiss (ZEO) and Mentha pulegium (MEO) have good potential for incorporation with carrageenan to generate antioxidant and antibacterial films for food applications [171]. The antimicrobial properties of films created by integrating essential oils were improved, and S. aureus was found to be the most sensitive, followed by B. cereus and E. coli. Around the films that were mixed with 3 percent ZEO, the maximum inhibition area of 544.05 mm2 for S. aureus was recorded. For S. Typhimurium, the total inhibitory region of 3 percent MEO films was 20.43 mm2 and for P. aeruginosa it was 10.15 mm2. These findings demonstrated that ZEO and MEO are promising antioxidant and antibacterial films in food technology when combined with carrageenan [171]. Carrageenan is commonly utilized in the food sector due to its good physical and functional capabilities, which include antioxidant activity, stabilizing ability, gelling, emulsifying, and thickening agent [136,172,173]. They can also be found in water-based foods, animal products (as an oxygen barrier for delayed fat oxidation), and dairy products [174]. Importantly, both in vitro and in the cell system, oligosaccharides carrageenan and its derivatives were found to have antioxidant activity [175]. Sun et al. [176] also found that the sugar concentration limit, which corresponds to the removal of polymerization from kappa carrageenan, had a substantial impact on the antioxidant activity of carrageenan oligosaccharides. However, because carrageenan is typically utilized as a coating, there is not a lot of literature on how to make edible films with it [173]. Pork sausage containing carrageenan–soy protein showed a significant reduction in rancidity and discoloration during 16 weeks of frozen storage [177].
Agar, a biopolymer derived mostly from red algae, is another potential biopolymer. Agar film is becoming a popular and sustainable alternative to plastic-based food packaging. Plain agar film, on the other hand, is brittle, has a high moisture permeability, and is thermally unstable. A lot of work has gone into refining the qualities of agar films so that they may be used in more places; for example, in nanomaterial reinforcement, merging with other biopolymers and integrating plasticizers, hydrophobic components, or antibacterial agents into the structure [178]. The impact of a bioactive film made of agar and containing green tea extract and probiotic bacteria on hake fillets were evaluated during 15 days of refrigerated storage. Throughout the storage period, the agar–green tea–probiotic strain film caused a drop in H2S-producing bacterium counts and total viable bacteria. The films influenced the fish quality indicators such as total volatile basic nitrogen (TVBN), trimethylamine nitrogen (TMA-N), and pH. For 15 days, the overall viable counts, H2S-producing bacteria, and TVB-N were below acceptable levels [179]. Agar containing alginate, collagen, silver nanoparticles, and grapefruit seed extract was discovered to be very transparent. The film had a superior water retention capacity and an outstanding UV-screening effect. Furthermore, composite films demonstrated excellent antibacterial action against Gram-positive (Listeria monocytogenes) and Gram-negative (Escherichia coli) food-borne pathogenic microorganisms. The microbiological, physical, and chemical characteristics of flounder fillets coated with agar–clove essential oil films were studied during 15 days at 5 °C. During storage, flounder fillet had low total viable bases, a low pH, and delayed development of H2S-producing bacteria [180]. According to Abdollahzadeh et al. [151], agar films infused with several essential oils (peppermint, chamomile, cumin, tarragon, dill, and cinnamon) can be effective against L. monocytogenes strains. Green grapefruit packed with the agar–zinc oxide nanoparticles film has great thermal stability and film thickness [181]. In ambient conditions, the packed green grape retained its fresh look for up to 14 days. The nanocomposite films’ thermal stability, elongation at break, and fruit preservation qualities were all improved. Green grapes wrapped in developed films kept their appearance over long periods under ambient storage. As a result, the nanocomposite film that was created might be employed as an effective packaging material for extending the shelf-life of green grapes. Current laboratory-scale agar film manufacturing has various issues, such as a long drying time, inability to manufacture continuous films, and imprecise thickness control, which need to be addressed before moving to an industrial scale. As a result, further research focusing on commercial sizes is needed to give more realistic information for commercializing agar-based food packaging materials [182].

Table 1.
Application of protein and polysaccharide biopolymers derived from marine resources in combination with active agents in food packaging.
Table 1.
Application of protein and polysaccharide biopolymers derived from marine resources in combination with active agents in food packaging.
| Marine Biopolymer | Food Products | Matrix Constituent | Packaging | Outcomes | Ref. |
|---|---|---|---|---|---|
| myofibrillar protein | bluefin tuna slices | myofibrillar protein–catechin–Kardon extract | film |
| [154] |
| gelatin | beef steak | chitosan–gelatin | film |
| [156] |
| gelatin | minced trout fillet | chitosan–gelatin–grape seed extract | film |
| [157] |
| gelatin | pork sausage | gelatin–sodium alginate | film |
| [183] |
| gelatin | refrigerated rainbow trout | chitosan–gelatin | coating and film |
| [159] |
| gelatin | shrimp | gelatin–essential oil | coating |
| [158] |
| chitosan | bread | chitosan–apricot kernel essential oil | film |
| [161] |
| chitosan | Nile tilapia fillets | chitosan–pomegranate peel extract | coating |
| [162] |
| chitosan | cherry tomato and grapes | chitosan–tannic acid | film |
| [163] |
| chitosan | pork sausages | chitosan–clove oil | coating |
| [164] |
| chitosan | pork fillets | chitosan–Origanum vulgare essential oil | coating |
| [184] |
| chitosan | chicken | chitosan–pink pepper extract–peanut skin extract | film |
| [185] |
| chitosan | chicken breast | chitosan–pomegranate juice–Zataria multiflora essential oil | coating |
| [186] |
| alginate | microwave food | alginate–salt | film |
| [166] |
| alginate | poached and deli turkey products | alginate–antimicrobials | coating |
| [167] |
| alginate | shiitake mushroom | alginate–nano–Ag | coating |
| [187] |
| alginate | fresh-cut pineapple | alginate–lemongrass essential oil | coating |
| [188] |
| carrageenan | papaya | carrageenan–glycerol | coating |
| [168] |
| carrageenan | pork sausage | carrageenan–soy protein | coating |
| [177] |
| carrageenan | encapsulated aroma compound | carrageenan–glycerol | film |
| [189] |
| carrageenan | fresh spinach | carrageenan–agar–konjac glucomannan | film |
| [41] |
| carrageenan | chicken breast | carrageenan–chitosan–allyl isothiocyanate–mustard extract | coating |
| [190] |
| agar | hake fillet | the agar–green tea–probiotic strain | film |
| [179] |
| agar | fresh potato | agar–alginate, collagen blend–silver nanoparticles–grapefruit seed extract | film |
| [191] |
| agar | flounder fillet | agar–fish protein hydrolysate–clove essential oil | film |
| [180] |
| agar | minced fish | agar–essential oil | film |
| [151] |
| agar | green grape | agar–zinc oxide nanoparticles | film |
| [181] |
| agar | fish oil | agar–gelatin–titanium dioxide nanoparticles | film |
| [182] |
6. Methods of Preparation of Edible Films and Coatings from Marine Biopolymers
Two important types of packaging are edible films and coatings. Edible films are used as primary edible packaging, wrapping around the food surface. The solubility of additives in biopolymers is a critical aspect of their effectiveness. The overall mechanical properties of the film are influenced by the cohesive forces between biopolymers. The casting (wet) method and the extrusion (dry) method are the two most common ways to make edible films [145,192]. The edible coating is a type of edible packaging that is put directly to the surface of fruits, vegetables, and other food items. Dipping, spraying, fluidized bed, and panning are the four basic coating processes. The coating process used is influenced by several elements, including the food product’s surface qualities and the coating layer’s purpose. Coating formation begins with the components being diffused on the food surface, followed by adhesion between the coating material and the food surface [192].
6.1. Film Fabrication Methods
6.1.1. Casting
The casting method is a frequent and inexpensive method of film preparation. At the laboratory and pilot scales, it is the most often employed approach for film formation. This procedure can be broken down into three stages. The first is the process of solubilization. The biopolymer substance is solubilized in a suitable solvent in this stage. Because the film is edible, the solvent must be non-toxic and edible as well. Ethyl alcohol and water are commonly employed as solvents. The casting solution is then poured into the predetermined mold in the second phase. The casted solution must be dried before it can be used. The solvent is removed from the casted film during drying, and the marine biopolymers form a gel-like structure, which is then dried into a film [193,194]. Figure 4 depicts all steps of the edible film preparation casting process. The film is dried using a vacuum drier, microwave, and air dryer. The drying period has an impact on the intramolecular bonding between the biopolymer chains as well as the microstructure of the film [194]. Quick-drying methods for casting the film have proved to have negative effects on the physical and structural qualities [195]. The amount of plasticizer determines the film’s gas and moisture barrier, as well as its mechanical properties, such as tensile strength and elongation [194]. The barrier characteristics, mechanical strength, and thermal properties of an edible film are all improved by increasing the concentration of plasticizers in the matrix [196,197]. The main benefit of the casting method of film formation is the ease with which it may be produced without specialist equipment and at a reasonable cost [198]. Because the casting method is a wet procedure, it results in greater particle-particle contact, resulting in more homogeneous particle packaging and fewer flaws [199]. Since most food-processing materials cannot be molded at higher degrees without causing irreversible structural changes, the lower temperature throughout processing steps can be an advantage. Furthermore, the film-casting method has several unique characteristics, including a high optical purity (no gels or particles), great transparency and low haze, low optical retardation, superb flatness, and isotropic orientation [200]. The many drawbacks of casting include the limited forms that can be created (typically sheets and tubes), the potential trapping of toxic solvents inside the polymer, denatured proteins and other molecules that are introduced into polymers by using solvents [198], evaporative levels, and temperatures that can change the film features, as well as a long process time [201,202].
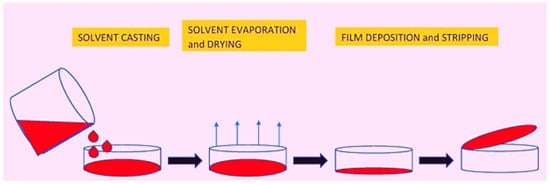
Figure 4.
Several steps in the casting procedure for edible film formation. Adopted from [194] with permission.
6.1.2. Extrusion
The extrusion process is used to make edible films, and it has the potential to expand commercial output. Extrusion is a dry technique since it utilizes very little or no solvent. Because there is little or no solvent, we do not have to wait for it to evaporate, and the drying period is eliminated. There are three zones in the extruder. The feeding zone is where biopolymer and additives are fed to the extruder for the first time. The next zone is the kneading zone, where components are correctly mixed with the assistance of an extruder screw, and the final zone is the heating zone, where some heat is delivered with the help of the oven. Here, biopolymer and additives are melted and mixed. A die is fixed at the extruder’s end, determining the shape and thickness of the extruded film. The biopolymer’s structure is altered by high temperatures, which improves the film’s overall qualities. The temperature-sensitive biopolymer cannot be extruded because it degrades at high temperatures [194,203]. The speed of the extruder screw, the quantity of heat, the length of the heating zone, and the solvent content, if any, are all critical parameters. They are crucial in determining the film’s mechanical and optical qualities.
Extrusion film production as a dry approach has several advantages over casting film formation, including a shorter processing time and lower energy consumption, as well as improved mechanical and optical qualities, such as the elongation and clarity of the edible film [204,205]. It is a high-performance, low-cost, and efficient technique that is employed in the food industry for commercial production [206,207]. Other benefits include the absence of solvents, ease of handling of high viscosity polymers, a wide range of processing settings (temperatures of 70–500 °C and pressures of 0–500 bar), and improved control of feed residence time and mixing degree [208]. The extrusion method of film manufacturing is also more efficient at controlling the mechanical characteristics of edible films and can produce a wider range of shapes than the solvent-casting method. Extrusion film manufacturing has several drawbacks, including the ability to process only temperature-tolerant and low-moisture raw material blends, which limits the use of specific polymers. The use of this procedure is also influenced by the greater initial cost of specialist equipment and the higher maintenance costs. A depiction of extrusion technology of film is presented in Figure 5. In addition, some examples of the film-formation methods used for food products from marine biopolymers are described in Table 2.
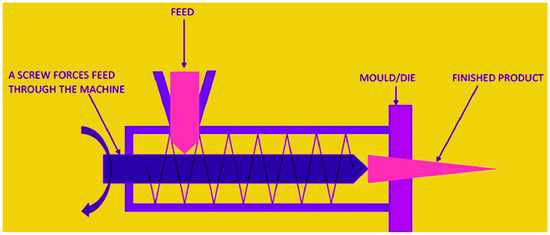
Figure 5.
Extrusion technology of film formation from marine biopolymers. Adopted from [194] with permission.

Table 2.
Film formation methods for food products from marine biopolymers.
Table 2.
Film formation methods for food products from marine biopolymers.
| Biopolymer Matrix | Food Product | Film Method | Conclusion on the Effectiveness of the Film | Ref. |
|---|---|---|---|---|
| chitosan–banana peel extract | apple | casting | the composite film has been hailed as a promising alternative for active packaging, and it is thought to be favorable to the valorization of banana peel by-products for other uses. | [209] |
| alginate gel–calcium | sausage | extrusion | the coating reduces or prevents white efflorescence on the surface of dry fermented sausages made with calcium alginate casing. | [210] |
| chitosan–syringic acid | quail eggs | casting | the film’s altered color, increased bacteriostatic and water-blocking characteristics, and minor changes in mechanical qualities all indicated that it could help extend the shelf-life of quail eggs. | [211] |
| agar–sodium–alginate–Stevia rebaudiana | cheese slice, sausage, meat slice, soluble coffee | casting | high solubility, homogeneity, regular margins, medium roughness, moderate strength, and flexibility were among the film’s best qualities for powder-type packaging. | [212] |
6.2. Coating Methods
6.2.1. Dipping
Fruits and vegetables are the most regularly dipped items. This procedure can be broken down into three stages. The first step is to completely immerse the food item in the coating-forming fluid. After that, the coating substance is applied to the food product’s surface. The solvent evaporates from the coating in the final phase, generating a solution and leaving a thin coating on the product’s surface. The dipping technique for the edible coating creation is depicted in Figure 6 [213]. Solvent evaporation can take place at room temperature or with the help of heat. Freshly cut fruits are soaked in an aqueous coating to create an antibacterial solution [194]. Chitosan coating was carried out on frozen salmon fish using the dipping method. This coating inhibited pathogenic microbe growth and extended the shelf-life of fish [214]. Fresh-cut mangoes were coated with alginate and then dipped in malic acid. This coating improved the hardness of coated foods and prevented quality degradation during storage [215]. The dipping method is the most common laboratory coating application method [216] because of its simplicity and low cost. The dipping method allows the food product to be fully coated on both sides [217]. It ensures good uniformity on the surface of food products with a rough and complex shape. This approach, in some situations, results in a thick coating, which may cause problems with food product respiration and storage [218]. The dipping procedure causes several challenges, including coating dilution, waste or dirt accumulation, and the development of microorganisms in the dipping vat. Furthermore, the dipping process has the disadvantage of diluting the exterior layer and reducing its functioning [219]; i.e., the natural wax coating of fruits and vegetables may be detached after dipping [220].
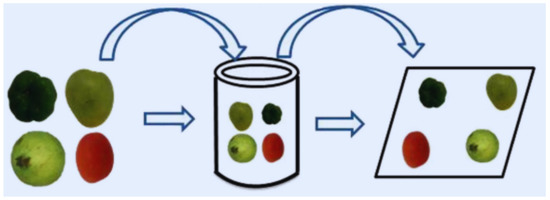
Figure 6.
Dipping method for applying an edible coating. Adopted from [194] with permission.
6.2.2. Spraying
A liquid solution is sprayed on the food product in this approach. The liquid solution is converted into small droplets when it is sprayed. These droplets will have a larger surface area for the same amount of liquid solution. As a result, droplets will cover a larger portion of the substance. For putting an edible coating onto food products, three types of spraying techniques have been employed in the industry: air spray atomization, air-aided airless atomization, and pressure atomization. High-speed air is used in air spray atomization to convert the liquid into droplets, while high pressure is used in pressure atomization to convert the liquid into droplets [194]. The spraying process for edible coatings is shown in Figure 7a. In air spray atomization, a high-velocity stream of air surrounds low-velocity fluid flowing from a tube. Fluid–air friction causes atomization by speeding up and disrupting the liquid fluid flow. It is also recognized as a low-cost spraying application method for applying edible coating on food products [221]. Air is employed for fine spraying of the droplet on food products in this approach. The air jet nozzle jounces are used to break down a cylindrical water jet (deflector) into small droplets for coating food products (Figure 7b). In air-aided airless atomization, with a unique fluid nozzle tip similar to a regular airless tip, air-assisted airless spray guns partially atomize the fluid. Second, they finish the atomization by using small volumes of pressurized air from the air nozzle’s face and/or horns (Figure 7c). As a result, the spray pattern is finely atomized and resembles that of a compressed air system. Many concerns related to the application of high-viscosity and high-solids coatings, as well as issues associated with heating and employing greater fluid pressures for the atomization of viscous materials, are solved using air-assisted airless atomization. This approach provides a high level of output and a high-quality finish [222].
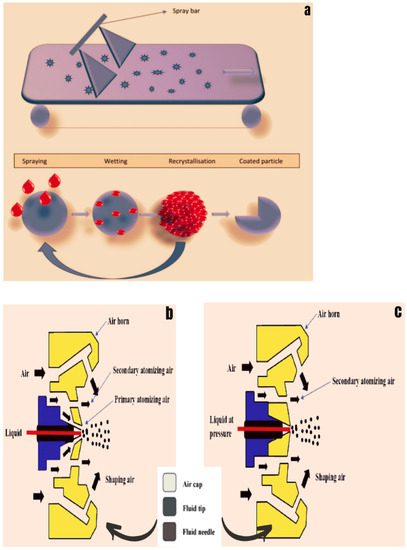
Figure 7.
(a) Spraying method for applying the edible coating. (b) Air spray atomization. (c) Air-aided airless atomization. Adopted from [194] with permission.
In pressure atomization, pressure is used to apply the edible coating on food goods. As a result of the lack of air in this process, it is also known as airless atomization. The small nozzles are utilized to pass high-pressure energy through the coating solution to provide surface tension and high viscosity for application in food products. To prevent the film-forming mechanism from being destroyed, the pressure is kept below 3.5 bars during the processing [219].
The spray piston and nozzle, temperature, air, and fluid flow of the polymer are all used to control the final drop size and hence the coating quality [223]. The standard spray system may produce a fine spray with a relative drop-sizing distribution of up to 20 μm, but electro-spraying products have been shown to produce uniform parts from polymer and biopolymer solutions of less than 100 nm. When creating polymeric films by spraying systems, various elements such as drying durations, drying temperatures, and the drying process, among others, are crucial [194]. After applying the initial coating and drying procedures to the top, the bottom surface of the product can be covered separately in this method. This method allows for a consistent coating with homogeneous thickness and multilayer applications, such as alternating sodium and calcium chloride solutions [218]. Spraying systems also prevent contamination of the layer, allow temperature control of the coating solution, and allow for automatic continuous production [219]. One main disadvantage of this strategy is that the highly viscous solution cannot be easily sprayed on food goods; therefore, only dipping methods may be used, resulting in a larger coating material thickness on the food product surface [146].
6.2.3. Panning
This process is ideal for coating foods and sweets. This approach may coat a large number of round or oval-shaped food items in a single batch. A pan, a large round ball, is rotated, while the food ingredients are rotated inside it. While the pan is spinning, the coat-forming solution is sprayed on the surface of the food product. The thickness of the final coating on the food product is determined by the volume of solution sprayed. The solvent evaporates and the coating dries with the help of air circulation [224]. Figure 8 depicts the panning coating process, in which the edible coating substance is sprayed onto the food products using a coating spray cannon. In a batch technique, the panning method is utilized to apply thin or thick layers to hard, virtually round particles. This is a coating technology used in the pharmaceutical, confectionery, and food-processing industries. Extruded items are ideal for panning since they may be produced in a round or oval shape, in a variety of sizes, and are generally easy to coat. Covering confectionery centers (peanuts and almonds) with polysaccharides, for example, provides a consistent base layer that also covers the hydrophilic/lipophilic surface, avoids moisture and fat displacement, and allows for additional flavor fusing [218]. This approach yields a glossy, extremely transparent edible covering with good elasticity [225]. This approach also has the advantage of being able to cover extruded food products of various shapes. It is simple to coat a large number of food items in a single batch with this procedure. Water must evaporate regularly throughout this operation to prevent food products from adhering and/or huge quantities of solution or suspension cannot be sprayed on food goods at random. As a result, the panning procedures take a long time and are inefficient [226].
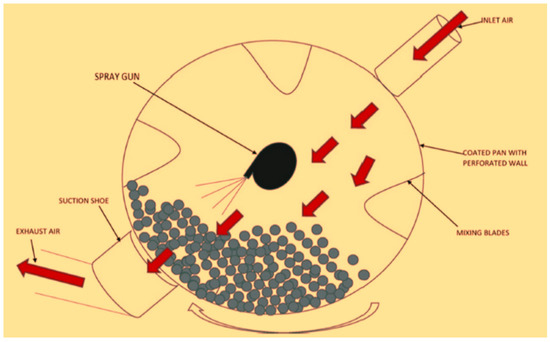
Figure 8.
Panning application method of an edible coating. Adopted from [194] with permission.
6.2.4. Fluidized Bed
This method allows a thin layer of coating to be applied to the surface of a dry food product of a very small size, such as wheat or nuts. The coating solution is sprayed using nozzles, which allow the sprayed solution to flow over tiny pieces of food. The solution begins to develop a shell on the food, which transforms into a coating over time. After that, the drying process begins. This approach is more expensive than other methods of coating [227]. This technique helps to minimize the rate of release to active chemicals by reducing the likelihood of agglomeration [228]. There are three types of fluidized-bed process methods: top spray, bottom spray, and rotating fluidized-bed. However, in the food business, the classic top spraying method outperforms the other methods [229]. Because the powders in the conventional bed do not fluidize stably or form excessive agglomerations in smaller sizes, the size of the particulate matter in the fluidized bed should be more than 100 m [230]. The fluidized bed coating technique’s particle aggregation improves the coating material’s dispersion and solubility. At the same time, a liquid binder is employed to improve the material’s stability; the process is carried out under heat treatment before being sprinkled on foods. The particles adhere, aggregate, and dry as a result of this process. It applies to both batch and continuous procedures [218]. Pneumatic or binary nozzles are often employed in the fluidized-bed coating because the fluid is fed at low pressure and the air is sheared into the nozzles. The fluidized bed is depicted schematically in the diagram (Figure 9). Fluidized bedding is used in the food sector to encapsulate a variety of foods and additives, including puffed wheat, almonds, and peanuts. This approach is also commonly used to protect seeds from mechanical and biological harm by coating them with pesticide slurry [218]. The fluidized-bed coating prevents the creation of coated product clusters, which is a typical problem in pan coating. Because of the loss on the column wall during spraying, the fluidized-bed method was first rejected by the food sector due to high costs. When compared to other coating methods, the fluidized-bed method requires a higher amount of coating solution. In the top spray design, the distance traveled by the droplets before reaching the substrates cannot be controlled. Premature evaporation can result in coating imperfections [230]. A list of application methods for edible coatings on food products prepared from marine biopolymers is presented in Table 3.
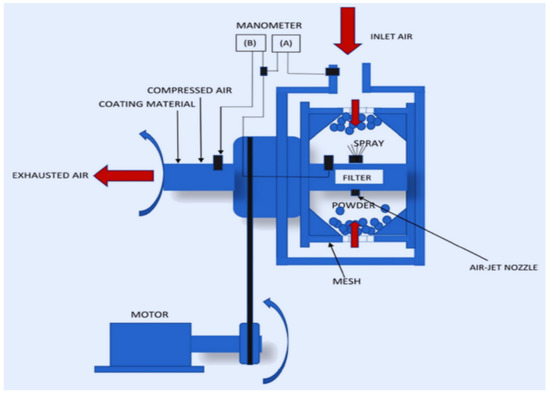
Figure 9.
Fluidized-bed application method for edible coatings. Adopted from [194] with permission.

Table 3.
List of application methods for edible coatings on food products prepared from marine biopolymers.
Table 3.
List of application methods for edible coatings on food products prepared from marine biopolymers.
| Biopolymer Matrix | Food Product | Coating Method | Conclusion on the Effectiveness of the Coating | Ref. |
|---|---|---|---|---|
| Alginate | Water melon | Dipping | Fresh-cut watermelon’s shelf-life was extended by a multilayered antibacterial covering. Coating prevented the growth of psychrotrophic bacteria, coliforms, yeast, and molds efficiently. In comparison to uncoated fruits, coated fruits retained their quality for 13–15 days at 4 °C (7 days). | [231] |
| Alginate–Chitosan | Bell pepper | Spraying | The coatings inhibited microbial growth and water loss while increasing hardness. Peppers that were coated retained their typical respiration and nutritional content. The shelf-life of peppers was extended. | [232] |
| Chitosan–nanoparticles | Tomato, chilly and brinjal | Dipping | In comparison to Amphotericin B, ChNP demonstrated superior antifungal efficacy against all selected infections. Antioxidant activity was determined to be significant. Vegetables coated with several concentrations of ChNP (1%, 2%, 3%, 4%, and 5%) shown lower weight loss as compared to the uncoated control. Because ChNP has low cytotoxicity, it is an excellent antifungal, antioxidant, and coating agent. | [233] |
| Alginate–lactate | Strawberry | Dipping | The application of strawberry coatings under the analyzed osmotic dehydration conditions was an efficient method for considerably reducing solids gain while maintaining water loss. Additionally, the presence of the coating had no detrimental effect on the drying rate of strawberries after later microwave drying. | [234] |
| Chitosan | Guava | Dipping | Chitosan improved the quality of guava fruit after it was harvested. Chitosan slows down the ripening of guava. Antioxidant activities were induced by the chitosan covering. | [235] |
| Chitosan–oxidized starch | papaya | Dipping | Edible coatings improved the shelf-life of papayas stored at room temperature, keeping their qualities for a longer period than uncoated fruits. Uncoated papayas reached a final stage of ripening after 5 days, whereas coated papayas reached this stage after 15 days at room temperature, demonstrating that coating aided to give bigger papaya pulp firmness. After 5 days, volatile chemicals associated with papaya fermentation, such as ethyl butanoate, developed, whereas coated fruits produced it after 10 days. Moreover, butyric acid production was nearly ten times higher in uncoated papayas than in coated papayas during 15 days of storage. | [236] |
| Gelatin–Mentha pulegium essential oil | Strawberries | Dipping | Coated strawberries had improved physicochemical and sensory qualities than the control. Gelatin coating on its own was ineffective compared to gelatin combined with essential oil. This combination might be an effective way to improve the shelf-life of strawberries while reducing pesticide use in postharvest treatments. | [237] |
| Collagen–cocoa butter | Rice crisp balls | Panning | The films were visually consistent, manageable, and adaptable. Shellac was replaced with a coating that could be applied on chocolate surfaces. The findings suggested that the films could be useful for covering chocolate products. | [238] |
7. Production, Drawbacks, and Resolution
Generally, films and coatings are developed from marine biopolymers and then applied to different food products to mimic the function of the usual plastic packaging. Despite the potentiality of marine biopolymers as a raw material for packaging, the extraction, isolation, and final application require a lot of treatments and changes to obtain packaging materials with the required properties [47,144,239,240]. Regarding marine protein biopolymers, a significant drawback that limits the application of muscle protein is the low technological strength resulting from the excessive protein–protein chain interactions in the film network [47,239,240]. As a solution, plasticizers are recommended while producing the film [154,241]. The plasticizers lower the protein–protein interactions, improving the film’s toughness, brittleness, and extensibility [242,243]. Collagen, another promising protein biopolymer, is also limited in application due to its low thermal stability and poor mechanical properties [244,245]. The collagen’s proposed resolution is to blend it with other biopolymers and chemical and enzymatic treatments to enhance its functional properties [245]. Gelatin, which is highly hygroscopic, is responsible for the sudden reduction in moisture barrier and technological strength [246]. To overcome this issue, the application of a multi-layer structure, crosslinking using nanofillers, and a plant extract are recommended [247,248]. The multi-layer form displays a compact and uniform system [248], crosslinking produces a modified network structure [249], and the plant extract improves the rheological and water-barrier properties [250]. The same strategies are also applied to other biopolymers. The application of other polysaccharides and nanoparticles in line with chitosan has been found effective in enhancing the mechanical and functional properties of the films [251,252]. Agar and carrageenan and their films and coatings can also be modified and enhanced with the incorporation of active agents, nanoparticles, and other polysaccharides.
8. Safety Concerns Related to Marine Biopolymer-Based Edible Packaging
Edible films and coatings are used to create biodegradable and environmentally friendly packaging; they are capable of obviating the necessity for basic food packaging. Edible films and coatings are used to protect food products and enhance their shelf-life, offering an advantage over conventional food packaging. A key disadvantage is the number of active agents released. These active compounds diffuse from packaging to food products due to the concentration gradient. The food product will be harmed if a high concentration of antimicrobial is released. The color and flavor of the food might change, and it can become dangerous and unsafe to eat. As a result, to accomplish their role, antimicrobial chemical discharge should be limited to a minimum [192]. Nanoparticles act as active agents while also enhancing the barrier properties. On food surfaces, nanoparticles act as antibacterial agents, and they may enter the human body after eating. Nanoparticles are present in large quantities in edible films and coatings, and they can enter the body if consumed. Because of their minuscule size, they can invade human organs and cause damage. They can cause genotoxicity by changing the DNA material. Human lungs have been discovered to be penetrated by silver nanoparticles with a diameter of 20 nm in experiments. The size and number of nanoparticles used in edible packaging are essential [227]. In Europe, the European framework law (2004/1935/EC) allows for the concept of active packaging, which allows for the intentional release of active agents [146]. All member states’ legislation was harmonized with the establishment of the European Union in order to create a united market and eliminate trade obstacles. Until now, EU food contact regulation has protected consumers’ health by ensuring that no object in touch with foodstuffs can produce a chemical reaction that modifies their composition or organoleptic properties. Regulation 1935/2004/EC repeals this law, allowing packaging to benefit from technological improvements. This was necessary for the EU since all packaging materials (including those that add substances to food on purpose) must meet all food-contact material criteria, including overall migration limits (OMLs) and specific migration limits (SMLs) [253]. Food additives are those that can be used in edible coating compositions and are listed in the additives for general use list. As long as the ‘quantum satis’ idea is followed, these coating-producing compounds can be used [254]. In addition, the Food Additive Purity Directive 2008/84/EC sets strict purity standards for food additives. The existence of these chemicals should be disclosed on the label because edible coatings may include beneficial components.
9. Conclusions and Future Perspectives
Marine sources are a natural factory of carbohydrate and protein biopolymers suitable for the formulation and production of advanced food packaging systems that can be ground-breaking in terms of generating revenue for food industries, improving food safety, meeting consumer demands, and reducing environmental issues imposed by many societies and institutions. This review focused on existing data on their properties, chemical composition, extraction methods, biodegradability, and applicability in food packaging applications. The number of marine sources and the by-products imposed by food-processing plants is enormous. In this case, the sources can be valorized to provide raw materials that are effective in food packaging, reducing the use of nonrenewable and nonbiodegradable materials while adhering to sustainability guidelines. In addition, active substances such as natural antioxidants and antimicrobials should be incorporated into food packaging applications with biopolymers. To build a sustainable food packaging system employing marine biopolymers, more research is needed to better understand the valorization perspectives, mechanism of action of active agents, new techniques to improve technological features, and cost competitiveness.
Author Contributions
Conceptualization, R.T, N.M. and J.I.; writing—original draft preparation, N.M. and J.I.; writing—review and editing, R.T, N.M. and J.I.; visualization, N.M. and J.I.; supervision, R.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are available within the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Abral, H.; Pratama, A.B.; Handayani, D.; Mahardika, M.; Aminah, I.; Sandrawati, N.; Sugiarti, E.; Muslimin, A.N.; Sapuan, S.M.; Ilyas, R.A. Antimicrobial edible film prepared from bacterial cellulose nanofibers/starch/chitosan for a food packaging alternative. Int. J. Polym. Sci. 2021, 2021, 1–11. [Google Scholar] [CrossRef]
- Gaikwad, K.K.; Singh, S.; Ajji, A. Moisture Absorbers for Food Packaging Applications. Environ. Chem. Lett. 2019, 17, 609–628. [Google Scholar] [CrossRef]
- Al-Tayyar, N.A.; Youssef, A.M.; Al-Hindi, R.R. Antimicrobial packaging efficiency of ZnO-SiO2 nanocomposites infused into PVA/CS film for enhancing the shelf life of food products. Food Packag. Shelf Life 2020, 25, 100523. [Google Scholar] [CrossRef]
- Youssef, A.M.; Assem, F.M.; Abdel-Aziz, M.E.; Elaaser, M.; Ibrahim, O.A.; Mahmoud, M.; Abd El-Salam, M.H. Development of bionanocomposite materials and its use in coating of ras cheese. Food Chem. 2019, 270, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Adrah, K.; Ananey-Obiri, D.; Tahergorabi, R. Development of bio-based and biodegradable plastics. In Handbook of Nanomaterials and Nanocomposites for Energy and Environmental Applications; Kharissova, O.V., Martínez, L.M.T., Kharisov, B.I., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–25. [Google Scholar] [CrossRef]
- Hahladakis, J.N.; Velis, C.A.; Weber, R.; Iacovidou, E.; Purnell, P. An overview of chemical additives present in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling. J. Hazard. Mater. 2018, 344, 179–199. [Google Scholar] [CrossRef] [PubMed]
- Luijsterburg, B.; Goossens, H. Assessment of plastic packaging waste: Material origin, methods, properties. Resour. Conserv. Recycl. 2014, 85, 88–97. [Google Scholar] [CrossRef]
- Padervand, M.; Lichtfouse, E.; Robert, D.; Wang, C. Removal of microplastics from the environment. A review. Environ. Chem. Lett. 2020, 18, 807–828. [Google Scholar] [CrossRef]
- Qasim, U.; Osman, A.I.; Al-Muhtaseb, A.H.; Farrell, C.; Al-Abri, M.; Ali, M.; Vo, D.-V.N.; Jamil, F.; Rooney, D.W. Renewable cellulosic nanocomposites for food packaging to avoid fossil fuel plastic pollution: A review. Environ. Chem. Lett. 2021, 19, 613–641. [Google Scholar] [CrossRef]
- Gaikwad, K.K.; Singh, S.; Lee, Y.S. Oxygen scavenging films in food packaging. Environ. Chem. Lett. 2018, 16, 523–538. [Google Scholar] [CrossRef]
- Global Plastic Production 1950–2020. Available online: https://www.statista.com/statistics/282732/global-production-of-plastics-since-1950/ (accessed on 27 November 2021).
- Biodegradable Plastic Packaging Market Value Worldwide 2026. Available online: https://www.statista.com/statistics/1136290/global-biodegradable-plastic-packaging-market-size/ (accessed on 28 November 2021).
- Biodegradable Plastics U.S. Market Value 2025. Available online: https://www.statista.com/statistics/1058523/us-biodegradable-plastic-market-value/ (accessed on 27 November 2021).
- Merino, D.; Gutiérrez, T.J.; Alvarez, V.A. Structural and thermal properties of agricultural mulch films based on native and oxidized corn starch nanocomposites. Starch-Stärke 2019, 71, 1800341. [Google Scholar] [CrossRef]
- Ruban, S. Biobased packaging—Application in meat industry. Vet. World 2009, 2, 79. [Google Scholar] [CrossRef]
- Souza, B.W.S.; Cerqueira, M.A.; Martins, J.T.; Casariego, A.; Teixeira, J.A.; Vicente, A.A. Influence of electric fields on the structure of chitosan edible coatings. Food Hydrocoll. 2010, 24, 330–335. [Google Scholar] [CrossRef]
- Mensitieri, G.; Di Maio, E.; Buonocore, G.G.; Nedi, I.; Oliviero, M.; Sansone, L.; Iannace, S. Processing and shelf life issues of selected food packaging materials and structures from renewable resources. Trends Food Sci. Technol. 2011, 22, 72–80. [Google Scholar] [CrossRef]
- Merino, D.; Gutiérrez, T.J.; Alvarez, V.A. Potential agricultural mulch films based on native and phosphorylated corn starch with and without surface functionalization with chitosan. J. Polym. Environ. 2019, 27, 97–105. [Google Scholar] [CrossRef]
- Rai, M.; Ingle, A.P.; Gupta, I.; Pandit, R.; Paralikar, P.; Gade, A.; Chaud, M.V.; dos Santos, C.A. Smart nanopackaging for the enhancement of food shelf life. Environ. Chem. Lett. 2019, 17, 277–290. [Google Scholar] [CrossRef]
- El-Sayed, S.M.; El-Sayed, H.S.; Ibrahim, O.A.; Youssef, A.M. Rational design of chitosan/guar gum/zinc oxide bionanocomposites based on roselle calyx extract for ras cheese coating. Carbohydr. Polym. 2020, 239, 116234. [Google Scholar] [CrossRef]
- De la Caba, K.; Guerrero, P.; Trung, T.S.; Cruz-Romero, M.; Kerry, J.P.; Fluhr, J.; Maurer, M.; Kruijssen, F.; Albalat, A.; Bunting, S.; et al. From seafood waste to active seafood packaging: An emerging opportunity of the circular economy. J. Clean. Prod. 2019, 208, 86–98. [Google Scholar] [CrossRef]
- Suresh, P.V.; Kudre, T.G.; Johny, L.C. Sustainable valorization of seafood processing by-product/discard. In Waste to Wealth; Energy, Environment, and Sustainability; Singhania, R.R., Agarwal, R.A., Kumar, R.P., Sukumaran, R.K., Eds.; Springer: Singapore, 2018; pp. 111–139. [Google Scholar] [CrossRef]
- Chitosan: Derivatives, Composites and Applications | Wiley. Available online: https://www.wiley.com/en-us/Chitosan%3A+Derivatives%2C+Composites+and+Applications-p-9781119364818 (accessed on 28 November 2021).
- Mathew, G.M.; Huang, C.C.; Sindhu, R.; Binod, P.; Sirohi, R.; Awsathi, M.K.; Pillai, S.; Pandey, A. Enzymatic approaches in the bioprocessing of shellfish wastes. 3 Biotech 2021, 11, 367. [Google Scholar] [CrossRef]
- Senturk Parreidt, T.; Lindner, M.; Rothkopf, I.; Schmid, M.; Müller, K. The development of a uniform alginate-based coating for cantaloupe and strawberries and the characterization of water barrier properties. Foods 2019, 8, 203. [Google Scholar] [CrossRef]
- Rios do Amaral, L.; Achaerandio Puente, M.I.; Benedetti, B.C.; Pujolà Cunill, M. The influence of edible coatings, blanching and ultrasound treatments on quality attributes and shelf-life of vacuum packaged potato strips. LWT Food Sci. Technol. 2017, 85, 449–455. [Google Scholar] [CrossRef]
- Rojas-Graü, M.A.; Raybaudi-Massilia, R.M.; Soliva-Fortuny, R.C.; Avena-Bustillos, R.J.; McHugh, T.H.; Martín-Belloso, O. Apple puree-alginate edible coating as carrier of antimicrobial agents to prolong shelf-life of fresh-cut apples. Postharvest Biol. Technol. 2007, 45, 254–264. [Google Scholar] [CrossRef]
- Chen, J.; Li, L.; Yi, R.; Xu, N.; Gao, R.; Hong, B. Extraction and characterization of acid-soluble collagen from scales and skin of tilapia (Oreochromis Niloticus). LWT Food Sci. Technol. 2016, 66, 453–459. [Google Scholar] [CrossRef]
- Sustainable Food Packaging Technology, 1st ed.; Athanassiou, A., Ed.; Wiley: Hoboken, NJ, USA, 2021. [Google Scholar] [CrossRef]
- Kafle, G.K.; Kim, S.H.; Sung, K.I. Ensiling of fish industry waste for biogas production: A lab scale evaluation of biochemical methane potential (BMP) and kinetics. Bioresour. Technol. 2013, 127, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Garrido, T.; Uranga, J.; Guerrero, P.; de la Caba, K. The potential of vegetal and animal proteins to develop more sustainable food packaging. In Polymers for Food Applications; Gutiérrez, T.J., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 25–59. [Google Scholar] [CrossRef]
- Vázquez, J.A.; Meduíña, A.; Durán, A.I.; Nogueira, M.; Fernández-Compás, A.; Pérez-Martín, R.I.; Rodríguez-Amado, I. Production of valuable compounds and bioactive metabolites from by-products of fish discards using chemical processing, enzymatic hydrolysis, and bacterial fermentation. Mar. Drugs 2019, 17, E139. [Google Scholar] [CrossRef]
- Murrieta-Martínez, C.L.; Soto-Valdez, H.; Pacheco-Aguilar, R.; Torres-Arreola, W.; Rodríguez-Felix, F.; Márquez Ríos, E. Edible protein films: Sources and behavior. Packag. Technol. Sci. 2018, 31, 113–122. [Google Scholar] [CrossRef]
- Wang, G.; Huang, D.; Ji, J.; Völker, C.; Wurm, F.R. Seawater-degradable polymers: Seawater-degradable polymers—Fighting the marine plastic pollution. Adv. Sci. 2021, 8, 2170004. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, S.; Wang, H. Scale-Up Preparation and characterization of collagen/sodium alginate blend films. J. Food Qual. 2017, 2017, e4954259. [Google Scholar] [CrossRef]
- Lionetto, F.; Esposito Corcione, C. Recent applications of biopolymers derived from fish industry waste in food packaging. Polymers 2021, 13, 2337. [Google Scholar] [CrossRef]
- Kozłowicz, K.; Nazarewicz, S.; Góral, D.; Krawczuk, A.; Domin, M. Lyophilized protein structures as an alternative biodegradable material for food packaging. Sustainability 2019, 11, 7002. [Google Scholar] [CrossRef]
- Goyal, N.; Rastogi, D.; Jassal, M.; Agrawal, A.K. Chitosan as a potential stabilizing agent for titania nanoparticle dispersions for preparation of multifunctional cotton fabric. Carbohydr. Polym. 2016, 154, 167–175. [Google Scholar] [CrossRef]
- Demitri, C.; Moscatello, A.; Giuri, A.; Raucci, M.G.; Esposito Corcione, C. Preparation and characterization of EG-chitosan nanocomposites via direct exfoliation: A green methodology. Polymers 2015, 7, 2584–2594. [Google Scholar] [CrossRef]
- Gokoglu, N. Innovations in seafood packaging technologies: A review. Food Rev. Int. 2020, 36, 340–366. [Google Scholar] [CrossRef]
- Rhim, J.-W.; Wang, L.-F. Mechanical and water barrier properties of agar/κ-carrageenan/konjac glucomannan ternary blend biohydrogel films. Carbohydr Polym. 2013, 96, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Asgher, M.; Qamar, S.A.; Bilal, M.; Iqbal, H.M.N. Bio-based active food packaging materials: Sustainable alternative to conventional petrochemical-based packaging materials. Food Res. Int. 2020, 137, 109625. [Google Scholar] [CrossRef] [PubMed]
- Bocqué, M.; Voirin, C.; Lapinte, V.; Caillol, V.; Robin, J.-J. Petro-based and bio-based plasticizers: Chemical structures to plasticizing properties. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 11–33. [Google Scholar] [CrossRef]
- Medina, E.; Caro, N.; Abugoch, L.; Gamboa, A.; Díaz-Dosque, M.; Tapia, C. Chitosan thymol nanoparticles improve the antimicrobial effect and the water vapour barrier of chitosan-quinoa protein films. J. Food Eng. 2019, 240, 191–198. [Google Scholar] [CrossRef]
- Gómez-Estaca, J.; Gómez-Guillén, M.C.; Fernández-Martín, F.; Montero, P. Effects of gelatin origin, bovine-hide and tuna-skin, on the properties of compound gelatin–chitosan films. Food Hydrocoll. 2011, 25, 1461–1469. [Google Scholar] [CrossRef]
- Bourbon, A.I.; Pereira, R.N.; Pastrana, L.M.; Vicente, A.A.; Cerqueira, M.A. Protein-based nanostructures for food applications. Gels 2019, 5, 9. [Google Scholar] [CrossRef]
- Zavareze, E.D.R.; Halal, S.L.M.E.; Marques e Silva, R.; Dias, A.R.G.; Prentice-Hernández, C. Mechanical, barrier and morphological properties of biodegradable films based on muscle and waste proteins from the whitemouth croaker (Micropogonias Furnieri). J. Food Proc. Prese. 2014, 38, 1973–1981. [Google Scholar] [CrossRef]
- Arfat, Y.A.; Jasim, A.; Nikhil, H.; Rafael, A.; Antony, J. Thermo-mechanical, rheological, structural and antimicrobial properties of bionanocomposite films based on fish skin gelatin and silver-copper nanoparticles. Food Hydrocoll. 2017, 62, 191–202. [Google Scholar] [CrossRef]
- Limpan, N.; Prodpran, T.; Benjakul, S.; Prasarpran, S. Properties of biodegradable blend films based on fish myofibrillar protein and polyvinyl alcohol as influenced by blend composition and pH level. J. Food Eng. 2010, 100, 85–92. [Google Scholar] [CrossRef]
- Nie, X.; Zhao, L.; Wang, N.; Meng, X. Phenolics-protein interaction involved in silver carp myofibrilliar protein films with hydrolysable and condensed tannins. LWT Food Sci. Tech. 2017, 81, 258–264. [Google Scholar] [CrossRef]
- Medina, I.; Pazos, M. Oxidation and protection of fish. In Oxidation in Foods and Beverages and Antioxidant Applications. Management in Different Industry Sectors; Elsevier: Amsterdam, The Netherlands, 2010; Volume 2, pp. 91–120. [Google Scholar]
- Kaewprachu, P.; Osako, K.; Rawdkuen, S. Effects of plasticizers on the properties of fish myofibrillar protein film. J. Food Sci. Technol. 2018, 55, 3046–3055. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.J.; Lonergan, S.M.; Huff-Lonergan, E. Huff-Lonergan. Myosin light chain 1 release from myofibrillar fraction during postmortem aging is a potential indicator of proteolysis and tenderness of beef. Meat Sci. 2012, 90, 345–351. [Google Scholar] [CrossRef]
- Bowker, B.C.; Fahrenholz, T.M.; Paroczay, E.W. Effect of hydrodynamic pressure processing and aging on the tenderness and myofibrillar proteins of beef strip loins. J. Muscle Foods 2008, 19, 74–97. [Google Scholar] [CrossRef]
- Della Malva, A.; Albenzio, M.; Santillo, A.; Russo, D.; Figliola, L.; Caroprese, M.; Marino, R. Methods for extraction of muscle proteins from meat and fish using denaturing and nondenaturing solutions. J. Food Qual. 2018, 2018, e8478471. [Google Scholar] [CrossRef]
- Coppola, D.; Oliviero, M.; Vitale, G.A.; Lauritano, C.; D’Ambra, I.; Iannace, S.; de Pascale, D. Marine collagen from alternative and sustainable sources: Extraction, processing and applications. Mar. Drugs 2020, 18, 214. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Bonilla, A.; Echeverria, C.; Sonseca, Á.; Arrieta, M.P.; Fernández-García, M. Bio-based polymers with antimicrobial properties towards sustainable development. Materials 2019, 12, E641. [Google Scholar] [CrossRef]
- Chinh, N.T.; Manh, V.Q.; Trung, V.Q.; Lam, T.D.; Huynh, M.D.; Tung, N.Q.; Trinh, N.D.; Hoang, T. Characterization of collagen derived from tropical freshwater carp fish scale wastes and its amino acid sequence. Nat. Product Comm. 2019, 14, 1934578X19866288. [Google Scholar] [CrossRef]
- Blanco, M.; Vázquez, J.A.; Pérez-Martín, R.I.G.; Sotelo, C. Collagen extraction optimization from the skin of the small-spotted catshark (S. canicula) by response surface methodology. Mar. Drugs 2019, 17, 40. [Google Scholar] [CrossRef]
- Song, Z.; Liu, H.; Chen, L.; Chen, L.; Zhou, C.; Hong, P.; Deng, C. Characterization and comparison of collagen extracted from the skin of the nile tilapia by fermentation and chemical pretreatment. Food Chem. 2021, 340, 128139. [Google Scholar] [CrossRef]
- Chen, S.; Tang, L.; Hao, G.; Weng, W.; Osako, K.; Tanaka, M. Effects of A1/A2 ratios and drying temperatures on the properties of gelatin films prepared from tilapia (Tilapia zillii) skins. Food Hydrocoll. 2016, 52, 573–580. [Google Scholar] [CrossRef]
- Chuaychan, S.; Benjakul, S.; Nuthong, P. Element distribution and morphology of spotted golden goatfish fish scales as affected by demineralisation. Food Chem. 2016, 197, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Guanghua, X.; Li, Y.; Shen, X.R.; Li, C. Comparison of characteristics and fibril-forming ability of skin collagen from barramundi (Lates calcarifer) and tilapia (Oreochromis niloticus). Int. J. Biol. Macromol. 2018, 107, 549–559. [Google Scholar] [CrossRef] [PubMed]
- León-López, A.; Morales-Peñaloza, A.; Martínez-Juárez, V.M.; Vargas-Torres, A.; Zeugolis, D.I.; Aguirre-Álvarez, G. Hydrolyzed collagen—Sources and applications. Molecules 2019, 24, 4031. [Google Scholar] [CrossRef] [PubMed]
- Sae-leaw, T.; Benjakul, S.; O’Brien, N.M. Effects of defatting and tannic acid incorporation during extraction on properties and fishy odour of gelatin from seabass skin. LWT Food Sci. Technol. 2016, 65, 661–667. [Google Scholar] [CrossRef]
- Tan, Y.; Chang, S.K.C. Isolation and characterization of collagen extracted from channel catfish (Ictalurus punctatus) skin. Food Chem. 2018, 242, 147–155. [Google Scholar] [CrossRef]
- Hanjabam, M.D.; Kannaiyan, S.K.; Kamei, G.; Jakhar, J.K.; Chouksey, M.K.; Gudipati, V. Optimisation of gelatin extraction from unicorn leatherjacket (Aluterus monoceros) skin waste: Response surface approach. J. Food Sci. Technol. 2015, 52, 976–983. [Google Scholar] [CrossRef] [PubMed]
- Pal, G.K.; Nidheesh, T.; Suresh, P.V. Comparative study on characteristics and in vitro fibril formation ability of acid and pepsin soluble collagen from the skin of catla (Catla catla) and rohu (Labeo rohita). Food Res. Int. 2015, 76, 804–812. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.; Getachew, A.T.; Cho, Y.-J.; Chun, B.-S. Application of bacterial collagenolytic proteases for the extraction of type I collagen from the skin of bigeye tuna (Thunnus obesus). LWT 2018, 89, 44–51. [Google Scholar] [CrossRef]
- Abdollahi, M.; Rezaei, M.; Jafarpour, A.; Undeland, I. Sequential extraction of gel-forming proteins, collagen and collagen hydrolysate from gutted silver carp (Hypophthalmichthys molitrix), a biorefinery approach. Food Chem. 2018, 242, 568–578. [Google Scholar] [CrossRef]
- Wang, L.; Liang, Q.; Chen, T.; Wang, Z.; Xu, J.; Ma, H. Characterization of collagen from the skin of amur sturgeon (Acipenser schrenckii). Food Hydrocoll. 2014, 38, 104–109. [Google Scholar] [CrossRef]
- Jeevithan, E.; Wu, W.; Nanping, W.; Lan, H.; Bao, B. Isolation, purification and characterization of pepsin soluble collagen Isolated from silvertip shark (Carcharhinus albimarginatus) skeletal and head bone. Process Biochem. 2014, 49, 1767–1777. [Google Scholar] [CrossRef]
- Zhang, F.; Xu, S.; Wang, Z. Pre-treatment optimization and properties of gelatin from freshwater fish scales. Food Bioprod. Process. 2011, 89, 185–193. [Google Scholar] [CrossRef]
- Azmi, N.S.; Kadir Basha, R.; Tajul Arifin, N.N.; Othman, S.H.; Mohammed, M.A.P. Functional properties of tilapia’s fish scale gelatin film: Effects of different type of plasticizers. Sains Malaysiana 2020, 49, 2221–2229. [Google Scholar] [CrossRef]
- The Potential of Proteins for Producing Food Packaging Materials: A Review—Gómez-Estaca 2016 Packaging Technology and Science—Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/10.1002/pts.2198 (accessed on 28 November 2021).
- De Melo Oliveira, V.; Assis, C.R.D.; Costa, B.D.A.M.; de Araujo Neri, R.C.; Monte, F.T.D.; da Costa Vasconcelos Freitas, H.M.S.; França, R.C.P.; Santos, J.F.; de Souza Bezerra, R.; Porto, A.L.F. Physical, biochemical, densitometric and spectroscopic techniques for characterization collagen from alternative sources: A review based on the sustainable valorization of aquatic by-products. J. Mol. Struct. 2021, 1224, 129023. [Google Scholar] [CrossRef]
- Pal, G.K.; Suresh, P.V. Sustainable valorisation of seafood by-products: Recovery of collagen and development of collagen-based novel functional food ingredient. Innov. Food Sci. Emerg. Technol. 2016, 37, 201–215. [Google Scholar] [CrossRef]
- Khrunyk, Y.; Lach, S.; Petrenko, I.; Ehrlich, H. Progress in modern marine biomaterials research. Mar. Drugs 2020, 18, 589. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Allan, C.R.; Hadwiger, L.A. The fungicidal effect of chitosan on fungi of varying cell wall composition. Exp. Mycol. 1979, 3, 285–287. [Google Scholar] [CrossRef]
- Industrial Prospects for Chitin and Protein from Shellfish Wastes: A Report on the First Marine Industries Business Strategy Program Marine Industry Advisory Service. Available online: https://repository.library.noaa.gov/view/noaa/9621 (accessed on 28 November 2021).
- Rathke, T.D.; Hudson, S.M. Determination of the degree of N-deacetylation in chitin and chitosan as well as their monomer sugar ratios by near infrared spectroscopy. J. Polym. Sci. Part A Polym. Chem. 1993, 31, 749–753. [Google Scholar] [CrossRef]
- Puvvada, Y.S.; Vankayalapati, S.; Sukhavasi, S. Extraction of chitin from chitosan from exoskeleton of shrimp for application in the pharmaceutical industry. Pharm. J. 2012, 1, 258–263. [Google Scholar] [CrossRef]
- Sugimoto, M.; Morimoto, M.; Sashiwa, H.; Saimoto, H.; Shigemasa, Y. Preparation and characterization of water-soluble chitin and chitosan derivatives. Carbohydr. Polym. 1998, 36, 49–59. [Google Scholar] [CrossRef]
- Hamed, I.; Özogul, F.; Regenstein, J.M. Industrial applications of crustacean by-products (chitin, chitosan, and chitooligosaccharides): A review. Trends Food Sci. Technol. 2016, 48, 40–50. [Google Scholar] [CrossRef]
- Rocha, M.A.M.; Coimbra, M.A.; Nunes, C. Applications of chitosan and their derivatives in beverages: A critical review. Curr. Opin. Food Sci. 2017, 15, 61–69. [Google Scholar] [CrossRef]
- Sinha, V.R.; Singla, A.K.; Wadhawan, S.; Kaushik, R.; Kumria, R.; Bansal, K.; Dhawan, S. Chitosan microspheres as a potential carrier for drugs. Int. J. Pharm. 2004, 274, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Chenite, A.; Buschmann, M.; Wang, D.; Chaput, C.; Kandani, N. Rheological characterisation of thermogelling chitosan/glycerol-phosphate solutions. Carbohydr. Polym. 2001, 46, 39–47. [Google Scholar] [CrossRef]
- Hou, Y.; Shavandi, A.; Carne, A.; Bekhit, A.A.; Ng, T.B.; Cheung, R.C.F.; Bekhit, A.E.A. Marine shells: Potential opportunities for extraction of functional and health-promoting materials. Crit. Rev. Environ. Sci. Technol. 2016, 46, 1047–1116. [Google Scholar] [CrossRef]
- Muxika, A.; Etxabide, A.; Uranga, J.; Guerrero, P.; de la Caba, K. Chitosan as a bioactive polymer: Processing, properties and applications. Int. J. Biol. Macromol. 2017, 105, 1358–1368. [Google Scholar] [CrossRef] [PubMed]
- Castillo, L.A.; Farenzena, S.; Pintos, E.; Rodríguez, M.S.; Villar, M.A.; García, M.A.; López, O.V. Active films based on thermoplastic corn starch and chitosan oligomer for food packaging applications. Food Packag. Shelf Life 2017, 14, 128–136. [Google Scholar] [CrossRef]
- Baron, R.D.; Pérez, L.L.; Salcedo, J.M.; Córdoba, L.P.; do Amaral Sobral, P.J. Production and characterization of films based on blends of chitosan from blue crab (Callinectes sapidus) waste and pectin from orange (Citrus Sinensis osbeck) peel. Int. J. Biol. Macromol. 2017, 98, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-Y.; Kuo, C.-H.; Wu, C.-H.; Ku, M.-W.; Chen, P.-W. Extraction of crude chitosans from squid (Illex argentinus) pen by a compressional puffing-pretreatment process and evaluation of their antibacterial activity. Food Chem. 2018, 254, 217–223. [Google Scholar] [CrossRef]
- Sedaghat, F.; Yousefzadi, M.; Toiserkani, H.; Najafipour, S. Bioconversion of shrimp waste Penaeus merguiensis using lactic acid fermentation: An alternative procedure for chemical extraction of chitin and chitosan. Int. J. Biol. Macromol. 2017, 104, 883–888. [Google Scholar] [CrossRef] [PubMed]
- Pusztahelyi, T. Chitin and chitin-related compounds in plant–fungal interactions. Mycology 2018, 9, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yun, S.; Song, L.; Zhang, Y.; Zhao, Y. The preparation and characterization of chitin and chitosan under large-scale submerged fermentation level using shrimp by-products as substrate. Int. J. Biol. Macromol. 2017, 96, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Shavandi, A.; Hu, Z.; Teh, S.; Zhao, J.; Carne, A.; Bekhit, A.; Bekhit, A.E.-D.A. Antioxidant and functional properties of protein hydrolysates obtained from squid pen chitosan Extraction effluent. Food Chem. 2017, 227, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Pachapur, V.L.; Guemiza, K.; Rouissi, T.; Sarma, S.J.; Brar, S.K. Novel biological and chemical methods of chitin extraction from crustacean waste using saline water. J Chem. Technol. Biotechnol. 2016, 91, 2331–2339. [Google Scholar] [CrossRef]
- Lopes, C.; Antelo, L.T.; Franco-Uría, A.; Alonso, A.A.; Pérez-Martín, R. Chitin production from crustacean biomass: Sustainability assessment of chemical and enzymatic processes. J. Clean. Prod. 2018, 172, 4140–4151. [Google Scholar] [CrossRef]
- El Knidri, H.; El Khalfaouy, R.; Laajeb, A.; Addaou, A.; Lahsini, A. Eco-friendly extraction and characterization of chitin and chitosan from the shrimp shell waste via microwave irradiation. Process Saf. Env. Prot. 2016, 104, 395–405. [Google Scholar] [CrossRef]
- Hosseinnejad, M.; Jafari, S.M. Evaluation of different factors affecting antimicrobial properties of chitosan. Int. J. Biol. Macromol. 2016, 85, 467–475. [Google Scholar] [CrossRef]
- Ibrahim, H.M.; El-Zairy, E.M.R. Chitosan as a biomaterial—Structure, properties, and electrospun nanofibers. Concepts Compd. Altern. Antibact. 2015, 81–101. [Google Scholar]
- Liu, Y.; Yuan, Y.; Duan, S.; Li, C.; Hu, B.; Liu, A.; Wu, D.; Cui, H.; Lin, L.; He, J.; et al. Preparation and characterization of chitosan films with three kinds of molecular weight for food packaging. Int. J. Biol. Macromol. 2020, 155, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Yang, L.; Kennedy, J.F.; Tian, S. Effects of chitosan and oligochitosan on growth of two fungal pathogens and physiological properties in pear fruit. Carb. Polym. 2010, 81, 70–75. [Google Scholar] [CrossRef]
- Hamdi, M.; Nasri, R.; Amor, I.B.; Li, S.; Gargouri, J.; Nasri, M. Structural features, anti-coagulant and anti-adhesive potentials of blue crab (Portunus segnis) chitosan derivatives: Study of the effects of acetylation degree and molecular weight. Int. J. Biol. Macromol. 2020, 160, 593–601. [Google Scholar] [CrossRef]
- Roy, J.C.; Salaün, F.; Giraud, S.; Ferri, A. Solubility of Chitin: Solvents, Solution Behaviors and Their Related Mechanisms; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef]
- Abdelmalek, B.E.; Sila, A.; Haddar, A.; Bougatef, A.; Ayadi, M.A. β-chitin and chitosan from squid gladius: Biological activities of chitosan and its application as clarifying agent for apple juice. Int. J. Biol. Macromol. 2017, 104, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Kariduraganavar, M.Y.; Kittur, A.A.; Kamble, R.R. Chapter 1—Polymer synthesis and processing. In Natural and Synthetic Biomedical Polymers; Kumbar, S.G., Laurencin, C.T., Deng, M., Eds.; Elsevier: Oxford, UK, 2014; pp. 1–31. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Richardson, S.W.; Kolbe, H.J.; Duncan, R. Potential of low molecular mass chitosan as a DNA delivery system: Biocompatibility, body distribution and ability to complex and protect DNA. Int. J. Pharm. 1999, 178, 231–243. [Google Scholar] [CrossRef]
- Issa, A.T.; Tahergorabi, R. Barrier, degradation, and cytotoxicity studies for chitin-chitosan bionanocomposites. In Chitin-and Chitosan-Based Biocomposites for Food Packaging Applications; CRC Press: Boca Raton, FL, USA, 2020; pp. 49–58. [Google Scholar]
- Venkatesan, J.; Bhatnagar, I.; Manivasagan, P.; Kang, K.-H.; Kim, S.-K. Alginate composites for bone tissue engineering: A review. Int. J. of Biol. Macromol. 2015, 72, 269–281. [Google Scholar] [CrossRef]
- Giri, S.; Dutta, P.; Kumarasamy, D.; Giri, T.K. Chapter 1—Natural polysaccharides: Types, basic structure and suitability for forming hydrogels. In Plant and Algal Hydrogels for Drug Delivery and Regenerative Medicine; Giri, T.K., Ghosh, B., Eds.; Woodhead Publishing Series in Biomaterials; Woodhead Publishing: Sawston, UK, 2021; pp. 1–35. [Google Scholar] [CrossRef]
- Rinaudo, M. Biomaterials based on a natural polysaccharide: Alginate. TIP Revista Especializada en Ciencias Químico-Biológicas 2014, 17, 92–96. [Google Scholar] [CrossRef]
- Ogaji, I.J.; Nep, E.I.; Audu-Peter, J.D. Advances in natural polymers as pharmaceutical excipients. Pharm. Anal. Acta 2012, 03. [Google Scholar] [CrossRef]
- Williams, P.A.; Campbell, K.T.; Gharaviram, H.; Madrigal, J.L.; Silva, E.A. Alginate-chitosan hydrogels provide a sustained gradient of sphingosine-1-phosphate for therapeutic angiogenesis. Ann. Biomed. Eng. 2017, 45, 1003–1014. [Google Scholar] [CrossRef]
- George, M.; Abraham, T.E. Polyionic hydrocolloids for the intestinal delivery of protein drugs: Alginate and chitosan—A review. J. Cont. Rel. 2006, 114, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Paul, W.; Sharma, C.P. Chitosan and alginate wound dressings: A short review. Trends Biomater. Artif. Organs 2004, 18, 18–24. [Google Scholar]
- Pawar, S.N.; Edgar, K.J. Alginate derivatization: A review of chemistry, properties and applications. Biomaterials 2012, 33, 3279–3305. [Google Scholar] [CrossRef]
- Hernández-Carmona, G.; McHugh, D.J.; López-Gutiérrez, F. Pilot plant scale extraction of alginates from macrocystis pyrifera. 2. Studies on extraction conditions andmethods of separating the alkaline-insoluble residue. J. Appl. Phycol. 1999, 11, 493–502. [Google Scholar] [CrossRef]
- Vauchel, P.; Arhaliass, A.; Legrand, J.; Kaas, R.; Baron, R. Decrease in dynamic viscosity and average molecular weight of alginate from laminaria digitata during alkaline extraction1. J. Phycol. 2008, 44, 515–517. [Google Scholar] [CrossRef]
- Peteiro, C. Alginate production from marine macroalgae, with emphasis on kelp farming. In Alginates and Their Biomedical Applications; Rehm, B.H.A., Moradali, M.F., Eds.; Springer Series in Biomaterials Science and Engineering; Springer: Singapore, 2018; pp. 27–66. [Google Scholar] [CrossRef]
- Hernández-Carmona, G.; Freile-Pelegrín, Y.; Hernández-Garibay, E. Conventional and alternative technologies for the extraction of algal polysaccharides. In Functional Ingredients from Algae for Foods and Nutraceuticals; Elsevier: Amsterdam, The Netherlands, 2013; pp. 475–516. [Google Scholar] [CrossRef]
- Silva, M.; Gomes, F.; Oliveira, F.; Morais, S.; Delerue-Matos, C. Microwave-assisted alginate extraction from Portuguese saccorhiza polyschides—Influence of acid pretreatment. Int. J. Biotechnol. Bioeng. 2015, 9, 30–33. [Google Scholar]
- Fertah, M.; Belfkira, A.; Dahmane, E.; Taourirte, M.; Brouillette, F. Extraction and characterization of sodium alginate from Moroccan laminaria digitata brown seaweed. Arab. J. Chem. 2017, 10, S3707–S3714. [Google Scholar] [CrossRef]
- Santagata, G.; Grillo, G.; Immirzi, B.; Tabasso, S.; Cravotto, G.; Malinconico, M. Non-conventional ultrasound-assisted extraction of alginates from Sargassum seaweed: From coastal waste to a novel polysaccharide source. In Proceedings of the International Conference on Microplastic Pollution in the Mediterranean Sea; Springer: Cham, Switzerland, 2018; pp. 211–217. [Google Scholar]
- Leal, D.; Matsuhiro, B.; Rossi, M.; Caruso, F. FT-IR spectra of alginic acid block fractions in three species of brown seaweeds. Carb. Res. 2008, 343, 308–316. [Google Scholar]
- Tapia, M.S.; Rojas-Graü, M.A.; Carmona, A.; Rodríguez, F.J.; Soliva-Fortuny, R.; Martin-Belloso, O. Use of alginate- and gellan-based coatings for improving barrier, texture and nutritional properties of fresh-cut papaya. Food Hydrocoll. 2008, 22, 1493–1503. [Google Scholar] [CrossRef]
- Martinsen, A.; Skjåk-Braek, G.; Smidsrød, O. Alginate as immobilization material: I. correlation between chemical and physical properties of alginate gel beads. Biotechnol. Bioeng. 1989, 33, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Grant, G.T.; Morris, E.R.; Rees, D.A.; Smith, P.J.C.; Thom, D. Biological interactions between polysaccharides and divalent cations: The egg-box model. FEBS Lett. 1973, 32, 195–198. [Google Scholar] [CrossRef]
- Seifert, D.B.; Phillips, J.A. Production of small, monodispersed alginate beads for cell immobilization. Biotechnol. Prog. 1997, 13, 562–568. [Google Scholar] [CrossRef]
- Olivas, G.I.; Barbosa-Cánovas, G.V. Alginate–calcium films: Water vapor permeability and mechanical properties as affected by plasticizer and relative humidity. LWT Food Sci. Technol. 2008, 41, 359–366. [Google Scholar] [CrossRef]
- Pereira, L.; Gheda, S.F.; Ribeiro-Claro, P.J.A. Analysis by vibrational spectroscopy of seaweed polysaccharides with potential use in food, pharmaceutical, and cosmetic industries. Int. J. of Carb. Chem. 2013, 2013, e537202. [Google Scholar] [CrossRef]
- Nussinovitch, A.; Gershon, Z. Physical characteristics of agar—Yeast sponges. Food Hydrocoll. 1997, 11, 231–237. [Google Scholar] [CrossRef]
- Hands, S.; Peat, S. Isolation of an anhydro L-galactose derivative from agar. Nature 1938, 142, 797. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Saurabh, C.K.; Tye, Y.Y.; Lai, T.K.; Easa, A.M.; Rosamah, E.; Fazita, M.R.N.; Syakir, M.I.; Adnan, A.S.; Fizree, H.M.; et al. Seaweed based sustainable films and composites for food and pharmaceutical applications: A review. Renew. Sustain. Energy Rev. 2017, 77, 353–362. [Google Scholar] [CrossRef]
- Xiao, Q.; Weng, H.; Ni, H.; Hong, Q.; Lin, K.; Xiao, A. Physicochemical and gel properties of agar extracted by enzyme and enzyme-assisted methods. Food Hydrocoll. 2019, 87, 530–540. [Google Scholar] [CrossRef]
- Lee, W.-K.; Lim, Y.-Y.; Leow, A.T.-C.; Namasivayam, P.; Ong Abdullah, J.; Ho, C.-L. Biosynthesis of agar in red seaweeds: A Review. Carbohydr. Polym. 2017, 164, 23–30. [Google Scholar] [CrossRef]
- Sousa, A.M.M.; Souza, H.K.S.; Liu, L.; Gonçalves, M.P. Alternative plasticizers for the production of thermo-compressed agar films. Int. J. Biol. Macromol. 2015, 76, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Campo, V.L.; Kawano, D.F.; DA Silva, D.B.; Carvalho, I. Carrageenans: Biological properties, chemical modifications and structural analysis—A review. Carb. Polym. 2009, 77, 167–180. [Google Scholar] [CrossRef]
- Nanaki, S.; Karavas, E.; Kalantzi, L.; Bikiaris, D. Miscibility study of carrageenan blends and evaluation of their effectiveness as sustained release carriers. Carb. Polym. 2010, 79, 1157–1167. [Google Scholar] [CrossRef]
- Recalde, M.P.; Canelón, D.J.; Compagnone, R.S.; Matulewicz, M.C.; Cerezo, A.S.; Ciancia, M. Carrageenan and agaran structures from the red seaweed Gymnogongrus tenuis. Carb. Polym. 2016, 136, 1370–1378. [Google Scholar]
- Gómez-Ordóñez, E.; Rupérez, P. FTIR-ATR spectroscopy as a tool for polysaccharide identification in edible brown and red seaweeds. Food Hydrocoll. 2011, 25, 1514–1520. [Google Scholar] [CrossRef]
- Bono, A.; Anisuzzaman, S.M.; Ding, O.W. Effect of process conditions on the gel viscosity and gel strength of semi-refined carrageenan (SRC) produced from seaweed (Kappaphycus alvarezii). J. King Saud Univ. Eng. Sci. 2014, 26, 3–9. [Google Scholar] [CrossRef]
- Kumar, L.; Ramakanth, D.; Akhila, K.; Gaikwad, K.K. Edible films and coatings for food packaging applications: A review. Environ. Chem. Lett. 2021, 1–26. [Google Scholar] [CrossRef]
- Díaz-Montes, E.; Castro-Muñoz, R. Edible films and coatings as food-quality preservers: An overview. Foods 2021, 10, 249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zou, X.; Zhai, X.; Huang, X.; Jiang, C.; Holmes, M. Preparation of an intelligent PH film based on biodegradable polymers and roselle anthocyanins for monitoring pork freshness. Food Chem. 2019, 272, 306–312. [Google Scholar] [CrossRef]
- Armentano, I.F.; Yoon, K.; Ahn, J.; Kang, S.; Kenny, J.M. Bio-based PLA_PHB plasticized blend films: Processing and structural characterization. LWT Food Sci. Technol. 2015, 64, 980–988. [Google Scholar] [CrossRef]
- López de Lacey, A.M.; López-Caballero, M.E.; Montero, P. Agar films containing green tea extract and probiotic bacteria for extending fish shelf-life. LWT Food Sci. Technol. 2014, 55, 559–564. [Google Scholar] [CrossRef]
- Sarwar, M.S.; Niazi, M.B.K.; Jahan, Z.; Ahmad, T.; Hussain, A. Preparation and characterization of PVA/nanocellulose/Ag nanocomposite films for antimicrobial food packaging. Carb. Polym. 2018, 184, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Abdollahzadeh, E.; Mahmoodzadeh Hosseini, H.; Imani Fooladi, A.A. Antibacterial activity of agar-based films containing nisin, cinnamon EO, and ZnO nanoparticles. J. Food Saf. 2018, 38, e12440. [Google Scholar] [CrossRef]
- Robles-Sánchez, R.M.; Rojas-Graü, M.A.; Odriozola-Serrano, I.; González-Aguilar, G.; Martin-Belloso, O. Influence of alginate-based edible coating as carrier of antibrowning agents on bioactive compounds and antioxidant activity in fresh-cut Kent mangoes. LWT Food Sci. Technol. 2013, 50, 240–246. [Google Scholar] [CrossRef]
- Rojas-Graü, M.A.; Avena-Bustillos, R.J.; Olsen, C.; Friedman, M.; Henika, P.R.; Martín-Belloso, O.; Pan, Z.; McHugh, T.H. Effects of plant essential oils and oil compounds on mechanical, barrier and antimicrobial properties of alginate-apple puree edible films. J. Food Eng. 2007, 81, 634–641. [Google Scholar] [CrossRef]
- Kaewprachu, P.; Osako, K.; Benjakul, S.; Suthiluk, P.; Rawdkuen, S. Shelf life extension for bluefin tuna slices (Thunnus thynnus) wrapped with myofibrillar protein film incorporated with catechin-kradon extract. Food Control 2017, 79, 333–343. [Google Scholar] [CrossRef]
- Ahmad, M.; Nirmal, N.P.; Chuprom, J. Molecular characteristics of collagen extracted from the starry triggerfish skin and its potential in the development of biodegradable packaging film. RSC Adv. 2016, 6, 33868–33879. [Google Scholar] [CrossRef]
- Cardoso, G.P.; Dutra, M.P.; Fontes, P.R.; Ramos, A.D.; de Miranda Gomide, L.A.; Ramos, E.M. Selection of a chitosan gelatin-based edible coating for color preservation of beef in retail display. Meat Sci. 2016, 114, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Kakaei, S.; Shahbazi, Y. Effect of chitosan-gelatin film incorporated with ethanolic red grape seed extract and ziziphora clinopodioides essential oil on survival of listeria monocytogenes and chemical, microbial and sensory properties of minced trout Fillet. LWT Food Sci. Technol. 2016, 72, 432–438. [Google Scholar] [CrossRef]
- Alparslan, Y.; Yapıcı, H.H.; Metin, C.; Baygar, T.; Günlü, A.; Baygar, T. Quality assessment of shrimps preserved with orange leaf essential oil incorporated gelatin. LWT Food Sci. Technol. 2016, 72, 457–466. [Google Scholar] [CrossRef]
- Nowzari, F.; Shábanpour, B.; Ojagh, S.M. Comparison of chitosan–gelatin composite and bilayer coating and film Effect on the quality of refrigerated rainbow trout. Food Chem. 2013, 141, 1667–1672. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, B.; Keshwani, A.; Kharkwal, H. Natural polymer based cling films for food packaging. Int. J. Pharm. Pharm. Sci. 2015, 7, 10–18. [Google Scholar]
- Priyadarshi, R.; Sauraj, K.B.; Deeba, F.; Kulshreshtha, A.; Negi, Y.S. Chitosan films incorporated with apricot (Prunus armeniaca) kernel essential oil as active food packaging material. Food Hydrocoll. 2018, 85, 158–166. [Google Scholar] [CrossRef]
- Alsaggaf, M.S.; Moussa, S.H.; Tayel, A.A. Application of fungal chitosan incorporated with pomegranate peel extract as edible coating for microbiological, chemical and sensorial quality enhancement of nile tilapia fillets. Int. J. Biol. Macromol. 2017, 99, 499–505. [Google Scholar] [CrossRef]
- Halim, A.L.A.; Kamari, A.; Phillip, E. Chitosan, gelatin and methylcellulose films incorporated with tannic acid for food packaging. Int. J. Biol. Macromol. 2018, 120, 1119–1126. [Google Scholar] [CrossRef]
- Lekjing, S. A Chitosan-based coating with or without clove oil extends the shelf life of cooked pork sausages in refrigerated storage. Meat Sci. 2016, 111, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Vu, C.H.T.; Won, K. Novel water-resistant UV-activated oxygen indicator for intelligent food packaging. Food Chem. 2013, 140, 52–56. [Google Scholar] [CrossRef]
- Albert, A.; Salvador, A.; Fiszman, S.M. A film of alginate plus salt as an edible susceptor in microwaveable food. Food Hydrocoll. 2012, 27, 421–426. [Google Scholar] [CrossRef]
- Juck, G.; Neetoo, H.; Chen, H. Application of an active alginate coating to control the growth of listeria monocytogenes on poached and deli turkey products. Intl J. Food Microbiol. 2010, 142, 302–308. [Google Scholar] [CrossRef]
- Hamzah, H.M.; Osman, A.; Tan, C.P.; Mohamad Ghazali, F. Carrageenan as an alternative coating for papaya (Carica papaya L. Cv. Eksotika). Postharvest Biol. Technol. 2013, 75, 142–146. [Google Scholar] [CrossRef]
- Seol, K.-H.; Lim, D.-G.; Jang, A.; Jo, C.; Lee, M. Antimicrobial effect of κ-carrageenan-based edible film containing ovotransferrin in fresh chicken breast stored at 5 °C. Meat Sci. 2009, 83, 479–483. [Google Scholar] [CrossRef]
- Kanmani, P.; Rhim, J.-W. Development and characterization of carrageenan/grapefruit seed extract composite films for active packaging. Int. J. Biol. Macromol. 2014, 68, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Shojaee-Aliabadi, S.; Hosseini, H.; Mohammadifar, M.A.; Mohammadi, A.; Ghasemlou, M.; Hosseini, S.M.; Khaksar, R. Characterization of κ-carrageenan films incorporated plant essential oils with improved antimicrobial activity. Carb. Polym. 2014, 101, 582–591. [Google Scholar] [CrossRef] [PubMed]
- Rhim, J.W. Physical-mechanical properties of agar/κ-carrageenan blend film and derived clay nanocomposite film. J. Food Sci. 2012, 77, N66–N73. [Google Scholar] [CrossRef] [PubMed]
- Tavassoli-Kafrani, E.; Shekarchizadeh, H.; Masoudpour-Behabadi, M. Development of edible films and coatings from alginates and carrageenans. Carbohydr. Polym. 2016, 137, 360–374. [Google Scholar] [CrossRef]
- Varela, P.; Fiszman, S.M. Hydrocolloids in fried foods. A Review. Food Hydrocoll. 2011, 25, 1801–1812. [Google Scholar] [CrossRef]
- Yuan, H.; Song, J.; Zhang, W.; Li, X.; Li, N.; Gao, X. Antioxidant activity and cytoprotective effect of κ-carrageenan oligosaccharides and their different derivatives. Bioorganic Med. Chem. Lett. 2006, 16, 1329–1334. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, B.; Wu, Y.; Liu, Y.; Gu, X.; Zhang, H.; Wang, C.; Cao, H.; Huang, L.; Wang, Z. Structural characterization and antioxidant activities of κ-carrageenan oligosaccharides degraded by different methods. Food Chem. 2015, 178, 311–318. [Google Scholar] [CrossRef]
- Ho, C.P.; Huffman, D.L.; Bradford, D.D.; Egbert, W.R.; Mikel, W.B.; Jones, W.R. Storage stability of vacuum packaged frozen pork sausage containing soy protein concentrate, carrageenan or antioxidants. J. Food Sci. 1995, 60, 257–261. [Google Scholar] [CrossRef]
- Mostafavi, F.S.; Zaeim, D. Agar-based edible films for food packaging applications—A review. Int. J. Biol. Macromol. 2020, 159, 1165–1176. [Google Scholar] [CrossRef]
- Mohamed, S.A.; El-Sakhawy, M.; El-Sakhawy, M.A.M. Polysaccharides, protein and lipid-based natural edible films in food packaging: A review. Carb. Polym. 2020, 238, 116178. [Google Scholar]
- Da Rocha, M.; Alemán, A.; Romani, V.P.; López-Caballero, M.E.; Gómez-Guillén, M.C.; Montero, P.; Prentice, C. Effects of agar films incorporated with fish protein hydrolysate or clove essential oil on flounder (Paralichthys orbignyanus) fillets shelf-life. Food Hydrocoll. 2018, 81, 351–363. [Google Scholar] [CrossRef]
- Kumar, S.; Boro, J.C.; Ray, D.; Mukherjee, A.; Dutta, J. Bionanocomposite films of agar incorporated with ZnO nanoparticles as an active packaging material for shelf life extension of green grape. Heliyon 2019, 5, e01867. [Google Scholar] [CrossRef]
- Vejdan, A.; Ojagh, S.M.; Abdollahi, M. Effect of gelatin/agar bilayer film incorporated with TiO2 nanoparticles as a UV absorbent on fish oil photooxidation. Int. J. Food Sci. Amp. Technol. 2017, 52, 1862–1868. [Google Scholar] [CrossRef]
- Liu, L.; Kerry, J.F.; Kerry, J.P. Application and assessment of extruded edible casings manufactured from pectin and gelatin/sodium alginate blends for use with breakfast pork sausage. Meat Sci. 2007, 75, 196–202. [Google Scholar] [CrossRef]
- Papparella, A.; Mazzarrino, G.; Chaves-López, C.; Rossi, C.; Sacchetti, G.; Guerrieri, O.; Serio, A. Chitosan boosts the antimicrobial activity of origanum vulgare essential oil in modified atmosphere packaged pork. Food Microbiol. 2016, 59, 23–31. [Google Scholar] [CrossRef]
- Serrano-León, J.S.; Bergamaschi, K.B.; Yoshida, C.M.P.; Saldaña, E.; Selani, M.M.; Rios-Mera, J.D.; Alencar, S.M.; Contreras-Castillo, C.J. Chitosan active films containing agro-industrial residue extracts for shelf life extension of chicken restructured product. Food Res. Int. 2018, 108, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Bazargani-Gilani, B.; Aliakbarlu, J.; Tajik, H. Effect of pomegranate juice dipping and chitosan coating enriched with zataria multiflora boiss essential oil on the shelf-life of chicken meat during refrigerated storage. Innov. Food Sci. Emerg. Technol. 2015, 29, 280–287. [Google Scholar] [CrossRef]
- Jiang, T.; Feng, L.; Wang, Y. Effect of alginate/nano-Ag coating on microbial and physicochemical characteristics of shiitake mushroom (Lentinus edodes) during cold storage. Food Chem. 2013, 141, 954–960. [Google Scholar] [CrossRef]
- Azarakhsh, N.; Osman, A.; Ghazali, H.M.; Tan, C.P.; Mohd Adzahan, N. Lemongrass essential oil incorporated into alginate-based edible coating for shelf-life extension and quality retention of fresh-cut pineapple. Postharvest Biol. Technol. 2014, 88, 1–7. [Google Scholar] [CrossRef]
- Hambleton, A.; Fabra, M.-J.; Debeaufort, F.; Dury-Brun, C.; Voilley, A. Interface and aroma barrier properties of iota-carrageenan emulsion–based films used for encapsulation of active food compounds. J. Food Eng. 2009, 93, 80–88. [Google Scholar] [CrossRef]
- Olaimat, A.N.; Fang, Y.; Holley, R.A. Inhibition of campylobacter jejuni on fresh chicken breasts by κ-carrageenan/chitosan-based coatings containing Allyl isothiocyanate or deodorized oriental mustard extract. Int. J. Food Microbiol. 2014, 187, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-F.; Rhim, J.-W. Preparation and application of agar/alginate/collagen ternary blend functional food packaging films. Int. J. Biol Macromol. 2015, 80, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, M.A.P.R.; Pereira, R.N.C.; da Silva Ramos, O.L.; Teixeira, J.A.C.; Vicente, A.A. (Eds.) Edible Food Packaging: Materials and Processing Technologies; Technology & Engineering: Boca Raton, FL, USA, 2017. [Google Scholar]
- Rhim, J.-W.; Mohanty, A.K.; Singh, S.P.; Ng, P.K.W. Effect of the processing methods on the performance of polylactide films: Thermocompression versus solvent casting. J. Appl. Polym. Sci. 2006, 101, 3736–3742. [Google Scholar] [CrossRef]
- Suhag, R.; Kumar, N.; Trajkovska Petkoska, A.; Upadhyay, A. Film formation and deposition methods of edible coating on food products: A review. Food Res. Int. 2020, 136, 109582. [Google Scholar] [CrossRef]
- Velaga, S.P.; Nikjoo, D.; Vuddanda, P.R. Experimental studies and modeling of the drying kinetics of multicomponent polymer films. AAPS Pharm. Sci. Tech. 2018, 19, 425–435. [Google Scholar] [CrossRef]
- Sothornvit, R.; Krochta, J.M. 23—Plasticizers in edible films and coatings. In Innovations in Food Packaging; Han, J.H., Ed.; Food Science and Technology; Academic Press: London, UK, 2005; pp. 403–433. [Google Scholar] [CrossRef]
- Sanyang, M.L.; Sapuan, S.M.; Jawaid, M.; Ishak, M.R.; Sahari, J. Effect of plasticizer type and concentration on tensile, thermal and barrier properties of biodegradable films based on sugar palm (Arenga pinnata) starch. Polymers 2015, 7, 1106–1124. [Google Scholar] [CrossRef]
- Chen, J.; Roether, A.; Boccaccini, A. Tissue engineering scaffolds from bioactive glass and composite materials. Top Tissue Eng. 2008, 4, 1–27. [Google Scholar]
- Yang, J.; Yu, J.; Huang, Y. Recent developments in gelcasting of ceramics. J. Euro. Ceram. Soc. 2011, 31, 2569–2591. [Google Scholar] [CrossRef]
- Siemann, U. Solvent cast technology—A versatile tool for thin film production. In Scattering Methods and the Properties of Polymer Materials; Progress in Colloid and Polymer Science; Stribeck, N., Smarsly, B., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 1–14. [Google Scholar] [CrossRef]
- Fakhouri, F.M.; Costa, D.; Yamashita, F.; Martelli, S.M.; Jesus, R.C.; Alganer, K.; Collares-Queiroz, F.P.; Innocentini-Mei, L.H. Comparative study of processing methods for starch/gelatin films. Carb. Polym. 2013, 95, 681–689. [Google Scholar] [CrossRef]
- Sait, H.H.; Ma, H.B. An experimental investigation of thin-film evaporation. Nanoscale Microscale Thermophys. Eng. 2009, 13, 218–227. [Google Scholar] [CrossRef]
- Cheng, Y.; Sun, C.; Zhai, X.; Zhang, R.; Zhang, S.; Sun, C.; Wang, W.; Hou, H. Effect of lipids with different physical state on the physicochemical properties of starch/gelatin edible films prepared by extrusion blowing. Int. J. Biol. Macromol. 2021, 185, 1005–1014. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Izquierdo, V.M.; Krochta, J.M. Thermoplastic processing of proteins for film formation—A review. J. Food Sci. 2008, 73, R30–R39. [Google Scholar] [CrossRef]
- Andreuccetti, C.; Carvalho, R.A.; Galicia-García, T.; Martinez-Bustos, F.; González-Nuñez, R.; Grosso, C.R.F. Functional properties of gelatin-based films containing yucca schidigera extract produced via casting, extrusion and blown extrusion processes: A preliminary study. J. Food Eng. 2012, 113, 33–40. [Google Scholar] [CrossRef]
- Krishna, M.; Nindo, C.I.; Min, S.C. Development of fish gelatin edible films using extrusion and compression molding. J. Food Eng. 2012, 108, 337–344. [Google Scholar] [CrossRef]
- Raghav, P.; Agarwal, N.; Saini, M. Edible coating of fruits and vegetables: A review. Education 2016, 1, 2455–5630. [Google Scholar]
- Liu, H.; Xie, F.; Yu, L.; Chen, L.; Li, L. Thermal processing of starch-based polymers. Prog. Polym. Sci. 2009, 34, 1348–1368. [Google Scholar] [CrossRef]
- Zhang, W.; Li, X.; Jiang, W. Development of antioxidant chitosan film with banana peels extract and its application as coating in maintaining the storage quality of apple. Int. J. Biol. Macromol. 2020, 154, 1205–1214. [Google Scholar] [CrossRef] [PubMed]
- Hilbig, J.; Hartlieb, K.; Herrmann, K.; Weiss, J.; Gibis, M. Influence of calcium on white efflorescence formation on dry fermented sausages with co-extruded alginate casings. Food Res. Int. 2020, 131, 109012. [Google Scholar] [CrossRef]
- Yang, K.; Dang, H.; Liu, L.; Hu, X.; Li, X.; Ma, Z.; Wang, X.; Ren, T. Effect of syringic acid incorporation on the physical, mechanical, structural and antibacterial properties of chitosan film for quail eggs preservation. Int. J. Biol. Macromol. 2019, 141, 876–884. [Google Scholar] [CrossRef]
- Puscaselu, R.; Gutt, G.; Amariei, S. Biopolymer-based films enriched with stevia rebaudiana used for the development of edible and soluble packaging. Coatings 2019, 9, 360. [Google Scholar] [CrossRef]
- Guerreiro, A.C.; Gago, C.M.L.; Faleiro, M.L.; Miguel, M.G.C.; Antunes, M.D.C. The use of polysaccharide-based edible coatings enriched with essential oils to improve shelf-life of strawberries. Postharvest Biol. Technol. 2015, 110, 51–60. [Google Scholar] [CrossRef]
- Soares, N.M.; Fernandes, T.A.; Vicente, A.A. Effect of variables on the thickness of an edible coating applied on frozen fish—Establishment of the concept of safe dipping time. J. Food Eng. 2016, 171, 111–118. [Google Scholar] [CrossRef][Green Version]
- Salinas-Roca, B.; Soliva-Fortuny, R.; Welti-Chanes, J.; Martín-Belloso, O. Combined effect of pulsed light, edible coating and malic acid dipping to improve fresh-cut mango safety and quality. Food Control 2016, 66, 190–197. [Google Scholar] [CrossRef]
- Atieno, L.; Owino, W.; Ateka, E.M.; Ambuko, J. Influence of coating application methods on the postharvest quality of cassava. Int. J. Food Sci. 2019, 2019, e2148914. [Google Scholar] [CrossRef]
- Lu, F.; Ding, Y.; Ye, X.; Liu, D. Cinnamon and nisin in alginate–calcium coating maintain quality of fresh northern snakehead fish fillets. LWT Food Sci. Technol. 2010, 43, 1331–1335. [Google Scholar] [CrossRef]
- Debeaufort, F.; Voilley, A. Lipid-based edible films and coatings. In Edible Films and Coatings for Food Applications; Huber, K.C., Embuscado, M.E., Eds.; Springer: New York, NY, USA, 2009; pp. 135–168. [Google Scholar] [CrossRef]
- Andrade, R.D.; Skurtys, O.; Osorio, F.A. Atomizing spray systems for application of edible coatings. Compr. Rev. Food Sci. Food Saf. 2012, 11, 323–337. [Google Scholar] [CrossRef]
- Lin, D.; Zhao, Y. Innovations in the development and application of edible coatings for fresh and minimally processed fruits and vegetables. Compr. Rev. Food Sci. Food Saf. 2007, 6, 60–75. [Google Scholar] [CrossRef]
- Valdés, A.; Ramos, M.; Beltrán, A.; Jiménez, A.; Garrigós, M.C. State of the art of antimicrobial edible coatings for food packaging applications. Coatings 2017, 7, 56. [Google Scholar] [CrossRef]
- Peretto, G.; Du, W.-X.; Avena-Bustillos, R.J.; De Berrios, J.; Sambo, P.; McHugh, T.H. Electrostatic and conventional spraying of alginate-based edible coating with natural antimicrobials for preserving fresh strawberry quality. Food Bioprocess Technol. 2017, 10, 165–174. [Google Scholar] [CrossRef]
- Bergeron, V.; Bonn, D.; Martin, J.Y.; Vovelle, L. Controlling droplet deposition with polymer additives. Nature 2000, 405, 772–775. [Google Scholar] [CrossRef] [PubMed]
- Campos, C.A.; Gerschenson, L.N.; Flores, S.K. Development of edible films and coatings with antimicrobial activity. Food Bioprocess Technol. 2011, 4, 849–875. [Google Scholar] [CrossRef]
- Jooyandeh, H. Whey protein films and coatings: A review. Pakistan J. Nut. 2011, 10, 296–301. [Google Scholar] [CrossRef]
- Mishra, P.; Handa, M.; Ujjwal, R.R.; Singh, V.; Kesharwani, P.; Shukla, R. Potential of nanoparticulate based delivery systems for effective management of alopecia. Colloids Surf. B Biointerfaces 2021, 208, 112050. [Google Scholar] [CrossRef] [PubMed]
- Chawla, R.; Sivakumar, S.; Kaur, H. Antimicrobial edible films in food packaging: Current scenario and recent nanotechnological advancements—A review. Carbohydr. Polym. Technol. Appl. 2021, 2, 100024. [Google Scholar] [CrossRef]
- Lipin, A.A.; Lipin, A.G. Prediction of coating uniformity in batch fluidized-bed coating process. Particuology 2022, 61, 41–46. [Google Scholar] [CrossRef]
- Jacquot, M.; Pernetti, M. Spray coating and drying processes. In Fundamentals of Cell Immobilisation Biotechnology; Focus on Biotechnology; Nedović, V., Willaert, R., Eds.; Springer: Dordrecht, The Netherlands, 2004; pp. 343–356. [Google Scholar] [CrossRef]
- Zank, J.; Kind, M.; Schlünder, E.-U. Particle growth and droplet deposition in fluidised bed granulation. Powder Technol. 2001, 120, 76–81. [Google Scholar] [CrossRef]
- Sipahi, R.E.; Castell-Perez, M.E.; Moreira, R.G.; Gomes, C.; Castillo, A. Improved multilayered antimicrobial alginate-based edible coating extends the shelf life of fresh-cut watermelon (Citrullus lanatus). LWT Food Sci. Technol. 2013, 51, 9–15. [Google Scholar] [CrossRef]
- Poverenov, E.; Zaitsev, Y.; Arnon, H.; Granit, R.; Alkalai-Tuvia, S.; Perzelan, Y.; Weinberg, T.; Fallik, E. Effects of a composite chitosan–gelatin edible coating on postharvest quality and storability of red bell peppers. Postharvest Biol. Technol. 2014, 96, 106–109. [Google Scholar] [CrossRef]
- Divya, K.; Smitha, V.; Jisha, M.S. Antifungal, antioxidant and cytotoxic activities of chitosan nanoparticles and its use as an edible coating on vegetables. Int. J. Biol. Macromol. 2018, 114, 572–577. [Google Scholar] [CrossRef]
- Gamboa-Santos, J.; Campañone, L.A. Application of osmotic dehydration and microwave drying to strawberries coated with edible films. Drying Technol. 2019, 37, 1002–1012. [Google Scholar] [CrossRef]
- Batista Silva, W.; Cosme Silva, G.M.; Santana, D.B.; Salvador, A.R.; Medeiros, D.B.; Belghith, I.; da Silva, N.M.; Cordeiro, M.H.M.; Misobutsi, G.P. Chitosan delays ripening and ROS production in guava (Psidium guajava L.) fruit. Food Chem. 2018, 242, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Escamilla-García, M.; Rodríguez-Hernández, M.J.; Hernández-Hernández, H.M.; Delgado-Sánchez, L.F.; García-Almendárez, B.E.; Amaro-Reyes, A.; Regalado-González, C. Effect of an edible coating based on chitosan and oxidized starch on shelf life of carica papaya L., and its physicochemical and antimicrobial properties. Coatings 2018, 8, 318. [Google Scholar] [CrossRef]
- Aitboulahsen, M.; Zantar, S.; Laglaoui, A.; Chairi, H.; Arakrak, A.; Bakkali, M.; Hassani Zerrouk, M. Gelatin-based edible coating combined with mentha pulegium essential oil as bioactive packaging for strawberries. J. Food Qual. 2018, 2018, e8408915. [Google Scholar] [CrossRef]
- Fadini, A.L.; Rocha, F.S.; Alvim, I.D.; Sadahira, M.S.; Queiroz, M.B.; Alves, R.M.V.; Silva, L.B. Mechanical properties and water vapour permeability of hydrolysed collagen–cocoa butter edible films plasticised with sucrose. Food Hydrocoll. 2013, 30, 625–631. [Google Scholar] [CrossRef]
- Romani, V.P.; Machado, A.V.; Olsen, B.D.; Martins, V.G. Effects of pH modification in proteins from fish (whitemouth croaker) and their application in food packaging films. Food Hydrocoll. 2018, 74, 307–314. [Google Scholar] [CrossRef]
- Araújo, C.S.; Rodrigues, A.M.C.; Peixoto Joele, M.R.S.; Araújo, E.A.F.; Lourenço, L.F.H. Optimizing process parameters to obtain a bioplastic using proteins from fish byproducts through the response surface methodology. Food Packag. Shelf Life 2018, 16, 23–30. [Google Scholar] [CrossRef]
- Muhoza, B.; Xia, S.; Zhang, X. Gelatin and high methyl pectin coacervates crosslinked with tannic acid: The characterization, rheological properties, and application for peppermint oil microencapsulation. Food Hydrocoll. 2019, 97, 105174. [Google Scholar] [CrossRef]
- Kang, Y.; Kim, H.-J.; Moon, C.-H. Eutrophication driven by aquaculture fish farms controls phytoplankton and dinoflagellate cyst abundance in the southern coastal waters of Korea. J. Mar. Sci. and Eng. 2021, 9, 362. [Google Scholar] [CrossRef]
- Arfat, Y.A.; Benjakul, S.; Prodpran, T.; Osako, K. Development and characterisation of blend films based on fish protein isolate and fish skin gelatin. Food Hydrocoll. 2014, 39, 58–67. [Google Scholar] [CrossRef]
- Sommer, A.; Dederko-Kantowicz, P.; Staroszczyk, H.; Sommer, S.; Michalec, M. Enzymatic and chemical cross-linking of bacterial cellulose/fish collagen composites—A comparative study. Int. J. Mol. Sci. 2021, 22, 3346. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Nirmal, N.P.; Danish, M.; Chuprom, J.; Jafarzedeh, S. Characterisation of composite films fabricated from collagen/chitosan and collagen/soy protein isolate for food packaging applications. RSC Adv. 2016, 6, 82191–82204. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Rezaei, M.; Zandi, M.; Farahmandghavi, F. Preparation and characterization of chitosan nanoparticles-loaded fish gelatin-based edible films. J. Food Process Eng. 2016, 39, 521–530. [Google Scholar] [CrossRef]
- Martucci, J.F.; Ruseckaite, R.A. Biodegradation of three-layer laminate films based on gelatin under indoor soil conditions. Polym. Degrad. Stabilit 2009, 94, 10307–11313. [Google Scholar] [CrossRef]
- Bae, H.J.; Darby, D.O.; Kimmel, R.M.; Park, H.J.; Whiteside, W.S. Effects of transglutaminase-induced cross-linking on properties of fish gelatin–nanoclay composite film. Food Chem. 2009, 114, 180–189. [Google Scholar] [CrossRef]
- Garavand, F.; Rouhi, M.; Razavi, S.H.; Cacciotti, I.; Mohammadi, R. Improving the integrity of natural biopolymer films used in food packaging by crosslinking approach: A review. Int. J. Biol. Macromol. 2017, 104, 687–707. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Guillén, M.C.; Pérez-Mateos, M.; Gómez-Estaca, J.; López-Caballero, E.; Giménez, B.; Montero, P. Fish gelatin: A renewable material for developing active biodegradable films. Trends Food Sci. Technol. 2009, 20, 3–16. [Google Scholar] [CrossRef]
- Esposito Corcione, C.; Striani, R.; Ferrari, F.; Visconti, P.; Rizzo, D.; Greco, A. An innovative method for the recycling of waste carbohydrate-based flours. Polymers 2020, 12, 1414. [Google Scholar] [CrossRef]
- Wang, H.; Qian, J.; Ding, F. Emerging chitosan-based films for food packaging applications. J. Agric. Food Chem. 2018, 66, 395–413. [Google Scholar] [CrossRef]
- Restuccia, D.; Spizzirri, U.G.; Parisi, O.I.; Cirillo, G.; Curcio, M.; Iemma, F.; Puoci, F.; Vinci, G.; Picci, N. New EU regulation aspects and global market of active and intelligent packaging for food industry applications. Food Control 2010, 21, 1425–1435. [Google Scholar] [CrossRef]
- Rojas-Graü, M.A.; Oms-Oliu, G.; Soliva-Fortuny, R.; Martín-Belloso, O. The use of packaging techniques to maintain freshness in fresh‐cut fruits and vegetables: A review. Int. J. Food Sci. Technol. 2009, 44, 875–889. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).