Electrochemical Performance of Iron-Doped Cobalt Oxide Hierarchical Nanostructure
Abstract
:1. Introduction
2. Materials and Methods
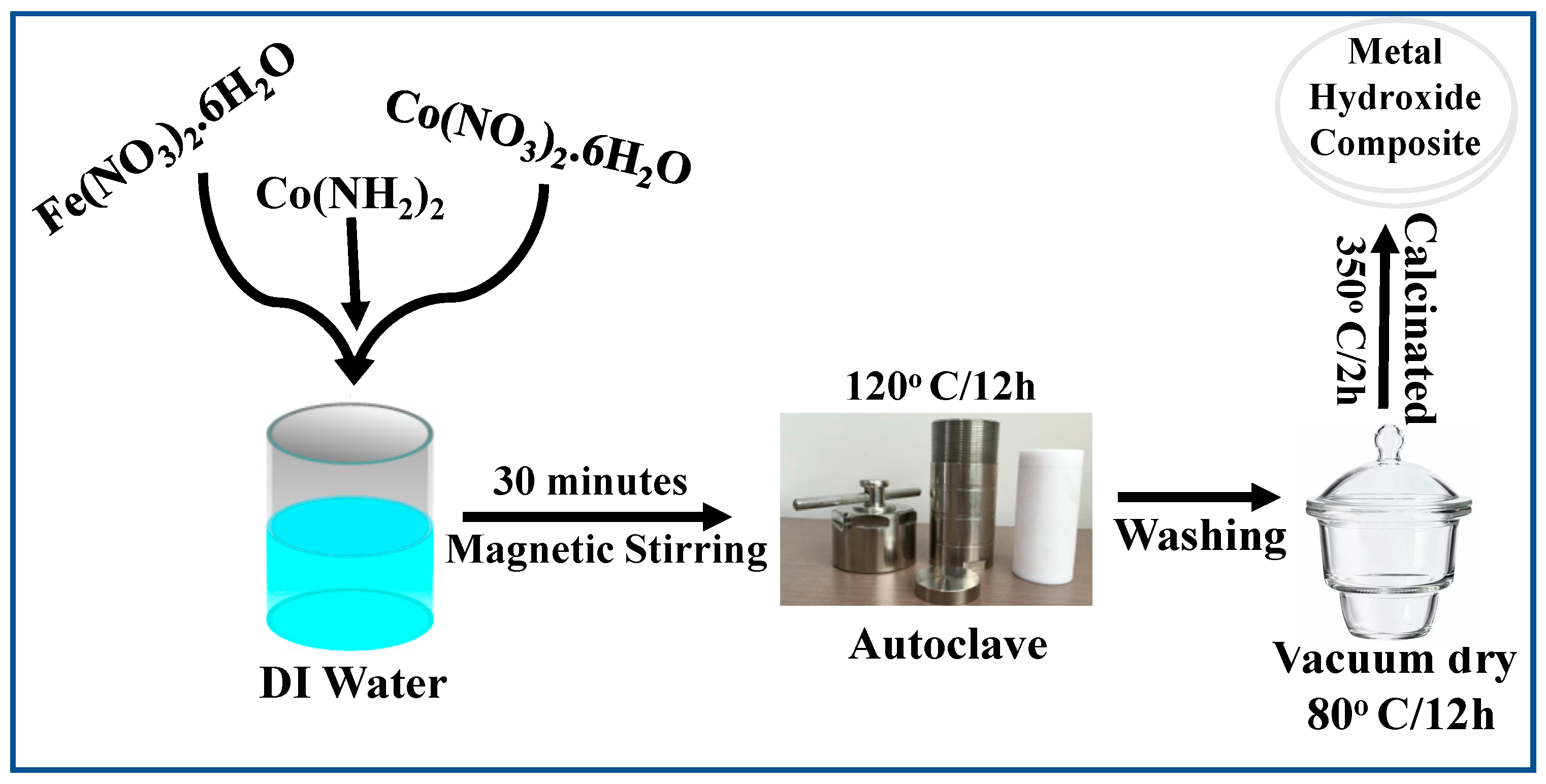
2.1. Synthesis
2.2. Characterization
2.3. Theoretical Study
3. Results and Discussion
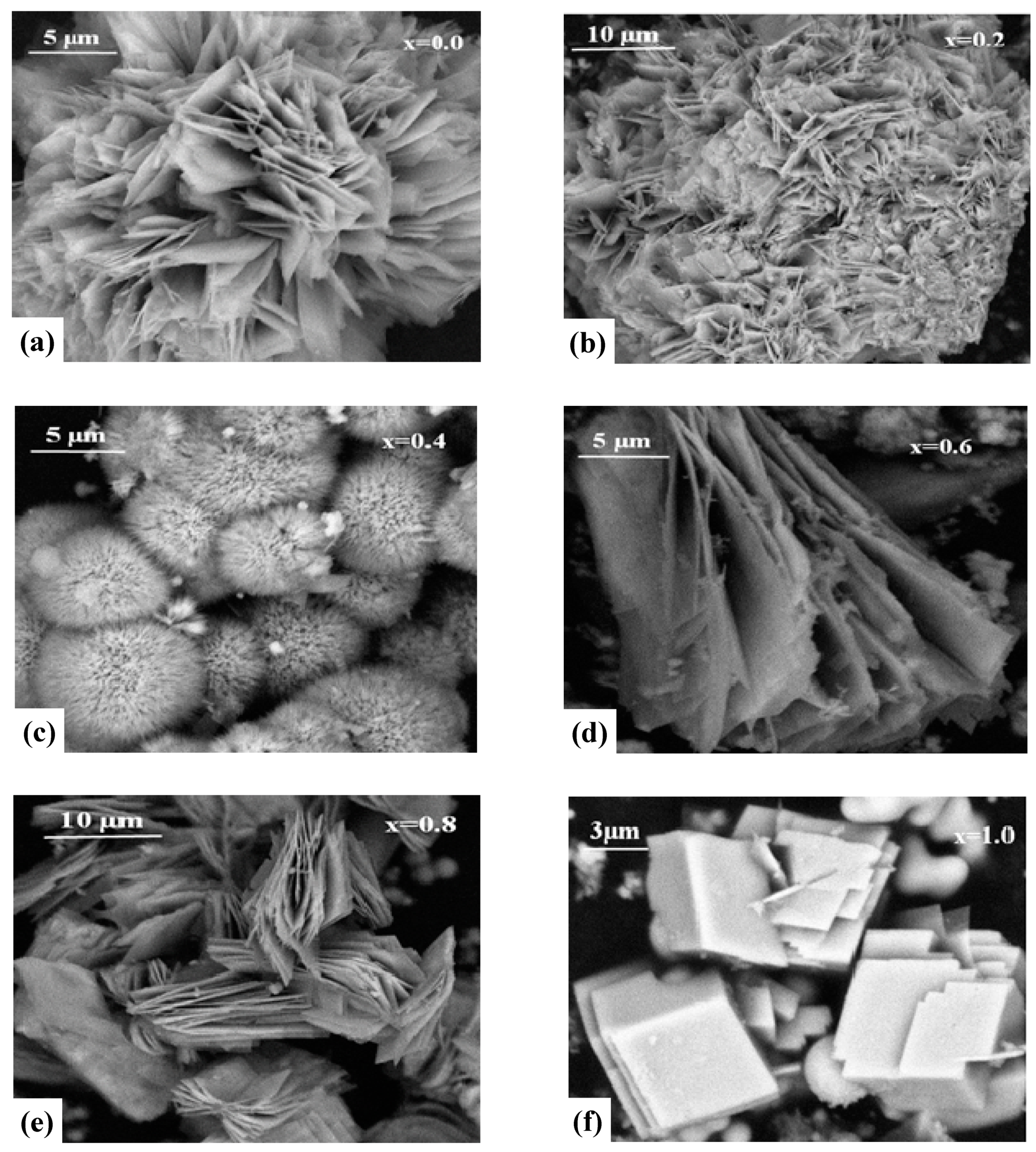
3.1. Structure and Morphology
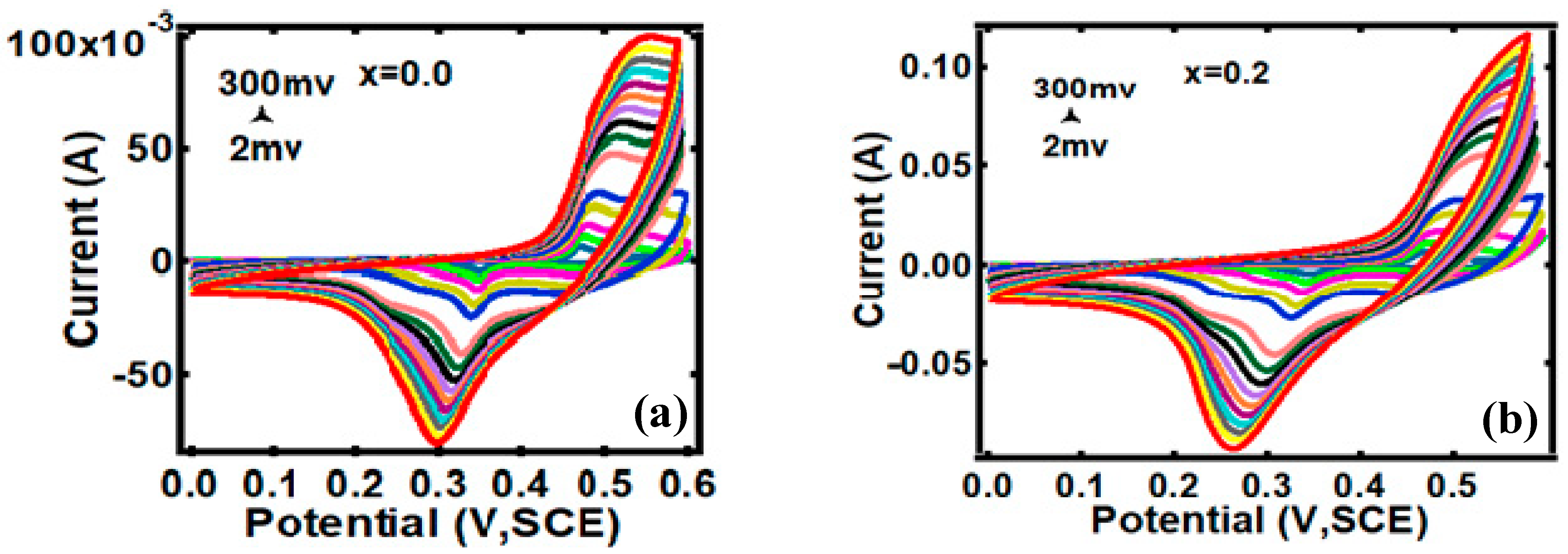
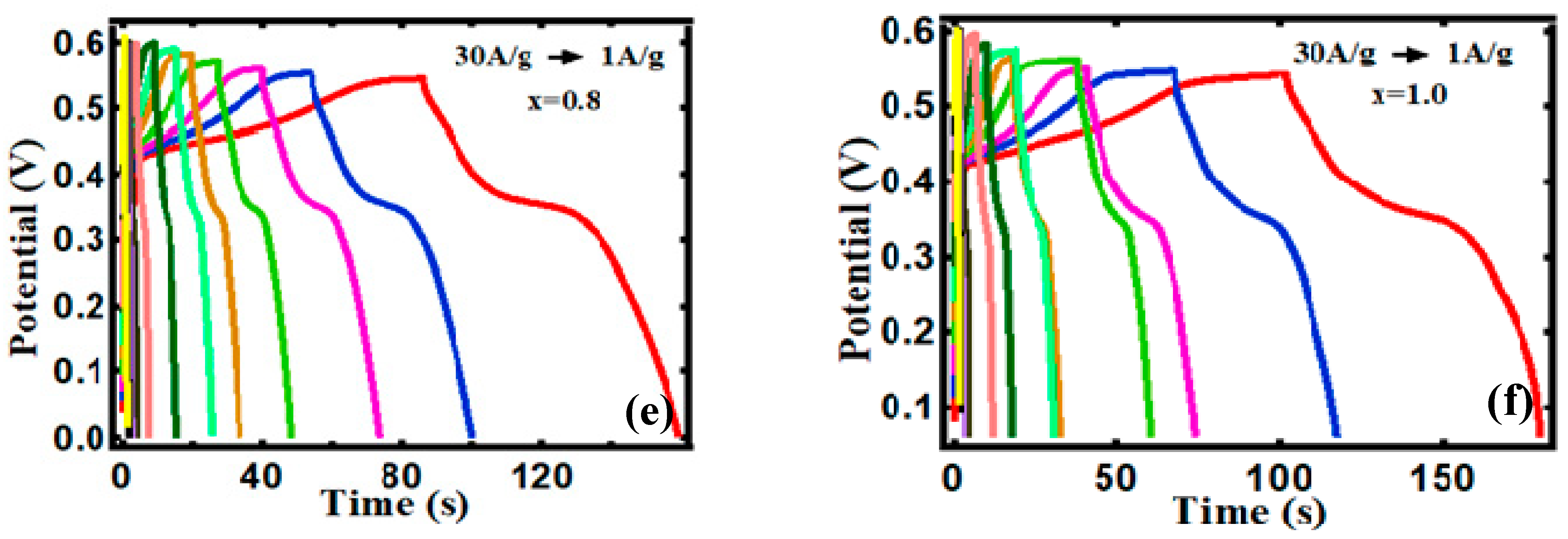
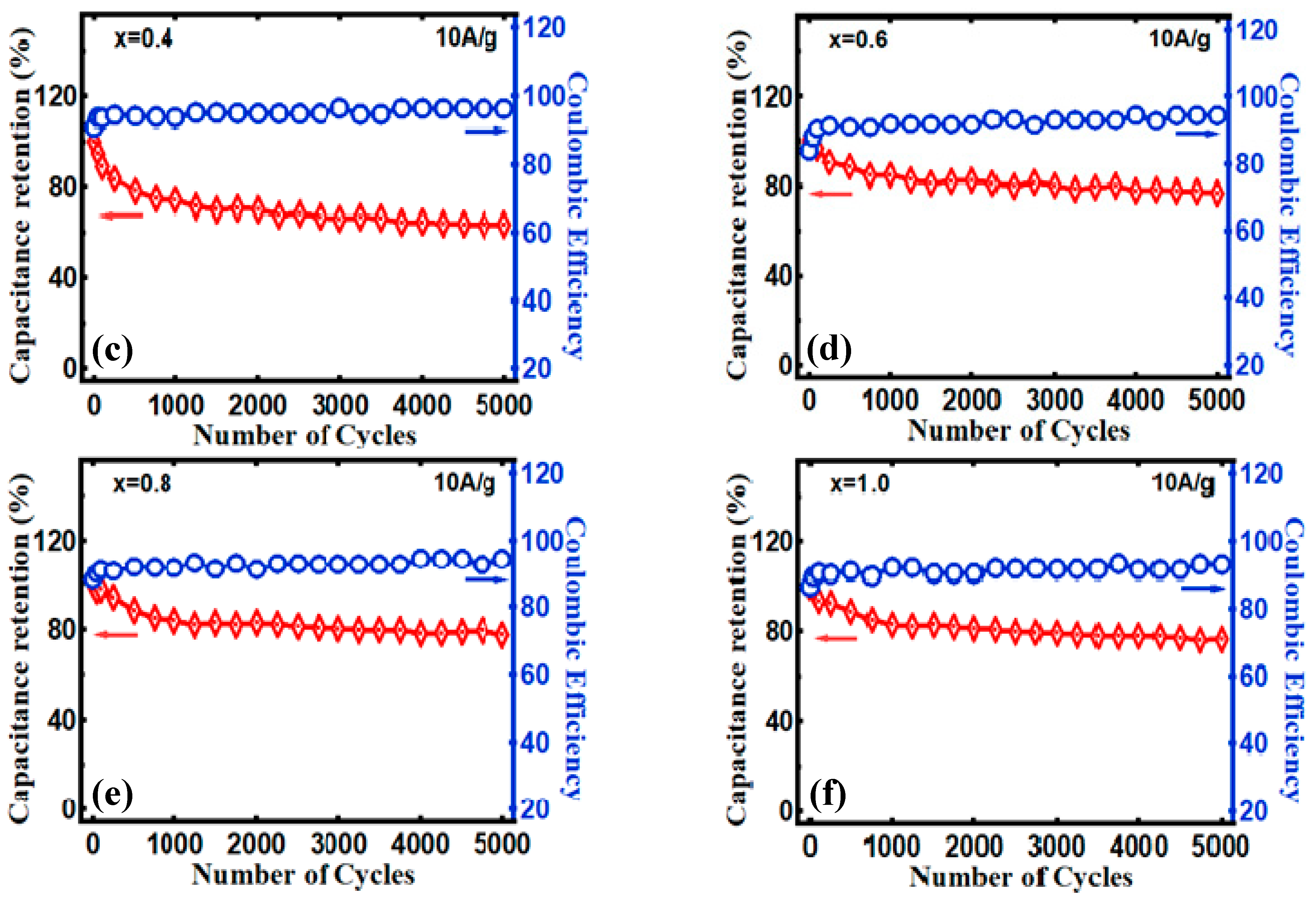
3.2. Electrocapacitive Study
| Catalysts | Sp. Capacitince (F/g)@1A/g | Stability % (Cycles) | Power Density (W/g) | Ref. |
|---|---|---|---|---|
| FexCo3-xO4 at (x = 1) | 149 | 82.7 (1000) | 0.27 | This work |
| FexCo3-xO4 at (x = 0) | 131 | 84.7 (1000) | 0.30 | This work |
| Co3O4 nanonet | 739 | 90.2 (1000) | -- | [64] |
| Co3O4//AC | 107.3 | 98 (1500) | 0.22 | [65] |
| Co3O4 nanocrystal | 284.2 | -- | -- | [66] |
| Fe3O4 | 67.9 | 83 (2000) | -- | [67] |
| Fe3O4@C | 74.4 | 95 (1500) | 0.3 | [67] |
| Porous Co3O4 | 150 | 100 (3400) | -- | [68] |
| Fe-Co3O4/graphene | 734 | 99.2 (1000) | -- | [20] |
| Co3O4 | 40 | -- | -- | [22] |
| Fe–Co3O4 | 69.8 | 72 | -- | [22] |
| Mn–Co3O4 | 773 | 73.9 (5000) | -- | [23] |
| Cd–Co3O4 | 737 | 90 (3500) | -- | [24] |
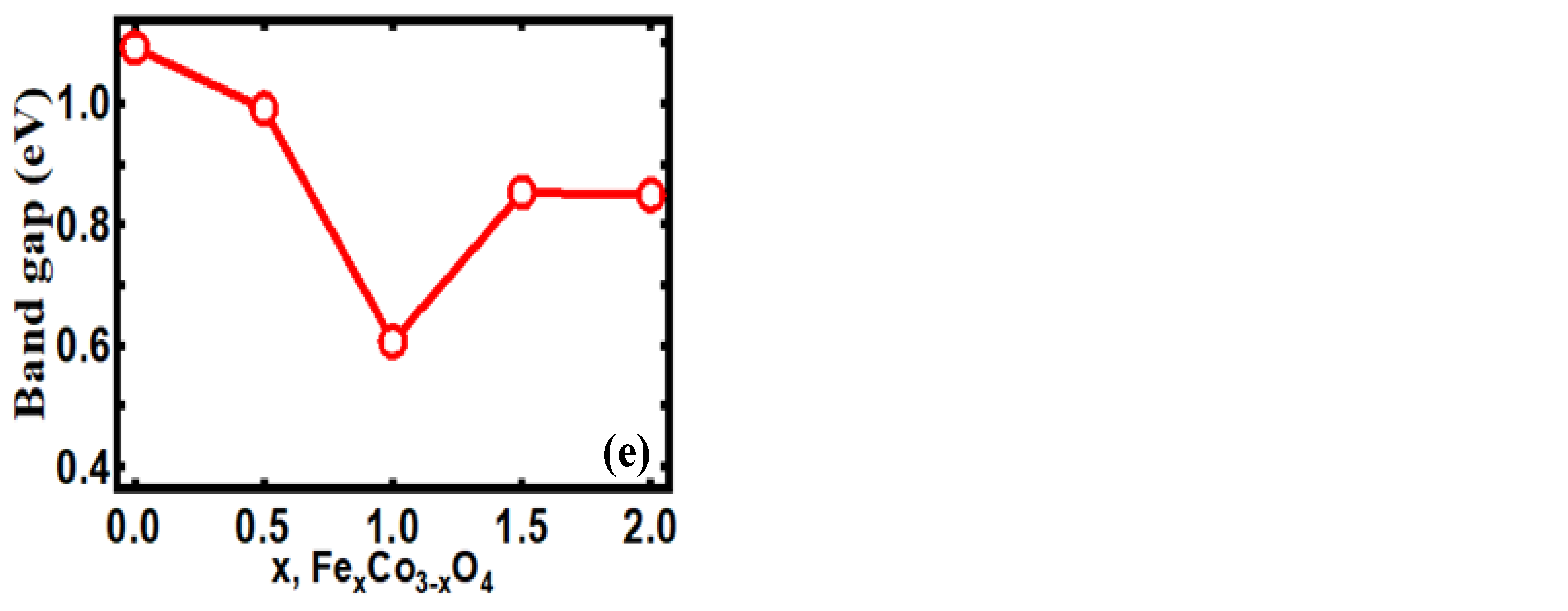
3.3. DFT Study
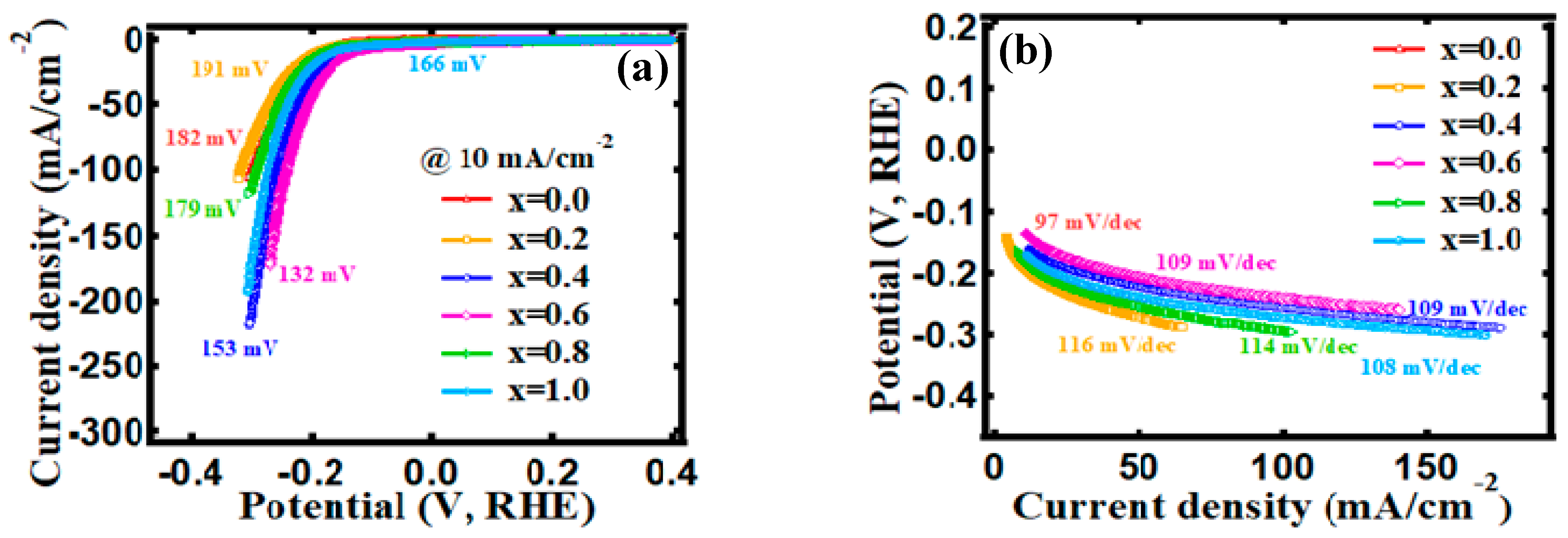
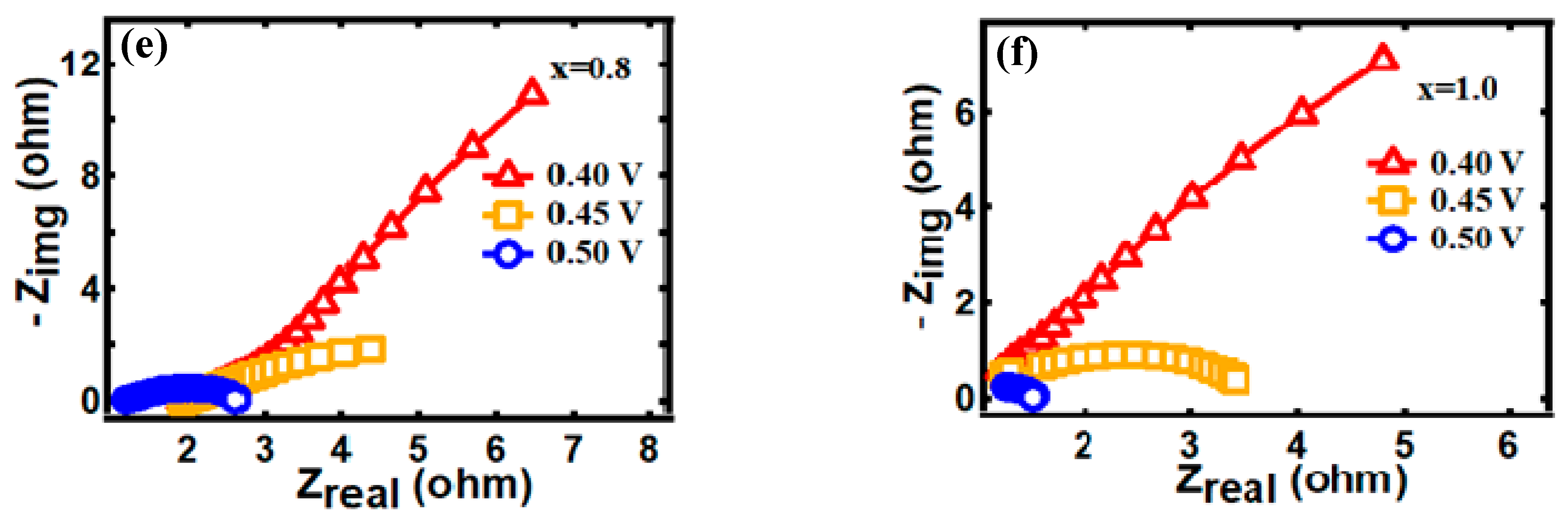
3.4. Electrocatalytic Behavior
| Catalyst | OER | HER | Ref. | ||
|---|---|---|---|---|---|
| Overpotential, η10 mV | Tafel Slope (mV/dec) | Overpotential, η10 mV | Tafel Slope (mV/dec) | ||
| Co3O4 | 423 | 69 | 351 | 91 | [78] |
| Fe–Co3O4 | 295 | 39 | 120 | -- | [79] |
| Fe–Co3O4 | 380 | 60 | -- | -- | [21] |
| NixCo3-xO4 | 320 | 38 | 170 | 98 | [80] |
| CoFe2O4 | 490 | 48 | -- | -- | [81] |
| Co3O4/C | 360 | -- | -- | -- | [82] |
| Fe–Co3O4 | 262 | 43 | -- | -- | [76] |
| Co3O4/Co–Fe oxide | 297 | 61 | -- | -- | [83] |
| Fe–Co–O NS | 260 | 53 | -- | -- | [84] |
| Co–Fe–O frame | 290 | 62 | -- | -- | [85] |
| Fe–CoS2 | -- | -- | 40 | 32 | [86] |
| FeCo2O4 | 510 | 237 | 400 | 215 | [33] |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weidner, J.; Barwe, S.; Sliozberg, K.; Piontek, S.; Masa, J.; Apfel, U.; Schuhmann, W. Cobalt–metalloid alloys for electrochemical oxidation of 5-hydroxymethylfurfural as an alternative anode reaction in lieu of oxygen evolution during water splitting. Beilstein J. Org. Chem. 2018, 14, 1436–1445. [Google Scholar] [CrossRef]
- Yue, Z.; Zhu, W.; Li, Y.; Wei, Z.; Hu, N.; Suo, Y.; Wang, J. Surface Engineering of a Nickel Oxide-Nickel Hybrid Nanoarray as a Versatile Catalyst for Both Superior Water and Urea Oxidation. Inorg. Chem. 2018, 57, 4693. [Google Scholar] [CrossRef]
- Esswein, A.J.; Nocera, D.G. Hydrogen Production by Molecular Photocatalysis. Chem. Rev. 2007, 107, 4022–4047. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Tang, J.; Chen, H.M.; Liu, B.; Yang, P. A fully integrated nanosystem of semiconductor nanowires for direct solar water splitting. Nano Lett. 2013, 13, 2989–2992. [Google Scholar] [CrossRef]
- Maeda, K.; Domen, K. Photocatalytic Water Splitting: Recent Progress and Future Challenges. J. Phys. Chem. Lett. 2010, 1, 2655–2661. [Google Scholar] [CrossRef]
- Zhang, M.; He, L.; Shi, T.; Zha, R. Nanocasting and direct synthesis strategies for mesoporous carbons as supercapacitor electrodes. Chem. Mater. 2018, 30, 7391–7412. [Google Scholar] [CrossRef]
- Tian, Q.; Wang, X.; Huang, Z.; Guo, X. Nanostructured (Co, Mn)3O4 for high capacitive supercapacitor applications. Nanoscale Res. Lett. 2017, 12, 214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Wu, Z.; Yin, Y. Hierarchical NiCo2S4@ PANI core/shell nanowires grown on carbon fiber with enhanced electrochemical performance for hybrid supercapacitors. Chem. Eng. J. 2017, 323, 330–339. [Google Scholar] [CrossRef]
- Xiong, S.; Yuan, C.; Zhang, X.; Xi, B.; Qian, Y. Controllable synthesis of mesoporous Co3O4 nanostructures with tunable morphology for application in super-capacitors. Chem. Eur. J. 2009, 15, 5320–5326. [Google Scholar] [CrossRef]
- Nkeng, P.; Koenig, J.F.; Gautier, J.I.; Chartier, P.; Poillerat, G. Enhancement of surface areas of Co3O4 and NiCo2O4 electrocatalysts prepared by spray pyrolysis. J. Electroanal. Chem. 1996, 402, 81–89. [Google Scholar] [CrossRef]
- Li, W.; Xu, L.; Chen, J. Co3O4 Nanomaterials in Lithium-Ion Batteries and Gas Sensors. Adv. Funct. Mater. 2005, 15, 851. [Google Scholar] [CrossRef]
- Choi, K.; Kim, H.; Kim, K.; Liu, D.; Cao, G.; Lee, J. C2H5OH sensing characteristics of various Co3O4 nanostructures prepared by solvothermal reaction. Sens. Actuators B 2010, 146, 183–189. [Google Scholar] [CrossRef]
- Kupfer, B.; Majhi, K.; Keller, D.A.; Bouhadana, Y.; Ruhle, S.; Barad, H.N.; Anderson, A.Y.; Zaban, A. Renewable Energy: Thin Film Co3O4/TiO2 Heterojunction Solar Cells. Adv. Energy Mater. 2015, 5, 1401007. [Google Scholar] [CrossRef]
- Mariammal, R.N.; Ramachandran, K.; Kalaiselvan, G.; Arumugam, S.; Renganathan, B.; Sastikumar, D. Effect of magnetism on the ethanol sensitivity of undoped and Mn-doped CuO nanoflakes. Appl. Surf. Sci. 2013, 270, 545–552. [Google Scholar] [CrossRef]
- Martin-Gonzalez, M.S.; Fernandez, J.F.; Rubio-Marcos, F.; Lorite, I.; Costa-Kramer, J.L.; Quesada, A.; Banares, M.A.; Fierro, J.L.G. Insights into the room temperature magnetism of ZnO/Co3O4 mixtures. J. Appl. Phys. 2008, 103, 083905. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.; Shkir, M.; Ashraf, I.M.; el-Toni, A.M.; Aldalbahi, A.; AlFaify, S. One-step straightforward synthesis of Tb-doped NiO nanocomposites using flash combustion method: Structural, optical, luminescent, and electrical switching properties. Ceram. Int. 2020, 46, 10678–10690. [Google Scholar] [CrossRef]
- Dong, Y.; Zhao, J.; Zhang, J.Y.; Chen, Y.; Yang, X.; Song, W.; Wei, L.; Li, W. Synergy of Mn and Ni enhanced catalytic performance for toluene combustion over Ni-doped α-MnO2 catalysts. Chem. Eng. J. 2020, 388, 124244. [Google Scholar] [CrossRef]
- Dong, C.; Qu, Z.; Jiang, X.; Ren, Y. Tuning oxygen vacancy concentration of MnO2 through metal doping for improved toluene oxidation. J. Hazard. Mater. 2020, 391, 122181. [Google Scholar] [CrossRef]
- Han, X.; Cui, Y.; Liu, H. Ce-doped Mn3O4 as high-performance anode material for lithium ion batteries. J. Alloys Compd. 2020, 814, 152348. [Google Scholar] [CrossRef]
- Wang, S.; Li, Q.; Chen, M.; Pu, W.; Wu, Y.; Yang, M. Electrochemical capacitance performance of Fe-doped Co3O4/graphene nanocomposite: Investigation on the effect of iron. Electrochim. Acta 2016, 215, 473–482. [Google Scholar] [CrossRef]
- Xiao, C.; Lu, X.; Zhao, C. Unusual Synergistic Effects upon Incorporation of Fe and/or Ni into Mesoporous Co3O4 for Enhanced Oxygen Evolution. Chem. Commun. Camb. UK 2014, 50, 10122–10125. [Google Scholar] [CrossRef] [PubMed]
- Manickam, M.; Ponnuswamy, V.; Sankar, C.; Suresh, R.; Mariappan, R.; Bose, A.C.; Chandrasekaran, J. Structural, optical, electrical and electrochemical properties of Fe:Co3O4 thin films for supercapacitor applications. J. Mater. Sci. Mater. Electron. 2017, 28, 18951–18965. [Google Scholar] [CrossRef]
- Chen, H.; Wang, J.; Liao, F.; Han, X.; Zhang, Y.; Xu, C.; Gao, L. Uniform and porous Mn-doped Co3O4 microspheres: Solvothermal synthesis and their superior supercapacitor performances. Ceram. Int. 2019, 45, 11876–11882. [Google Scholar] [CrossRef]
- Deng, S.; Xiao, X.; Chen, G.; Wang, L.; Wang, Y. Cd doped porous Co3O4 nanosheets as electrode material for high performance supercapacitor application. Electrochim. Acta 2016, 196, 316–327. [Google Scholar] [CrossRef]
- Zhu, Y.P.; Guo, C.; Zheng, Y.; Qiao, S.Z. Surface and interface engineering of noble-metal-free electrocatalysts for efficient energy conversion processes. Acc. Chem. Res. 2017, 50, 915e923. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, J.; Zhang, B.; Ruan, Y.; Lv, L.; Ji, X.; Xu, K.; Miao, L.; Jiang, J. Hierarchical NiCo2S4@ NiFe LDH heterostructures supported on nickel foam for enhanced overall-water-splitting activity. ACS Appl. Mater. Interfaces 2017, 9, 15364e15372. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Pu, X.; Song, W.; Wu, Z.; Zhou, Z.; He, X.; Lu, F.; Jing, M.; Tang, B.; Ji, X. High capacity NiCo2O4 nanorods as electrode materials for supercapacitor. J. Alloys Compd. 2014, 617, 988–993. [Google Scholar] [CrossRef]
- Zou, R.; Xu, K.; Wang, T.; He, G.; Liu, Q.; Zhang, Z.; Hu, J. Chain-like NiCo2O4 nanowires with different exposed reactive planes for high-performance supercapacitors. J. Mater. Chem. A 2013, 1, 8560–8566. [Google Scholar] [CrossRef]
- Guragain, D.; Zequine, C.; Gupta, R.K.; Mishra, S.R. Facile Synthesis of Bio-Template Tubular MCo2O4 (M= Cr, Mn, Ni) Microstructure and Its Electrochemical Performance in Aqueous Electrolyte. Processes 2020, 8, 343. [Google Scholar] [CrossRef] [Green Version]
- Hao, C.; Zhou, S.; Wang, J.; Wang, X.; Gao, H.; Ge, C. Preparation of hierarchical spinel NiCo2O4 nanowires for high-performance supercapacitors. Ind. Eng. Chem. Res. 2018, 57, 2517–2525. [Google Scholar] [CrossRef]
- Guo, J.; Yin, Z.; Zang, X.; Dai, Z.; Zhang, Y.; Huang, W.; Dong, X. Facile one-pot synthesis of NiCo2O4 hollow spheres with a controllable number of shells for high-performance supercapacitors. Nano Res. 2017, 10, 405–414. [Google Scholar] [CrossRef]
- Guo, X.; Yang, C.; Huang, G.; He, B. High-performance supercapacitors based on flower-like FexCo3-xO4 electrodes. J. Alloys Compd. 2018, 735, 184–192. [Google Scholar] [CrossRef]
- Kundu, A.; Robby, A.; Shit, A.; Jo, H.J.; Park, S.Y. Construction of FeCo2O4@N-Doped Carbon Dots Nanoflowers as Binder Free Electrode for Reduction and Oxidation of Water. Materials 2020, 13, 3119. [Google Scholar] [CrossRef]
- Guragain, D.; Bhattarai, R.; Choi, J.; Lin, W.; Gupta, R.K.; Mishra, S.R.; Shen, X.; Perez, F. Electrochemical Performance of Aluminum Doped Ni1−xAlxCo2O4 Hierarchical Nanostructure: Experimental and Theoretical Study. Processes 2021, 9, 1750. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [Green Version]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Heyd, J.; Scuseria, G.E.; Ernzerhof, M. Hybrid functionals based on a screened Coulomb potential. J. Chem. Phys. 2003, 118, 8207–8215. [Google Scholar] [CrossRef] [Green Version]
- Guragain, D.; Zequine, C.; Poudel, T.; Neupane, D.; Gupta, R.K.; Mishra, S.R. Facile Synthesis of Bio-Templated Tubular Co3O4 Microstructure and Its Electrochemical Performance in Aqueous Electrolytes. J. Nanosci. Nanotechnol. 2020, 20, 3182–3194. [Google Scholar] [CrossRef] [PubMed]
- Sumadiyasa, M.; Manuaba, I.B.S. Determining Crystallite Size Using Scherrer Formula, Williamson-Hull Plot, and Particle Size with SEM. Bul. Fis. 2018, 19, 28–34. [Google Scholar] [CrossRef] [Green Version]
- Zhi, J.; Wang, Y.; Deng, S.; Hu, A. Study on the relation between pore size and supercapacitance in mesoporous carbon electrodes with silica-supported carbon nanomembranes. RSC Adv. 2014, 4, 40296–40300. [Google Scholar] [CrossRef]
- Kondrat, S.; Perez, C.R.; Presser, V.; Gogotsi, Y.; Kornyshev, A.A. Effect of pore size and its dispersity on the energy storage in nanoporous supercapacitors. Energy Environ. Sci. 2012, 5, 6474–6479. [Google Scholar] [CrossRef]
- Jayanthi, K.; Chawla, S.; Sood, K.N.; Chhibara, M.; Singh, S. Dopant induced morphology changes in ZnO nanocrystals. Appl. Surf. Sci. 2009, 255, 5869–5875. [Google Scholar] [CrossRef]
- Adhikari, H.; Ghimire, M.; Ranaweera, C.K.; Bhoyate, S.; Gupta, R.K.; Alam, J.; Mishra, S.R. Synthesis and electrochemical performance of hydrothermally synthesized Co3O4 nanostructured particles in presence of urea. J. Alloys Compd. 2017, 708, 628–638. [Google Scholar] [CrossRef]
- Pendashteh, A.; Rahmanifar, M.S.; Kaner, R.B.; Mousavi, M.S. Facile synthesis of nanostructured CuCo2O4 as a novel electrode material for high-rate supercapacitors. Chem. Commun. 2014, 50, 1972–1975. [Google Scholar] [CrossRef] [PubMed]
- Guragain, D.; Zequine, C.; Poudel, T.; Neupane, D.; Gupta, R.K.; Mishra, S.R. Influence of Urea on the Synthesis of NiCo2O4 Nanostructure: Morphological and Electrochemical Studies. J. Nanosci. Nanotechnol. 2020, 20, 2526–2537. [Google Scholar] [CrossRef]
- Liu, X.; Long, Q.; Jiang, C.; Zhan, B.; Li, C.; Liu, S.; Zhao, Q.; Dong, X. Facile and green synthesis of mesoporous Co3O4 nanocubes and their applications for supercapacitors. Nanoscale 2013, 5, 6525–6529. [Google Scholar] [CrossRef]
- Ren, J.; Bai, W.; Guan, G.; Zhang, Y.; Peng, H. Flexible and weaveable capacitor wire based on a carbon nanocomposite fiber. Adv. Mater. 2013, 25, 5965–5970. [Google Scholar] [CrossRef]
- Xu, P.; Gu, T.; Cao, Z.; Wei, B.; Yu, J.; Li, F.; Byun, J.H.; Lu, W.; Li, Q.; Chou, T.W. Carbon Nanotube Fiber-Based Stretchable Wire-Shaped Supercapacitors. Adv. Energy Mater. 2014, 4, 1300759. [Google Scholar] [CrossRef]
- Guragain, D.; Zequine, C.; Gupta, R.K.; Mishra, S.R. Effect of dopant on the morphology and electrochemical performance of Ni1-xCaxCo2O4 (0≤ x ≤ 0.8) oxide hierarchical structures. MRS Adv. 2020, 5, 2487–2494. [Google Scholar] [CrossRef]
- Karna, S.; Neupane, D.; Mishra, S.R.; Choi, J.; Gupta, R.K.; Mishra, S.R.; Karna, P. Electrochemical Behavior of S and C Mono-Doped Sodium Tantalate Photocatalysts. J. Electron. Mater. 2021, 50, 7133–7142. [Google Scholar] [CrossRef]
- Augustyn, V.; Come, J.; Lowe, M.A.; Kim, J.W.; Taberna, P.L.; Tolbert, S.H.; Abruña, H.D.; Simon, P.; Dunn, B. High-rate electrochemical energy storage through Li+ intercalation pseudocapacitance. Nat. Mater. 2018, 12, 518. [Google Scholar] [CrossRef]
- Zhai, S.; Karahan, H.E.; Wang, C.; Pei, Z.; Wei, L.; Chen, Y. 1D supercapacitors for emerging electronics: Current status and future directions. Adv. Mater. 2019, 1902387. [Google Scholar] [CrossRef]
- Kirubasankar, B.; Vijayan, S.; Angaiah, S. Sonochemical synthesis of a 2D–2D MoSe2/graphene nanohybrid electrode material for asymmetric supercapacitors. Sustain. Energy Fuels 2019, 3, 467–477. [Google Scholar] [CrossRef]
- Muhammad, H.; Tahiri, I.A.; Muhammad, M.; Masood, Z.; Versiani, M.A.; Khaliq, O.; Latif, M.; Hanif, M. A comprehensive heterogeneous electron transfer rate constant evaluation of dissolved oxygen in DMSO at glassy carbon electrode measured by different electrochemical methods. J. Electroanal. Chem. 2016, 775, 157–162. [Google Scholar] [CrossRef]
- Ali, G.A.; Fouad, O.A.; Makhlouf, S.A.; Yusoff, M.M.; Chong, K.F. Co3O4/SiO2 nanocomposites for supercapacitor application. J. Solid State Electrochem. 2014, 18, 2505–2512. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Polleux, J.; Lim, J.; Dunn, B. Pseudocapacitive contributions to electrochemical energy storage in TiO2 (anatase) nanoparticles. J. Phys. Chem. C 2007, 111, 14925–14931. [Google Scholar] [CrossRef]
- Fu, H.; Liu, Y.; Chen, L.; Shi, Y.; Wong, W.; Hou, J.; Yu, F.; Wei, T.; Wang, H.; Guo, X. Designed formation of NiCo2O4 with different morphologies self-assembled from nanoparticles for asymmetric supercapacitors and electrocatalysts for oxygen evolution reaction. Electrochim. Acta 2019, 296, 719–729. [Google Scholar] [CrossRef]
- Qing, X.; Liu, S.; Huang, K.; Lv, K.; Yang, Y.; Lu, Z.; Fang, D.; Liang, X. Facile synthesis of Co3O4 nanoflowers grown on Ni foam with superior electrochemical performance. Electrochim. Acta 2011, 56, 4985–4991. [Google Scholar] [CrossRef]
- Kim, B.K.; Sy, S.; Yu, A.; Zhang, J. Electrochemical Supercapacitors for Energy Storage and Conversion. In Handbook of Clean Energy Systems; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015. [Google Scholar] [CrossRef]
- Lakehal, A.; Bouaza, A.; Benrabah, B.; Dalache, C.; Benhebal, H. Characterization of Mn-Doped Co3O4 Thin Films Prepared by Sol Gel-Based Dip-Coating Process. High Temp. Mater. Process. 2019, 38, 237–247. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.; Jamal, A.; Khan, S.B.; Faisal, M. Fabrication of Highly Sensitive Ethanol Chemical Sensor Based on Sm-Doped Co3O4 Nanokernels by a Hydrothermal Method. J. Phys. Chem. C 2011, 115, 9503–9510. [Google Scholar] [CrossRef]
- Hitkari, G.; Sandhya, S.; Gajanan, P.; Shrivash, M.; Kumar, D. Synthesis of Chromium Doped Cobalt Oxide (Cr:Co3O4) Nanoparticles by Co-Precipitation Method and Enhanced Photocatalytic Properties in the Visible Region. J. Mater. Sci. Eng. 2018, 7, 419. [Google Scholar] [CrossRef]
- Wang, Y.; Lei, Y.; Li, J.; Gu, L.; Yuan, H.; Xiao, D. Synthesis of 3D-Nanonet Hollow Structured Co3O4 for High Capacity Supercapacitor. ACS Appl. Mater. Interfaces 2014, 6, 6739–6747. [Google Scholar] [CrossRef]
- Tao, J.; Liu, N.; Li, L.; Su, J.; Gao, Y. Hierarchical nanostructures of polypyrrole@MnO2 composite electrodes for high performance solid-state asymmetric supercapacitors. Nanoscale 2014, 6, 2922. [Google Scholar] [CrossRef]
- Sun, D.; He, L.; Chen, R. Biomorphic composites composed of octahedral Co3O4 nanocrystals and mesoporous carbon microtubes templated from cotton for excellent supercapacitor electrodes. Appl. Surf. Sci. 2019, 465, 232–240. [Google Scholar] [CrossRef]
- Pu, J.; Shen, L.; Zhu, S.; Wang, J.; Zhang, W.; Wang, Z. Fe3O4@C core–shell microspheres: Synthesis, characterization, and application as supercapacitor electrodes. J. Solid State Electrochem. 2013, 18, 1067–1076. [Google Scholar] [CrossRef]
- Meng, F.; Fang, Z.; Li, Z.; Xu, W.; Wang, M.; Liu, Y.; Zhang, J.; Wang, W.; Zhao, D.; Guo, X.; et al. Porous Co3O4 materials prepared by solid-state thermolysis of a novel Co-MOF crystal and their superior energy storage performances for supercapacitors. Chem. A 2013, 1, 7235. [Google Scholar] [CrossRef]
- Singh, V.; Kosa, M.; Majhi, K.; Major, D.T. Putting DFT to the test: A first-principles study of electronic, magnetic, and optical properties of Co3O4. J. Chem. Theory Comput. 2015, 11, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Amaral, L.; Cardoso, D.; Santos, D.; Sljukic, B.; Sequeira, C. Room temperature ionic liquids as electrolyte additives for the HER in alkaline media. J. Electrochem. Soc. 2017, 164, F427. [Google Scholar] [CrossRef]
- Armstrong, R.; Henderson, M. Impedance plane display of a reaction with an adsorbed intermediate. J. Electroanal. Chem. Interfacial Electrochem. 1972, 39, 81–90. [Google Scholar] [CrossRef]
- Krstajic, N.; Popovic, M.; Grgur, B.; Vojnovic, M.; Sepa, D. On the kinetics of the hydrogen evolution reaction on nickel in alkaline solution: Part I. The mechanism. J. Electroanal. Chem. 2001, 512, 16–26. [Google Scholar] [CrossRef]
- Mehta, N.K. Equivalent Circuit Model: Electrolytic Damage Assessment of the Scribed Coated Steel Surface Embedded with Corrosion Inhibitor Capsules. Am. J. Mater. Sci. 2013, 3, 149–161. [Google Scholar] [CrossRef]
- Matasso, A.; Wong, D.; Wetz, D.; Liu, F. Effects of High-Rate Cycling on the Bulk Internal Pressure Rise and Capacity Degradation of Commercial LiCoO2 Cells A. J. Electrochem. Soc. 2015, 162, A885–A891. [Google Scholar] [CrossRef]
- Wei, Z.; Ren, Y.; Wang, M.; He, J.; Huo, W.; Tang, H. Improving the Conductivity of Solid Polymer Electrolyte by Grain Reforming. Nanoscale Res. Lett. 2020, 15, 122. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.I.; Guan, B.Y.; Lu, X.F.; Xi, S.; Du, Y.; Lou, X.W. Metal Atom-doped Co3O4 hierarchical nanoplates for electrocatalytic Oxygen Evolution. Adv. Mater. 2020, 32, 2002235. [Google Scholar] [CrossRef]
- Zhong, X.; Yi, W.; Qu, Y.; Zhang, L.; Bai, H.; Zhu, Y.; Wan, Y.; Chen, S.; Yang, M.; Huang, L.; et al. Co Single-atom Anchored on Co3O4 and Nitrogen-Doped Active Carbon toward Bifunctional Catalyst for Zinc-Air Batteries. Appl. Catal. B 2020, 260, 118188. [Google Scholar] [CrossRef]
- Liu, H.; Xia, G.; Zhang, R.; Jiang, P.; Chen, J.; Chen, Q. MOF-derived RuO2/Co3O4 heterojunctions as highly efficient bifunctional electrocatalysts for HER and OER in alkaline solutions. RSC Adv. 2017, 7, 3686–3694. [Google Scholar] [CrossRef] [Green Version]
- Sheng, Y.; Botero, M.L.; Manuputty, M.Y.; Kraft, M.; Xu, R. Co3O4 and FexCo3–xO4 Nanoparticles/Films Synthesized in a Vapor-Fed Flame Aerosol Reactor for Oxygen Evolution. ACS Appl. Energy Mater. 2018, 1, 655–665. [Google Scholar] [CrossRef]
- Bao, J.; Liu, W.; Xie, J.; Xu, L.; Guan, M.; Lei, F.; Zhao, Y.; Huang, Y.; Xia, J.; Li, H. NixCo3-xO4 Nanoneedle Arrays Grown on Ni Foam as an Efficient Bifunctional Electrocatalyst for Full Water Splitting. Chem. Asian J. 2019, 14, 480–485. [Google Scholar] [CrossRef]
- Indra, A.; Menezes, P.W.; Sahraie, N.R.; Bergmann, A.; Das, C.; Tallarida, M.; Schmeißer, D.; Strasser, P.; Driess, M. Unification of Catalytic Water Oxidation and Oxygen Reduction Reactions: Amorphous Beat Crystalline Cobalt Iron Oxides. J. Am. Chem. Soc. 2014, 136, 17530–17536. [Google Scholar] [CrossRef]
- Zhang, C.; Antonietti, M.; Fellinger, T.P. Blood Ties: Co3O4 Decorated Blood Derived Carbon as a Superior Bifunctional Electrocatalyst. Adv. Funct. Mater. 2014, 24, 7655–7665. [Google Scholar] [CrossRef]
- Wang, X.; Yu, L.; Guan, B.Y.; Song, S.; Lou, X.W. Metal–Organic Framework Hybrid-Assisted Formation of Co3O4/Co-Fe Oxide Double-Shelled Nanoboxes for Enhanced Oxygen Evolution. Adv. Mater. 2018, 30, 1801211. [Google Scholar] [CrossRef]
- Wang, Q.; Xue, X.; Lei, Y.; Wang, Y.; Feng, Y.; Xiong, X.; Wang, D.; Li, Y. Engineering of Electronic States on Co3O4 Ultrathin Nanosheets by Cation Substitution and Anion Vacancies for Oxygen Evolution Reaction. Small 2020, 16, 2001571. [Google Scholar] [CrossRef]
- Nai, J.; Zhang, J.; Lou, X.W. Construction of Single-Crystalline PrussianBlue Analog Hollow Nanostructures with Tailorable Topologies. Chem 2018, 4, 1967–1982. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; He, J.; Zhao, S.; Liu, Y.; Zhao, Z.; Luo, J.; Hu, G.; Sun, X.; Ding, Y. Self-powered H2 production with bifunctional hydrazine as sole consumable. Nat. Commun. 2018, 9, 4365. [Google Scholar] [CrossRef] [PubMed]











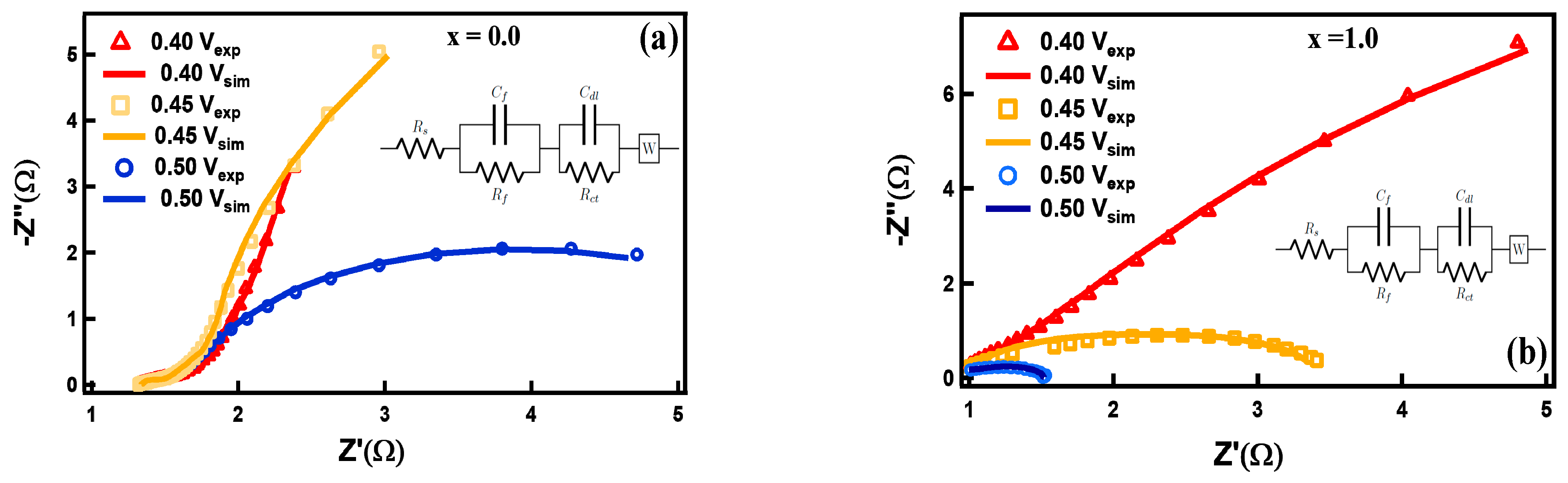
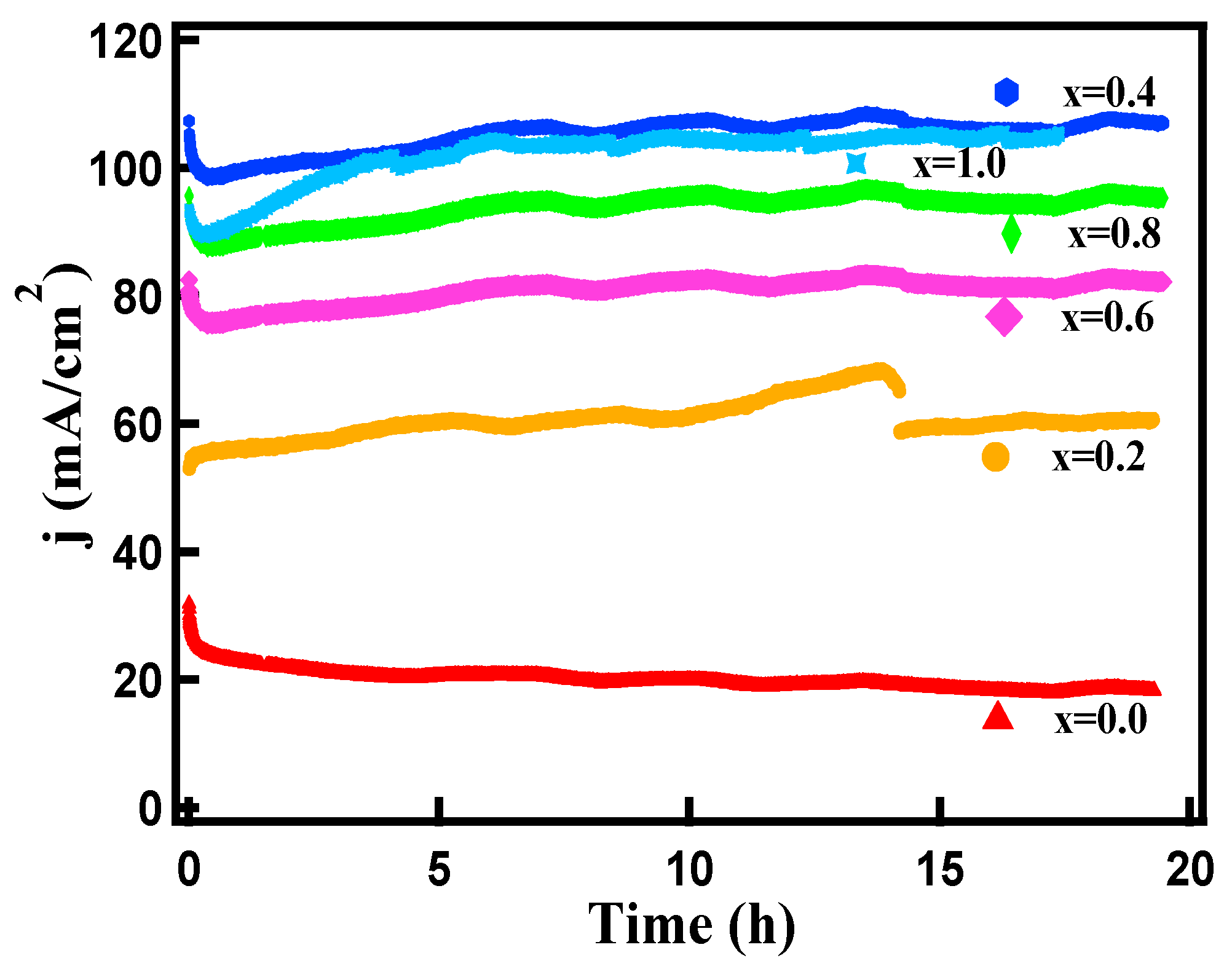
| FexCo3-xO4 x | Co(NO3)2.6H2O (g) | Fe(NO3)2.6H2O (g) | ν1 (cm−1) | ν2 (cm−1) |
|---|---|---|---|---|
| 0.0 | 3.6265 | 0.0000 | 538.060 | 653.295 |
| 0.2 | 3.3934 | 0.3365 | 544.557 | 655.710 |
| 0.4 | 3.1591 | 0.6747 | 546.877 | 656.918 |
| 0.6 | 2.9237 | 1.0146 | 549.661 | 657.120 |
| 0.8 | 2.6870 | 1.3564 | 550.125 | 657.716 |
| 1.0 | 2.4490 | 1.6998 | 551.517 | 658.081 |
| FexCo3-xO4 x | BET Surface Area(m2/g) | BJH Surface area (m2/g) | BJH Avg. Pore Radius (nm) | BJH Avg. Pore Volume (cc/g) | Crystallite Size (nm) | Lattice Parameter, a (nm) |
|---|---|---|---|---|---|---|
| 0.0 | 59.47 | 81.48 | 1.432 | 0.202 | 19.4349 | 8.06235 |
| 0.2 | 64.02 | 83.43 | 1.270 | 0.186 | 16.9104 | 8.05657 |
| 0.4 | 76.22 | 75.85 | 1.522 | 0.201 | 18.3513 | 8.05746 |
| 0.6 | 68.36 | 108.93 | 0.531 | 0.213 | 17.2522 | 8.06047 |
| 0.8 | 70.74 | 75.78 | 3.228 | 0.191 | 18.4247 | 8.05297 |
| 1.0 | 87.45 | 94.41 | 1.511 | 0.189 | 20.0733 | 8.06054 |
| Content, x. | OER | HER | ||
|---|---|---|---|---|
| Onset Overpotential (η0) | Overpotential (η10) | Onset Overpotential (η0) | Overpotential (η10) | |
| 0.0 | 297 mV | 329 mV | 81 mV | 182 mV |
| 0.2 | 248 mV | 268 mV | 81 mV | 191 mV |
| 0.4 | 248 mV | 268 mV | 17 mV | 153 mV |
| 0.6 | 303 mV | 320 mV | 1 mV | 132 mV |
| 0.8 | 249 mV | 270 mV | 30 mV | 179 mV |
| 1.0 | 245 mV | 266 mV | 2 mV | 166 mV |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guragain, D.; Karna, S.; Choi, J.; Bhattarai, R.; Poudel, T.P.; Gupta, R.K.; Shen, X.; Mishra, S.R. Electrochemical Performance of Iron-Doped Cobalt Oxide Hierarchical Nanostructure. Processes 2021, 9, 2176. https://doi.org/10.3390/pr9122176
Guragain D, Karna S, Choi J, Bhattarai R, Poudel TP, Gupta RK, Shen X, Mishra SR. Electrochemical Performance of Iron-Doped Cobalt Oxide Hierarchical Nanostructure. Processes. 2021; 9(12):2176. https://doi.org/10.3390/pr9122176
Chicago/Turabian StyleGuragain, Deepa, Sunil Karna, Jonghyun Choi, Romakanta Bhattarai, Tej P. Poudel, Ram Krishna Gupta, Xiao Shen, and Sanjay R. Mishra. 2021. "Electrochemical Performance of Iron-Doped Cobalt Oxide Hierarchical Nanostructure" Processes 9, no. 12: 2176. https://doi.org/10.3390/pr9122176
APA StyleGuragain, D., Karna, S., Choi, J., Bhattarai, R., Poudel, T. P., Gupta, R. K., Shen, X., & Mishra, S. R. (2021). Electrochemical Performance of Iron-Doped Cobalt Oxide Hierarchical Nanostructure. Processes, 9(12), 2176. https://doi.org/10.3390/pr9122176








