Abstract
Algae-assisted microbial desalination cells represent a sustainable technology for low-energy fresh water production in which microalgae culture is integrated into the system to enhance oxygen reduction reaction in the cathode chamber. However, the water production (desalination rate) is low compared to conventional technologies (i.e., reverse osmosis and/or electrodialysis), as biocathodes provide low current generation to sustain the desalination process. In this sense, more research efforts on this topic are necessary to address this bottleneck. Thus, this study provides analysis, from the electrochemical point of view, on the cathode performance of an algae-assisted microbial desalination cell (MDC) using Chlorella vulgaris. Firstly, the system was run with a pure culture of Chlorella vulgaris suspension in the cathode under conditions of an abiotic anode to assess the cathodic behavior (i.e., cathode polarization curves in light-dark conditions and oxygen depletion). Secondly, Geobacter sulfurreducens was inoculated in the anode compartment of the MDC, and the desalination cycle was carried out. The results showed that microalgae could generate an average of 9–11.5 mg/L of dissolved oxygen during the light phase, providing enough dissolved oxygen to drive the migration of ions (i.e., desalination) in the MDC system. Moreover, during the dark phase, a residual concentration of oxygen (ca. 5.5–8 mg/L) was measured, indicating that oxygen was not wholly depleted under our experimental conditions. Interestingly, the oxygen concentration was restored (after complete depletion of dissolved oxygen by flushing with N2) as soon as microalgae were exposed to the light phase again. After a 31 h desalination cycle, the cell generated a current density of 0.12 mA/cm2 at an efficiency of 60.15%, 77.37% salt was removed at a nominal desalination rate of 0.63 L/m2/h, coulombic efficiency was 9%, and 0.11 kWh/m3 of electric power was generated. The microalgae-assisted biocathode has an advantage over the air diffusion and bubbling as it can self-sustain a steady and higher concentration of oxygen, cost-effectively regenerate or recover from loss and sustainably retain the system’s performance under naturally occurring conditions. Thus, our study provides insights into implementing the algae-assisted cathode for sustainable desalination using MDC technology and subsequent optimization.
1. Introduction
A little over a decade ago, a group of researchers from Tsinghua University (China) and Penn State University (USA) introduced the first microbial desalination cells (MDC) concept as a new method for water desalination using a bioelectrochemical strategy [1]. This technology is, therefore, capable of simultaneous treatment of wastewater, desalination, and electricity production. The MDC system operates with electroactive bacteria capable of generating a flux of electrons to the anode from the oxidation of organic content in the wastewater. An electrical current is generated, and energy is produced as the electrons flow from the anode to the cathode through an external load, where the reduction reaction occurs. The gradient created in the anode and cathode chamber causes the migration of ions across anion- and cation-exchange membranes in the middle compartment, and the desalination process is produced [2,3,4,5,6,7]. The basis of this technology is similar to other microbial electrochemical technologies (METs), but in the case of MDC, an additional desalination compartment is implemented between anodic and cathodic compartments [2,8]. The technology’s sustainable and promising merits have received a great deal of attention, as a significant contribution to the development of a sustainable application of MDC in desalination has been reported [9,10,11] (pp. 175–220 in [9]).
However, more understanding and advancement are needed [6,12,13,14,15] as some limitations challenge the microbial and electrochemical processes. In this sense, the main drawback for scaling up and implementing MDC technology could be cathodic limitations, resulting in low freshwater production and partial salt removal.
Different studies have widely explored cathodic limitations in MDC due to their significant influence on the desalination performance, current generation, and electricity production [16]. The presence of an electron acceptor (e.g., oxygen as in Equation (1)) in the cathode chamber enables the reduction process to complete oxidation reaction (by the anode in Equation (2)), considering acetate oxidation by Geobacter sulfurreducens) in MDC to generate electricity (i.e., ΔG = −nF (E0cathode − E0anode) < 0, spontaneous process):
Cathode reaction: O2 + 4H+ 4e−→ 2H2O; (E0cathode = 1.229 V)
Anode reaction: C2H4O2 + 2H2O → 2CO2 + 8H+ + 8e−; (E0anode = −0.290 V)
However, the main limitation in MDC systems is related to slow kinetics associated with this oxygen reduction reaction at neutral or biological pHs (pH = 7–10), which is commonly used for growing electroactive microorganisms. For these reasons, electrochemical reduction reactions with a high redox potential are suitable to be used in MDC systems. Therefore, various compounds including permanganate, ferricyanide, ferric iron, manganese dioxide, nitrate, persulfate, sodium hypochlorite [7], and oxygen have been successfully used as electron acceptors used in this technology [17,18,19,20]. Table S1 (in Supplementary Materials) shows the different electron acceptors (in catholyte) introduced and used in previous studies. Comparison of their results confirms that the nature (abiotic or biotic), type, and chemical composition of the catholyte has a significant influence on the performance of the cathode [21,22]. Unfortunately, the use of such compounds implies the replacement when depleted, decreasing the sustainability of the process.
Biocathodes use microorganisms to mediate reduction reactions (for example, nitrate reduction) instead of noble or non-noble catalysts for oxygen reduction in the cathode chamber [14]. Studies have confirmed that biocathodes are of low cost, easy to use, and have natural buffering capacity, which can boost high salt removal rates and effective wastewater treatments, thus making it the right candidate for MDC development and scale-up [12]. Nevertheless, the limitation of the low current generation and desalination rate in MDC using biocathodes compared with abiotic cathodes persists, so more research efforts on this topic are necessary to address this bottleneck. Since the introduction of algae-assisted MDC systems in 2013, there has not been any significant improvement in its mechanism and performance. Arana and Gude studied the role of using sodium bicarbonate (NaHCO3) to increase the production of dissolved oxygen and ionic concentration difference in an algae-assisted MDC. They recommended a thorough analysis of algae-assisted MDC systems from the electrochemical point of view to improve the understanding of the process and its optimization as the way forward for biocathodes implementation on MDC systems [23].
Thus, this study analyses the cathode performance of an algae-assisted microbial desalination cell (MDC) using Chlorella vulgaris. Firstly, the cathode oxygen reduction reaction (from the dissolved oxygen produced by the microalgae) was examined in different experimental conditions (i.e., light and dark phase, operation condition) to understand its electrochemical behavior. Then, the MDC performance (i.e., current density, specific energy production, COD removal rate, current efficiency, desalination process, nominal desalination rate, and coulombic efficiency (CE)) of algae assisted MDC is presented and discussed in comparison with analogous MDC systems from the literature using air-diffusion cathodes and/or air bubbling. The results of this study could help to understand the performance of algae-assisted MDC systems from an electrochemical behavior point of view and significantly contribute to further development, design, and optimization.
2. Materials and Methods
2.1. Microbial Culture: Algae (Photobioreactor) and Electroactive Biofilm (Anode)
A pure culture of Chlorella vulgaris (Figure S1 in Supplementary Materials) was cultivated under isolated conditions using Bold’s Basal Media (BBM) as culturing media. Studies have proved that BBM is suitable for the best specific growth rate, biomass productivity, lipid yield/productivity and best strategy to induce high lipid in Chlorella vulgaris [24] and therefore the appropriate choice for this study. BBM was prepared by addition to distilled of 10 mL of the next solutions per 1 L of culture medium: 25 g/L NaNO3, 7.5 g/L MgSO4.7H2O, 2.5 g/L NaCl, 7.5 g/L K2HPO4, 17.5 g/L KH2PO4, and 2.5 g/L CaCl2.2H2O. Additionally, 1 mL of the next solutions per 1 L of culture medium: Alkaline EDTA solution (50 g/L EDTA, 31 g/L KOH); Acidified Iron solution (4.98 g/L FeSO4.7H2O, 1.0 mL H2SO4 conc.); Boron solution (11.42 g/L H3BO3); Trace Metals solution (8.82 g/L ZnSO4.7H2O, 1.44 g/L MnCl2.4H2O, 0.71 g/L MoO3, 1.57 g/L CuSO4.5H2O, 0.49 g/L Co(NO3)2.6H2O). 10 mL of Chlorella vulgaris was inoculated in 1 L media and was cultivated under the temperature of 26.5 °C. As shown in Figure S1 in Supplementary Materials, the culture was kept in aerobic conditions (air pump), continuous agitation (magnetic stirrer, IKA Topolino), and a continuous supply of light in an incubator. At the same time, the growth was monitored using absorbance at a wavelength of 680 nm. After a couple of days, the algal suspension was ready to be used as a catholyte (Figure S1 in Supplementary Materials) in the MDC system. The catholyte tank (i.e., photobioreactor) was filled with 2 L of microalgae suspension (at a continuous supply of light or 16/8, 26 ± 1 °C), which supplied the cathode chamber at a continuous fed-batch mode.
Additionally, a pure culture of Geobacter sulfurreducens (strain DL1) was cultured (in freshwater media (FWM) with Sodium acetate (NaC2H3O2 20 mM) as electron donor and fumarate (C4H2Na2O4, 40 mM) as a sole electron acceptor under anaerobic conditions (80:20 N2/CO2 gas atmosphere) till reach late exponential phase. The 200 mL of such culture (OD = 0.6) was introduced into the anode chamber to facilitate the formation of an electrogenic biofilm, as previously reported [25]. The anolyte solution used at IMDEA Water consisted of FWM containing 0.1 g/L, KCl, 2.5 g/L NaHCO3, 0.6 g/L KH2PO4, 0.5 g/L NH4Cl, 10 mL/L of trace element solution, 1 mL/L of Wolfe’s vitamins solution, and 20 mM sodium acetate as organic substrate.
2.2. MDC Construction and Setup
The MDC reactor used in this study had three distinct compartments with a cross-section of 100 cm2, thus a ratio of 1:1:1. Details of the components of the reactor, chambers, and operations conditions are summarized in Table 1. The reactor and its components were sterilized before use.

Table 1.
Microbial desalination cell (MDC) components.
Carbon felt were used as the electrodes in both anode and cathode chambers coupled with isostatic graphite plates as electron collectors (see Table 1 for details). Anion exchange membrane (Neosepta AMX) and cation exchange membrane (Neosepta CMX) separated the anode, desalination, and cathode chamber, respectively. They were held in place inside the reactor, fitting between rubber gaskets to prevent leakages. Both membranes were immersed in a 5% sodium chloride solution for 24 h and then washed adequately with deionized water before installing it into the reactor.
2.3. Start-Up and Operation Conditions
Figure 1A shows the cross-section of the MDC cell and the main reactions occurring in the individual chambers. All solutions in the reservoirs were continuously agitated to maintain uniform distribution of light and mixing conditions. Before microalgae were into the MDC, an abiotic configuration test was carried out to test the system’s capability for the desalination process as introduced previously [7]. In addition, a Biofilm of Geobacter sulfurreducens on the anode surface was grown as reported elsewhere [7]. All measurements and tests were done at a temperature of 25 °C ± 1. Data acquisition was carried out with a Visual Basic program and ModBus modules (ICP-DAS).
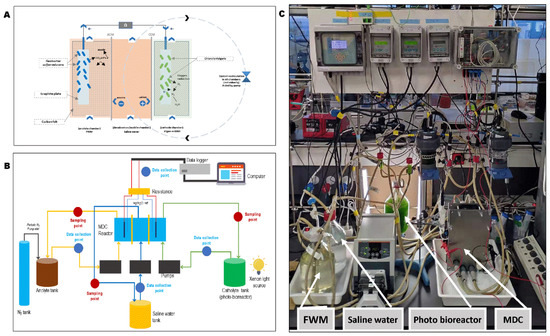
Figure 1.
(A) Details of reactions and compartment of MDC cell in this study; (B) diagram showing the experimental setup and flow pattern; (C) actual picture of experimental setup.
Electric conductivity measurements were carried out using GLP 31 conductivity meter (CRISON) and pH with pH meter-28 controller (CRISON). Two reference electrodes (Ag/AgCl KCl 3.5 M, CRISON) were placed in the middle of the anode and cathode chamber (near the electrodes) using an agar-KCL salt bridge to boost precision in electrode potential analysis. External voltage was applied using a Laboratory Power Supply EA-PS 3016-40 B (EA-Elektro-Automatik, Viersen, Germany). Two 12-inch fluorescent white light bars were used as a source of light for the microalgae (Inspire, LED W13-349MM-C, Zhejiang, China). The microalgae growth was monitored during the culturing stage to assess microalgae growth in the medium by analyzing 4 mL of the microalgae suspension daily using a spectrophotometer (SpectroQuant HARO 1000 Merck) at 680 nm absorbance. Dissolved oxygen (DO) was measured with a Fiber-Optic Oxygen Meter and a Pyro Oxygen Logger Software (FireSting O2, Aachen, Germany). All electrolytes and saline water were recirculated using a 3-channelled PD 5201 pump drive (Heidolph Instruments, Schwabach, Germany) at a constant pumping rate of 95 mL/min. The complete setup for all the stages/phases of this study is shown in Figure 1B,C.
2.4. Parameter Calculations
To determine the performance of the MDC, the following equations were used to calculate the main parameters:
where ci = initial saline concentration (mol/m3), cf = final saline concentration (mol/m3), Qt = volume of the saline tank (L), Am = effective electrode surface area (cm2), td = desalination time, thus when conductivity is 1 mS/cm2 (h), V = voltage readings (mV), Rext = external resistance (Ω), ΔCOD = changes in COD concentration (mg/L), VA = volume of liquid in the anode compartment (m3), v and z represent the stoichiometric coefficient and the valence of the salt ions, respectively and F is the Faraday constant (96,485 C mol−1).
3. Results
This study was conducted in two main phases, as summarized in Figure S2 in Supplementary Materials. Previously, the MDC reactor was operated under abiotic conditions and an external power supply to confirm the system’s feasibility as an electrochemical device for the desalination process [7] (Figure S3 in Supplementary Materials). Then, the reactor was first run with an abiotic anode and microalgae in the cathode to study algae-assisted cathode’s electrochemical performance (Phase 1). Under this phase, the system was operated non-spontaneously by applying an external power range of 0–1.2 V. Secondly, a desalination cycle (initial conductivity of 9.98 mS/cm) was performed with a bionaode (Geobacter sulfurreducens) and cathode (Chlorella vulgaris) (Phase 2). Power density, current density, salt removal, nominal desalination rate, and COD removal were measured to assess the microalgae-assisted MDC’s performance, understand the microalgae’s behavior and growth condition, and outline the merits of this system in contributing to the sustainable scaling of this technology.
3.1. Algae Growth and the Electrochemical Behaviour of Algae Assisted Cathode
A pure culture of Chlorella vulgaris was prepared under isolated conditions using Bold Basal Media (BBM) as culturing media. Figure 2 shows the absorbance of the culture at a wavelength of 680 nm as an indirect measurement of the efficiency of biomass growth of the microalgae, which is associated with chlorophyll absorption [26]. After 25 days, the absorbance showed that steady growth and reached a plateau phase, thus capable of sustaining oxygen generation in the MDC system. After the MDC system was tested under abiotic conditions to check performance as an electrochemical desalination device, the cathode circuit (chamber, tubes, and reservoir) was sterilized. Then, the catholyte tank was substituted by the pre-cultured microalgae solution (in steady-state conditions).
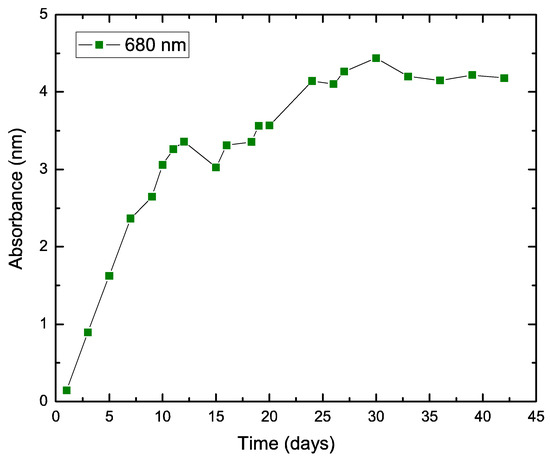
Figure 2.
Growth curve of the microalgae culture according to absorbance at 680 nm.
The results showed that the microalgae produced varying oxygen concentrations in the cathode under both the light and dark phases (Figure 3A). Oxygen concentration was higher during the light phase (mainly photosynthesis)—ranging between 9–11.5 mg/L- and lowered during the dark phase (respiration)—in the range of 5.5–8 mg/L. Moreover, the decrease during the dark phase was due to oxygen consumption in the cathode due to the lack of photosynthesis or electrochemical consumption.
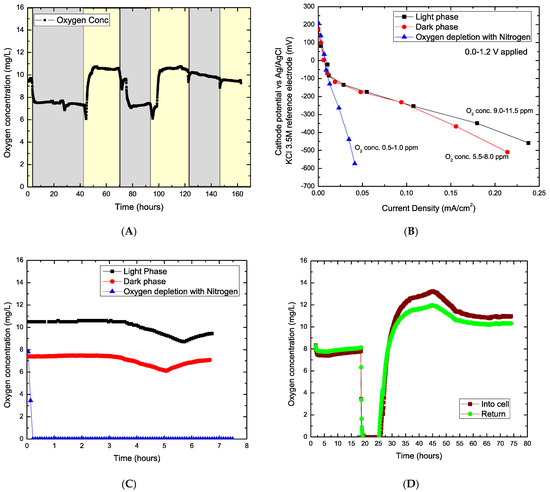
Figure 3.
(A) Dissolved oxygen (DO) generated by microalgae in light (yellow background) and dark phase (grey background); (B) Cathodic behavior of algae assisted MDC under different conditions for light phase, dark phase, and O2 depletion with N2, (C) DO concentration levels under the conditions of the light phase, dark phase and oxygen depletion and; (D) algae self-recovery capacity; DO before and after nitrogen gas was pumped in. NOTE: (A) corresponds to an independent experiment. (B,C) were registered simultaneously during the same experiment. Figure 3D test was carried out after experiment related to (B,C).
In Figure 3B, the electrochemical response of the system to variations in the cathodic potential was studied under three distinct conditions: (i) light phase, (ii) dark phase, and (iii) no oxygen (replaced with N2). Our results revealed that oxygen concentration in the catholyte influences cathodic potential. Figure 3D shows the oxygen depletion with N2 gas and self-recovery by algae in the cell (before this, the system was left under a dark phase for 24 h). After oxygen was depleted (0 ppm), the algae self-recovered the oxygen concentration (rise from 0 ppm) within 1 h after the N2 pump was stopped. After 18 h of continuous operation in the light phase condition, the DO was fully recovered and stable.
In a closed or open bioreactor, maintaining continuous microalgae growth is essential for current generation and desalination. To sustain algae biomass growth, it is necessary to maintain favorable conditions, including light/dark phases, carbon source, air, and conducive pH and temperature. Studies have shown that metabolism and respiration of photosynthetic algae occur during the light and dark phases, respectively. Meanwhile, optimal biomass growth rate takes place under suboptimal conditions, thereby making the respiration process during the dark phase significantly important for the photobioreactor [27,28]. The photosynthetic efficiency is critically reduced when light and dark cycles are not optimum [29]. In the microalgae suspension (aqueous solution), microalgae culture provides enough O2 (i.e., from photosynthesis) to maintain cathode potential and drive the desalination process. The oxygen reduction reaction (ORR) process in the cathode compartment occurs by the direct transfer pathway of 4-electrons from O2 to H2O (O2 + 2H2O + 4e− → 4 OH−, E0′ = 0.815 V, pH = 7) [30]. At lower current densities, near open-circuit voltage, cathode potential under the three conditions showed a less significant difference, unlike at higher current densities (i.e., 0.15–0.20 mA/cm2) where potential under light and dark phase generated higher current compared to the O2 depleted phase.
Due to the mass transfer limitation process, O2 should be readily available in the catholyte to enhance ORR. Therefore, the cathode potential depends on the availability of oxygen in the solution [31]. According to the results, microalgae can continuously provide O2, and a higher DO concentration than air bubbling and air cathodes. The oxygen concentration generated in the results of this study is averagely higher than observed in other studies using microalgae as a catholyte in MDC [32,33,34,35] and comparatively higher in concentration and stability with air diffusion cathodes [36,37,38]. The results also observed that microalgae could maintain an optimum DO concentration and self-regenerate depleted DO in the catholyte (Figure 3D). Sustaining a high concentration of dissolved oxygen in the reservoir can affect biomass growth productivity [39]. Nevertheless, this phenomenon is vital for the ORR process at the cathode.
The average concentration of DO recorded in this study to support the MDC operation under stable favorable conditions is higher than other studies using microalgae biocathode [14,32,34,40,41,42]. This could be attributed to the effective pre-culturing process of the microalgae before the operation. This process is significant to skip the microalgae lag phase and maximize performance when introduced into the reactor.
3.2. Desalination and COD Removal
In phase 2, 200 mL of pre-cultured Geobacter sulfurreducens was inoculated into the anode chamber with FWM as anolyte and allowed to adhere to the carbon felt for 96 h before the first desalination cycle (Figure S2 in Supplementary Materials). Electrolytes and saline water were replaced once the electrogenic biofilm was adequately developed on the anode surface (electrical current production). The system was connected to an external resistance of 2.5 Ω. Geobacter sulfurreducens is specialized in degrading acetate under anaerobic conditions and transferring electrons directly to electrodes as part of extracellular respiration [43]. Therefore, the cathode potential is significant for desalination as it could limit the performance in MDCs.
3.2.1. Oxygen Concentration and Electric Current Generation
This section discusses the capacity of Chlorella vulgaris to sustain the current production by generating enough oxygen for the reduction process and then provide potential to drive the desalination process in the MDC. Figure 4A shows the oxygen concentration vs. time for the desalination experiment during the desalination period. The results showed an average concentration of 9–10 ppm in the influent (thus measured in the microalgae suspension just before entering the cathode compartment) and an average of 8 ppm in the effluent (thus measured in the microalgae suspension just after leaving the cell compartment) throughout the operation of the system. It is observed that in the presence of favorable conditions (suitable media-BBM, temperature, pH, light/dark cycles, open system for aeration), microalgae are capable of generating a consistent concentration of oxygen to sustain the processes of the MDC reactor. Figure 4B presents the anode and cathode potentials measured against the Ag/AgCl reference electrode over the desalination period. A steady potential difference was observed between the anode and the potential, which creates a gradient to drive the desalination process in the MDC. The slight decrease of cathode potential during the experiment could be attributed to the increase of pH in the catholyte tank (see Figure 4B).
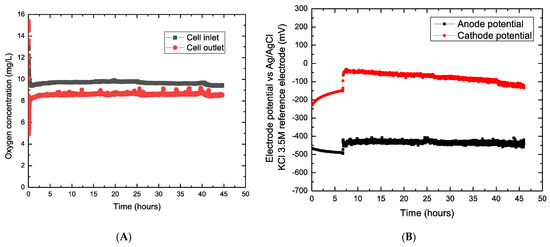
Figure 4.
(A) Inlet and outlet oxygen concentration vs. time in the cathodic chamber (B) Cathode and anode potential vs. time during desalination. NOTE: Experimental data from (A,B) were registered simultaneously.
During the experiment, the current density generated was stable in 0.10–0.12 mA/cm2. From Figure 4A, it can be observed that the steady generation of oxygen by the microalgae was higher than the oxygen consumption as the cathode and enough to generate current within the desalination period. Thus, it could be considered that microalgae culture provides enough oxygen (i.e., from photosynthesis) to maintain cathode potential and drive the desalination process, as indicated in Figure 4B (red curve). In this sense, if microalgae culture were not providing enough oxygen to the catholyte solution, the potential would be dramatically decreased, as suggested by Figure 3B (see cathode curve for oxygen depletion). However, compared to other studies [23], the current density generated was relatively low, attributed to the low initial salinity concentration. Saline concentration in the middle compartment can significantly regulate the internal resistance and the conductivity (i.e., ionic exchange) within the MDC system, thereby affecting the electric current [26,44,45] (pp. 15–40 in [9]).
3.2.2. Desalination and COD Degradation Performance
Table 2 shows the conductivity changes during the experiment in the three chambers. Saline water has an initial conductivity of 7.93 mS/cm (i.e., brackish water) and is reduced by 88% after 31 h of continuous batch recirculation. Thus, a nominal desalination rate (NDR) of 0.623 L/m2/h was obtained. The desalination cycle was stopped when the conductivity was <1 mS/cm, i.e., reaching the standard drinkable conductivity levels. Regarding organic matter degradation, 28% of COD in the anolyte was removed in the desalination cycle. Due to the continuous fed-batch flow mode adopted in this process, the pH changes in all the chambers were relatively insignificant to adversely impact the system’s performance. As reported in the literature, Chlorella vulgaris cultures seem not to be negatively affected below pH = 12 [45], and a slightly alkaline medium could improve algae performance (i.e., biomass production, pH = 9.3) [46]. In this sense, as the pH of algal catholyte was in the range pH = 9.5–10.3 during the desalination experiment, it could be estimated that Chlorella vulgaris culture was not affected by pH values during the desalination cycle in this study. This reflects that pH and other factors (including temperature, carbon source) selected in our assay are conducive for biofilm growth and essential for the current generation, which has been confirmed by several studies [34,47,48,49].

Table 2.
Electric conductivity, COD removal in the anolyte tank, and pH changes during the desalination cycle (desalination time = 31 h) *.
The results showed a current efficiency of 60.15%, which depicts the percentage of electric current used for desalination (i.e., migration of ions). As previously reported in the literature [6], the back diffusion of ions from anolyte/catholyte into the saline compartment reduces the effective migration of ions from the saline compartment to the adjacent one. Therefore, the specific energy production of 0.11 kWh/m3 was attained during the desalination process. It is important to note that the energy required for brackish water desalination with similar salt content (7.9 mS/cm) using conventional electrodialysis is approximately 1.69 kW/m3 [50], demonstrating the sustainability of algae-assisted MDC.
Coulombic efficiency (EC) is a measure of the number of electrons recovered by the electroactive biofilm of Geobacter suflurredecuens from the substrate to generate electrical current, which was about 9% (see Table 3). In this sense, low coulombic efficiency has been attributed to oxygen diffusion into the anode compartment, methane production associated with mixed culture biofilm, and organics’ complexity in wastewater [51]. Nonetheless, the electric current generation (i.e., 0.09–0.10 mA/cm2) confirms that the anaerobic biofilm was actively degrading the acetate in the FWM used as the anolyte. Additionally, the COD removal rate of 6.38 kg/m3/day indicates the proper operation of the MDC as a wastewater treatment device.

Table 3.
Performance parameter for the algae-assisted MDC *.
As presented in the results in this study, the micro-algae culture could maintain enough oxygen concentration in the catholyte solution to support the desalination process, even at moderate current densities obtained during the process (i.e., 0.09–0.12 mA/cm2) for this kind of microbial electrochemical devices. Moreover, under similar conditions, the microalgae cathode strategy has the upper hand over the air cathode (or air bubbling) as it showed better performance in terms of current efficiency, nominal desalination rate and coulombic efficiency, as also confirmed by Kokabian and Gude [14], as a higher concentration of dissolved oxygen is reached since microalgae can release pure oxygen directly into the solution (i.e., oxygen concentration in air is 21%).
Finally, Table 4 shows a comparison in the performance of air bubbling, air diffusion, and microalgae assisted cathode MDC (in other studies and this current study) in terms of current density, current efficiency, desalination, coulombic efficiency, and nominal desalination rate considering initial conditions of salinity concentration, COD, and desalination period.

Table 4.
Electrochemical performance of cathode comparison.
As mentioned in Section 3.1, though other studies have proven the merits of microalgae over the use of air bubbling and air diffusion cathodes, this study discussed the detailed electrochemical behavior of a microalgae-assisted biocathode. Additionally, the study outlined a proper pre-culturing protocol to boost the general growth and performance of the microalgae, as shown clearly in the results presented in this paper. Nevertheless, studies have proven the slow kinetics of this reaction at neutral pH and ambient oxygen conditions. However, other factors such as the electrode properties could contribute to this effect [52,53]. Modification of the electrode material and efficient pre-treatment can boost the kinetics of the algae-assisted cathode. From the analysis in Section 3.1, micro-algae can self-sustain a high concentration of oxygen, which eliminates the high energy needed in mechanical oxygen supply, unlike that presented before [16,54].
Finally, regarding the long-run performance of algae-assisted MDCs, it is important to indicate that biomass production in the cathode could be an important issue for a continuous system, and regular removal of algal biomass would be necessary to maintain the steady-state. In this sense, algae biomass production in such systems could be part of the benefit, as it could be valorized as fertilizers, feedstock, or energy production (i.e., anaerobic digestion), increasing the sustainability of the process.
4. Conclusions
This study presented a fundamental electrochemical analysis of algae-assisted cathode in an MDC reactor and its performance in a desalination process. The results proved that microalgae could generate enough dissolved oxygen to support the ORR process at the cathode under ambient conditions. Furthermore, microalgae can self-maintain a steady concentration of oxygen, regenerate or recover from loss and sustainably retain the system’s performance. This is vital in implementing the algae-assisted cathode for sustainable desalination using MDC technology and subsequent optimization. The results of this study could help to understand the microbial electrochemical performance of algae-assisted MDC systems and significantly contribute to further development, design, and optimization. Future studies can focus on cost-effective cathode plating or modification and chemically enhancing algae solution (media) to enhance the current generation’s desalination rate and compete with the liquid cathodes.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/pr9112011/s1, Table S1. Various electron acceptors (catholyte) are used in MDC and MFC; Figure S1: (A) Culture setup plan, (B) Culturing of algae culture for photobioreactor, (C) Newly inoculated BBM for culturing (right) and photobioreactor (left), (D) Microscopic view of Chlorella vulgaris; Figure S2: Experimental phases; Figure S3: Abiotic test: (A) Electric conductivity in the anolyte, saline, and catholyte; (B) potential of a cell, anode electrode and cathode electrode against the Ag/AgCl reference electrode.
Author Contributions
Conceptualization, D.E.-M. and J.M.O.; Methodology, D.E.-M., P.R. and M.R.-M.; software, D.E.-M. and P.R.; validation, D.E.-M., J.H. and J.M.O.; formal analysis, D.E.-M. and M.R.-M.; investigation, D.E.-M. and L.K.C.; resources, D.E.-M. and P.R.; data curation, D.E.-M., M.R.-M. and L.K.C.; writing—original draft preparation, D.E.-M. and L.K.C.; writing—review and editing, D.E.-M. and J.M.O.; visualization, D.E.-M.; supervision, A.E.-N. and J.H.; project administration, J.M.O.; funding acquisition, A.E.-N. All authors have read and agreed to the published version of the manuscript.
Funding
Marina Ramirez-Moreno acknowledges the financial support of the Consejería de Educación e Investigación, de la Comunidad de Madrid and Fondo Social Europeo (Ref: PEJD-2018-PRE/AMB-8721). Pau Rodenas acknowledges the financial support of Project “MIDES H2020”. This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 685793. Juan M. Ortiz acknowledges the financial support of the Agencia Estatal de Investigación (AEI) and the Fondo Europeo de Desarrollo Regional (FEDER) (Proyecto BioDES, CTM2015-74695-JIN).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cao, X.; Huang, X.; Liang, P.; Xiao, K.; Zhou, Y.; Zhang, X.; Logan, B.E. A New Method for Water Desalination Using Microbial Desalination Cells. Environ. Sci. Technol. 2009, 43, 7148–7152. [Google Scholar] [CrossRef]
- Jingyu, H.; Ewusi-Mensah, D.; Norgbey, E. Microbial desalination cells technology: A review of the factors affecting the process, performance and efficiency. Desalin. Water Treat. 2017, 87, 140–159. [Google Scholar] [CrossRef]
- Luo, H.; Xu, P.; Ren, Z. Long-term performance and characterization of microbial desalination cells in treating domestic wastewater. Bioresour. Technol. 2012, 120, 187–193. [Google Scholar] [CrossRef]
- Ge, Z.; Dosoretz, C.G.; He, Z. Effects of number of cell pairs on the performance of microbial desalination cells. Desalination 2014, 341, 101–106. [Google Scholar] [CrossRef]
- Luo, H.; Xu, P.; Roane, T.M.; Jenkins, P.E.; Ren, Z. Microbial desalination cells for improved performance in wastewater treatment, electricity production, and desalination. Bioresour. Technol. 2012, 105, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Moreno, M.; Rodenas, P.; Aliaguilla, M.; Bosch-Jimenez, P.; Borràs, E.; Zamora, P.; Monsalvo, V.; Rogalla, F.; Ortiz, J.M.; Esteve-Núñez, A. Comparative Performance of Microbial Desalination Cells Using Air Diffusion and Liquid Cathode Reactions: Study of the Salt Removal and Desalination Efficiency. Front. Energy Res. 2019, 7, 135. [Google Scholar] [CrossRef] [Green Version]
- Borjas, Z.; Esteve-Núñez, A.; Ortiz, J.M. Strategies for merging microbial fuel cell technologies in water desalination processes: Start-up protocol and desalination efficiency assessment. J. Power Sources 2017, 356, 519–528. [Google Scholar] [CrossRef]
- Ragab, M.; Elawwad, A.; Abdel-Halim, H. Evaluating the performance of Microbial Desalination Cells subjected to different operating temperatures. Desalination 2019, 462, 56–66. [Google Scholar] [CrossRef]
- Microbial Desalination Cells for Low Energy Drinking Water; Salinas-Rodríguez, S.G.; Arévalo, J.; Ortiz, J.M.; Borràs-Camps, E.; Monsalvo-Garcia, V.; Kennedy, M.D.; Esteve-Núñez, A. (Eds.) IWA Publishing: London, UK, 2021; ISBN 9781789062120. [Google Scholar]
- Ramírez-Moreno, M.; Esteve-Núñez, A.; Ortiz, J.M. Desalination of brackish water using a microbial desalination cell: Analysis of the electrochemical behaviour. Electrochim. Acta 2021, 388, 138570. [Google Scholar] [CrossRef]
- Dargam, F.; Perz, E.; Bergmann, S.; Rodionova, E.; Sousa, P.; Souza, F.A.A.; Matias, T.; Ortiz, J.M.; Esteve-Nuñez, A.; Rodenas, P.; et al. Supporting Operational Decisions on Desalination Plants from Process Modelling and Simulation to Monitoring and Automated Control with Machine Learning. In Lecture Notes in Business Information Processing; Springer: Cham, Switzerland, 2020; Volume 384, pp. 150–164. ISBN 9783030462239. [Google Scholar]
- Ebrahimi, A.; Najafpour, G.D.; Yousefi Kebria, D. Performance of microbial desalination cell for salt removal and energy generation using different catholyte solutions. Desalination 2018, 432, 1–9. [Google Scholar] [CrossRef]
- Rahimnejad, M.; Ghoreyshi, A.A.; Najafpour, G.; Jafary, T. Power generation from organic substrate in batch and continuous flow microbial fuel cell operations. Appl. Energy 2011, 88, 3999–4004. [Google Scholar] [CrossRef]
- Kokabian, B.; Gude, V.G. Photosynthetic microbial desalination cells (PMDCs) for clean energy, water and biomass production. Environ. Sci. Process. Impacts 2013, 15, 2178–2185. [Google Scholar] [CrossRef]
- Rismani-Yazdi, H.; Carver, S.M.; Christy, A.D.; Tuovinen, O.H. Cathodic limitations in microbial fuel cells: An overview. J. Power Sources 2008, 180, 683–694. [Google Scholar] [CrossRef]
- Lu, M.; Li, S.F.Y. Cathode Reactions and Applications in Microbial Fuel Cells: A Review. Crit. Rev. Environ. Sci. Technol. 2012, 42, 2504–2525. [Google Scholar] [CrossRef]
- Li, Y.; Lu, A.; Ding, H.; Jin, S.; Yan, Y.; Wang, C.; Zen, C.; Wang, X. Cr(VI) reduction at rutile-catalyzed cathode in microbial fuel cells. Electrochem. Commun. 2009, 11, 1496–1499. [Google Scholar] [CrossRef]
- Oh, S.; Min, B.; Logan, B.E. Cathode Performance as a Factor in Electricity Generation in Microbial Fuel Cells. Environ. Sci. Technol. 2004, 38, 4900–4904. [Google Scholar] [CrossRef]
- ter Heijne, A.; Hamelers, H.V.M.; de Wilde, V.; Rozendal, R.A.; Buisman, C.J.N. A Bipolar Membrane Combined with Ferric Iron Reduction as an Efficient Cathode System in Microbial Fuel Cells. Environ. Sci. Technol. 2006, 40, 5200–5205. [Google Scholar] [CrossRef] [PubMed]
- Ucar, D.; Zhang, Y.; Angelidaki, I. An Overview of Electron Acceptors in Microbial Fuel Cells. Front. Microbiol. 2017, 8, 643. [Google Scholar] [CrossRef] [Green Version]
- Lefebvre, O.; Al-Mamun, A.; Ng, H.Y. A microbial fuel cell equipped with a biocathode for organic removal and denitrification. Water Sci. Technol. 2008, 58, 881–885. [Google Scholar] [CrossRef]
- Sun, Y.; Wei, J.; Liang, P.; Huang, X. Microbial community analysis in biocathode microbial fuel cells packed with different materials. AMB Express 2012, 2, 21. [Google Scholar] [CrossRef] [Green Version]
- Arana, T.J.; Gude, V.G. A microbial desalination process with microalgae biocathode using sodium bicarbonate as an inorganic carbon source. Int. Biodeterior. Biodegrad. 2018, 130, 91–97. [Google Scholar] [CrossRef]
- Wong, Y. Growth Medium Screening for Chlorella vulgaris Growth and Lipid Production. J. Aquac. Mar. Biol. 2017, 6, 143. [Google Scholar] [CrossRef] [Green Version]
- Borjas, Z.; Ortiz, J.; Aldaz, A.; Feliu, J.; Esteve-Núñez, A. Strategies for Reducing the Start-up Operation of Microbial Electrochemical Treatments of Urban Wastewater. Energies 2015, 8, 14064–14077. [Google Scholar] [CrossRef]
- Khazraee Zamanpour, M.; Kariminia, H.R.; Vosoughi, M.; Zamanpour, M.K.; Kariminia, H.R.; Vosoughi, M.; Khazraee Zamanpour, M.; Kariminia, H.R.; Vosoughi, M.; Zamanpour, M.K.; et al. Electricity generation, desalination and microalgae cultivation in a biocathode-microbial desalination cell. J. Environ. Chem. Eng. 2017, 5, 843–848. [Google Scholar] [CrossRef] [Green Version]
- Edmundson, S.J.; Huesemann, M.H. The dark side of algae cultivation: Characterizing night biomass loss in three photosynthetic algae, Chlorella sorokiniana, Nannochloropsis salina and Picochlorum sp. Algal Res. 2015, 12, 470–476. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.-S.; Parameswaran, P.; Kato-Marcus, A.; Torres, C.I.; Rittmann, B.E. Evaluation of energy-conversion efficiencies in microbial fuel cells (MFCs) utilizing fermentable and non-fermentable substrates. Water Res. 2008, 42, 1501–1510. [Google Scholar] [CrossRef]
- Sforza, E.; Simionato, D.; Giacometti, G.M.; Bertucco, A.; Morosinotto, T. Adjusted light and dark cycles can optimize photosynthetic efficiency in algae growing in photobioreactors. PLoS ONE 2012, 7, e38975. [Google Scholar] [CrossRef]
- Si, F.; Zhang, Y.; Yan, L.; Zhu, J.; Xiao, M.; Liu, C.; Xing, W.; Zhang, J. Electrochemical Oxygen Reduction Reaction. In Rotating Electrode Methods and Oxygen Reduction Electrocatalysts; Elsevier: Amsterdam, The Netherlands, 2014; pp. 133–170. ISBN 9780444632784. [Google Scholar]
- Ling, J.; Xu, Y.; Lu, C.; Lai, W.; Xie, G.; Zheng, L.; Talawar, M.P.; Du, Q.; Li, G. Enhancing Stability of Microalgae Biocathode by a Partially Submerged Carbon Cloth Electrode for Bioenergy Production from Wastewater. Energies 2019, 12, 3229. [Google Scholar] [CrossRef] [Green Version]
- Jaroo, S.S.; Jumaah, G.F.; Abbas, T.R. Photosynthetic Microbial Desalination Cell to Treat Oily Wastewater Using Microalgae Chlorella Vulgaris. Civ. Eng. J. 2019, 5, 2686–2699. [Google Scholar] [CrossRef] [Green Version]
- Bahareh Kokabian, V.G.G. Beneficial Bioelectrochemical Systems for Energy, Water, and Biomass Production. J. Microb. Biochem. Technol. 2013, S6, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Hui, W.J.; David, E.-M.M.; Huang, J. Using C. vulgaris assisted microbial desalination cell as a green technology in landfill leachate pre-treatment: A factor-performance relation study. J. Water Reuse Desalin. 2020, 10, 1–16. [Google Scholar] [CrossRef]
- Kakarla, R.; Min, B. Photoautotrophic microalgae Scenedesmus obliquus attached on a cathode as oxygen producers for microbial fuel cell (MFC) operation. Int. J. Hydrogen Energy 2014, 39, 10275–10283. [Google Scholar] [CrossRef]
- Lee, M.; Kondaveeti, S.; Jeon, T.; Kim, I.; Min, B. Influence of Humidity on Performance of Single Chamber Air-Cathode Microbial Fuel Cells with Different Separators. Processes 2020, 8, 861. [Google Scholar] [CrossRef]
- Lee, M.; Kakarla, R.; Min, B. Performance of an air-cathode microbial fuel cell under varied relative humidity conditions in the cathode chamber. Bioprocess Biosyst. Eng. 2019, 42, 1247–1254. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, S.; Uemura, S.; Imanishi, N.; Hirai, S. Oxygen concentration measurement in the porous cathode of a lithium-air battery using a fine optical fiber sensor. Mech. Eng. Lett. 2019, 5, 19-00095. [Google Scholar] [CrossRef] [Green Version]
- Kazbar, A.; Cogne, G.; Urbain, B.; Marec, H.; Le-Gouic, B.; Tallec, J.; Takache, H.; Ismail, A.; Pruvost, J. Effect of dissolved oxygen concentration on microalgal culture in photobioreactors. Algal Res. 2019, 39, 101432. [Google Scholar] [CrossRef] [Green Version]
- Kokabian, B.; Gude, V.G. Sustainable photosynthetic biocathode in microbial desalination cells. Chem. Eng. J. 2015, 262, 958–965. [Google Scholar] [CrossRef]
- González del Campo, A.; Cañizares, P.; Rodrigo, M.A.; Fernández, F.J.; Lobato, J. Microbial fuel cell with an algae-assisted cathode: A preliminary assessment. J. Power Sources 2013, 242, 638–645. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Z.; Zheng, Y.; Xiao, Y.; Yang, Z.; Zhao, F. Light intensity affects the performance of photo microbial fuel cells with Desmodesmus sp. A8 as cathodic microorganism. Appl. Energy 2014, 116, 86–90. [Google Scholar] [CrossRef]
- Lovley, D.R.; Ueki, T.; Zhang, T.; Malvankar, N.S.; Shrestha, P.M.; Flanagan, K.A.; Aklujkar, M.; Butler, J.E.; Giloteaux, L.; Rotaru, A.-E.; et al. Geobacter: The microbe electric’s physiology, ecology, and practical applications. Adv. Microb. Physiol. 2011, 59, 1–100. [Google Scholar] [CrossRef]
- Jafary, T.; Daud, W.R.W.; Aljlil, S.A.; Ismail, A.F.; Al-Mamun, A.; Baawain, M.S.; Ghasemi, M. Simultaneous organics, sulphate and salt removal in a microbial desalination cell with an insight into microbial communities. Desalination 2018, 445, 204–212. [Google Scholar] [CrossRef]
- Vandamme, D.; Foubert, I.; Fraeye, I.; Meesschaert, B.; Muylaert, K. Flocculation of Chlorella vulgaris induced by high pH: Role of magnesium and calcium and practical implications. Bioresour. Technol. 2012, 105, 114–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ihnken, S.; Beardall, J.; Kromkamp, J.; Gómez Serrano, C.; Torres, M.; Masojídek, J.; Malpartida, I.; Abdala, R.; Jerez, C.; Malapascua, J.; et al. Light acclimation and pH perturbations affect photosynthetic performance in Chlorella mass culture. Aquat. Biol. 2014, 22, 95–110. [Google Scholar] [CrossRef] [Green Version]
- An, Z.; Zhang, H.; Wen, Q.; Chen, Z.; Du, M. Desalination combined with hexavalent chromium reduction in a microbial desalination cell. Desalination 2014, 354, 181–188. [Google Scholar] [CrossRef]
- Venkata Mohan, S.; Sunita, V.; Pandey, A. (Eds.) Microbial Electrochemical Technology, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9780444640529. [Google Scholar]
- Toczyłowska-Mamińska, R. Limits and perspectives of pulp and paper industry wastewater treatment—A review. Renew. Sustain. Energy Rev. 2017, 78, 764–772. [Google Scholar] [CrossRef]
- Ortiz, J.; Exposito, E.; Gallud, F.; Garciagarcia, V.; Montiel, V.; Aldaz, A. Desalination of underground brackish waters using an electrodialysis system powered directly by photovoltaic energy. Sol. Energy Mater. Sol. Cells 2008, 92, 1677–1688. [Google Scholar] [CrossRef]
- Fornero, J.J.; Rosenbaum, M.; Angenent, L.T. Electric Power Generation from Municipal, Food, and Animal Wastewaters Using Microbial Fuel Cells. Electroanalysis 2010, 22, 832–843. [Google Scholar] [CrossRef]
- Gadhamshetty, V.; Belanger, D.; Gardiner, C.-J.; Cummings, A.; Hynes, A. Evaluation of Laminaria-based microbial fuel cells (LbMs) for electricity production. Bioresour. Technol. 2013, 127, 378–385. [Google Scholar] [CrossRef]
- Rana, A.; Baig, N.; Saleh, T.A. Electrochemically pretreated carbon electrodes and their electroanalytical applications—A review. J. Electroanal. Chem. 2019, 833, 313–332. [Google Scholar] [CrossRef]
- Zhao, F.; Harnisch, F.; Schröder, U.; Scholz, F.; Bogdanoff, P.; Herrmann, I. Challenges and Constraints of Using Oxygen Cathodes in Microbial Fuel Cells. Environ. Sci. Technol. 2006, 40, 5193–5199. [Google Scholar] [CrossRef]
- Wen, Q.; Zhang, H.; Chen, Z.; Li, Y.; Nan, J.; Feng, Y. Using bacterial catalyst in the cathode of microbial desalination cell to improve wastewater treatment and desalination. Bioresour. Technol. 2012, 125, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, K.S.; Drew, D.M.; He, Z. Efficient salt removal in a continuously operated upflow microbial desalination cell with an air cathode. Bioresour. Technol. 2011, 102, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Mehanna, M.; Saito, T.; Yan, J.; Hickner, M.; Cao, X.; Huang, X.; Logan, B.E. Using microbial desalination cells to reduce water salinity prior to reverse osmosis. Energy Environ. Sci. 2010, 3, 1114. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).