1. Introduction
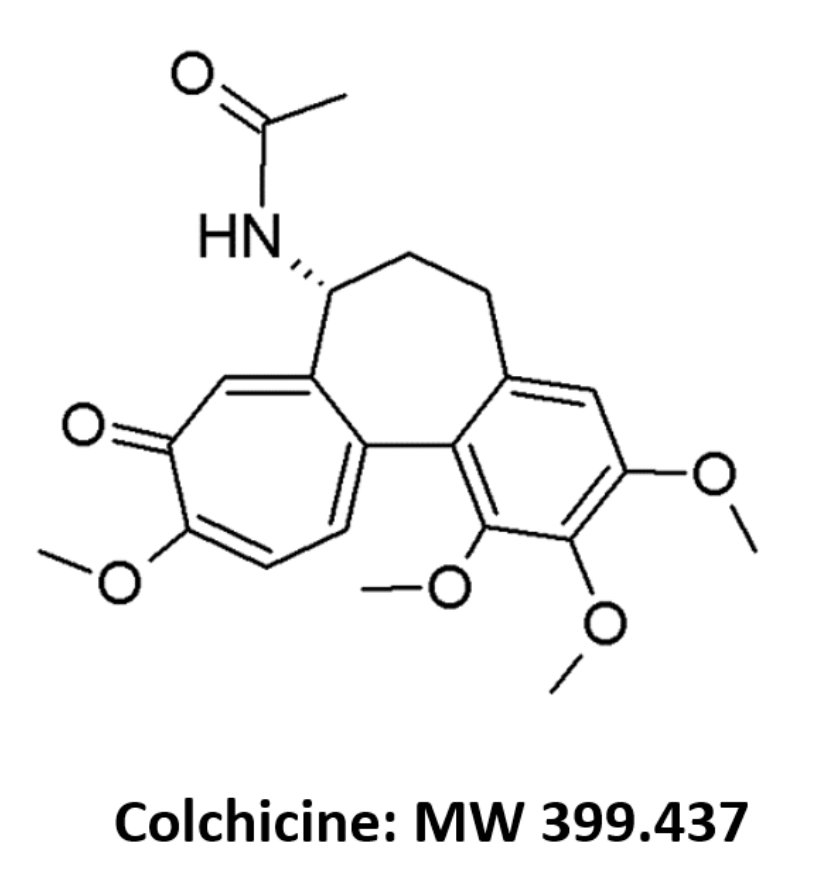
Colchicine (COL;
Figure 1) is a tricyclic alkaloid (logP 1.2) derived from the plant
Colchicum autumnale. It possesses anti-inflammatory and antimitotic effects and since ancient times has been known to be beneficial in the treatment of gout [
1,
2]. It also has utility as prophylaxis against acute attacks of familial Mediterranean fever [
3]. More recently, COL has been investigated for its possible amelioration of the “cytokine storm” associated with serious cases of SARS-Covid-19 [
4,
5]. Experimentally COL has also been used to impair the formation of intestinal lymph after intraperitoneal injection in rodents [
6,
7].
Clinically, COL is used at relatively low dose levels, with usual maximum doses of 1.2 mg/d, or about 0.017 mg/kg/d in a 70 kg subject, which makes its quantitation in blood fluids challenging even where volumes as high as 0.5 to 1 mL are obtainable. This issue becomes even more problematic in studies involving smaller species such as rats, where sample volume size may be particularly limited. Although several methods are available for the measurement of COL in biological specimens, most of these methods have only been validated for use in human samples [
8,
9,
10,
11,
12,
13,
14].
Studies where COL was used to impair the formation of lymph flow in rodents involved doses far in excess of human doses and were associated with significant toxicity [
6,
7,
15]. The ability of the drug to be effective at blocking lymph flow at lower nontoxic doses is unknown. Furthermore, the sensitivity of most existing assays in rats poses limits on the ability to measure systemic exposure with lower doses [
16,
17]. To permit an evaluation of systemic exposure with lower doses, here we opted to develop and validate a more sensitive method for the measure of COL in rat specimens. Hence, here we describe a rapidly eluting, specific, and sensitive liquid chromatographic tandem mass spectrometric (LC–MS/MS) method for measurement of COL in rat specimens. The assay has been applied to assay plasma, urine, and whole blood concentrations of COL from male Sprague-Dawley rats given COL in doses as low as 0.1 mg/kg orally and intravenously.
2. Materials and Methods
2.1. Chemicals and Materials
Reference compounds COL (≥95 % purity, catalog no. C9754) and the internal standard (IS), COL-D6 (99.94% purity, catalog no. C640003) were purchased from Sigma Aldrich (Oakville, ON, Canada) and Toronto Research Chemicals (Toronto, ON, Canada), respectively. HPLC-grade methanol and water were procured from Fisher Scientific (Ottawa, ON, Canada). Ammonium acetate (≥ 97% purity) was obtained from BDH Inc. (Toronto, ON, Canada). Liquid chromatography–mass spectrometry−grade formic acid (>99.7% purity) was purchased from Fisher Scientific (Ottawa, ON, Canada). HPLC−grade hexane, isopropanol, and dichloromethane (≥99.5% purity) were obtained from Caledon (Georgetown, ON, Canada). Nylon membrane 0.45 μM filters from Millipore Sigma (Cork, Ireland) was used for the filtration of the mobile phase. Sprague-Dawley rat plasma (containing sodium heparin) was purchased from Innovative Research (Novi, MI, USA) while rat blood and urine was obtained from rats purchased from the University of Alberta Health Sciences Laboratory Animal Services. Blood was used freshly, plasma stored at −20 °C until used.
2.2. Liquid Chromatographic and Mass Spectrometric Conditions
The LC−MS/MS system comprised a system controller (CBM-20A), degasser unit (DGU-20A 5R), an autosampler (SIL-30 AC), a binary pump (LC-30 AD), a column oven (CTO-20 AC) and a triple quadrupole mass spectrometry (MS/MS) detector with unit resolution of 0.7 µm (LCMS-8050) using electrospray ionization (ESI) (Shimadzu, Kyoto, Japan). The chromatographic separation was carried out using a 100 × 3 mm i.d., 4 μm particle size Genesis C18 column (Brockville, ON, Canada) attached to Agilent Zorbax Reliance guard-column (4.6 × 3 mm, 5 µm particle size) (Mississauga, ON, Canada). The mobile phase, a combination of formic acid:10 mM ammonium acetate:methanol [1:49:75,
v/
v/
v], was pumped at a set flow rate of 0.5 mL/min. Autosampler injection volume ranged from 5-40 μL for high to low concentrations. The temperature of autosampler and column oven were retained at 4 and 40 °C, respectively. The detector was operated at unit resolution in a positive multiple reaction monitoring (MRM) mode. COL was monitored using the transition
m/
z 400.30→358.30, and for IS,
m/
z 406.30→362.30. Data acquisition and chromatographic integration was accomplished with LabSolutions software (Shimadzu version 5.91, 2018a). Instrument conditions are summarized in the Appendix (
Table A1).
2.3. Compounds Optimization
Compound-dependent parameters including MRM mass transitions, Q1/Q3 pre-bias and collision energy were optimized automatically and manually using LabSolutions software by flow injecting a mixture of COL and IS. The final optimization was associated with highest sensitivity for the most abundant product ions in Q3 MS spectra for COL and IS
2.4. Extraction Procedure
Liquid–liquid extraction was used to help isolate COL from rat specimens. After adding 10 µL volume of the IS stock solution and 0.1 mL of 2 M KH2PO4 (pH 8.4) to the rat specimen, 3 mL of n-hexane:dichloromethane:isopropanol [20:10:1, v/v/v] was added. This was followed by vortex-mixing of tubes for 30 s, shaking 10 min using test tube shaker at 1000 rpm then, centrifuging at 3000× g for 10 min. The organic solvent layer was transferred by pipette to new tubes and evaporated to dryness in vacuo. The dried residues were reconstituted using 0.1 mL of HPLC grade methanol: water [10:90, v/v] with up to 40 µL being injected into the LC-MS/MS system. The same procedure was applied to each specimen assayed (blood, plasma, and urine) and volume (0.1 or 0.5 mL).
2.5. Method Validation
Development and validation procedures were guided by recommendations published by the European Medicines Agency (EMA) [
18] and the US Food and Drug Administration [
19].
2.5.1. Calibration, Linearity, Accuracy and Precision
A standard stock solution of COL (48 µg/mL) was prepared in methanol. The working stocks were prepared daily from the standard solution by serial dilution with methanol: HPLC grade water [10:90 v/v] to provide final concentrations of 4.8, 0.48, 0.048, and 0.0048 µg/mL COL. Calibration standards and validation samples were prepared by spiking blank plasma with working solutions. The internal standard (IS) stock solution (100 µg/mL) was prepared by dissolving 1 mg of COL-d6 in 10 mL of methanol. Its working solution (10 ng/mL) was obtained by appropriate dilution of the stock solution in methanol: water [10:90 v/v]. All standard stock solutions were stored at −20° between use.
Male Sprague-Dawley rats were the source of all biologic specimens used. Six blank plasma samples from different rats were prepared independently as drug-free samples to assure there were no significant interference of endogenous components with eluted COL and IS. The LLOQ was defined as the lowest concentration that provided acceptable accuracy and precision (within a range of 20%) [
19]. A blank calibrator (specimen without IS) and a zero COL calibrator (blank specimen with IS only) were added to each analytical run.
For the construction of the calibration curve included in each analytical run, volumes of the specimens (i.e., rat plasma, blood, or urine) were spiked with known concentrations of COL, with the lower concentration being half of the lowest concentration meeting acceptable validation criteria and above the highest concentration tested for validation. Each sample spiked with the drug also had a set volume of IS added. Each calibration curve included eight concentrations of COL. The linearity of the method was established by analysis of six standard curves containing the eight nominal COL concentrations. Linear weighted (1/concentration2) regression of the peak area ratios response for COL to IS versus nominal COL concentration were used to establish slope, intercept, and linearity (correlation coefficient r2).
To determine the bias and precision of the method, a minimum of four concentrations for each specimen and volume of sample were tested, including those representing a lower limit of quantitation (LLOQ), low quantifiable concentration (LQC), medium quantifiable concentration (MQC), and high quantifiable concentration (HQC). Each of these were prepared from stock solutions of COL. Intraday, accuracy, and precision of the assay were calculated based on the included COL concentrations. For the 0.1 mL plasma volume the assay was fully validated, with the assay being repeated on three separate days using four concentrations. For each daily run, a calibration curve independent of the samples used for validation was included and used to obtain the standard curve used to quantify the concentrations of COL in the samples. Precision was assessed by calculating the percentage coefficient of variation (CV%), while accuracy was represented by determining the mean percent error of the measured versus nominal concentration.
Similar to 0.1 mL of plasma, standard curves were prepared multiple times to assure linearity using volumes of 0.1 mL whole blood and urine, and for a higher 0.5 mL volume of plasma. This was followed by intraday validation using four or five concentrations with five replicates of each, and an independent standard curve of the same matrix type and volume prepared alongside the validation samples.
2.5.2. Recovery, Carryover and Matrix Effect
The recovery of COL and IS after liquid-liquid extraction (LLE) was determined by comparing the mean area ratios of samples spiked before extraction to those of plasma extracts spiked with the compounds after extraction at three (low, medium, and high) concentrations. Carryover from one sample to the next was assessed by the presence of chromatographic peaks in blank samples injected immediately after a highest calibrator concentration. Matrix effect, expressed as matrix factor (MF), was assessed by calculating the ratio of the peak area of each analyte in blank plasma spiked after extraction, to the peak area response of solutions prepared in water. MFs based on the ratios of COL to IS were also calculated to assess the variability of the assay due to matrix effects [
18]. The MF was estimated for low and high levels of concentration with six replicates each of three-fold LLOQ and near upper validated concentration (1.5 and 40 ng/mL, respectively).
2.5.3. Selectivity and Sensitivity
Six blank rat plasma samples were prepared independently as blank calibrators to assess any interfering endogenous components. The LLOQ was defined as a concentration on the calibration curve with acceptable precision and accuracy (±20%) [
19]. The analyte responses at the LLOQ were ≥5 fold the analyte response in the analyte-free calibrator samples [
19].
2.5.4. Stability
The standard stock solution of COL was evaluated at −20 °C. The stability of analytes in rat plasma was evaluated under various storage conditions. This included autosampler stability (24 h in the autosampler rack at 4 °C), bench top stability (up to 20 h at room temperature and at refrigerated temperature 4 °C), short term stability (−20 °C for 14 days), and freeze thaw stability (three cycles of freeze thawing from −20 °C). Three replicates of low and high concentrations (1.5 and 40 ng/mL) were subjected to the above processing. The analyte stability in spiked plasma samples was evaluated by measuring the peak area ratio response (COL/IS) of stability samples against freshly prepared standards having identical concentration.
2.6. Applicability
The use of rats for these experiments was approved by the University of Alberta Health Sciences Animal Care and Use Committee Committee. All rats were housed one per cage in temperature-controlled room with 12 h dark/light cycle. Free access to food and fluids was permitted throughout the study period.
2.6.1. Pharmacokinetic Protocol with Serial Blood Sampling
To test the applicability of the assay in a pharmacokinetic study, three male Sprague-Dawley rats with surgically-implanted jugular vein cannulas purchased from Charles River Laboratories (260–300 g) were dosed with intravenous doses of COL 0.1 mg/kg. After dosing, the blood samples (volumes 0.1 to 0.25 mL) were serially collected into heparinized tubes from the cannulas (at 5 min, 20 min, 40 min, and 1, 1.5, 2, 3, 4, 6, 9, 24 h after each dose). The rats were placed in metabolic cages for 24 h after each dose, which allowed for the collection of urine at intervals of 0 to 1.5 h, 2.5 to 5 h, 5 to 10 h, and from 10 to 24 h after each dose. All blood fluid and urine specimens were frozen and later assayed using the described method.
Noncompartmental analysis was used to determine the pharmacokinetic parameters. The terminal phase rate constant (λz) was estimated by applying exponential linear regression to the concentrations in the terminal phase, and the terminal half-life (t½) was estimated as 0.693/λz. The linear trapezoidal rule was used to measure the area under the concentration vs. time curve from the time of injection to the last (Clast) measured concentration (AUC0-tlast). The AUC extrapolated to infinity (AUC0–∞) was estimated as the sum of AUC0-tlast and Clast//λz. Total body clearance (CL) was calculated as the ratio of the dose to the AUC0–∞. The area under the first moment concentration vs. time curve (AUMC) was estimated as the sum of the area under the C × t vs. t curve from time zero to Clast, plus the ratios of Clast × t/λz and Clast/λz2. The mean residence time (MRT) was calculated as the ratio of AUMC to AUC0–∞). The volume of distribution of steady-state (Vdss)was calculated as the product of CL × MRT. All of these were based on the whole blood concentration measures.
2.6.2. Blood-to-Plasma Ratio
Three Sprague-Dawley rats were administered COL orally. Then, under isoflurane anesthesia, blood was obtained collected via cardiac puncture into heparinized tubes at 2, 4, or 6 h post-dose. Part of the blood was directly assayed, and the remainder of the blood was centrifuged and the corresponding plasma assayed.
In vitro, whole blood was collected from isoflurane anesthetized Sprague-Dawley rats (not given COL) by cardiac puncture into heparinized tubes. Then, a volume of 0.5 mL blood was immediately transferred to clean tubes and spiked with 200 ng/mL COL. The COL was allowed to equilibrate with the blood cells for 1 h at 37 °C in a shaking water bath. 0.1 mL of blood was then transferred to new tubes. The rest of the blood sample was centrifuged at 3000× g for 10 min to obtain the plasma. Afterwards, equal volumes of blood and plasma (0.1 mL) were extracted and assayed for COL.
3. Results
The chromatographic peaks for COL and IS eluted within 3 min after injection, with elution taking about 0.5 min to complete once the peak started to appear (
Figure 2,
Figure 3,
Figure 4 and
Figure 5). The chromatograms showed peak shapes of each component (COL and IS) that were symmetrical and devoid of interference with endogenous components within blood fluids and urine. The average recoveries were essentially complete (≥96% in each matrix). Excellent linearity was present between the peak area drug to IS ratios and the tested range of concentration, in blood, plasma, and urine (r
2 > 0.99). This linearity was established over the concentration ranges of 0.25 to 50 ng/mL in 0.1 mL of plasma or blood, 0.25 to 200 ng/mL in 0.1 mL of rat urine, and 0.05 to 500 ng/mL in 0.5 mL of plasma. In each case, the linearity of peak area ratios to concentration was excellent (r
2 ≥ 0.999).
The validation assessments exhibited acceptable measures of precision and accuracy (
Table 1 and
Table 2). All intra- and inter-day CV% were ˂15% in 0.1 mL volumes of plasma and urine across the concentrations assessed (
Table 1). These measures fell out of the ranges of acceptable performance with concentrations below 0.5 ng/mL in 0.1 mL of specimen. A one-day validation of a larger volume of plasma (0.5 mL) showed that the LLOQ could be improved five-fold (
Table 2), in a linear manner, compared to a volume of 0.1 mL (
Table 1). Whole blood was shown to be an alternate fluid to plasma for COL quantitation, with the same measures of accuracy and precision compared to an equivalent volume of plasma (
Table 1 and
Table 2). The peak area responses at the LLOQ were at least five-fold higher than the associated response at the same elution time in the analyte-free plasma samples [
19]. Using the monitoring conditions for IS, its response in the blank did not exceed 5% of the average IS responses of the calibrators which represents the assay selectivity.
The stability of COL was evaluated under various conditions of time and temperature (
Table 3). The changes in the concentration of COL were less than 10% after freeze and thaw (−20 °C), placement of sample in the autosampler (4 °C), benchtop (4 and 25 °C), and short-term stability at −20 °C. The COL MF ratio was 1.02 for 40 ng/mL, 1.3 for 1.5 ng/mL, and for IS, 1.05. The analyte responses at the LLOQ were at least five-fold higher than the associated response at the same elution time in the analyte-free blank plasma samples [
19]. Using the monitoring conditions for IS, its response in the blank did not exceed 5% of the average IS responses of the calibrators which represent the assay selectivity. The absence of any peak in a drug- and IS-free plasma sample injected immediately after injection of a 0.5 mL sample containing 50 ng COL/mL and IS confirmed the absence of carryover in the instrument.
When tested for applicability in rat blood in a pharmacokinetic experiment using 0.1 mg/kg doses, COL was quantifiable for at least 24 h after an intravenous dose (
Figure 6). The maximum blood concentrations measured at 5 min after the injection were between 285 and 450 ng/mL. After the dose of COL, the drug displayed properties consistent with a high extent of distribution to tissues, and relatively long t½ (
Table 4). The percent of the dose recovered in urine over 24 h was 23.3 ± 9.32% (
Figure 7), with renal clearance 1.38 mL/min. There was no evidence of endogenous interference in the eluted COL peaks in any of the samples assayed from the rats. There were no signs of adverse effects noticed (no diarrhea, a common side effect), and the rats continued to increase normally in their body weights for the next seven days while being monitored.
In the three rats given COL orally for determination of the blood-to-plasma ratio (chromatograms in plasma shown in
Figure 4), the ratios ranged from 1.17 to 1.63 (1.36 ± 0.24). In plasma spiked with COL, the in vitro blood-to-plasma ratio was 1.23 ± 0.12.
4. Discussion
In the laboratory, rats are commonly used to gain insight into the pharmacokinetics of drugs. COL is known to have a narrow therapeutic index, with significant toxicity ensuing with doses of higher than 0.5 mg/kg [
20]. In rats given oral doses from 10 to 30 mg, there were decreases in food intake, decreases in body weight, and mortality, each of which worsened with increased dosage [
15]. Possibly due to limitations in assay sensitivity, previous studies performed in rats to measure COL in blood fluids have been injected, usually via the intraperitoneal route, with doses sufficient to cause significant morbidity and even mortality [
21,
22]. Such adverse effects could have a profound effect on the interpretation of the study results. Several studies involving rats have relied on high performance liquid chromatography HPLC or thin layer chromatography to quantify COL concentrations [
16,
23]. The previously published methods had relatively poor sensitivity with LLOQ of 1000 ng/mL [
16,
17]. These studies tended to involve administration of higher, potentially toxic doses of COL intravenously (1 to 2 mg/kg) or intraperitoneally (10 mg/kg), perhaps in part to boost the concentrations to quantifiable levels [
16,
17]. There have been no specific methods reported for the measure of COL at very low doses and concentration in blood fluids of rats.
Electrospray ionization (ESI) in positive ion mode was chosen since COL and the IS possess a greater capacity for protonation in positive mode than deprotonation in the negative mode. This occurs due to the presence of a heteroatom secondary amine group (
Figure 1). To ensure the optimal choice for ionization source, we examined the effect of ESI, atmospheric pressure chemical ionization alone and/or a combination of the two (dual-ionization source) on the intensity of product. ESI exhibited the highest sensitivity and optimal linearity in the calibration curves for COL. Protonated precursor [M + H]+ ions for COL and IS were determined at
m/
z 400.3 and 406.3. The most abundant product ions under the optimized conditions for COL were found at
m/
z 358.3 and at
m/
z 362.3 for IS. This corresponded to a reduction in
m/
z of about 42 mass units, which is in line with the fragmentation expected of the acetanilide functional group [
24].
Several other methods have used LLE for the assay of COL, with recoveries from 78 to over 90% being reported [
8,
10,
11,
12,
16,
17]. We adopted the organic solvent mixture for extraction from Jiang et al. [
12], who reported that the extraction of COL from human plasma was virtually complete. We likewise found the same to be the case in extraction of COL from rat specimens. Using dichloromethane alone shifts the biological matrix to the upper layer which makes transferring process of the organic layer difficult. Shah et al. reported that solid phase extraction (SPE) provides a superior recovery to LLE [
25]. However, LLE may be more cost effective than SPE [
26] while still providing excellent recovery. Although we found an expected increase in MF with a decrease in concentration, the assay was still highly linear and met all standards of acceptable accuracy and precision (
Table 1 and
Table 2). Recently another group reported an LC-MS/MS assay for COL in rat plasma with a slightly lower limit of quantitation of 0.25 ng/mL [
27]. However, they validated and assayed COL only in plasma, and in their application they administered COL doses 20 to 50-fold greater than we did, well within the range associated with significant toxicities. [
6,
7,
15,
23]
The described method performed well in terms of stability, linearity, accuracy, and precision. It is the first assay to determine COL levels in a variety of biological specimens from rats (plasma, urine and whole blood) using volume as small as 0.1 mL (
Table 1 and
Table 2). Thus, the assay has utility in measurement of the drug in serial blood sampling protocols involving cannulated rats. The assay was also capable of studying the kinetics of COL excretion in urine. In some study designs such as terminal collection of blood and tissues, larger volumes of specimens might be available for assay, which was also validated here. There was a proportional increase in the sensitivity of the assay compared to smaller volumes where, as the specimen volume increased from 0.1 mL to 0.5 mL, the assay provided a five-fold decrease in the LLOQ.
The assay was validated in plasma, blood, and urine. Given the desired aim of measuring COL with these low, nontoxic dose levels to rats, it would be of benefit to use specimens with higher concentrations. The finding of a blood to plasma concentrations ratio of over one indicates that in performing studies in rats, blood is the preferred specimen. This allows the ability to draw roughly half as much blood per sample, and also allows a direct measure of the CL of COL in relation to eliminating organ blood flows, such as the liver [
28,
29]. Given the low recovery in the urine, this becomes particularly relevant for COL since this suggests liver metabolism as a primary pathway for elimination of the drug.