1. Introduction
Cyanobacteria are prokaryote that have a simple cellular structure, and photosynthesis-like plants but don’t have plant cell walls as with primitive bacteria. Cyanobacteria are considered as valuable sources of natural bioactive compounds, such as phenolic, flavonoids, terpenoids, proteins, lipids, vitamins, polysaccharides with considerable nutritional and medicinal value [
1,
2,
3,
4,
5].
Nostoc genus is considered an edible source, and the genus belongs to the Nostocaceae family division of Cyanophyta. It forms spherical cells, which link together, forming filaments. Heterocysts design homogeneous cells that make up the filament and distance between vegetative cells [
6,
7]. Extracts from
Nostoc biomass have been used in the medical field for fistula treatment, cancer therapy, anti-inflammatory, immune-boosting, and blood pressure control [
8]. Moreover,
Nostoc sp. creates numerous compounds with an antimicrobial and antiviral activity, which significantly improved its cultivation [
9]. A potential protein molecule, cyanovirin, generated by
Nostoc sp., has been shown to obtain a pronounced effect in treatment of HIV and also influenza A virus [
10,
11,
12,
13,
14,
15,
16,
17,
18,
19,
20,
21,
22,
23,
24,
25].
Nostoc sp. includes polyunsaturated fatty acids that contain essential fatty acids, such as octadecatetraenoic, linoleic, α-linolenic, γ-linolenic acid, and eicosapentaenoic acid [
26]. Many reports have been identified algal antioxidants such as glutathione, tocopherols and ascorbate [
16,
17,
18,
19,
20,
21,
22,
23,
24]. Additionally, the algal extracts have proven antimicrobial, antiviral, and antitumor activities [
25,
26].
Different types of environmental stress (biotic and abiotic) lead to the disruption of homeostasis due to stressor application. As cells acclimate and strive to restore equilibrium, stress responses cause alterations in cell metabolism. Regulation, alarming stage, and adaptability are various steps of stress responses [
27]. Stress strategies have been used to enhance high synthesis of active compounds using individual stress parameters, such as dietary factors (e.g., nitrogen, and carbon) and environmental factors (e.g., pH, temperature, light intensities, and salinity) [
28,
29,
30]. Cu and Zn at high concentration has been shown to produce oxidative stress in an alga test by enhancing lipid peroxidation, membrane permeability, and lowering sulfhydryl level [
30].
There is a lack of information regarding the influence of chemicals and biological responses manipulating conditions for growth. This study aimed to identify the impact of raising levels of copper and/or zinc in the Nostoc linckia culture medium (BG-110) on growth and secondary metabolite production and to assess the antioxidant and anticancer activity of the extracts.
4. Discussion
A greater understanding of cyanobacteria’s heavy metal response mechanisms could lead to the development of these microorganisms for usage in the field of biologically active natural products. In our present work (
Table 3,
Table 4 and
Table 5), the resistance of cyanobacteria to the high concentration of Cu can explain the increased growth rate at 0.079 mg/L Cu. For certain biomass enzymes, copper appears to control functional activity as a concentration factor [
60]. In response to a wide range of divalent metal ion concentrations, some strains release mRNA for the stress protein GroEL and metallothionein’s metal-binding protein. Following exposure to divalent cations at values ranging from 10 μM to 100 μM for Cu and Zn, a high transcription rate is established within 15 min. After one hour of exposure to every cation, transcript levels revert to normal. Within 15 min of these exposures, metallothionein operon is also induced. These resistance systems, we assume, operate collectively to protect the cell from injury [
61]. Metallothioneins are cysteine-rich proteins that bind metal ions, reducing their cellular availability and ultimately detoxifying them [
62]. Our findings were also consistent with those of Mamboya et al. [
63], who found that copper is highly poisonous
to Padina boergesenii at doses greater than 500 mg/L. A low concentration of copper, on the other hand, is harmful after 21 days of exposure. Because zinc works as a prosthetic moiety of several key enzymes that can readily impact metabolism, the observed differences in the growth rate of
Nostoc linckia cultured under varying zinc concentrations (of BG-110 media) may be understood [
64]. The noticed high growth rate of the cultures at 0.22 mg/L may be due to the tolerance of
Nostoc linckia to this concentration. A fructose bisphosphate aldolase, DNA primase, carbonic anhydrase, alkaline phosphatase, and RNA polymerase are just a few of the zinc-dependent or binding proteins found in E.coli. TonB-dependent outer membrane proteins that implicated in the active transport of zinc or zinc chelates across membrane. Metals are also required as cofactors by around 40% of all enzymes, ranking from Mg (16%), Zn (9%), Fe (8%), Mn (6%), Ca (2%), Co and Cu (1%) [
65]. On the other hand, zinc accumulation onto cells could explain the declined growth of cyanobacteria at higher zinc concentrations. In the end experiment time, this accumulation greatly affected/reduced the available zinc ion concentration and its toxicity to algal survival cells, which matched with the results [
66].
Similarly, our findings (
Table 5) revealed that a copper–zinc combination had a growth inhibiting impact when compared to control. The accumulation of metals (zinc or copper) onto cyanobacteria cells at higher concentrations significantly reduces the growth. At this concentration, zinc and copper ions led to toxicity to cyanobacterial cells during the final experiment period [
66]. Moreover, increased zinc and copper concentration resulted in a gradual decrease in water-soluble pigments contents. Heavy metals have also been shown to disrupt a range of photosynthetic activities [
67]. Under standard conditions (molar concentrations; pH 7) and a half-cell potential (Eo) ≤816 mV, electron transfer from NADH (Eo = ⫽≥320 mV) releases around ≥238 kJ/mol enough to conserve three ATP/electron pairs transmitted. By electron transfer-dependent phosphorylation, no other respiratory electron acceptor allows for such significant energy conservation. We can conclude from our results that, all the metabolic processes are affected due to accumulation of Cu and Zn that reach the toxic level, the energy supply consequently decrease the growth rate decreased.
In cyanobacteria, phycobiliproteins are a category of colorful proteins that operate as light harvesters. Phycobiliproteins are water-soluble compounds made up of proteins and phycobilins, which are covalently bound via cysteine. Phycoerythrin, phycocyanin, phycoerythrocyanin, and allophycocyanin are the four primary types. Phycocyanin, phycoerythrin, and allo-phycocyanin are the most prevalent phycobiliproteins in cyanobacteria quantitatively [
68]. In our present work (
Table 6) the low concentration of Cu and Zn metals resulted in significant high production of phycobiliproteins. The membrane-bound P-type ATPases CtaA and PacS, as well as the metallochaperone Atx1, supply Cu to plastocyanin for photosynthesis and cytochrome c oxidase for respiration in Synechocystis PCC 6803 thylakoids [
69]. Copper is required in cyanobacteria’s thylakoids for type 1 copper site of plastocyanin, which is involved in photosynthetic electron transfer, as well as the CuA and CuB centers of cytochrome c oxidase, which is a terminal electron acceptor in aerobic respiration. The zinc exporter ZiaA (ZiaANmetal-binding)’s domain MBD has a nearly identical Cu(I) affinity. Thermodynamics of zinc removal from the cell may be influenced by the binding of potentially competing zinc to trafficking proteins, with ZiaAN having the highest Zn(II) affinity. Our results revealed that elevated concentrations of either Cu or Zn resulted in significant decrease in phycobiliproteins and the mechanism of metal toxicity, which depends mainly on the growth stage could explain that results. Our results agreed with many reports, they tested the effect of various concentrations of Pb(II) on
Spirulina platensis conducted in Zarrouk liquid medium [
70,
71]. Phycobiliprotein contents were found to have decreased with the increased Pb(II) concentration at the end of 10-day culture period. A decrease in phycobiliprotein might be due to a change in the arrangement and structure of the photosystem II induced by led ions. The multiprotein complex (phycobilisome structure), which is localized in the thylakoid membrane, is composed of different phycobiliproteins. At a higher concentration of Pb
2+ the biosynthesis of membrane proteins dramatically affected, and this could be the possible reason for the decrease of phycobiliprotein contents. Similarly, heavy metals Cu and Zn may affect the phycobiliprotein in cyanobacterium culture. As copper is a heavy metal, the elevated concentration can act strongly on chromatin, photosynthetic apparatus, growth, pigments, and senescence processes.
Furthermore, Hemlata and Fatma discovered that in the presence of many metals (Pb
2+, Cr
6+, Cu
2+, Zn
2+, Ni
2+, and Cd
2+), the formation of phycobiliproteins by Anabaena NCCU-9 diminishes. The necessity for metabolic energy and amino acids for enzymatic defense explains this [
72]. Fe and Cu can also generate Fenton and Fenton-like reactions, according to O’Halloran and Culotta [
73], especially in oxygen-producing cyanobacteria. Metal chaperones are tiny proteins that bind transition metal cations in the cytoplasm or periplasm to reduce harmful reactions and interference with other metals, as well as to offer cations as cofactors to their specialized enzymes. Because copper is at the top of Irving-Williams family, plus a powerful thiol-binding metal and a toxic Fenton reagent, copper chaperones sequester copper in the cytoplasm of many species. This clearly explains our results by which the high decrease in phycobiliprotein content was recorded in elevated Cu treatments [
74].
The level of secondary metabolites (
Table 7) (phenolic, flavonoids and tannins) was elevated significantly at levels of 0.316 and 0.44 mg/L of Cu and Zn respectively. These results may be due to the effect of these metals concentrations on gene expression of
Nostoc linckia cells, which combined with enhancing biosynthesis of different secondary metabolites, mainly carotenoids, phenolic compounds and flavonoids. These findings corroborated those of Sakihama et al. [
75], who discovered that these chemicals may serve as antioxidants as well as chelating agents [
76]. These observations agreed with the previously published results [
54,
57,
77]. Shafiq et al. [
78] confirmed similar findings, stating that there was a strong link between antioxidant activity and total phenolic compound levels. Although secondary metabolites in cyanobacteria are widely studied, the effect of transitional metals, especially copper and zinc, still needs further research.
Antioxidant activity results of extracts in this study (
Table 8 and
Table 9) were parallel with that of El-fayoumy et al. [
5], who found that extract from Nostoc sp was the most effective extract, exhibiting a significant increase in antioxidant activity based on DPPH and ABTS assays (88.18 0.64% and 84.20 1.01%, respectively). Martel et al. [
79] also tested DPPH radical scavenging capacity of different microalgae and cyanobacteria species in methanolic and aqueous extracts. They discovered that
Euglena cantabrica has the strongest antiradical scavenging activity due to its high phenolic content. Moreover, Prabakaran et al. [
80] evaluated the quantitative phytochemical profile of
C. vulgaris. In total, seven phytochemicals were estimated quantitatively from methanolic extract, and phenols were found to be higher followed by the alkaloid, terpenoid and glycoside and the tannin were found to be lesser amount. Takyar et al. [
81] examined the antioxidant activity of alcoholic extract of
C. vulgaris as 42.96% at 200 ppm, while
Spirulina platensis showed antioxidant activity (as 62.46% at 400 ppm) using DPPH method. These findings may be explained by heavy metals’ mechanism and action as an inducer of oxidative stress in microalgal cells, which activated antioxidative enzymes as SOD, GST, CAT, and APX to reduce ROS [
82]. According to [
83,
84], these biological reactions can be viewed as a tolerant mechanism. Antioxidant activity of active components in the effective extract of
Nostoc linckia (0.44 mg/L Zn) might be attributed to several functional groups such as hydroxyl, sulfhydryl, and unsaturated bonds, which have a strong potential to scavenge free radicals and inhibit oxidation processes. The results of the extract’s GC-MS analysis are associated with the data in
Table 7, indicating that there is a strong relationship between antioxidant activity of effective extracts and flavonoids and phenolic compounds concentrations in
Table 8. Siloxanes (cyclononasiloxane and cyclodecasiloxane), eneon compounds, esters, and phenyl esters are all found in the discovered substance. All those compounds were excepted an antioxidant activity.
The cyanobacteria grown under 0.44 mg/L Zn generated 21.05 ± 0.92mg/g flavonoids and 89.29 ± 1.46 mg/g phenolic content, which recorded a higher combined antioxidant activity. Our findings matched those of Nedamani et al. [
85], who looked at the antioxidant activity of individuals and combined rosemary and oak fruit extracts to detect possible interactions in their antioxidant activity. They found that there is a good relationship between total phenols and antioxidant activity. Rosemary extract exhibited a higher antioxidant activity (with higher entire phenol content) than oak extract. The combined extracts showed different behaviors with all three kinds of interactions (synergistic, antagonistic and additive effect). Singprecha et al. [
86] investigated the effect of ginger extract and ascorbic acid on antioxidant activity, implying that an antagonistic interaction between ascorbic acid and ginger, or herbal extracts, should be considered to avoid any potential health consequences. El-Beltagi et al. [
87], found that a mixture of Spirulina platensis and pomegranate juice ameliorate the rat hepatic harm caused by CCl
4 through its antioxidant activity.
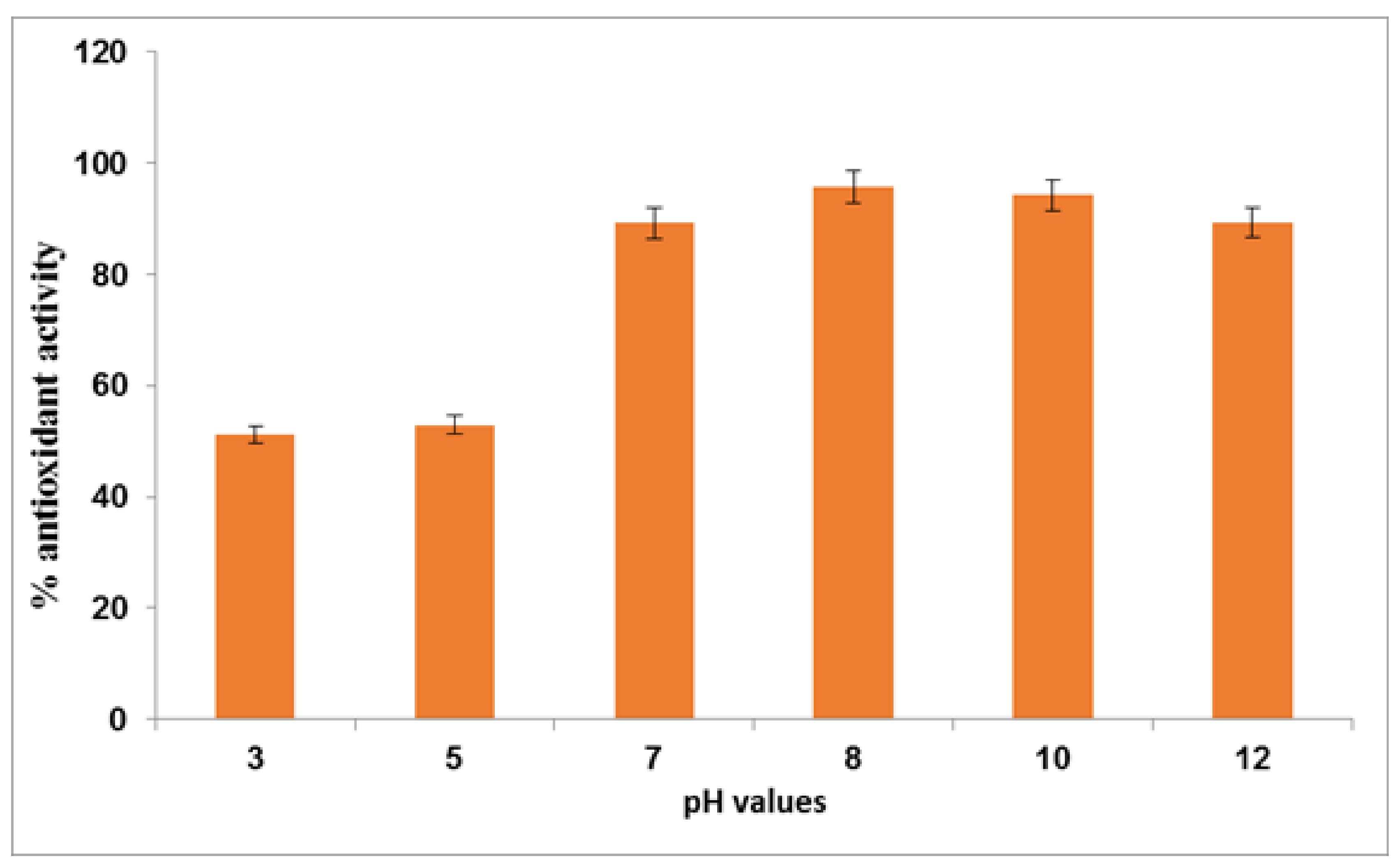
In a pH range of 3–12, the extract’s antioxidant activity was examined (
Figure 1). Because of the presence of phenolic OH group, these compounds behaved as good antioxidants in solution. The primary mechanism of the chain-breaking action was linked to the hydrogen atom transfer (HAT) from the phenolic OH to peroxyl radicals since their antioxidant activity was inversely proportional to magnitude of their O−H bond dissociation enthalpies (BDE). The antioxidant activity of buffer solution was greatly influenced by its pH. All phenolic acids or esters were modest inhibitors of peroxidation in acid conditions (pH 3–5), but their antioxidant activity rose significantly with increasing pH. The enhanced activity reported near the pKa value corresponding to the ionization of one of phenolic hydroxyl groups at pH 8-12 has been attributed to phenolate anion’s high antioxidant activity [
88].
Data showed that all fractions revealed lower activity than that exhibited by the crude extract (
Table 12). Lower activities may be due to the antagonistic effect of crude extract components that cause an increment in ROS scavenging efficiency responsible for antioxidant activity. These results were in agreement with several reports [
4,
89,
90]. The obtained data in
Table 11 could be explained if we answer two questions. First, what is the relationship between the heavy metals’ (Cu/Zn) stress and the antioxidants enzymes? The second question, how to join between lipid peroxidation and the levels of heavy metals and antioxidant enzymes? Many studies were joined between the metal ions and activity of antioxidant enzymes in cyanobacteria. The evolution of superoxide dismutase enzymes (SODs), which can remove superoxide free radicals, can be used to estimate when cyanobacteria first appeared. SODs containing copper and zinc cofactors were employed by cyanobacteria (CuZnSOD). Carotenoids, tocopherol, and antioxidant enzymes such as peroxidases, catalases, superoxide reductases (SORs), and superoxide dismutases (SODs) are used by cyanobacteria to eliminate ROS. SORs and SODs remove superoxide free radicals (O
2.−), whereas peroxidases and catalases speed up the removal of peroxides (such as H
2O
2 and R-O-O-H). Photosynthetic and respiratory electron transport chains 12, as well as extracellular processes on the cell surface, produce O
2.− as a consequence [
91,
92,
93,
94]. They can also help with iron acquisition, cell signaling, and growth, but if O
2.− is allowed to build up inside the cell, it reacts with solvent-exposed 4Fe-4S clusters in proteins, such as those involved in amino acid biosynthesis and photosynthesis, resulting in Fenton reaction reactants, which can cause extensive DNA damage. Organisms must keep control over their abundance to balance the good effects of O
2.− with the damage caused by overexposure. SODs and SORs have been detected in all three domains of life—Eukarya, Archaea, and Bacteria—possibly because of this. For the first time, our phylogenetic analyses reveal CuZnSOD to be antioxidant enzyme that protects photosynthetic repair systems from oxidative damage. The reduced (thiol) sulfur atom (SH) of the cysteinyl residue of GSH catalyzes the nucleophilic attack on the electrophilic center of various hydrophobic compounds (R-X) by the GST superfamily (EC 2.5.1.18) [
95]. Deglutathionylation has been discovered to play a role in photosynthetic organisms’ redox regulation, protection, and recovery of oxidized enzymes in cyanobacterial cells. Lopez et al. [
96] published findings that explained the effect of Cu on lipid peroxidation. Cu’s propensity to cycle between oxidation states makes it a perfect cofactor in redox reactions, but it also allows Cu to catalyze the formation of reactive oxygen species via the metal catalyzed Haber-Weiss reaction, according to the researchers.
•OH is produced as a result, causing severe lipid, protein, and DNA damage. The Irving-Williams series also predicts an alternative route of Cu toxicity, which is related to Cu’s great capacity to bind organic compounds, which means Cu can displace other metals from their associated binding sites within metalloproteins [
97,
98]. Similarly, This interpretation applies to zinc because both Zn and Cu which are divalent cations have almost the same redox potential.
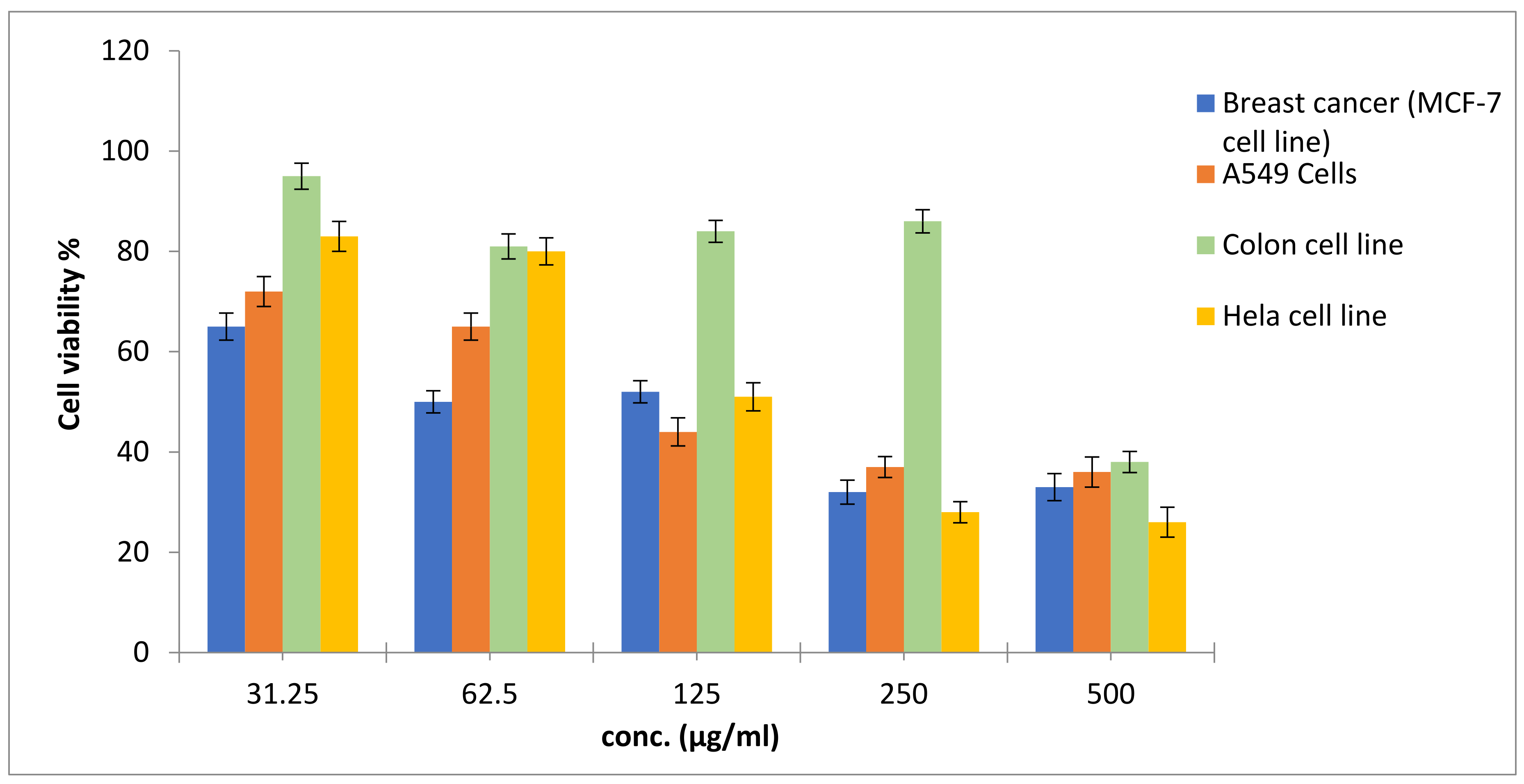
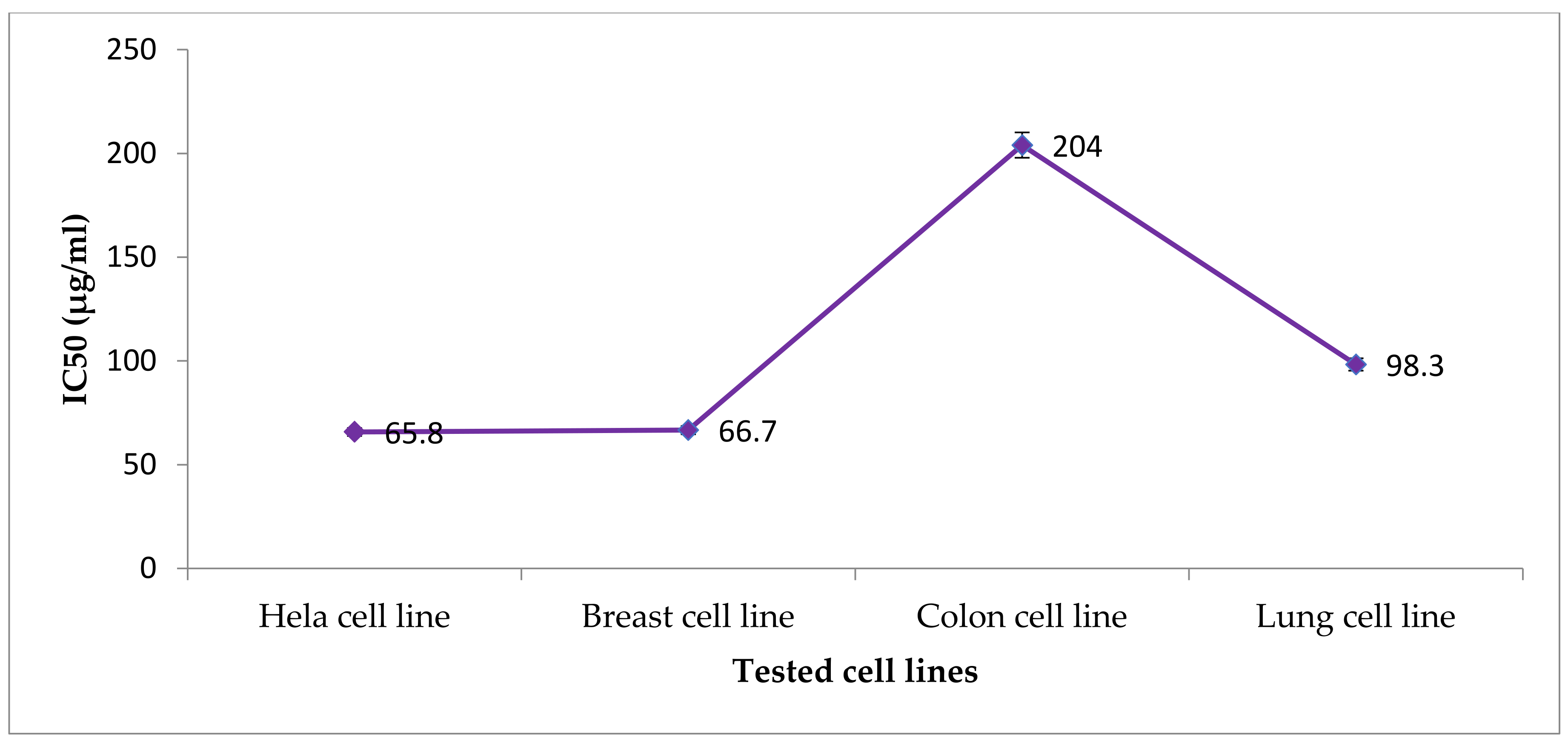
From the obtained data in
Figure 2 and
Figure 3, the antitumor pattern of the
Nostoc linckia crude extract of 0.44 mg/L Zn was similar in MCF-7, A549, and HeLa cell lines, while differrent on HCT 116 cells. The effect of the crude extract is concentration-dependent (31.25–500 g/mL), according to these findings. For these cells, the effect can be described as receptor-independent, as reported [
99]. Furthermore, as previously noted in
Table 10, the anticancer character of the crude extract may be related to its abundance of bioactive compounds. Anticancer compounds such as cycloheptasiloxane, hexadecanol, hexadecenoic acid, hexadecen, and octadecatrienoic acid methyl ester were abundant in cyanobacteria grown at 0.44 mg/L Zn [
100,
101,
102,
103]. The obtained results in
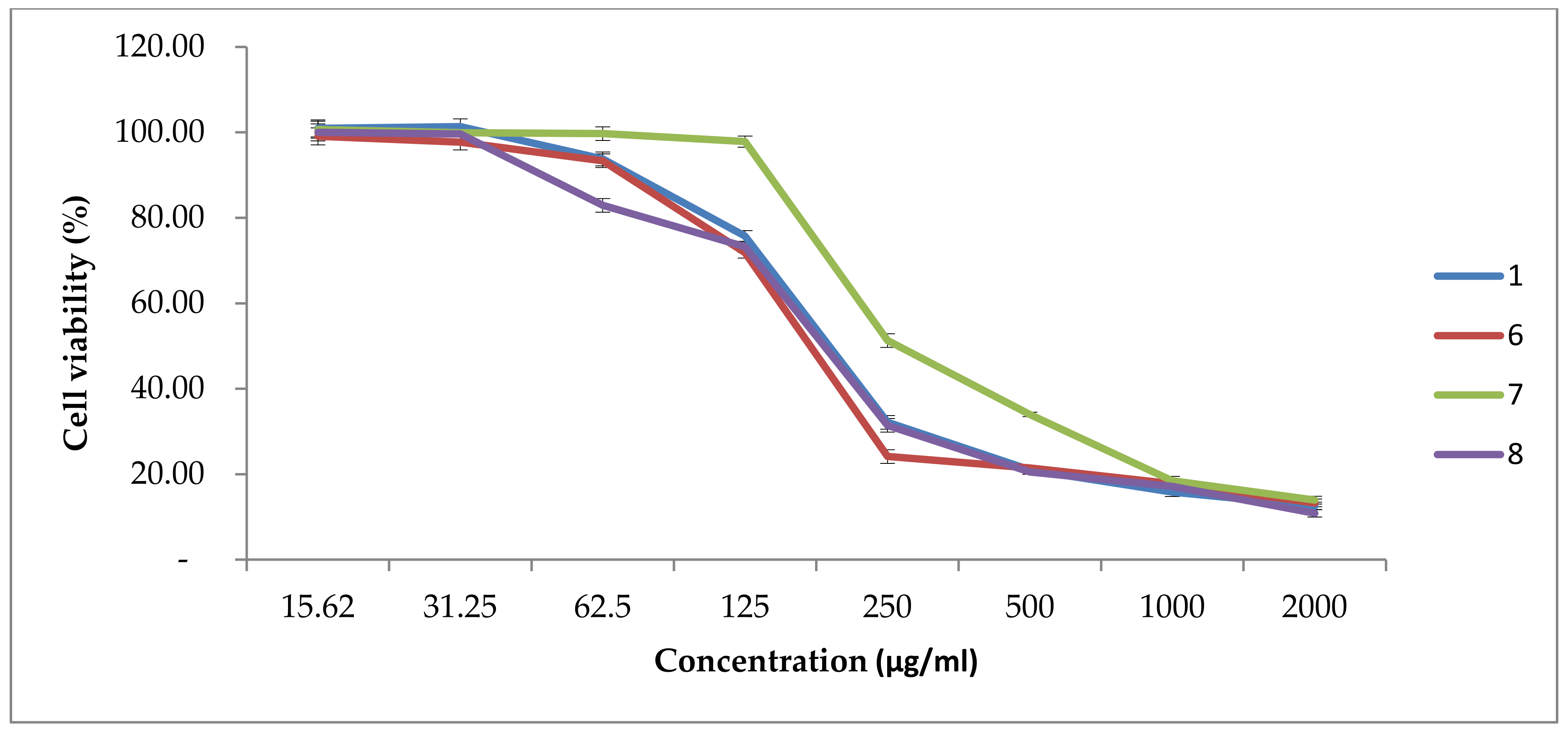
Figure 4 revealed that the four promising fractions (1, 6, 7 and 8) from 0.44m g/L (Zn) of
Nostoc linckia against the promising cell line (Hela cell line) have high antitumor effect ranged from 50 to 90% at 130 and 2000 μg/mL, respectively, with no significant difference between each of tested fraction toward Hela cell line. The antitumor activity revealed in this investigation was similar to that identified by El-fayoumy et al. [
5], who discovered that a crude extract of
Nostoc linckia had anticancer activity against the HCT116 cell line with an IC
50 of 155 μg/mL. Moreover, Alghazeer et al. [
104] who determined anticancer activity of crude extracts of some macroalgae species such as
Ulva lactuca,
Cystoseira stricta and
Sargassum vulgare they reported that phenolic compounds (polyphenols and flavonoids), which are rich in crude promising extracts showed high antioxidant activity and antiradical characteristics. After exposing varied extract concentrations to CaCO
2 cells, anticancer activity was observed, and this activity was linked to polyphenol and flavonoid content. Obviously, both anticancer and antioxidant activity of crude extract of 0.44 mg/L Zn showed the highest activity compared to their promising fractions. Different behaviors of extract and its fractions can be attributed to the compounds’ chemical properties, nature, and reactivity that may suffer polymerization, leading to structural changes responsible for the antioxidant activity.