Use of Biochar-Based Cathodes and Increase in the Electron Flow by Pseudomonas aeruginosa to Improve Waste Treatment in Microbial Fuel Cells
Abstract
:1. Introduction
1.1. Biochar: A Promising Electrode Material
1.2. MFCs and Solid Organic Residues
1.3. Principle of Electron Transfer
1.4. Acidogenic and Acetogenic Microflora in Organic Waste
1.5. Aim of the Research
2. Materials and Methods
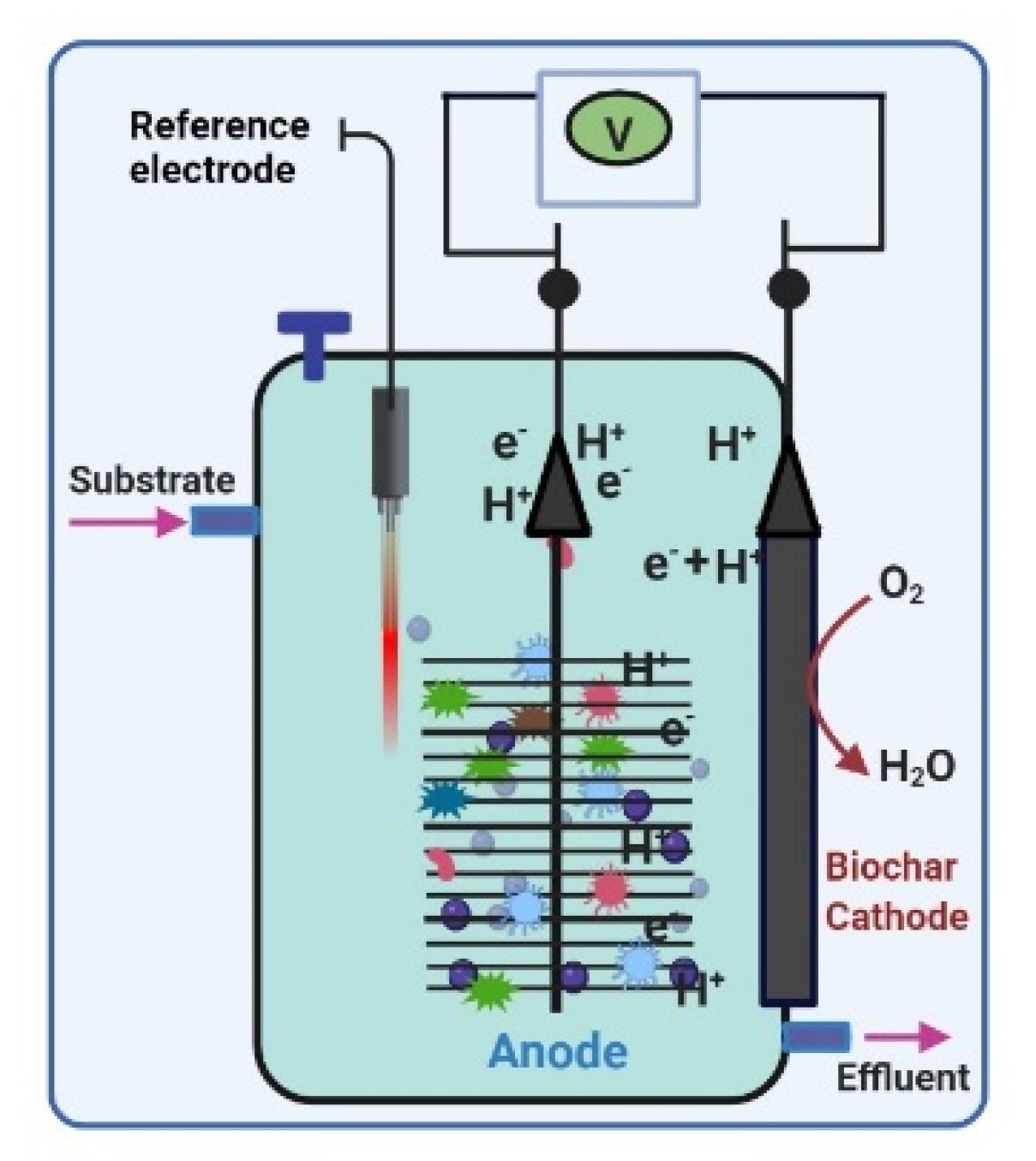
2.1. Biochar-Based Cathode Constructing and Testing
2.2. MFCs Assembly
2.3. MFCs Feedstock and Operation
2.4. Effect of P. aeruginosa on MFCs Performance
- organic residues feedstock
- autoclaved organic residues feedstock + P. aeruginosa ATCC 15692
- organic residues feedstock + P. aeruginosa ATCC 15692
3. Results
3.1. Biochar Analyses
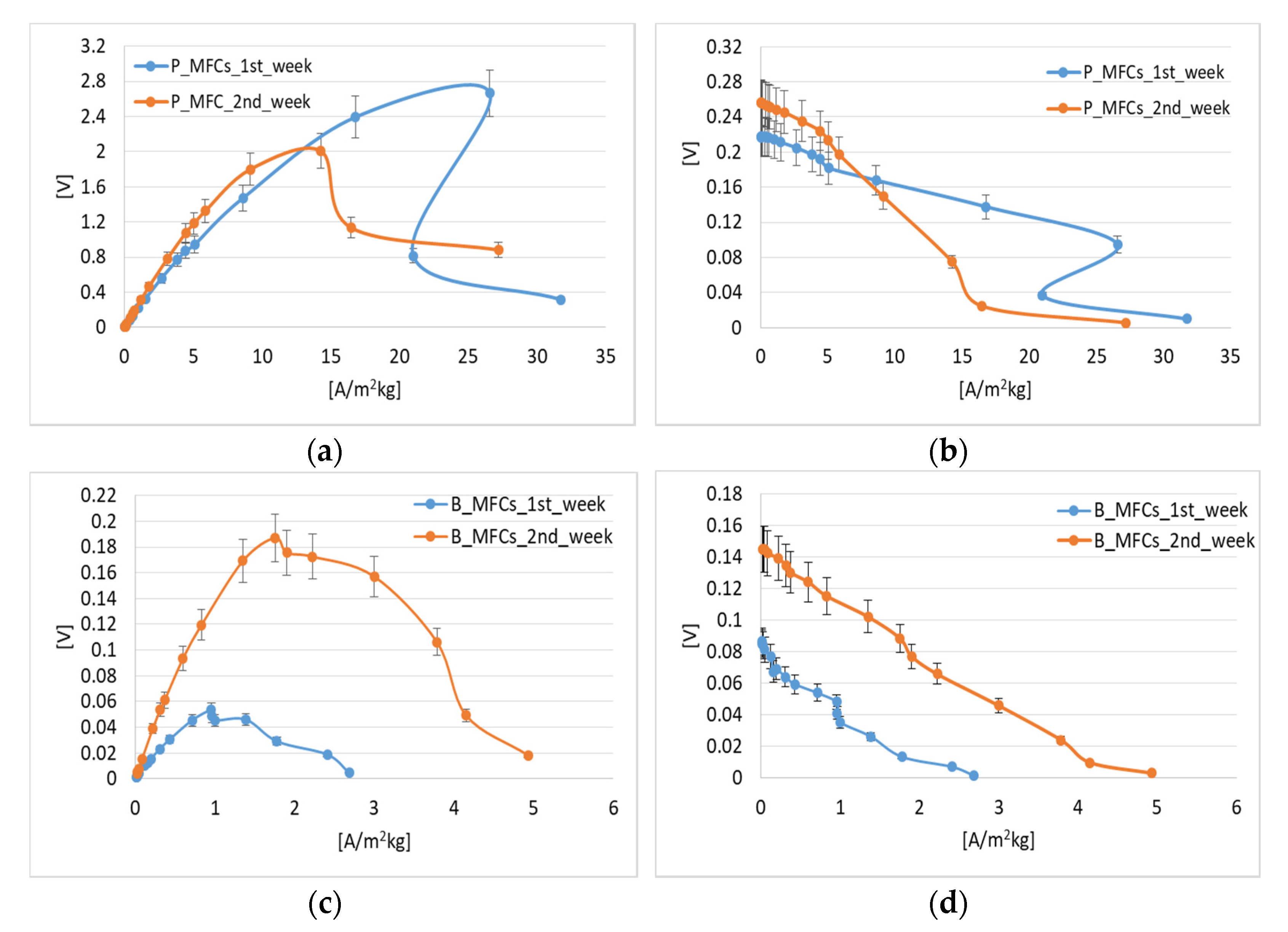
3.2. Polarization Behaviour
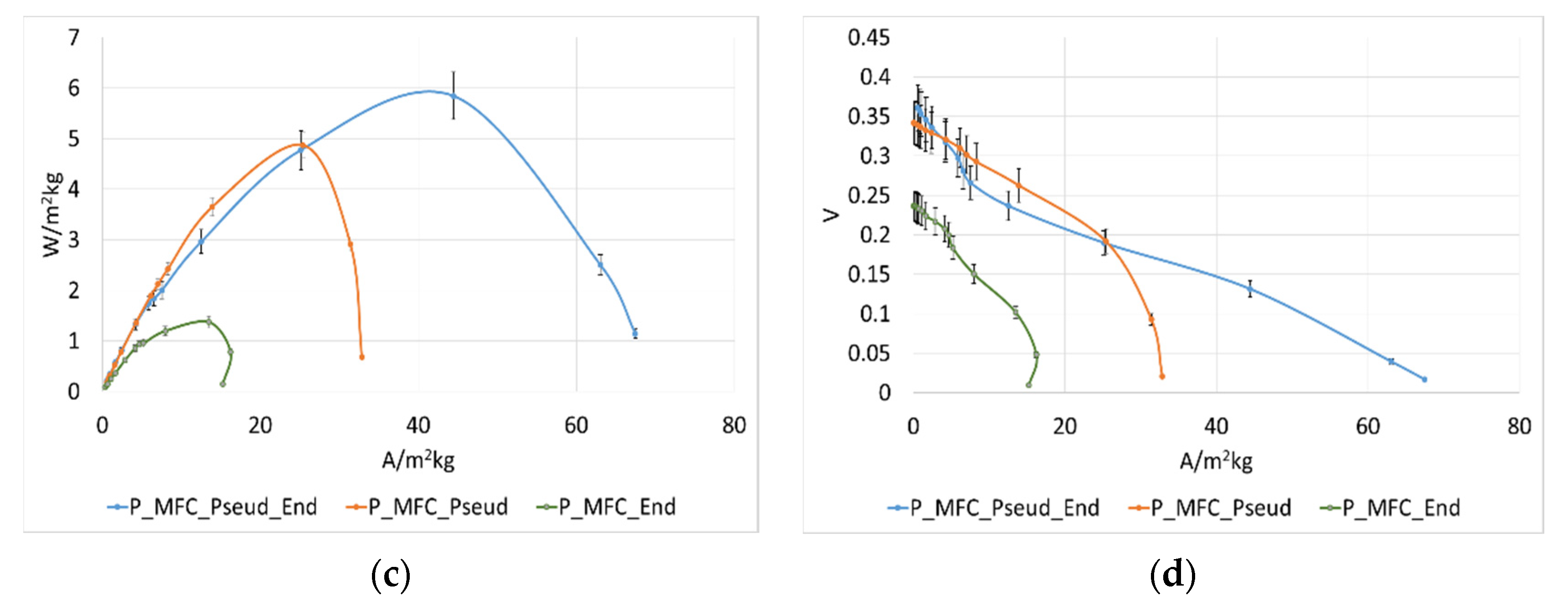
3.3. Influence of Pseudomonas aeruginosa on MFC Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Endreny, T.; Avignone-Rossa, C.; Nastro, R.A. Generating electricity with urban green infrastructure microbial fuel cells. J. Clean. Prod. 2020, 263, 121337. [Google Scholar] [CrossRef]
- Chandrasekhar, K.; Kadier, A.; Kumar, G.; Nastro, R.A.; Jeevitha, V. Challenges in Microbial Fuel Cells and Future Scope. In Microbial Fuel Cell: A Bioelectrochemical System That Convert Waste into Watts; Capital/Publishing Company: New Delhi, India; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Nastro, R.A.; Falcucci, G.; Minutillo, M.; Jannelli, E. Microbial Fuel Cells in Solid Waste Valorization: Trends and Applications. In Recent Trends in Management of Solid and Hazardous Waste; Springer Nature: Cham, Switzerland, 2016. [Google Scholar]
- Winfield, J.; Gajda, I.; Greenman, J.; Ieropoulos, I. A review into the use of ceramics in microbial fuel cells. Bioresour. Technol. 2016, 215, 296–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jannelli, N.; Nastro, R.A.; Cigolotti, V.; Minutillo, M.; Falcucci, G. Low pH, high salinity: Too much for microbial fuel cells? Appl. Energy 2017, 192, 543–550. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.D.; Khan, N.; Sultana, S.; Joshi, R.; Ahmed, S.; Yu, E.; Scott, K.; Ahmad, A.; Khan, M.Z. Bioelectrochemical conversion of waste to energy using microbial fuel cell technology. Process. Biochem. 2017, 57, 141–158. [Google Scholar] [CrossRef]
- Ahmad, J.; Patuzzi, F.; Rashid, U.; Shahabz, M.; Ngamcharussrivichai, C.; Baratieri, M. Exploring untapped effect of process conditions on biochar characteristics and applications. Environ. Technol. Innov. 2021, 21, 101310. [Google Scholar] [CrossRef]
- Gambino, E.; Toscanesi, M.; Del Prete, F.; Flagiello, F.; Falcucci, G.; Minutillo, M.; Trifuoggi, M.; Guida, M.; Nastro, R.A.; Jannelli, E. Polycyclic aromatic hydrocarbons (PAHs) degradation and detoxification of water environment in single-chamber air-cathode microbial fuel cells (MFCs). Fuel Cells 2017, 17, 618–626. [Google Scholar] [CrossRef]
- Di Ilio, G.; Falcucci, G. Multiscale methodology for microbial fuel cell performance analysis. Int. J. Hydrogen Energy 2021, 46, 20280–20290. [Google Scholar] [CrossRef]
- Chandrasekhar, K.; Kumar, A.N.; Kumar, G.; Kim, D.H.; Song, Y.C.; Kim, S.H. Electro-fermentation for biofuels and biochemicals production: Current status and future directions. Bioresour. Technol. 2021, 323, 124598. [Google Scholar] [CrossRef]
- Florio, C.; Nastro, R.A.; Flagiello, F.; Minutillo, M.; Pirozzi, D.; Pasquale, V.; Ausiello, A.; Toscano, G.; Jannelli, E.; Dumontet, S. Biohydrogen production from solid phase-microbial fuel cell spent substrate: A preliminary study. J. Clean. Prod. 2019, 227, 506–511. [Google Scholar] [CrossRef]
- Kumar, P.; Chandrasekhar, K.; Kumari, A.; Sathiyamoorthi, E.; Kim, B.S. Electro-fermentation in aid of bioenergy and biopolymers. Energies 2018, 11, 343. [Google Scholar] [CrossRef] [Green Version]
- Schievano, A.; Pant, D.; Puig, S. Microbial synthesis, gas-fermentation and bioelectroconversion of CO2 and other gaseous streams. Front. Energy Res. 2019, 7, 110. [Google Scholar] [CrossRef]
- Sasaki, K.; Sasaki, D.; Tsuge, Y.; Morita, M.; Kondo, A. Enhanced methane production from cellulose using a two-stage process involving a bioelectrochemical system and a fixed film reactor. Biotechnol. Biofuels 2021, 14, 7. [Google Scholar] [CrossRef]
- Im, C.H.; Kim, C.; Song, Y.E.; Oh, S.E.; Jeon, B.H.; Kim, J.R. Electrochemically enhanced microbial CO conversion to volatile fatty acids using neutral red as an electron mediator. Chemosphere 2018, 191, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tian, Y.; Zuo, W.; Zhang, J.; Pan, X.; Li, L.; Su, X. Electricity generation from food wastes and characteristics of organic matters in microbial fuel cell. Bioresour. Technol. 2016, 205, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Rabaey, K.; Rozendal, R. Microbial electrosynthesis—Revisiting the electrical route for microbial production. Nat. Rev. Microbiol. 2010, 8, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Sivagurunathan, P.; Kuppam, C.; Mudhoo, A.; Saratale, G.D.; Kadier, A.; Zhen, G.; Chatellard, L.; Trably, E.; Kumar, G. A comprehensive review on two-stage integrative schemes for the valorization of dark fermentative effluents. Crit. Rev. Biotechnol. 2018, 38, 868–882. [Google Scholar] [CrossRef] [PubMed]
- Nastro, R.A.; Leccisi, E.; Toscanesi, M.; Liu, G.; Trifuoggi, M.; Ulgiati, S. Exploring avoided environmental impacts as well as energy and resource recovery from microbial desalination cell treatment of brine. Energies 2021, 14, 4453. [Google Scholar] [CrossRef]
- Flagiello, F.; Gambino, E.; Nastro, R.A.; Chandrasekhar, K. Harvesting Energy Using Compost as a Source of Carbon and Electrogenic Bacteria. In Bioelectrochemical Systems; Springer Nature: Cham, Switzerland, 2020. [Google Scholar]
- Jung, S.; Lee, J.; Park, Y.K.; Kwon, E.E. Bioelectrochemical systems for a circular bioeconomy. Bioresour. Technol. 2020, 300, 122748. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumar, K.; Naveenkumar, M. Enhanced performance study of microbial fuel cell using waste biomass-derived carbon electrode. Biomass Conv. Bioref. 2021, 1–9. [Google Scholar] [CrossRef]
- Rahman, M.Z.; Edvinsson, T.; Kwong, P. Biochar for electrochemical applications. Curr. Opin. Green Sustain. Chem. 2020, 23, 25–30. [Google Scholar] [CrossRef]
- Zha, Z.; Zhang, Z.; Xiang, P.; Zhu, H.; Zhou, B.; Sun, Z.; Zhou, S. One-step preparation of eggplant-derived hierarchical porous graphitic biochar as efficient oxygen reduction catalyst in microbial fuel cells. RSC Adv. 2021, 11, 1077–1085. [Google Scholar] [CrossRef]
- Li, S.; Ho, S.H.; Hua, T.; Zhou, Q.; Li, F.; Tang, J. Sustainable biochar as an electrocatalysts for the oxygen reduction reaction in microbial fuel cells. Green Energy Environ. 2020. [Google Scholar] [CrossRef]
- Minutillo, M.; Flagiello, F.; Nastro, R.A.; Di Trolio, P.; Jannelli, E.; Perna, A. Performance of two different types of cathodes in microbial fuel cells for power generation from renewable sources. Energy Procedia 2018, 148, 1129–1134. [Google Scholar] [CrossRef]
- Chakraborty, I.; Sathe, S.M.; Dubey, B.K.; Ghangrekar, M.M. Waste-derived biochar: Applications and future perspective in microbial fuel cells. Bioresour. Technol. 2020, 312, 123587. [Google Scholar] [CrossRef] [PubMed]
- Joseph, S.; Husson, O.; Graber, E.R.; van Zwieten, L.; Taherymoosavi, S.; Thomas, T.; Nielsen, S.; Ye, J.; Pan, G.; Chia, C.; et al. The electrochemical properties of biochars and how they affect soil redox properties and processes. Agronomy 2015, 5, 322. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Song, X.; Li, Q.; Bai, H.; Zhu, C.; Weng, B.; Yan, D.; Bai, J. Bioenergy generation and degradation pathway of phenanthrene and anthracene in a constructed wetland-microbial fuel cell with an anode amended with nZVI. Water Res. 2019, 150, 340–348. [Google Scholar] [CrossRef]
- Mian, M.; Liu, G.; Fu, B. Conversion of sewage sludge into environmental catalyst and microbial fuel cell electrode material: A review. Sci. Total Environ. 2019, 666, 525–539. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Ngo, H.H.; Guo, W.; Chang, W.S.; Nguyen, D.D.; Li, J.; Ly, Q.V.; Nguyen, T.A.H.; Tran, V.S. Applying a new pomelo peel derived biochar in microbial fell cell for enhancing sulfonamide antibiotics removal in swine wastewater. Bioresour. Technol. 2020, 318, 123886. [Google Scholar] [CrossRef]
- Huggins, T.M.; Latorre, A.; Biffinger, J.C.; Ren, Z.J. Biochar based microbial fuel cell for enhanced wastewater treatment and nutrient recovery. Sustainability 2016, 8, 169. [Google Scholar] [CrossRef] [Green Version]
- Ayyappan, C.S.; Bhalambaal, V.M.; Kumar, S. Effect of biochar on bio-electrochemical dye degradation and energy production. Bioresour. Technol. 2018, 251, 165–170. [Google Scholar] [CrossRef]
- Ganesh, K.; Jambeck, J.R. Treatment of landfill leachate using microbial fuel cells: Alternative anodes and semi-continuous operation. Bioresour. Technol. 2013, 139, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Nastro, R.A.; Falcucci, G.; Toscanesi, M.; Minutillo, M.; Pasquale, V.; Trifuoggi, M.; Dumontet, S.; Jannelli, E. Performances and Microbiology of a Microbial Fuel Cell (MFC) Fed with the Organic Fraction of Municipal Solid Waste (OFMSW). In Proceedings of the EFC2015 European Fuel Cell Technology & Applications Conference—Piero Lunghi Conference, Naples, Italy, 15–18 December 2015. [Google Scholar]
- Chandrasekhar, K.; Amulya, K.; Venkata Mohan, S. Solid phase bio-electrofermentation of food waste to harvest value-added products associated with waste remediation. Waste Manag. 2015, 45, 57–65. [Google Scholar] [CrossRef]
- Budihardjo, M.A.; Effendi, A.J.; Hidayat, S.; Purnawan, C.; Lantasi, A.I.D.; Muhammad, F.I.; Ramadan, B.S. Waste valorization using solid-phase microbial fuel cells (SMFCs): Recent trends and status. J. Environ. Manag. 2021, 277, 111417. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Luo, H.; Fallgren, P.H.; Jin, S. Bioelectrochemical system platform for sustainable environmental remediation and energy generation. Biotechnol. Adv. 2015, 33, 317–334. [Google Scholar] [CrossRef]
- Di Ilio, G.; Nastro, R.A.; Di Trolio, P.; Falcucci, G. A Comprehensive Multiscale Methodology to Investigate Microbial Fuel Cell Performance. In Proceedings of the ECOS 2019—32nd International Conference on Efficiency, Cost, Optimization, Simulation and Environmental Impact of Energy Systems, Wroclaw, Poland, 23–28 June 2019; pp. 3841–3857. [Google Scholar]
- Zheng, T.; Li, J.; Ji, Y.; Zhang, W.; Fang, Y.; Xin, F.; Dong, W.; Wei, P.; Ma, J.J.M. Progress and prospects of bioelectrochemical systems: Electron transfer and its applications in the microbial metabolism. Front. Bioeng. Biotechnol. 2020, 8, 10. [Google Scholar] [CrossRef] [Green Version]
- Rabaey, K.; Boon, N.; Höfte, M.; Verstraete, W. Microbial phenazine production enhances electron transfer in biofuel cells. Environ. Sci. Technol. 2005, 39, 3401–3408. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.J.; Qiao, Y.; Zou, L.; Wu, X.S.; Liu, J.H. Biofilm promoted current generation of Pseudomonas aeruginosa microbial fuel cell via improving the interfacial redox reaction of phenazines. Bioelectrochemistry 2017, 117, 34–39. [Google Scholar] [CrossRef]
- Pham, H.T.; Boon, N.; De Maeyer, K.; Höfte, M.; Rabaey, K.; Verstraete, W. Use of Pseudomonas species producing phenazine-based metabolites in the anodes of microbial fuel cells to improve electricity generation. Appl. Microbiol. Biotechnol. 2008, 80, 985–993. [Google Scholar] [CrossRef] [PubMed]
- Marone, A.; Izzo, G.; Mentuccia, L.; Massini, G.; Paganin, P.; Rosa, S.; Varrone, C.; Signorini, A. Vegetable waste as substrate and source of suitable microflora for bio-hydrogen production. Renew. Energy 2014, 68, 6–13. [Google Scholar] [CrossRef]
- Panin, S.; Setthapun, W.; Sinsuw, A.A.E.; Sintuya, H.; Chu, C.Y. Biohydrogen and biogas production from mashed and powdered vegetable residues by an enriched microflora in dark fermentation. Int. J. Hydrogen Energy 2021, 46, 14073–14082. [Google Scholar] [CrossRef]
- Paquete, C.M. Electroactivity across the cell wall of gram-positive bacteria. Comput. Struct. Biotechnol. J. 2020, 18, 3796–3802. [Google Scholar] [CrossRef]
- Brooijmans, R.J.W.; de Vos, W.M.; Hugenholtz, J. Lactobacillus plantarum WCFS1 electron transport chains. Appl. Environ. Microbiol. 2009, 75, 3580–3585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar physicochemical properties: Pyrolysis temperature and feedstock kind effects. Rev. Environ. Sci. Biotechnol. 2020, 19, 191–215. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Xu, X.; Cao, L.; Ok, Y.S.; Cao, X. Characterization and quantification of electron donating capacity and its structure dependence in biochar derived from three waste biomasses. Chemosphere 2018, 211, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Yaqoob, A.A.; Mohamad, I.M.N.; Rafatullah, M.; Chua, Y.S.; Ahmad, A.; Umar, K. Recent advances in anodes for microbial fuel cells: An overview. Materials 2020, 13, 2078. [Google Scholar] [CrossRef] [PubMed]
- Mallik, A.; Ray, B.C. Evolution of principle and practice of electrodeposited thin film: A review on effect of temperature and sonication. Int. J. Electrochem. 2011, 2011, 16. [Google Scholar] [CrossRef] [Green Version]
- Falcucci, G.; Amati, G.; Fanelli, P.; Krastev, V.K.; Polverino, G.; Porfiri, M.; Succi, S. Extreme flow simulations reveal skeletal adaptations of deep-sea sponges. Nature 2021, 595, 537–541. [Google Scholar] [CrossRef]
- Krastev, V.K.; Falcucci, G. Evaluating the electrochemical and power performances of microbial fuel cells across physical scales: A novel numerical approach. Int. J. Hydrogen Energy 2019, 44, 4468–4475. [Google Scholar] [CrossRef]

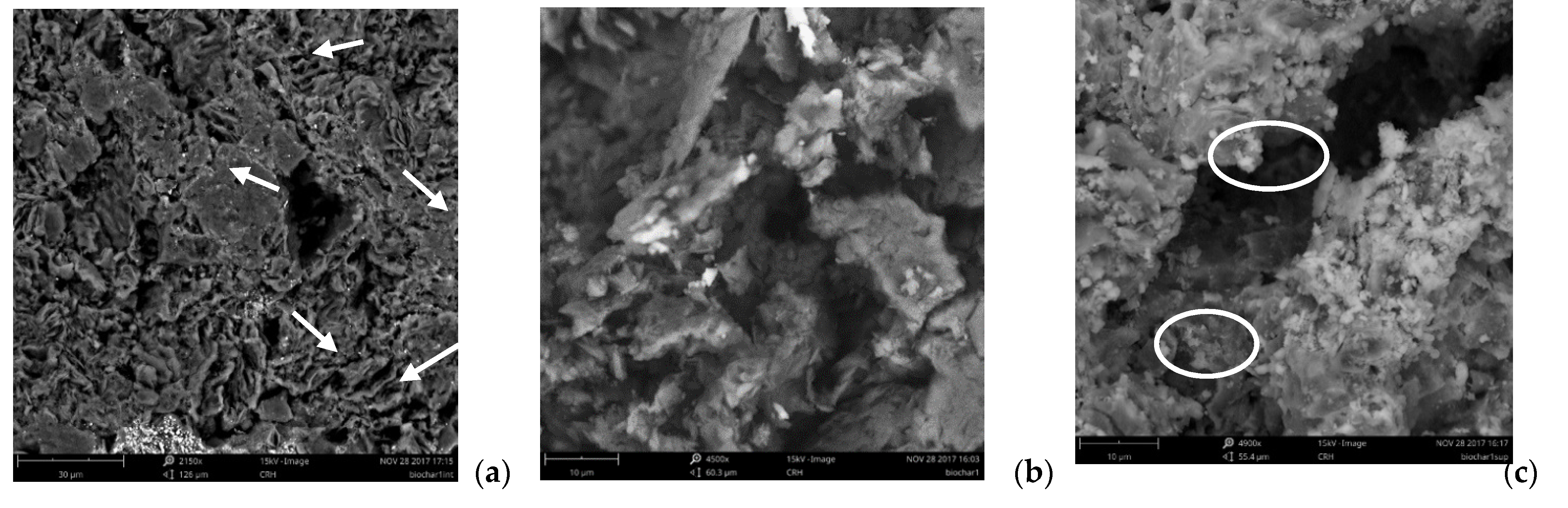
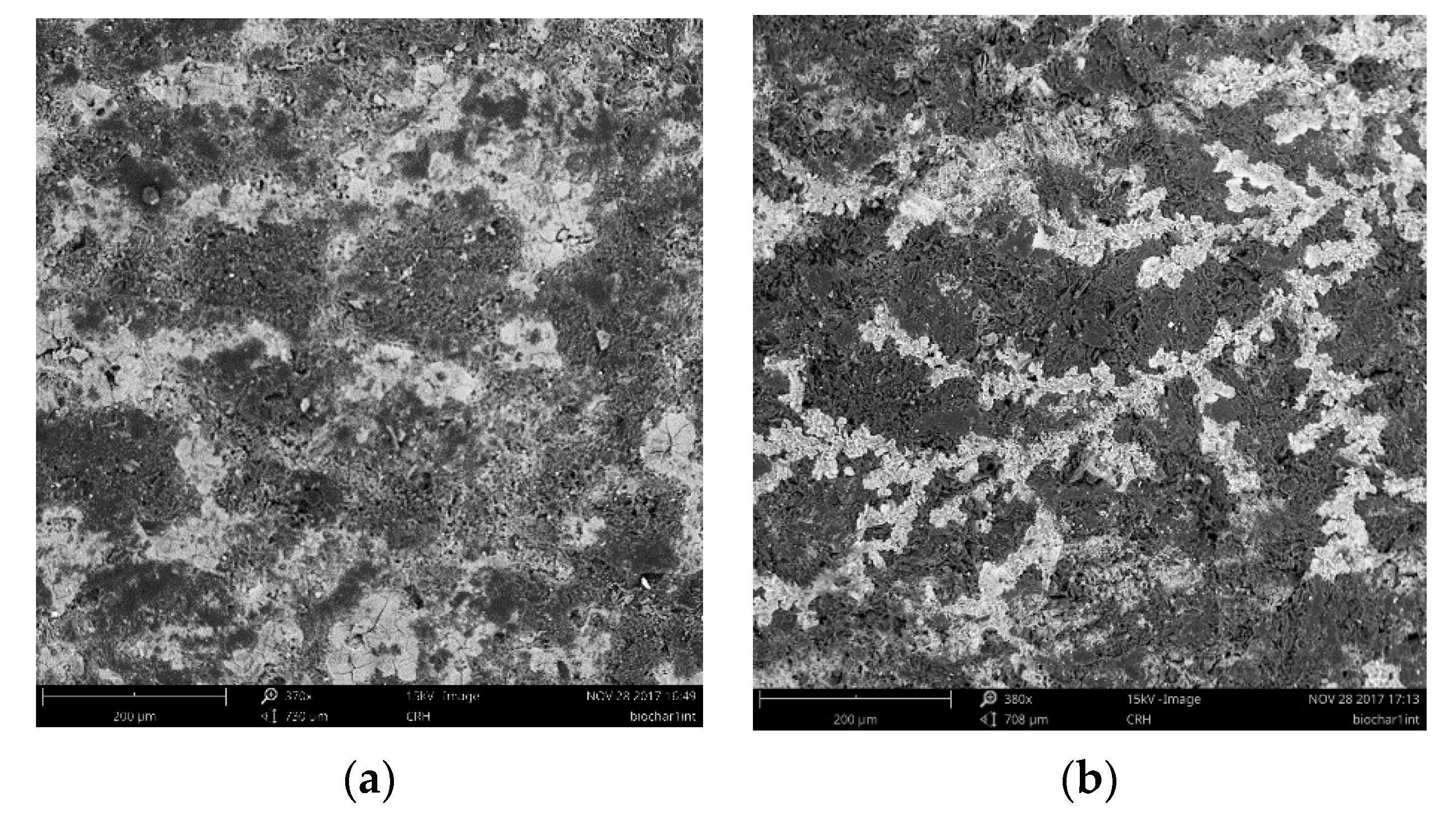





| Chemical Elements | Biochar (Clear Zones) | Biochar (Graphite Like Structures) | Inner Surface * (Figure 4a) | Outer Surface * (Figure 4a) |
|---|---|---|---|---|
| C | 51.7 | 72.1 | 68.7 | 64.6 |
| O | 33.8 | 23.3 | 12.2 | 5.7 |
| P | 4.6 | 0.8 | 0.5 | n.a. |
| Ca | 5.6 | 2.0 | 0.7 | n.a. |
| Na | 1.6 | 0.8 | n.a. | 18.9 |
| Cl | 0.4 | 0.4 | 1.0 | 10.9 |
| Si | 0.5 | 0.4 | 0.4 | n.a. |
| Mg | 1.4 | n.a. | n.a. | n.a. |
| Mo | n.a. | n.a. | 0.2 | n.a. |
| K | 0.4 | n.a. | n.a. | n.a. |
| Fe | n.a. | n.a. | 9.4 | n.a. |
| Zn | n.a. | n.a. | 6.5 | n.a. |
| In | n.a. | n.a. | 0.3 | n.a. |
| Pseud_End/End | Pseud_End/Pseud | Pseud/End | |
|---|---|---|---|
| Test t | Test t | Test t | |
| CD | 0.119 | 0.316 | 0.417 |
| PD | 0.0001 * | 0.131 | 0.0218 * |
| Test F | Test F | Test F | |
| CD | 0.0001 * | 0.022 * | 0.063 |
| PD | 2.59 × 10−1 * | 0.736 | 4.97 × 10−10 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nastro, R.A.; Flagiello, F.; Silvestri, N.; Gambino, E.; Falcucci, G.; Chandrasekhar, K. Use of Biochar-Based Cathodes and Increase in the Electron Flow by Pseudomonas aeruginosa to Improve Waste Treatment in Microbial Fuel Cells. Processes 2021, 9, 1941. https://doi.org/10.3390/pr9111941
Nastro RA, Flagiello F, Silvestri N, Gambino E, Falcucci G, Chandrasekhar K. Use of Biochar-Based Cathodes and Increase in the Electron Flow by Pseudomonas aeruginosa to Improve Waste Treatment in Microbial Fuel Cells. Processes. 2021; 9(11):1941. https://doi.org/10.3390/pr9111941
Chicago/Turabian StyleNastro, Rosa Anna, Fabio Flagiello, Nicandro Silvestri, Edvige Gambino, Giacomo Falcucci, and Kuppam Chandrasekhar. 2021. "Use of Biochar-Based Cathodes and Increase in the Electron Flow by Pseudomonas aeruginosa to Improve Waste Treatment in Microbial Fuel Cells" Processes 9, no. 11: 1941. https://doi.org/10.3390/pr9111941
APA StyleNastro, R. A., Flagiello, F., Silvestri, N., Gambino, E., Falcucci, G., & Chandrasekhar, K. (2021). Use of Biochar-Based Cathodes and Increase in the Electron Flow by Pseudomonas aeruginosa to Improve Waste Treatment in Microbial Fuel Cells. Processes, 9(11), 1941. https://doi.org/10.3390/pr9111941







