Evaluation of Thermal Hazard Properties of Low Temperature Active Azo Compound under Process Conditions for Polymer Resin in Construction Industries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Thermogravimetric Analyzer
2.3. Basic Theory of Kinetic Model
2.4. Extending from the Analysis of Material Dynamics to the Thermal Balance of the System
2.5. Safety Operation and Related Evaluation Parameters of Actual Manufacturing Process
2.6. Thermal Stability on Storage Condition
3. Results
3.1. Experiment Data Curve Fitting and Derivation of Related Reaction Kinetic Parameters
3.2. Thermal Hazard Evaluation
3.3. Runaway Reaction of AIBA
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, S.H.; Cao, C.R.; Lin, W.C.; Shu, C.M. Experimental and numerical simulation study of the thermal hazards of four azo compounds. J. Hazard. Mater. 2019, 365, 164–177. [Google Scholar] [CrossRef]
- Dubikhin, V.; Knerel’man, E.; Manelis, G.; Nazin, G.; Prokudin, V.; Stashina, G.; Chukanov, N.; Shastin, A. Thermal decomposition of azobis (isobutyronitrile) in the solid state. Cage effect. Recombination and disproportionation of cyanoisopropyl radicals. Dokl. Phys. Chem. 2012, 446, 171–175. [Google Scholar] [CrossRef]
- Dou, Z.; Jiang, J.C.; Wang, Z.R.; Zhao, S.P.; Yang, H.Q.; Mao, G.B. Kinetic analysis for spontaneous combustion of sulfurized rust in oil tanks. J. Loss Prev. Process Ind. 2014, 32, 387–392. [Google Scholar] [CrossRef]
- Dou, Z.; Jiang, J.C.; Zhao, S.P.; Zhang, W.X.; Ni, L.; Zhang, M.G.; Wang, Z.R. Analysis on oxidation process of sulfurized rust in oil tank. J. Therm. Anal. Calorim. 2017, 128, 125–134. [Google Scholar] [CrossRef]
- Liu, S.H.; Lin, W.C.; Hou, H.Y.; Shu, C.M. Comprehensive runaway kinetic analysis and validation of three azo compounds using calorimetric approach and simulation. J. Loss Prev. Process Ind. 2017, 49, 970–982. [Google Scholar] [CrossRef]
- Kandelhard, F.; Georgopanos, P. Predici as a Polymer Engineers’ Tool for the Synthesis of Polymers via Anionic Polymerization. Ind. Eng. Chem. Res. 2021, 60, 11373–11384. [Google Scholar] [CrossRef]
- Berdouzi, F.; Villemur, C.; Olivier Maget, N.; Gabas, N. Dynamic simulation for risk analysis: Application to an exothermic reaction. Process Saf. Environ. Prot. 2018, 113, 149–163. [Google Scholar] [CrossRef] [Green Version]
- Cao, C.R.; Liu, S.H.; Shu, C.M. Reaction simulation of multistage evaluations for AMBN based on DSC experiments. Thermochim. Acta 2018, 661, 18–26. [Google Scholar] [CrossRef]
- Liu, S.H.; Lin, W.C.; Xia, H.; Hou, H.Y.; Shu, C.-M. Combustion of 1-butylimidazolium nitrate via DSC, TG, VSP2, FTIR, and GC/MS: An approach for thermal hazard, property and prediction assessment. Process Saf. Environ. Prot. 2018, 116, 603–614. [Google Scholar] [CrossRef]
- Kandelhard, F.; Schuldt, K.; Schymura, J.; Georgopanos, P.; Abetz, V. Model-Assisted Optimization of RAFT Polymerization in Micro-Scale Reactors—A Fast Screening Approach. Macromol. React. Eng. 2021, 15, 2000058. [Google Scholar] [CrossRef]
- Chiang, C.L.; Liu, S.H.; Cao, C.R.; Hou, H.Y.; Shu, C.M. Multiapproach thermodynamic and kinetic characterization of the thermal hazards of 2, 2′-azobis (2-methylpropionate) alone and when mixed with several solvents. J. Loss Prev. Process Ind. 2018, 51, 150–158. [Google Scholar] [CrossRef]
- Liu, S.H.; Cao, C.R.; Lin, Y.C.; Shu, C.M. Using thermal analysis and kinetic calculation method to assess the thermal stability of 2,2′-azobis-(2-methylbutyronitrile). J. Therm. Anal. Calorim. 2018, 131, 545–553. [Google Scholar] [CrossRef]
- Martín-Lara, M.; Blázquez, G.; Zamora, M.; Calero, M. Kinetic modeling of torrefaction of olive tree pruning. Appl. Therm. Eng. 2017, 113, 1410–1418. [Google Scholar] [CrossRef]
- Li, X.R.; Koseki, H.; Iwata, Y.; Mok, Y.S. Decomposition of methyl ethyl ketone peroxide and mixtures with sulfuric acid. J. Loss Prev. Process Ind. 2004, 17, 23–28. [Google Scholar] [CrossRef]
- Zhu, H.; Liu, N. Kinetic analysis based on the kinetic compensation effect and optimization calculation. Thermochim. Acta 2020, 690, 178686. [Google Scholar] [CrossRef]
- Gonzales, N.O.; Levin, M.E.; Zimmerman, L.W. The reactivity of sodium borohydride with various species as characterized by adiabatic calorimetry. J. Hazard. Mater. 2007, 142, 639–646. [Google Scholar] [CrossRef]
- Liu, S.H.; Shu, C.M. Advanced technology of thermal decomposition for AMBN and ABVN by DSC and VSP2. J. Therm. Anal. Calorim. 2015, 121, 533–540. [Google Scholar] [CrossRef]
- Liu, S.H.; Hou, H.Y.; Shu, C.M. Thermal hazard evaluation of the autocatalytic reaction of benzoyl peroxide using DSC and TAM III. Thermochim. Acta 2015, 605, 68–76. [Google Scholar] [CrossRef]
- Chen, W.C.; Shu, C.M. Prediction of thermal hazard for TBPTMH mixed with BPO through DSC and isoconversional kinetics analysis. J. Therm. Anal. Calorim. 2016, 126, 1937–1945. [Google Scholar] [CrossRef]
- Kossoy, A.A.; Sheinman, I.Y. Evaluating Thermal Explosion Hazard by Using Kinetics-Based Simulation Approach. Process Saf. Environ. Prot. 2004, 82, 421–430. [Google Scholar] [CrossRef]
- Kossoy, A.A.; Benin, A.I.; Akhmetshin, Y.G. An advanced approach to reactivity rating. J. Hazard. Mater. 2005, 118, 9–17. [Google Scholar] [CrossRef]
- ChemInform Saint-Petersburg (CISP), L. Thermal Safety Software, Retrieved. 2021. Available online: http://www.cisp.spb.ru (accessed on 27 October 2021).
- Andreozzi, R.; Caprio, V.; Somma, I.D.; Sanchirico, R. Kinetic and safety assessment for salicylic acid nitration by nitric acid/acetic acid system. J. Hazard. Mater. 2006, 134, 1–7. [Google Scholar] [CrossRef]
- Kossoy, A.A.; Akhmetshin, Y.G. Identification of kinetic models for the assessment of reaction hazards. Process Saf. Prog. 2007, 26, 209–220. [Google Scholar] [CrossRef]
- Wang, S.Y.; Kossoy, A.A.; Yao, Y.D.; Chen, L.P.; Chen, W.H. Kinetics-based simulation approach to evaluate thermal hazards of benzaldehyde oxime by DSC tests. Thermochim. Acta 2017, 655, 319–325. [Google Scholar] [CrossRef]
- Roduit, B.; Folly, P.; Sarbach, A.; Berger, B.; Brogli, F.; Mascarello, F.; Schwaninger, M.; Glarner, T.; Irle, E.; Tobler, F. Estimation of time to maximum rate under adiabatic conditions (TMRad) using kinetic parameters derived from DSC-investigation of thermal behavior of 3-methyl-4-nitrophenol. Chem. Propel. Polym. Mater. 2011, 1, 84–93. [Google Scholar]
- United Nations. Recommendations on the Transport of Dangerous Goods: Model Regulations, 20th Revised ed.; United Nations Publications: New York, NY, USA, 2018; Volume 1. [Google Scholar]
- United Nations. Recommendations on the Transport of Dangerous Goods: Manual of Tests and Criteria, 6th Revised ed.; United Nations Publications: New York, NY, USA, 2018. [Google Scholar] [CrossRef]
- Kossoy, A.A.; Sheinman, I.Y. Comparative analysis of the methods for SADT determination. J. Hazard. Mater. 2007, 142, 626–638. [Google Scholar] [CrossRef]
- Steensma, M.; Schuurman, P.; Malow, M.; Krause, U.; Wehrstedt, K.D. Evaluation of the validity of the UN SADT H.4 test for solid organic peroxides and self-reactive substances. J. Hazard. Mater. 2005, 117, 89–102. [Google Scholar] [CrossRef]
- Lv, J.; Chen, L.; Chen, W.; Gao, H.; Peng, M. Kinetic analysis and self-accelerating decomposition temperature (SADT) of dicumyl peroxide. Thermochim. Acta 2013, 571, 60–63. [Google Scholar] [CrossRef]
- Malow, M.; Wehrstedt, K.D. Prediction of the self-accelerating decomposition temperature (SADT) for liquid organic peroxides from differential scanning calorimetry (DSC) measurements. J. Hazard. Mater. 2005, 120, 21–24. [Google Scholar] [CrossRef]
- Li, X.R.; Koseki, H. SADT prediction of autocatalytic material using isothermal calorimetry analysis. Thermochim. Acta 2005, 431, 113–116. [Google Scholar] [CrossRef]
- Kossoy, A.A.; Belokhvostov, V.M.; Koludarova, E.Y. Thermal decomposition of AIBN: Part D: Verification of simulation method for SADT determination based on AIBN benchmark. Thermochim. Acta 2015, 621, 36–43. [Google Scholar] [CrossRef]
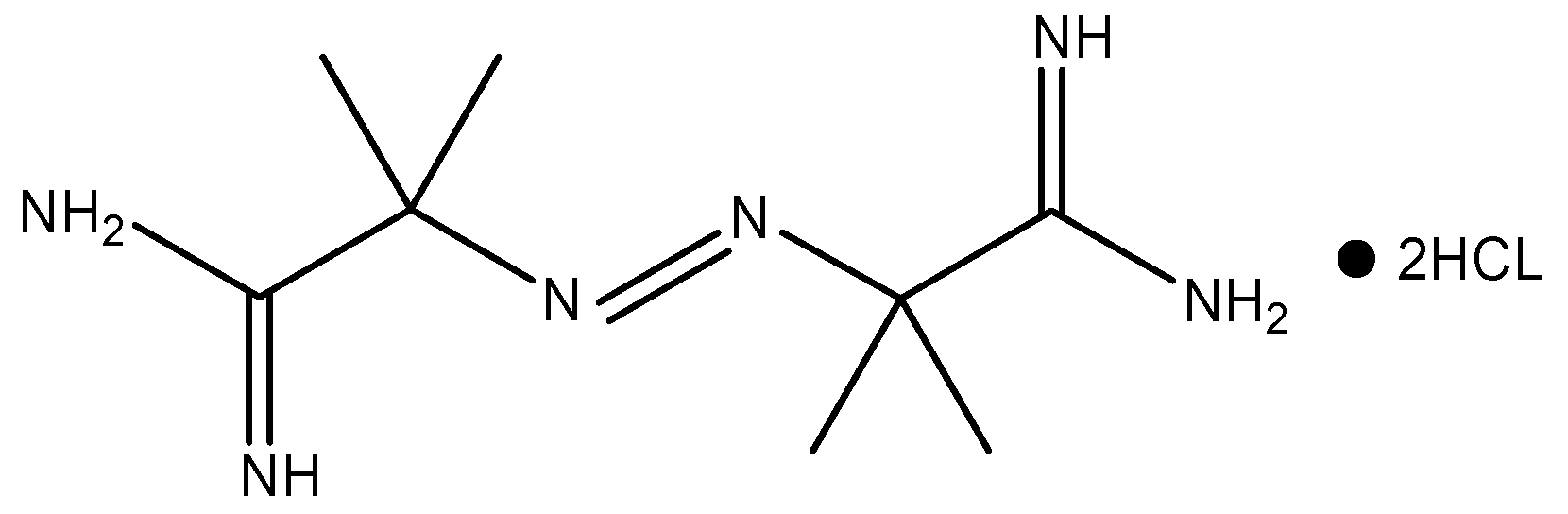

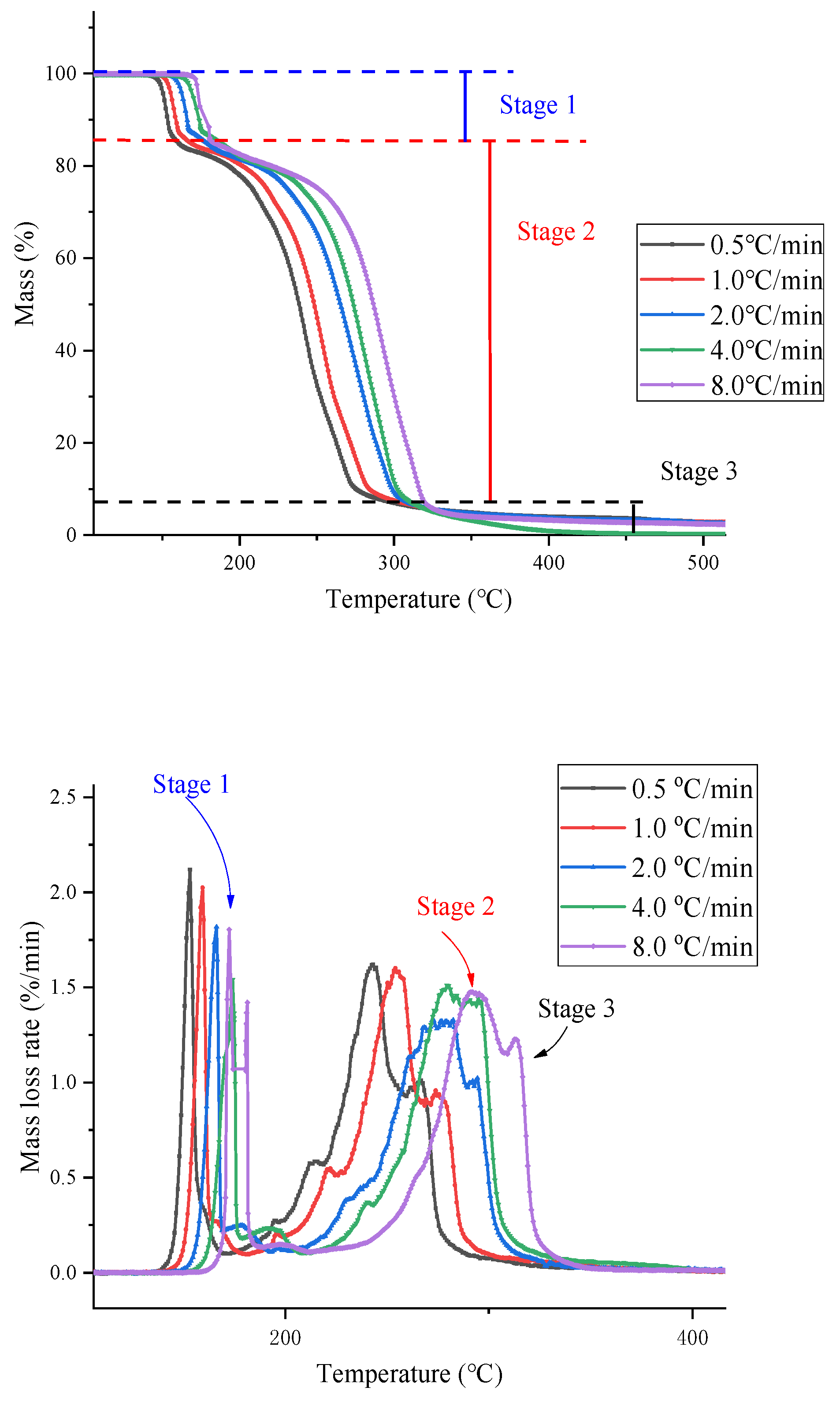



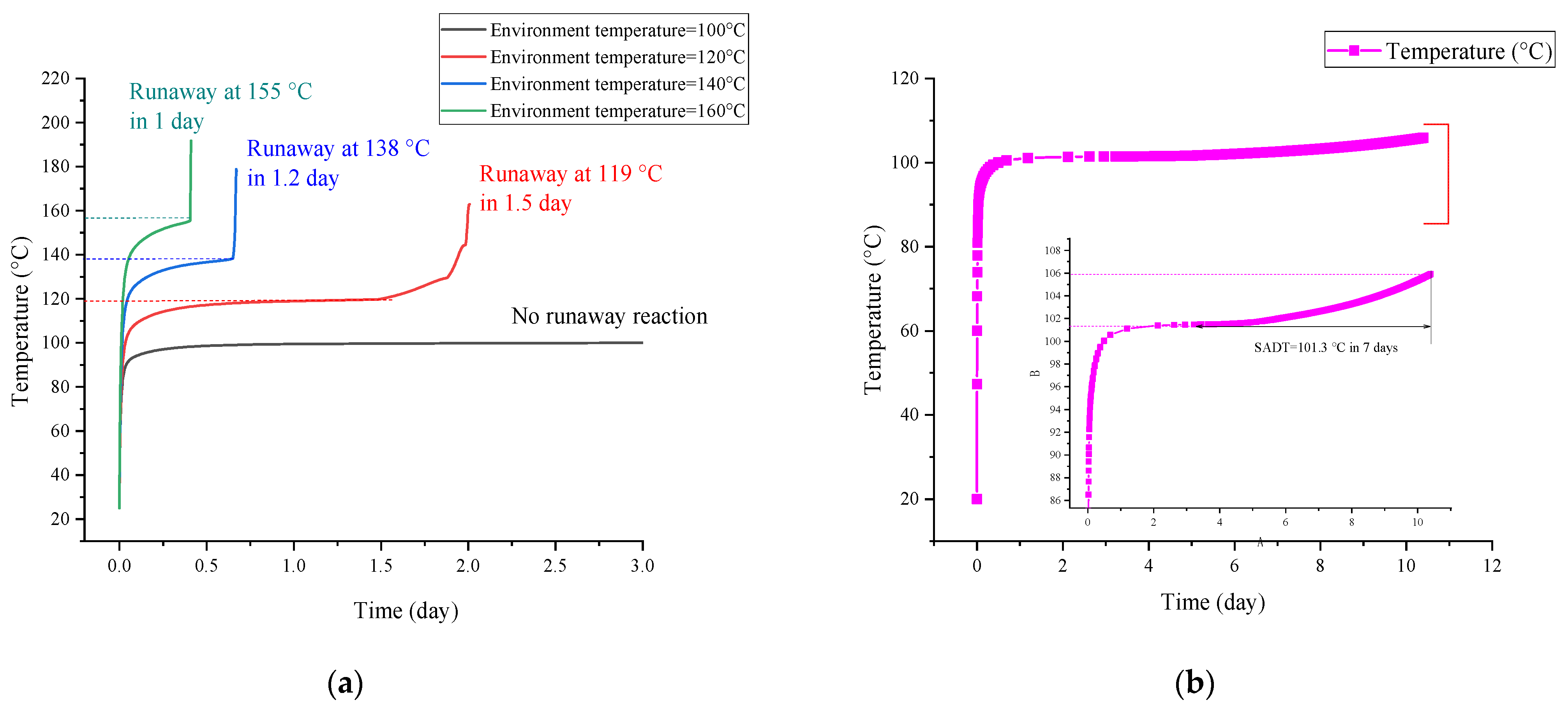
| AIBA | β(°C/min) | T0 for 1st Reaction (°C) | T0 for 2nd Reaction (°C) | T0 for 3rd Reaction (°C) |
| 0.5 | 146 | 158 | 276 | |
| 1.0 | 150 | 166 | 289 | |
| 2.0 | 152 | 180 | 306 | |
| 4.0 | 156 | 181 | 308 | |
| 8.0 | 158 | 186 | 319 |
| Substance | Reaction Form | Parameter | ln (k0) | Ea | n1 | n2 | TG |
|---|---|---|---|---|---|---|---|
| Units | ln (1/s) | kJ/mol | Dimensionless | % | |||
| AIBA | Auto | 1st stage | 47.43 ± 0.1 | 188.32 ± 0.03 | 1.70 ± 0.01 | 0.69 ± 0.01 | 17.92 ± 0.5 |
| 2nd stage | 36.42 ± 0.1 | 155.17 ± 0.03 | 7.35 ± 0.01 | 7.18 ± 0.01 | 34.81 ± 0.5 | ||
| 3rd stage | 21.18 ± 0.1 | 122.73 ± 0.03 | 0.75 ± 0.01 | 0.08 ± 0.01 | 46.35 ± 0.5 | ||
| Material | Size (cm) | Shell Thickness (mm) | Filling Height (cm) | Density (g/cm) | Specific Heat Capacity (J/(g K)) | Thermal Conductivity Coefficient (W/(m K)) | Heat Transfer Coefficient (W/(m2·K)) |
|---|---|---|---|---|---|---|---|
| AIBA | - | - | - | 0.735 | 2.0 | 0.95 | 10 |
| 25 kg Box | L × W × H 29 × 39 × 46 | 5.0 | 35 | 0.75 | 1.7 | 0.3 | 2.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, C.-F.; Wen, I.-J. Evaluation of Thermal Hazard Properties of Low Temperature Active Azo Compound under Process Conditions for Polymer Resin in Construction Industries. Processes 2021, 9, 1934. https://doi.org/10.3390/pr9111934
Tsai C-F, Wen I-J. Evaluation of Thermal Hazard Properties of Low Temperature Active Azo Compound under Process Conditions for Polymer Resin in Construction Industries. Processes. 2021; 9(11):1934. https://doi.org/10.3390/pr9111934
Chicago/Turabian StyleTsai, Chia-Feng, and I-Jyh Wen. 2021. "Evaluation of Thermal Hazard Properties of Low Temperature Active Azo Compound under Process Conditions for Polymer Resin in Construction Industries" Processes 9, no. 11: 1934. https://doi.org/10.3390/pr9111934
APA StyleTsai, C.-F., & Wen, I.-J. (2021). Evaluation of Thermal Hazard Properties of Low Temperature Active Azo Compound under Process Conditions for Polymer Resin in Construction Industries. Processes, 9(11), 1934. https://doi.org/10.3390/pr9111934






