Abstract
The use of nanofluids for heat transfer has been examined in recent years as a potential method for augmentation of heat transfer in different systems. Often, the use of nanoparticles in a working fluid does not disrupt the system in significant ways. As a result of this general improvement of a system’s heat transfer capabilities with relatively few detrimental factors, nanofluids and hybrid nanofluids have become an area of considerable research interest. One subcategory of this research area that has been under consideration is the concentration of each of the nanoparticles, leading to either successful augmentation or hindrance. The focus of the current experimental investigation was to examine the resulting impact on heat transfer performance as a result of each nanofluid implemented in an identical three-channel heat exchanger. This work examined the experimental impacts of 0.5 wt% titania (TiO2), 1 wt% titania, a mixture of 0.5 wt% titania and 0.5% silica, and a 0.5 wt% hybrid nanofluid of titania synthetically modified with copper-based nanostructures (Cu + TiO2). The experimental work examined a range of heat flux densities from 3.85 W cm−2 to 7.51 W cm−2, and varying flow rates. Each of the nanoparticles were suspended in distilled water and then mixed using an ultrasonic water bath. The performances of each nanofluid were determined using the local Nusselt number to evaluate the possible thermal enhancement offered by each nanofluid mixture. While the 0.5 wt% Cu + TiO2 hybrid nanofluid did significantly increase performance, the use of a 0.5 wt% TiO2/SiO2 double nanofluid in a three-channel heat exchanger exhibited the greatest performance enhancement, with an average increase of 37.3% as compared to water.
1. Introduction
Improving a system’s thermal performance is always a consideration in heat transfer. The study and implementation of nanofluids and their synthesis can provide insight into cost-effective methods of drastically improving a system’s thermal performance while presenting relatively few detrimental impacts. The limitations introduced (such as the increase in viscous effects as compared to the pure liquid, which then introduce an added strain on the pumping power for the system) are areas in need of further understanding and improvement. The improvement potential of hybrid nanofluids and new nanofluids grows much more prominent as concentration is increased, as with increases in concentration, an increase in the thermal enhancement often follows.
Jumpholkul et al. [1] conducted an experimental investigation into the impact of aqueous SiO2 nanofluids on pressure drop and heat transfer when used in a horizontal tube. For their nanofluid preparation, they conducted a two-stage process to suspend their particles. First, they used commercially available nanoparticles that were then mixed at their target particle concentration and suspended in deionized water, then submerged in an ultrasonic bath for two hours to ensure particle dispersion. Their test section was a tube with a total length of two meters and an internal diameter of 7.1 mm filled with a free swirl generator that acted as a twisted surface to encourage fluid mixture. Over the length of their test section, they applied a heat flux using a DC powered heater that was then insulated using fiberglass cloth. Over the length of the tube, they placed nine thermocouples evenly spaced along the length to measure the temperature of the wall. They had two additional thermocouples, one at the inlet and one at the outlet, to measure the temperature of the water before and after the application of heat flux. They concluded that the inclusion of their SiO2 nanofluid led to a 545% increase in the Nusselt number when compared to the base fluid on its own.
Attalla [2] examined the impact of a water-based SiO2 nanofluid and its resulting impact on heat transfer and friction factor when passed through different conduits at a fixed heat flux. For their nanofluid synthesis, they used commercially available nanoparticles suspended in distilled water and submerged the working fluid in an ultrasonic bath to ensure particle dispersion. The thermophysical properties of the mixture were estimated empirically. Their experimental setup consisted of a heated test section that was then insulated to limit thermal losses to the environment. They placed six thermocouples along the length of the test section with additional thermocouples located at the inlet and outlet of the test section. They also determined the pressure difference across the test’s sections with a pressure loop. They examined the thermal performance of the system by examining the local Nusselt number, they noted that the inclusion of their nanofluid at an optimal concentration of 3 vol.% led to a maximum performance increase of 18.7% when compared to water. However, they noted that, due to the inclusion, the friction factor of the working fluid was increased by 19.6% when compared to the base fluid.
Other nanofluids used in heat enhancement include TiO2 or titania. Khanlari et al. [3], in their work, examined the impact of TiO2 nanoparticles suspended in water when used in a heat exchanger with corrugated thin plates. Their experimental apparatus consisted of test section lines with thermocouples and their steel plates with a corrugation angle of 60°. They used an atmospheric burner to provide the required heat flux for the system. They also carried out a numerical investigation, in which they created their model using Ansys fluent 16 and found that the results from both methods were in good agreement. Their nanofluid did implement a surfactant to ensure particle distribution within the base fluid and to limit any possible sedimentation. They noted that the inclusion of a 2 wt% TiO2 nanofluid saw an average increase of 6% in the heat transfer coefficient as compared to the base fluid.
Additional research into the impact of TiO2 was conducted by Mukherjee et al. [4], in which they examined the impact of five different concentrations varying from 1 wt% to 0.01 wt%. They investigated a commercially available lab-grade nanofluid that was then dispersed in water at varying concentrations. They did not implement a surfactant in their work to ensure nanoparticle stability. They then conducted viscosity measurements and conductivity measurements and noted that their findings were all less than 2.04% of the anticipated results for the examined temperatures. They also examined and measured the pumping power required for each of the different nanoparticle concentrations and developed relations. They noted that TiO2 nanofluids on their own demonstrated good stability in water and found that lower concentrations exhibited better particle dispersion in the liquid than higher concentration TiO2 mixtures. They also observed that an increase in nanoparticle concentration resulted in an increase in the thermal conductivity of the nanofluid, noting that, at a certain concentration, the increase in particles present in the working fluids resulted in poor stability.
Other areas of study for the application of nanofluids include flat plate solar collectors, such as the work examined by Bazdidi-Tehrani et al. [5], in which they conducted a numerical investigation into the impact of TiO2/water in a ribbed flat-plate solar collector. They noted that the inclusion of the TiO2 nanofluid resulted in an increase in the efficiency of their system by 10% and that the use of a copper thermofluidic might lead to further enhancement.
Ahmed et al. [6] also examined the impact of TiO2 nanofluids. In their work, they examined the impact of nanofluids (suspended in water and suspended with a surfactant) in a car radiator. They noted that, with the inclusion of the nanofluid in a car radiator, the resulting fluid was able to outperform the base fluid in all cases, with an optimal concentration of nanofluid of 0.2% volume and a possible enhancement increase to 47%.
Another nanofluid that is currently being examined for possible improvements to heat transfer is alumina, or Al2O3. An example can be seen in the work of Ho et al. [7,8,9] and Hader and Al-Kouz [10], in which they examined a large range of nanofluid concentrations from 0–10% and a range of applied Reynolds numbers. They used commercially available nanoparticles which they then mixed in water at their desired concentrations for the given experiment. They examined the flow through a circular tube and noted that the use of their nanofluid saw a maximum increase in overall efficiency of 2.84% with a concentration of 10%, noting that the increase in the concentration resulted in an increase in the heat transfer effectiveness ratio.
A next step in examining the possible performance of each nanofluid was to subject them to the same conditions and compare the results. An example of this can be seen in the work of Meisam et al. [11], who compared the results of Al2O3, TiO2, and SiO2 nanofluids suspended in water at three concentrations, 0.05, 0.1, and 0.2 wt%. Each of the nanofluids examined were created by suspending commercially available nanofluids and then ensuring the dispersion of particles by submerging the nanofluids in an ultrasonic vibrator. They observed that each of the nanofluids examined saw an increase in the heat transfer coefficient as the concentration of the nanofluid was increased. They noted a maximum 182% increase in the heat transfer enhancement for SiO2. They also noted that the maximum observed increase in the heat transfer coefficient of each of the nanofluids was observed for 0.1 wt% spare TiO2, whereas the maximum was observed at 0.1 wt%, suggesting a more ideal concentration. Another example of this can be seen in the work of Bakhti and Si-Ameur [12]. In their work, they compared the performance of three separate nanofluids (TiO2, Al2O3, and Cu), with each of them suspended in water with varying concentrations from 2–10% volume, all of which were compared to the performance of the base fluid. They implemented an experimental model using the SIMPLE algorithm, which demonstrated that the friction factor of the TiO2 nanofluid was nearly identical to that of water on its own, with the alumina nanofluid showing only a slight increase in their model, and the copper nanofluid resulting in the largest increase in experienced friction factor. They noted that, as the concentration of each nanofluid increased, the resulting Nusselt number decreased—decreasing the possible enhancement. They found that the TiO2 nanofluid had the lowest overall Nusselt number, with copper producing the largest.
Alkasmoul et al. [13] also conducted a comparison between multiple nanofluids. In their work, they compared Al2O3, TiO2, and CuO nanofluids with varying concentrations. They examined forced convection in the turbulent regime and noted that pressure drops in the nanofluids (as the flow rate increased) rose significantly alongside the increase in concentration. They concluded that the nanofluids examined under their conditions were not ideal and did not result in any increase in the possible enhancement of the heat transfer performance. Bioucas et al. [14] also conducted a comparison of TiO2, SiO2, and polystyrene nanoparticles with varied concentrations of up to 0.31 vol.%. They used a steady state parallel-plate heater to ensure that the working temperature was within 298 and 323 K. They compared their numerical model results to their experimental results and found that they were in good agreement. They noted that both their TiO2 and SiO2 nanofluids were able to produce enhancement, as compared to water.
Saghir and Rahman [15] examined the impact of using two distinct nanoparticles suspended in water in an experimental study. In their work, they found that, of their examined nanofluids (consisting of Al2O3-Cu, TiO2-SiO2, FWCNT-Fe3O4, and ND-Fe3O4), the best performance was obtained using ND-Fe3O4 nanoparticles when suspended in a water-ethylene glycol mix. Arsana et al. [16] examined a similar nanofluid. In their work, they examined a SiO2-TiO2 core-shell nanoparticles at low concentrations in a water/ethylene glycol mixture. Their concentrations varied from 0 to 0.025% mass. They created their own nanoparticles experimentally, unlike most of the above, which used commercially made powders. They then carried out their own analyses on the nanoparticles to determine the size and characteristics of the particles. They noted that, with their overall lower concentration of 0.025%, a maximum increase in the heat transfer effectiveness of 43.75% was achieved without a significant increase in the viscosity of the working fluid.
Hybridization was always the next step to further improve the possible performance offered by different nanofluids. This resulted in the creation of more complicated nanoparticles to offer further enhancement in the thermal performance offered by the resulting nanofluid. An example of this can be seen in the work of Gulzar et al. [17]. In their work, they examined TiO2, Al2O3, and a hybrid Al2O3-TiO2 nanofluid using Therminol-55 as the base fluid in concentrating solar collectors. They noted that the inclusion of nanofluid in their system resulted in a maximum increase in the thermal conductivity of 8.3% for the hybrid Al2O3-TiO2. They also observed that the inclusion of nanofluid in their collectors presented the possibility of a reduction in the size requirements of the system along with an increase in performance.
Further work with hybrid nanofluids was conducted by Rahman et al. [18], who examined an Al2O3-Cu nanoparticles in water when examined in a rectotrapezoidal enclosure. They conducted a numerical study using COMSOL multiphysics to examine their system. They noted that a lower Rayleigh number resulted in a higher thermal efficiency index for the hybrid nanofluid. They also observed that the use of their hybrid nanofluid resulted in accelerated nanoparticle loading, as a result of which an increase in the rate of heat transfer occurred. This increase led to an increase in the friction factor of the working fluid, which then resulted in higher required pumping power for the system.
Hamid et al. [19] examined hybrid nanofluids with an investigation into TiO2-SiO2 used in conjunction with wire coil inserts. They examined a range of Reynolds numbers from 2300 to 12,000 in their experiment to help identify their possible impact on thermal performance. Their nanofluid was created using commercially available nanoparticles which they then placed in suspension in a water/ethylene glycol mixture with five different concentrations from 0.5 vol.% to 3 vol.%. They noted that their TiO2-SiO2 nanofluid at 2.5 vol.% resulted in a 254.4% increase in the maximum heat transfer enhancement as compared to the base fluid. They noted that the friction factor of the nanofluid increased from 1.76 to 6.38 when the wire coil was present in the test section. Ramadhan et al. [20] also examined a hybrid TiO2-SiO2 nanoparticles in a water/EG mixture. They noted that they experienced the largest values of Nusselt number for their 3 vol.% concentration but observed an increase in the friction factor as a result of its inclusion.
Martin et al. [21] examined a different hybrid nanofluid for their work in a plain heat pipe. They examined a Fe-CuO nanofluid at a 2% mass concentration with 50% contribution of each component. They implemented a surfactant in the aqueous solution to help ensure the nanoparticle suspension in water at a concentration of 0.2% mass. They compared their results to water using the same experimental conditions. They observed a 72.63% increase in the thermal efficiency of the heat pipe compared to distilled water.
2. Materials and Methods
The experimental work for this study was carried out using the apparatus depicted in Figure 1. The apparatus used the following parts for this experiment: an array of 9 T-type thermocouples (one for measuring the inlet temperature, one for the outlet temperature of the fluid, and the remaining 7 used for the experiment), a digital flow meter, two pumps working in parallel, an AC voltage regulator, three resistance-based barrel heaters, a copper helical heat exchanger, and a calibrated water bath. The test area used was designed to have the same heated surface area as an intel i7 computer processor. The applied heat flux was measured using the applied power over the area of the test section used. To carry this out, the voltage regulator was fed into an ammeter and a voltmeter to find the power applied to the test section. The heated plate for this experimental investigation was square, with a side length of 3.75 cm and a height of 1.27 cm. The plate was heated from the bottom using the resistance-based barrel heaters, similar to the work of Welsford et al. [22]. To limit thermal contact resistance between the heated plate and the three-channel block, a thermal paste was included. The flow meter and thermocouples were connected to the data acquisition system (National instruments) to allow for temperature reading along the length of the heated channels, the inlet and outlet regions, and the fluid flow rate before entering the test section.
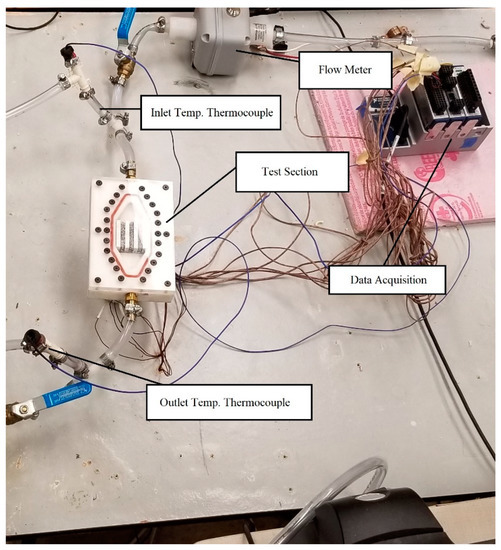
Figure 1.
Experimental apparatus.
2.1. Nanofluid Synthesis
Nanostructured titania (P25, ThermoFisher Scientific, Waltham, MA, USA, No. AC384290010, 30–50 nm) and silica (Sigma-Aldrich, Darmstadt, Germany, No. 637238, 10–20 nm) were obtained from commercial suppliers and suspended in distilled water by ultrasonication. Since the nanoparticles implemented were obtained from a manufacturer, the nanoparticles were amorphous and were of a non-uniform spheroid morphology. For the hybrid Cu + TiO2 nanofluid, commercial titania (P25) was synthetically modified by photochemical reduction of copper (II) sulfate pentahydrate in the presence of titania and a suitable UVA photo-initiator, similar to the procedure used to prepare a copper-alumina nanocomposite in our previous work [23]. In brief, 1 equivalent of copper salt (11.65 millimolar, mM), 2 equivalents of 2-hydroxy-4′-(2-hydroxyethoxy)-2-methylpropiophenone (Irgacure-2959™, 23.31 mM), and 3.5 wt% titania were dissolved in Milli-Q water (18.2 MΩ cm at 25 °C) and nitrogen gas was bubbled through the solution for 2 h while stirring. The solution was then irradiated under inert atmosphere for 72 h in a UVA photoreactor (365 nm, 100 W/m2) while stirring. Radical-mediated photoreduction of the copper salt led to in situ formation of copper nanostructures supported on nanoscale titania, as evidenced by a color change from white to pink. The absorption spectrum of a diluted sample of the Cu + TiO2 further demonstrated the presence of copper-based nanoparticles, which extended the absorption of the nanocomposite from the UV (titania alone) into the visible region (see Figure 2). The nanocomposite was subsequently diluted to 0.5 wt% using distilled water and sonicated prior to thermal fluid experimentation. The nanostructures were subjected to SEM and STEM and were not observable, which is common for nanoparticles synthesized through photochemical particle synthesis [24].
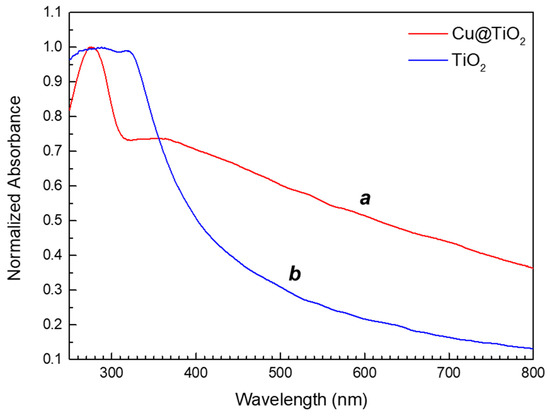
Figure 2.
Absorption spectra of (a) Cu + TiO2 hybrid and (b) titania in distilled water.
2.2. Experimental Procedure
For this work, a range of flow rates were examined, ranging from 0.2 USGPM to 0.3 USGPM (with 0.2 USGPM being equivalent to 0.16043 m/s). The applied heat flux density was an additional variable ranging from 3.86 W/cm−2 to 7.51 W/cm−2, respectively. The experiment was carried out in the following manner: the three-channel block was inserted into the apparatus with a thin layer of thermal paste applied to the heated block. At that point, the lid of the apparatus was secured, and the working fluid was circulated without any applied heat flux. The working fluid was allowed to circulate freely to remove all air from the pipes and the test section. When the working fluid reached a steady state temperature—as determined through the data acquisition system (DAQ) using a thermocouple output in the heated block located in the middle of the block 0.1 cm under the surface against the total time elapsed—the heat flux was applied by turning the voltage regulator to the desired first heat flux. This heat flux was maintained until a steady state was observed, again through the use of the DAQ output of temperature vs total time elapsed for that test. After steady state was observed for the temperature along the channel, the data was recorded for that run. Then, the run was concluded, the next desired heat flux density was targeted, and the heat flux was removed so that the system could reach steady state for cooling. Once the entire range of testing was concluded for each nanofluid, the system was flushed with distilled water to remove any contamination from the nanofluid when changing fluid types and concentrations. To measure temperature along the length of the channel, a series of 7 thermocouples were spaced evenly along the length 0.1 cm below the surface at 0.46875 cm, 0.9375 cm, 1.40631 cm, 1.875 cm, 2.3438 cm, 2.8125 cm, and 3.2813 cm, respectively, as shown as a blue dot in Figure 3a. Figure 3b presents the insert used in our experiment. The insert is a square cavity with 3.87 cm length. The height of the insert is 1.27 cm, and the channel width is 0.535 cm.
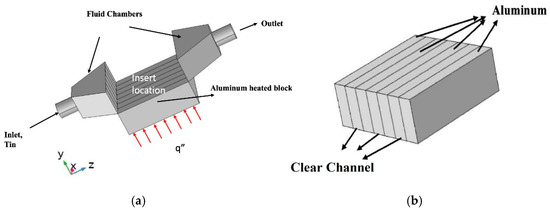
Figure 3.
Schematic of the test section (a) Test Section; (b) Channel insert.
2.3. Measurement Error Analysis
To successfully conduct experimental work, an understanding of the sources of error is critical to identify possible ways to reduce the experienced error of an experiment. In this work, the uncertainty was created from the propagation of errors arising from the local Nusselt number. This error was evaluated using the Taylor uncertainty approach, as follows:
The terms of the which are, x, y, and z, which represent the directional coordinate system used for each of the terms, and Nu, which is the local Nusselt number determined from the thermocouples. For this work the dimensions used are in the terms of x, y, and z, which are representative of measured quantities, such as flow rate, and temperature. The uncertainty of such terms would be represented using , , , and using such, we were able to determine the uncertainty of the local Nusselt number. The uncertainty of the individual parts used to measure and calculate the values in this work were identified as the T-Type thermocouples, with a known uncertainty of 0.75% as indicated by the manufacturer, calibrated uncertainty of the T-Type thermocouples of 3.87%, and the flow meter of 0.44%, as determined through the use of the calibration software in the data acquisition system included by the manufacture. The combined uncertainty was obtained by determining the systematic error sources as well as the sources of random error. This resulted in a combined uncertainty of 3.96%. For further details as to the determination of the uncertainty values, see our previous work [24].
2.4. Thermal Losses
The thermal losses of the system were measured by comparing the measured heat flux values to that of the applied heat flux. The applied heat flux was achieved by using Equation (2), in which the applied heat flux was obtained by multiplying the applied current with the voltage over the surface area of the heated surface.
This was then compared to the measured heat flux obtained using Equation (3). The average thermal losses for the system were determined to be 1.46 W/cm2.
Here H is the test section height (12.7 mm) and L is the test section length (where the fluid will travel), equal to 3.78 cm.
3. Results and Discussion
The aim of this experimental study was to examine the performance of multiple nanofluids and hybrid nanofluids and compare their effectiveness to one another using deionized water as a benchmark. The working fluids were all subjected to the same applied heat flux densities, experienced flow rates, and test area. For this work, a three-channel configuration was used with a channel geometry of 0.93 cm height, 0.535 cm in width, and a length of 3.75 cm, and the thickness of the plate where it contacts the heated surface was 0.027 cm thick.
3.1. Surface Temperature Distribution
For this experimental work, the applied heat flux densities remained constant between the different nanofluids as a result of using the AC voltage regulator. The applied heat flux densities were 3.85 W cm−2, 5.17 W cm−2, and 7.51 W cm−2, with three different flow rates implemented (0.1USGPM, 0.2USPGM, and 0.3USPGM). The following temperature distribution plots were a result of the 7 thermocouples located 0.1 cm below the heated surface along the centerline of the block. The y-axis of the plot used the notation of T-Tin, where T is the temperature of that given thermocouple, and Tin represents the temperature measured at the inlet of the test section. The measured inlet temperature was observed to vary between 14.06 °C and 18.65 ℃ for different cases; however, this has been observed previously and was found to be in good agreement with the anticipated results. With the temperature plots, a weak parabolic trend was observable when the nanofluids were examined on their own. However, this parabolic nature grew much more pronounced when the nanofluids were all on a single graph. This parabolic shape, seen in every nanofluid temperature difference, demonstrated that there was an increase in the heat transfer in the inlet and outlet regions of the test section.
Possible sources of losses in this experimental investigation include thermal, in that the housing of the test was made of Delrin, which provided insulation for the heated portion. However, there were still losses even with the insulation provided. Another area of loss was the port for the thermocouples, which were insulated in rockwool. The hole, while small and insulated, could still be a source of thermal losses. Another area of possible deviation may be contamination of the nanofluids. Due to the nature of the study (a comparison between multiple nanofluids), the same apparatus was used for all nanofluids to ensure that the testing environments were as close to identical as possible. After each nanofluid test, the apparatus was cleaned for the next test; however, there will always be a chance of contamination due to fluids or sedimentation left behind in the testing environment. Another area of consideration is sedimentation of the nanoparticles. With nanofluids, there is always a balance between the particles in the fluid. When this balance is disturbed, the particles will agglomerate and fall out of suspension in the working fluid. While this was not observed when the nanofluids were in motion, there was sedimentation observed in the fluids when they were placed into storage after use. With all these areas of possible concern considered, the data was still deemed acceptable and able to offer a better understanding into the behaviour of nanofluids.
3.2. Three-Channel Temperature Distribution
The following figures depict the temperature distributions obtained from the difference in the inlet temperature reading and each thermocouple reading along the test sections length. Each of the figures were obtained from the three-channel experimental data. Initially, each data set was of a parabolic nature with a decrease in the inlet and outlet region—a result of the boundary layer effect which led to an increase in experienced heat transfer in the regions identified.
The boundary layer effect is a result of the interaction of the fluid flow when it enters a channel. Upon entering the channel, a thin layer is formed on the channel walls, and there is an increase in overall fluid flow. Due to the increase in fluid flow, the temperature difference in the thin layer is increased. As a result of this increase in fluid flow and temperature difference, there is a subsequent increase in the experienced heat transfer in this region. Then, as the flow becomes more developed, there is an observable reduction in impact until the flow reaches the fully developed region. The boundary layer thickness is inversely proportional to the local Nusselt number, as noted by Bayomy et al. [25]. The following table and its fluid physical properties were obtained from literature and can be found in citation [26,27,28].
In Figure 4, the temperature difference for the 3.85 W cm−2 case with an applied flow rate of 0.2 USGPM is presented. In this case, it was observable that the temperature differences for the 0.5 wt% and 1 wt% TiO2 nanofluids were slightly greater than that of the base fluid and resulted in an average increase of 0.15% for the 0.5 wt% TiO2 and a decrease of −2.23% for the 1 wt% TiO2. This demonstrated that the nanofluids were in nonideal conditions, as the inclusion of nanofluid often leads to a notable increase in the overall performance (whereas in this instance there was a decrease) as compared to water. Regardless of the poor performance of the TiO2 nanofluids, the Cu + TiO2 and TiO2/SiO2 nanofluids were both able to offer an average improvement over the base fluid of 12.8% and 16.6%, respectively.
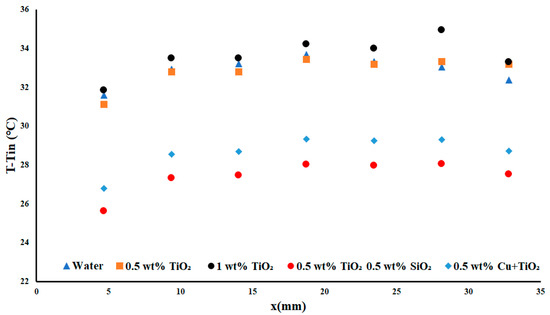
Figure 4.
Temperature difference with a heat flux density of 3.85 W cm−2 and flow rate of 0.2 USGPM.
The Reynolds numbers for each of the nanofluids examined are summarized in the table below and were determined using Equation (4) with the values in Table 1.
where is the density of the fluid, u is the velocity of the fluid, De is the characteristic diameter of the channel equal to 1.87 cm, and is the dynamic viscosity of the fluid.

Table 1.
Physical Properties.
It is of note that the nanofluids examined were subjected to a range of Reynolds flow regimes, both transitional and turbulent as seen in Table 2. This Reynolds number was measured at the cross section of all channels. Thus, it decreased as it entered the channels, due to the fluid flow being uniformly distributed between the three channels.

Table 2.
Reynolds number for each fluid.
The improvement for each of the nanoparticles examined was evaluated using a percent change calculation, which can be seen in the following, where Tnf is the temperature of the nanofluid at that stage and Tw is the temperature of the water under the same conditions.
As compared to Figure 4, in Figure 5, it is observable that the temperature difference for water was greater than all the examined nanofluids, showing that the inclusion of the TiO2, when subjected to an increased heat flux, resulted in an increase in the thermal performance of the fluid. The inclusion of the TiO2 nanofluid led to decreases in the temperature distribution of 6.5% and 6.92%, on average, for the 0.5 wt% and 1 wt% TiO2, respectively. This is the first case of flow rate examination wherein the inclusion of the TiO2 nanofluids resulted in a visible improvement to the thermal performance of the system. Overall, this suggested that, when subjected to an applied heat flux density of 3.85 W cm−2, the nanofluid was unable to achieve an impactful amount of heat transfer. The difference in performance between the copper hybrid nanofluid and the two concentrations of TiO2 represented an average difference of 16%. This difference between the two concentrations was significant, showing that the inclusion of the hybrid Cu + TiO2 had significantly greater impact than the TiO2 on its own. Lastly, the inclusion of the TiO2/SiO2 nanoparticles led to an increase of 2.49% over the copper hybrid, showing that there may be a nanofluid with even more potential than the hybrid nanofluid.
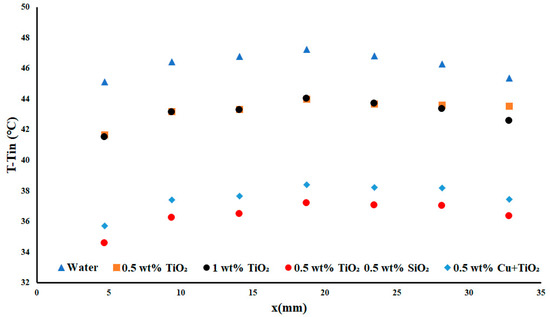
Figure 5.
Temperature difference with a heat flux density of 5.1 W cm−2 and flow rate of 0.2 USGPM.
As previously shown in Figure 6, we noted a further increase in the performance difference between each of the nanofluids. The change in the temperature difference between water and both TiO2 nanofluids was around 7 °C, whereas, in Figure 3, the difference was 3 °C on average. This difference represented an increase in the average performance (as compared to water) of 8.9% and 9.25%, respectively, for the 0.5 wt% and 1 wt% TiO2. Again, the performance of the hybrid nanofluid (as compared to water) was very significant; at an 18.72% decrease in the temperature difference at 0.5 wt%, TiO2/SiO2 was able to offer even greater possible enhancement at an average increase of 24.47%.
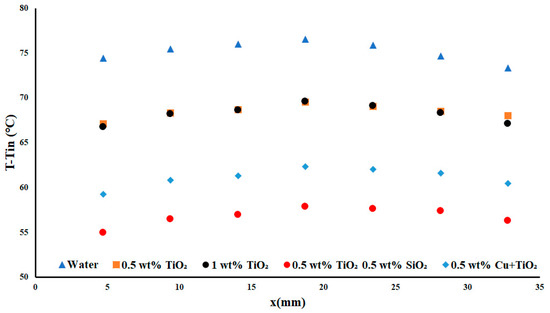
Figure 6.
Temperature difference with a heat flux density of 7.5 W cm−2 and flow rate of 0.2 USGPM.
The temperature differences shown in Figure 7 demonstrated the temperature along the test section for each of the examined nanofluids when subjected to the lowest applied heat flux density of 3.85 watts per square centimetre at the highest fluid flowrate of 0.3 USGPM. We noted that the largest temperature difference between water and 1 wt% TiO2 was around 3 °C, a difference of 6.92% between the two fluids. The next improvement was of 0.5 wt% TiO2 over water, at 11.35%, or a 4.43% improvement over the 1 wt% TiO2. This demonstrated that the ideal concentration of TiO2 was under 1 wt% for this system. Interestingly, the copper nanofluid was outperformed by the double-monodispersed nanofluid (TiO2-SiO2) by a margin of 5.7% for the high-flow, low-applied heat flux density case. When examining all the working fluids in this case, there was an observable decrease in the temperature difference of nearly 10 °C, or 23.43%. This change in temperature difference was deemed significant for a small inclusion of nanoparticles.
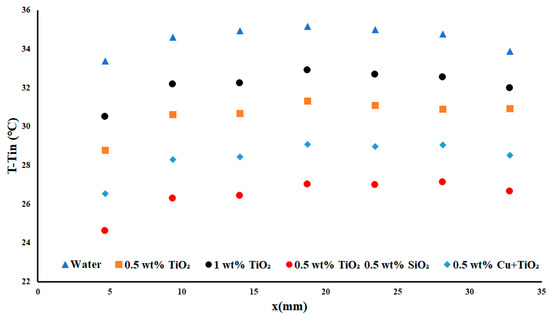
Figure 7.
Temperature difference with a heat flux density of 3.85 W cm−2 and flow rate of 0.3 USGPM.
An observation of note is that the performance difference between the 0.5 wt% TiO2 nanoparticles and the 1 wt% TiO2 resulted in a significant decrease in the performance as the particle concentration was increased. This was a result of the increase in the particle concentration which, in turn, resulted in an increase in the viscous effects in the working fluid, yielding an overall decrease in the performance. This observation represents a key point of nanofluid experimentation, as an increase in nanoparticle concentration does not always result in an increase in the system performance.
As the applied heat flux density was increased to 5.1 W cm−2, it was noted that the parabolic shape was maintained along with the temperature difference. Figure 8 shows that the change between the water temperature difference and that of the first nanofluid increased from the first figure of approximately 3 °C to nearly 5 °C. This increase in the disparity between the water and the nanofluids showed the impact of the nanoparticle inclusion on the experienced heat transfer under the same conditions. Interestingly, the change in the temperature difference between the two concentrations of TiO2 has essentially been removed—that is to say that they were both performing at nearly the same efficiency, with only a 0.23% difference in their average improvement over water. Copper performed very well but was outperformed by the TiO2-SiO2 nanofluid. These results suggested that doubly-monodispersed nanoparticles were better suited for this experiment.
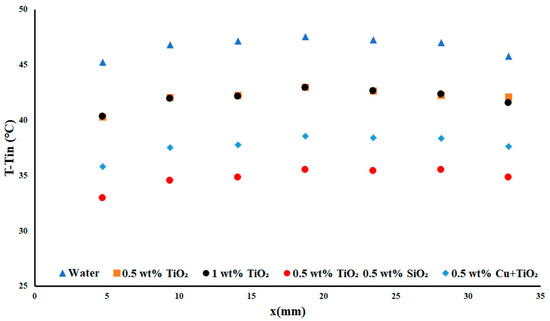
Figure 8.
Temperature difference with a heat flux density of 5.1 W cm−2 and flow rate of 0.3 USGPM.
In the above Figure 9, the highest applied heat flux density case of 7.51 W cm−2 was presented. The inclusion of TiO2 at a concentration of 1 wt% led to a decrease in the temperature difference of nearly 10 °C, and a decrease of 25 °C with the highest performing nanofluid. This difference represented a 28.84% decrease in the temperature difference for TiO2-SiO2 compared to water. This change in the temperature difference represented a significant improvement, with all nanofluids showing an average improvement ranging from 6.92–28.84% for the highest flow and applied heat flux density cases.
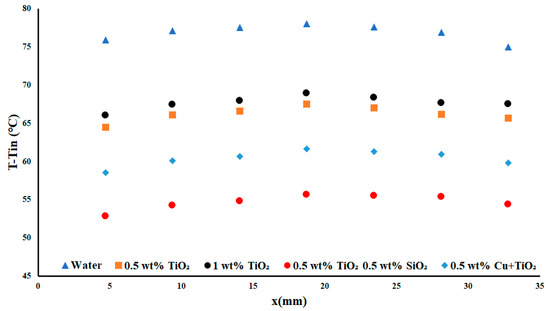
Figure 9.
Temperature difference with a heat flux density of 7.5 W cm−2 and flow rate of 0.3 USGPM.
3.3. Local Nusselt Number
A common method for determining the experienced heat enhancement is through the use of the local Nusselt number. To determine the degree of enhancement, the variation of the local Nusselt number along the length of the test section and how the Nusselt number changes with position with a known heat flux density must be measured. For this work, the local Nusselt number was determined through the hydraulic diameter, which was determined to be De = 0.01879 m, as a product with the convective heat transfer coefficient (W/m °C) over the conductivity of water kw (W/m/K). The equations below display the formulation for the Nusselt number and that of the heat convection coefficient.
The following figures show the local Nusselt number over the length of the test section for each of the examined nanofluids. When the working fluid enters the channels, there is an increase in the experienced heat transfer in that region as a result of the boundary layer effect. Figure 10, Figure 11, Figure 12 and Figure 13 depict the 0.2 and 0.3 USGPM flow rate cases for the three-channel heat exchanger with a range of applied heat flux densities.
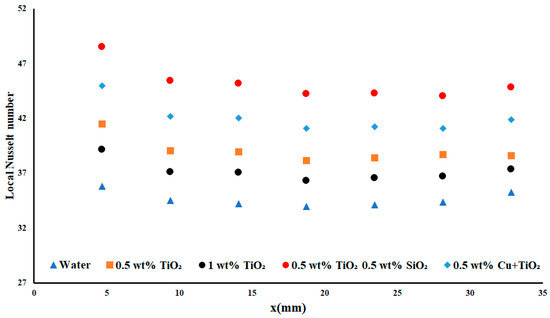
Figure 10.
Local Nusselt number with a heat flux density of 3.85 W cm−2 and flow rate of 0.3 USGPM.
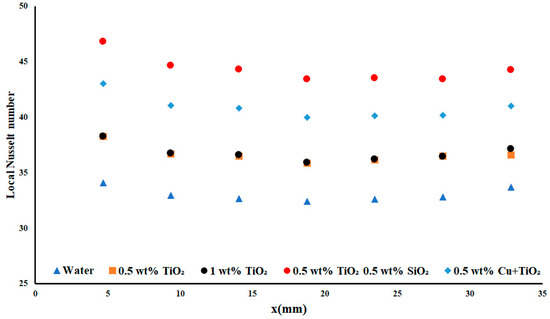
Figure 11.
Local Nusselt number with a heat flux density of 5.1 W cm−2 and flow rate of 0.3 USGPM.
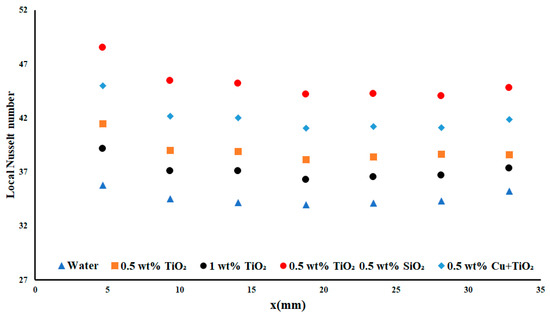
Figure 12.
Local Nusselt number with a heat flux density of 7.5 W cm−2 and flow rate of 0.3 USGPM.
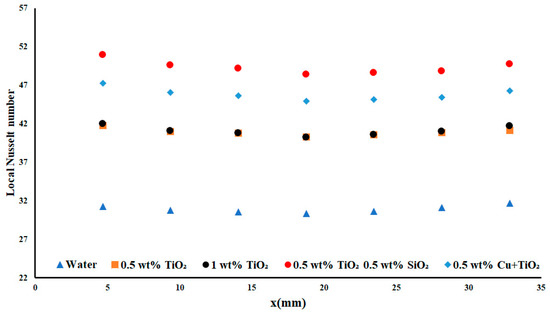
Figure 13.
Local Nusselt number with a heat flux density of 7.5 W cm−2 and flow rate of 0.2 USGPM.
In Figure 10, the results of the 3.85 W cm−2 case for the local Nusselt numbers for each of the nanofluids are presented. In this, it was of note that the water case saw an average local Nusselt number of 34.6 over the length of the test. When the water entered the channel, the local Nusselt number increased to 35.79 a gain of 3.4% over the average. This slight increase in the performance in the entry region was a result of the boundary layer effect. The first nanofluid encountered after the water results was the 1 wt% TiO2. This result—the 0.5 wt% TiO2 performing better than the 1 wt% TiO2—was previously seen in analyses of temperature difference, pointing once more to the optimal concentration of TiO2 being less than 1 wt%. The average local Nusselt number for the 1 wt% TiO2 was 37.18, an increase of 6.6% compared to that of water. The difference between the 0.5 wt% TiO2 nanoparticles and the 1 wt% was a further increase of 4.41% for the average local Nusselt number. This difference between the two concentrations was significant compared to the anticipated increase in performance as nanoparticle concentration was increased. The Cu + TiO2 nanoparticles were able to produce an average local Nusselt number of 42.07, an average increase of 17.35%, Interestingly, the optimal results were obtained from the double-monodispersed TiO2/SiO2 nanofluid, which saw an average local Nusselt number of 45.2, or an increase of 23.03% over water.
Note that, in Figure 11, as the applied heat flux density increased to 7.51 W cm−2, the difference between the two TiO2 nanofluids decreased, as was also observed in the temperature difference section. The difference between the local Nusselt numbers of water and TiO2 decreased as the applied heat flux density increased. This case held true for each of the applied nanofluids. As the applied heat flux density increased, the local Nusselt number decreased; however, when examining the increase of flow rate while maintaining the applied heat flux density, a resulting increase in the local Nusselt number was observed. This reaction demonstrated the dependence of the local Nusselt number on the applied flow rate—and the dependence of the Reynolds number on the non-Darcy regime. In this case, the best average local Nusselt number was achieved with the TiO2/SiO2 nanofluid, with an average increase of 25.22% compared to water.
Figure 12 displays the highest applied heat flux density and flow rate case. Similarly, to what was observed before, the performances of both concentrations of TiO2 yielded similar results, with the 0.5 wt% TiO2 nanofluid performing 0.27% better, on average. The difference between water and the TiO2/SiO2 nanoparticles saw an increase of 28.63%. This increase was rather significant and demonstrated the impact of the TiO2/SiO2 double-monodispersed nanoparticles. This performance suggested that the presence of each nanofluid led to augmentation of the performance of the overall fluid to a greater degree than the nanoparticles on their own (as seen when examining the performance of TiO2 on its own).
There was a slight increase in the heat absorption in the exit region of the test section, as evidenced by the increase in the local Nusselt number in this region. This indicated that there was also an increase in the heat enhancement experienced in this region, as the possible heat enhancement was proportional to the heat flux density. The parabolic shape was clearest for the TiO2/SiO2 nanofluid, in which a strong curve was visible in the entry region, demonstrating the largest impact of the boundary layer effect. A smaller (but still clear) curve was observed on the outlet regime, again pointing to a boundary layer effect, but in a reduced capacity.
Another possible reason for the observed increase in the heat absorption in the exit region may be the sedimentation of the nanoparticles when they move toward the exit region of the channels. This sedimentation would result in additional time for the absorption of heat by the nanoparticles before they re-entered the working fluid, thus increasing the heat absorption in that region.
As shown in Figure 13, the 0.2 USGPM flow rate for the 7.5 W cm−2 resulted in a maximum improvement of 38.68% over distilled water for the local Nusselt number, and an average improvement of 37.31% over water. This was an increase of 4.77% over the hybrid copper and TiO2 nanofluid. This sharp increase in the local Nusselt number performance over distilled water for the same experimental apparatus and testing conditions was deemed significant as it demonstrated the importance of nanoparticle concentration and selection for each apparatus.
The greatest impact of the nanoparticles’ inclusion, when compared to the performance of water, was observed when comparing the lowest average increase in the nanofluid performance. It still yielded an increase of 24.42% in the local Nusselt number. This increase showcased the improvements in cooling made possible by nanofluids, as often, even a suboptimal nanofluid selection can still result in a net improvement to the possible heat transfer of the system. Pressure drop was not examined along the length of the test section. However, when increasing a nanoparticle concentration in the working fluid, the experienced viscous effects will increase, and subsequently have a detrimental impact on the system performance.
A comparison of the performance of all of the examined nanoparticles and their concentrations was determined to be useful. In Figure 13, note that the flow rate of 0.2 USGPM yielded the optimal results for each of the nanofluids, with the largest value obtained when examining the largest highest applied heat flux. This was an interesting result, as the largest applied flow rate would suggest the ability to provide greater possible heat removal due to the greater mass flow of the working fluid. However, the largest value was obtained at 0.2 USGPM flow rate, with an improvement of approximately 19 (a 23% increase) in the average local Nusselt number for the TiO2/SiO2 nanofluid over the 0.3USGPM flow. This demonstrated that flow rate had a significant effect on the potential performance of the system. Overall, similar improvements were observed between the 0.3 USGPM flow rate and the 0.2 USGPM flow rate, with one notable exception: the performance of the 0.5 wt% and 1 wt% TiO2 nanofluid for the 0.2USGPM flow rate yielded results near and below those found for water. This indicated that the nanofluid was in non-ideal operating conditions when under the lowest applied heat flux density situation. As the applied heat flux density was increased, the performance of both concentrations of nanofluids increased above that of water by at least 6.9%, on average.
4. Conclusions
The above discussion outlined potential improvements offered by aqueous 0.5 wt% TiO2, 1 wt% TiO2, 0.5% SiO2, 0.5 wt% TiO2/SiO2, and 0.5 wt% Cu+TiO2 when subjected to the same applied heat flux densities and flow rates in the same apparatus. There was an observable increase between the different nanofluids examined as well as a difference in concentration for the two examined TiO2 nanofluids and the hybrid Cu+TiO2 nanofluid. The nearly identical performance of the higher concentration TiO2 nanofluid demonstrated the importance of concentrations. Increases in concentration could result in increased viscosity without any significant increases in performance to offset them. In this work, the use of a 0.5 wt% TiO2/SiO2 doubly-dispersed nanofluid saw an average increase of 37.3% in the local Nusselt number when compared to the base fluid. This work was conducted as an experimental investigation and resulted in the following conclusions:
- The 0.5 wt% TiO2/SiO2 nanofluid resulted in an average increase in the local Nusselt number for a three-channel heat exchanger. This increase was equal to 37.3%, as compared to distilled water subjected to the same conditions.
- Increased concentration of the TiO2 nanofluids yielded near-identical performances, with a slight improvement observed for 0.5 wt% TiO2. This demonstrated the importance of nanoparticle concentration for the given application; while the concentration may have yielded a similar performance to the 0.5 wt% nanofluid, increases in concentration could lead to an increase in the detrimental viscous effects experienced.
Author Contributions
Conceptualization, M.Z.S.; methodology, all authors were involved in the methodology; validation, M.Z.S. and R.P.; investigation R.P.; writing initial draft, R.P.; writing-review editing all authors were involved. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Qatar Foundation, grant number NPRP12S-0123-190011.
Acknowledgments
This research was funded by the Natural Sciences and Engineering Research Council of Canada, the Ryerson University Faculty of Engineering and Architecture (NSERC Discovery Grant program), the Ryerson University Faculty of Science Dean’s Research Fund and [Qatar Foundation] grant number [NPRP12S-0123-190011].
Conflicts of Interest
The authors declare that they have no conflict of interest.
Nomenclature
| Hydraulic diameter (m) | |
| Tnf | Nanofluid temperature |
| Tout | Outlet temperature in °C |
| Conductivity of water (W/m K) | |
| density (kg/m3) | |
| viscosity of the fluid (kg/m s) | |
| Length of the channel (cm) | |
| Nu | Local Nusselt number |
| Area of test section (cm2) | |
| Heat flux (W/cm2) | |
| Sample standard deviation | |
| SE | Standard error |
| Measured heat flux | |
| Temperature of fluid (°C) | |
| TW | Water temperature |
| Tin | Inlet temperature in °C |
| Voltage (V) | |
| Current (A) | |
| H | Height of the channel (cm) |
| h | Heat convection coefficient in W/m2/K |
| Nux | Local Nusselt number in x direction |
| Reynolds number | |
| u | Velocity in m/s |
| n | sample |
| Applied heat flux | |
| USGPM | US gallon per minute |
References
- Chaiwat, J.; Godson, A.; Selim, D.; Omid, M.; Seon, A.; Dong-Wook, J.; Somchai, W. Experimental investigation of the heat transfer and pressure drop characteristics of SiO2/water nanofluids flowing through a circular tube equipped with free rotat-ing swirl generators. Heat Mass Transf. 2019, 150, 119302. [Google Scholar]
- Attalla, M. Experimental investigation of heat transfer and pressure drop of SiO2/water nanofluid through conduits with altered cross-sectional shapes. Heat Mass Transf. 2019, 55, 3427–3442. [Google Scholar] [CrossRef]
- Khanlari, A.; Sözen, A.; Variyenli, H.İ. Simulation and experimental analysis of heat transfer characteristics in the plate type heat exchangers using TiO2/water nanofluid. Int. J. Numer. Method Heat Fluid Flow 2018, 29, 1343–1362. [Google Scholar] [CrossRef]
- Mukherjee, S.; Panda, S.R.; Mishra, P.C.; Chaudhuri, P. Enhancing Thermophysical Characteristics and Heat Transfer Potential of TiO2/Water Nanofluid. Int. J. Thermophys. 2020, 41, 1–33. [Google Scholar] [CrossRef]
- Bazdidi-Tehrani, F.; Khabazipur, A.; Vasefi, S.I. Flow and heat transfer analysis of TiO2/water nanofluid in a ribbed flat-plate solar collector. Renew. Energy 2018, 122, 406–418. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Ozkaymak, M.; Sözen, A.; Menlik, T.; Fahed, A. Improving car radiator performance by using TiO2-water nanofluid. Eng. Sci. Technol. Int. J. 2018, 21, 996–1005. [Google Scholar] [CrossRef]
- Ho, C.; Cheng, C.; Yang, T.; Rashidi, S.; Yan, W. Experimental study on cooling performance of nanofluid flow in a hori-zontal circular tube. Int. J. Heat Mass Trans. 2021, 169, 120961. [Google Scholar] [CrossRef]
- Ho, C.; Liao, J.-C.; Li, C.-H.; Yan, W.-M.; Amani, M. Experimental study of cooling performance of water-based alumina nanofluid in a minichannel heat sink with MEPCM layer embedded in its ceiling. Int. Commun. Heat Mass Transf. 2019, 103, 1–6. [Google Scholar] [CrossRef]
- Ho, C.; Liao, J.-C.; Li, C.-H.; Yan, W.-M.; Amani, M. Experimental study of cooling characteristics of water-based alumina nanofluid in a minichannel heat sink. Case Stud. Therm. Eng. 2019, 14, 100418. [Google Scholar] [CrossRef]
- Hader, M.; Al-Kouz, W. Performance of a hybrid photovoltaic/thermal system utilizing water-Al2O3 nanofluid and fins. Int. J. Energy Res. 2018, 43, 219–230. [Google Scholar] [CrossRef] [Green Version]
- Meisam, A.; Ahmad, A.; Hamed, M. Experimental Investigation of Metal Oxide Nanofluids in a Plate Heat Exchanger. J. Thermophys. Heat Transf. 2019, 33, 994–1005. [Google Scholar] [CrossRef]
- Bakhti, F.Z.; Si-Ameur, M. A comparison of mixed convective heat transfer performance of nanofluids cooled heat sink with circular perforated pin fin. Appl. Therm. Eng. 2019, 159, 113819. [Google Scholar] [CrossRef]
- Alkasmoul, F.; Al-Asadi, M.; Myers, T.; Thompson, H.; Wilson, M. A practical evaluation of the performance of Al2O3-water, TiO2-water and CuO-water nanofluids for convective cooling. Int. J. Heat Mass Trans. 2018, 126, 639–651. [Google Scholar] [CrossRef] [Green Version]
- Bioucas, F.E.B.; Rausch, M.H.; Schmidt, J.; Bück, A.; Koller, T.M.; Fröba, A.P. Effective Thermal Conductivity of Nanofluids: Measurement and Prediction. Int. J. Thermophys. 2020, 41, 1–27. [Google Scholar] [CrossRef] [Green Version]
- Saghir, M.; Rahman, M. Forced Convection of Al2O3–Cu, TiO2–SiO2, FWCNT–Fe3O4, and ND–Fe3O4 Hybrid Nanofluid in Porous Media. Energies 2020, 13, 2902. [Google Scholar] [CrossRef]
- Arsana, I.M.; Muhimmah, L.C.; Nugroho, G.; Wahyuono, R.A. Enhanced Heat Transfer Effectiveness Using Low Concentration SiO2–TiO2 Core–Shell Nanofluid in a Water/Ethylene Glycol Mixture. J. Eng. Phys. Thermophys. 2021, 94, 423–430. [Google Scholar] [CrossRef]
- Gulzar, O.; Qayoum, A.; Gupta, R. Experimental study on thermal conductivity of mono and hybrid Al2O3–TiO2 nanofluids for concentrating solar collectors. Int. J. Energy Res. 2020, 45, 4370–4384. [Google Scholar] [CrossRef]
- Rahman, M.; Saghir, Z.; Pop, I. Free convective heat transfer efficiency in Al2O3–Cu/water hybrid nanofluid inside a rec-totrapezoidal enclosure. Int. J. Numer. Method Heat Fluid Flow 2020, 45, 4370–4384. [Google Scholar]
- Hamid, K.A.; Azmi, W.; Mamat, R.; Sharma, K. Heat transfer performance of TiO2–SiO2 nanofluids in a tube with wire coil inserts. Appl. Therm. Eng. 2019, 152, 275–286. [Google Scholar] [CrossRef]
- Ramadhan, A.; Azmi, W.; Mamat, R.; Hamid, K. Experimental and numerical study of heat transfer and friction factor of plain tube with hybrid nanofluids. Case Stud. Therm. Eng. 2020, 22, 100782. [Google Scholar] [CrossRef]
- Martin, K.; Sözen, A.; Çiftçi, E.; Ali, H.M. An Experimental Investigation on Aqueous Fe–CuO Hybrid Nanofluid Usage in a Plain Heat Pipe. Int. J. Thermophys. 2020, 41, 1–21. [Google Scholar] [CrossRef]
- Welsford, C.A.; Delisle, C.S.; Plant, R.D.; Saghir, M.Z. Effects of nanofluid concentration and channeling on the thermal effectiveness of highly porous open-cell foam metals: A numerical and experimental study. J. Therm. Anal. Calorim. 2019, 140, 1507–1517. [Google Scholar] [CrossRef]
- Plant, R.; Hodgson, G.K.; Impellizzeri, S.; Saghir, M.Z. Experimental and numerical investigation of heat enhancement us-ing a hybrid nanofluids of copper oxide/alumina nanoparticles in water. J. Therm. Anal. Calorim 2019, 141, 1951–1968. [Google Scholar] [CrossRef]
- Hodgson, G.K.; Scaiano, J.C. Heterogeneous Dual Photoredox-Lewis Acid Catalysis Using a Single Bifunctional Nanomaterial. ACS Catal. 2018, 8, 2914–2922. [Google Scholar] [CrossRef]
- Bayomy, A. Electronic Cooling Using ERG Aluminum foam Subjected to Steady/Pulsating Water and γ-Al2O3-Water Nanofluid Flows: Experimental and Numerical Approach. Ph.D. Thesis, Ryerson University, Toronto, ON, Canada, 2017. [Google Scholar]
- Ghadikolaei, S.; Yassari, M.; Sadeghi, H.; Hosseinzadeh, K.; Ganji, D. Investigation on thermophysical properties of TiO2–Cu/H2O hybrid nanofluid transport dependent on shape factor in MHD stagnation point flow. Powder Technol. 2017, 322, 428–438. [Google Scholar] [CrossRef]
- Madhash, D.; Parameshwaran, R.; Kalaiselvam, S. Experimental investigation on convective heat transfer and rheological characteristics of Cu-TiO2 hybrid nanofluids. Exp. Therm. Fluid Sci. 2014, 52, 104–115. [Google Scholar] [CrossRef]
- Hamid, K.A.; Azmi, W.; Nabil, M.; Mamat, R.; Sharma, K. Experimental investigation of thermal conductivity and dynamic viscosity on nanoparticle mixture ratios of TiO2-SiO2 nanofluids. Int. J. Heat Mass Transf. 2018, 116, 1143–1152. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).