3.1. Catalyst Characterization
Table 1 shows the chemical composition measured by XRF and textural properties measured by N
2 physisorption of the catalysts and their respective supports. It was observed that measured and nominal compositions are similar, considering the semiquantitative nature of the analysis, because fluorescent emissions from elements can interfere with each other.
There was a decrease in BET surface area by the addition of NiO and the promoters (CeO2 and La2O3). This tendency was expected due to the low BET area of these oxides and because its addition decreases the alumina content in the catalyst composition. Moreover, a decrease in pore volume was observed by ceria and lanthana addition from 0.37 to 0.26–0.25 cm3 g−1, which may indicate a recovering of alumina pores during the wet impregnation. Lanthana addition led to the highest decrease in BET area, from 201 to 88.5 m2 g−1, indicating a higher dispersion of this promoter on alumina surface.
Mortola et al. [
18] also reported a decrease in BET surface area and pore volume by the addition of 12 wt.% La
2O
3 and 12 wt.% CeO
2 on alumina support due to the blockage of alumina smaller pores and the incorporation of lanthanum or cerium into the alumina network. In their study, the addition of lanthana caused the greatest decrease in surface area, from 182 to 104 m
2 g
−1, and in pore volume, from 0.29 to 0.12 cm
3 g
−1, which is in accordance with this work. They explained this behavior by a strong interaction between alumina and lanthana during synthesis. Mazumder et al. [
19] attributed the blockage of alumina pores to the formation of LaAlO
3 species due to solid interaction at high temperatures between alumina and lanthana.
Ceria and lanthana incorporation on alumina also significantly increased mean pore size, from 81.7 to 177.4 and 186.0 Å, respectively. This behavior may be explained by the larger atomic radii of cerium and lanthanum, compared to aluminum, which promotes an expansion of the pores during impregnation and calcination. In addition, the nickel incorporation in the promoted supports also caused an additional increase in pore size, especially for the NiLaAl catalyst. Mazumder et al. [
19] also reported an increase in pore diameter from 73.64 to 136.22 Å after 20 wt.% Ni loading on 10 wt.% La
2O
3–Al
2O
3 support. They attributed this effect to the thermal sintering and inclusion of large-size metal particles into the support pores.
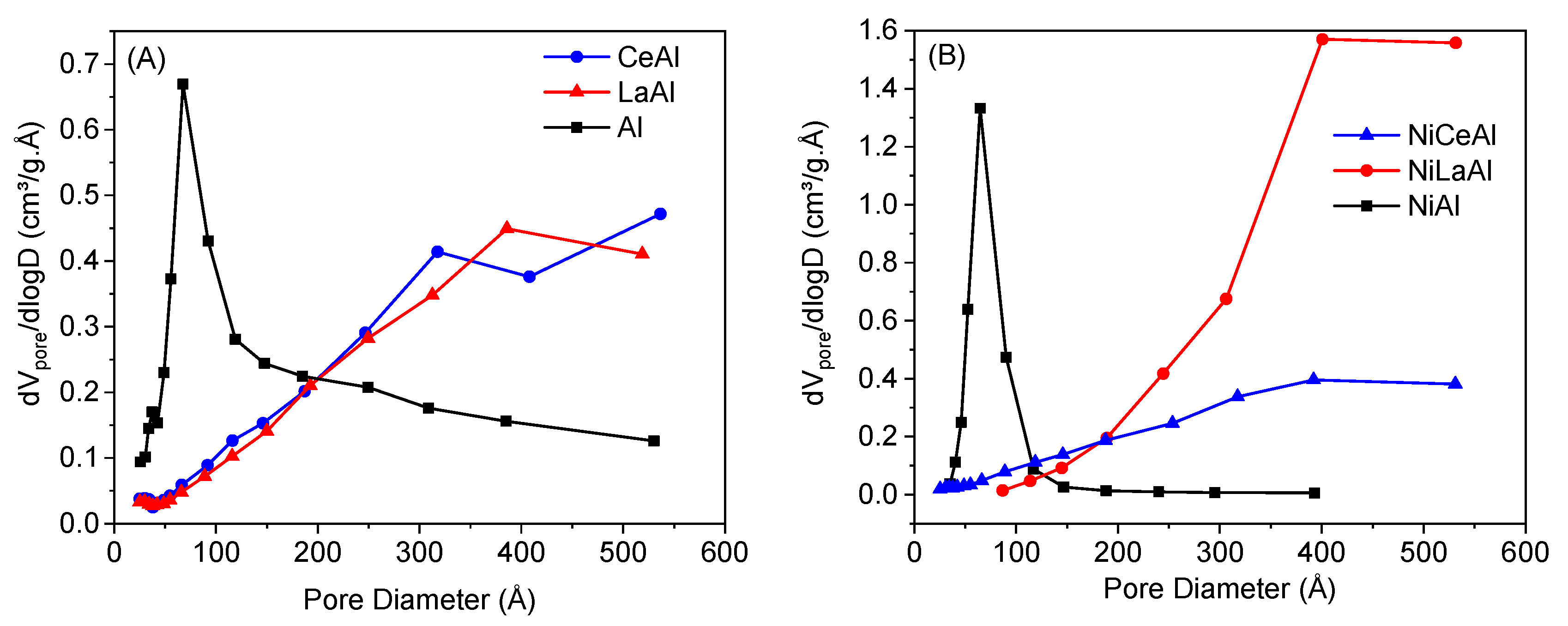
Figure 1 shows the distribution of pore size for the supports and catalysts. It is observed that the nonpromoted catalyst and support presented a narrower distribution of pores, mostly centered between 0 and 100 Å. The addition of both promoters shifted the distribution towards larger pore sizes, which corroborates the hypothesis that small alumina pores are clogged by ceria and lanthana. Moreover, there was an expansion of pores, mainly by nickel incorporation on lanthana-modified support, as mentioned earlier.
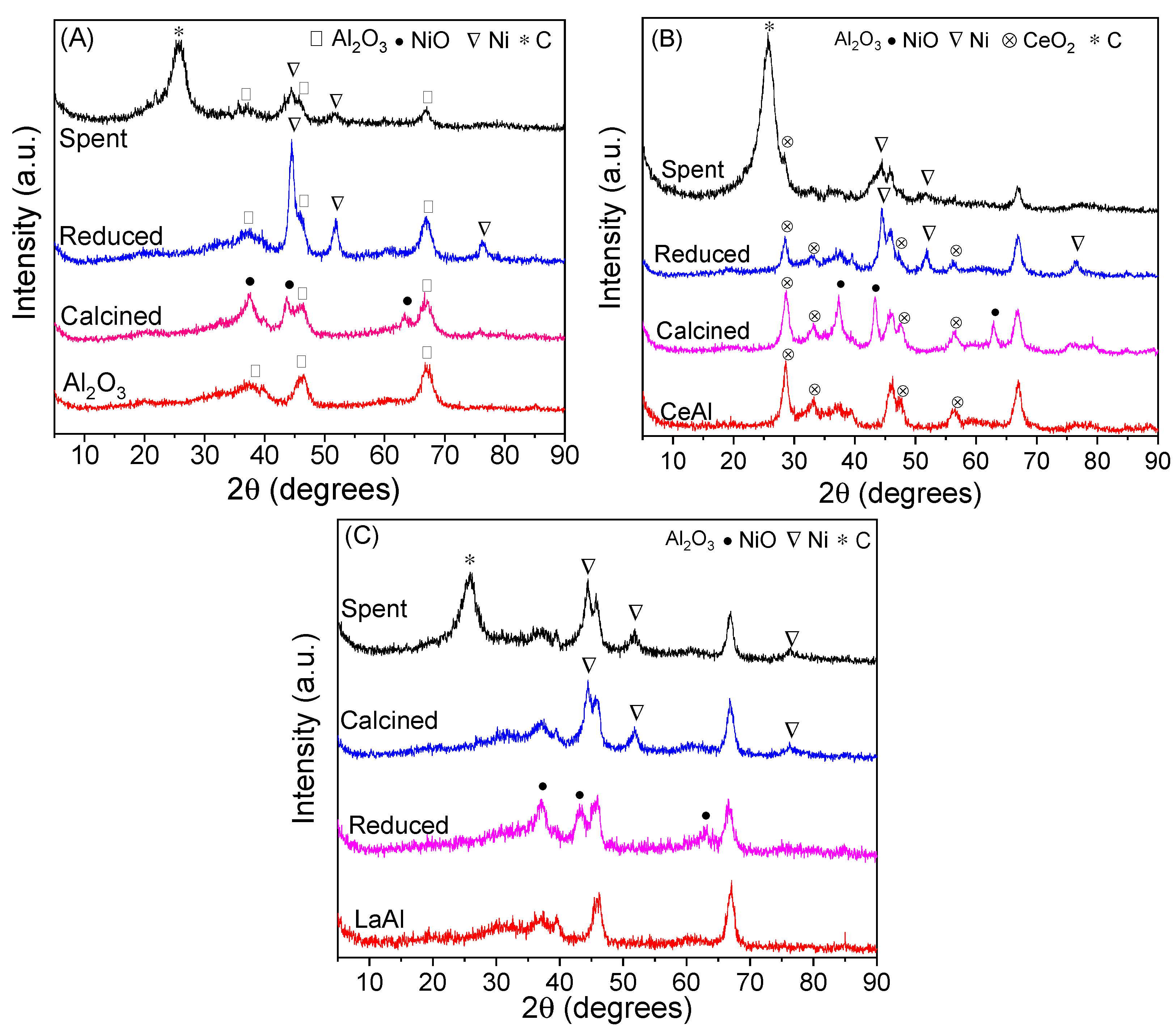
Figure 2 presents the XRD diffractograms of the supports, calcined, reduced, and spent catalysts. Peaks related to alumina (γ-Al
2O
3) are observed in all diffractograms at 2θ equal to 37.6 (3 1 1), 45.9 (4 0 0), and 66.1° (4 4 0) (JCPDS 10-425). It is also observed NiO peaks at 2θ equal to 37.3 (1 0 1), 43.3 (0 1 2), and 62.9° (1 0 4) (JCPDS 47-1049) in all calcined catalysts and peaks related with Ni metallic phase at 44.5 (1 1 1), 51.8 (2 0 0), and 76.5° (2 2 0) (JCPDS 04-0850) in all reduced catalysts. This indicates a good efficiency of calcination and reduction steps. Spent catalysts presented the peaks related to nickel phase and no peaks related to NiO, indicating a good stability of Ni phase during the reaction. Furthermore, a peak at 26° (1 1 1) (JCPDS 75-0444) on the spent catalysts is associated with carbon deposits produced during the reaction. This peak is most intense for NiCeAl catalyst, which suggests a high coke deposit, corroborated by TGA analysis. Coke crystallite size, reported in
Table 2, is similar for all catalysts.
Peaks related to CeO
2 at 28.6 (1 1 1), 33.2 (2 0 0), 47.8 (2 2 0) and 56.4° (3 1 1) (JCPDS 34-0394) are observed in all stages of NiCeAl catalyst presented in
Figure 2B. In contrast, no peaks associated with lanthana were detected in NiLaAl profiles, reported in
Figure 2C. This result corroborates a better dispersion of lanthana on alumina in comparison with ceria. Mazumder et al. [
19] also did not observe any peaks related to lanthana on a 10 wt.% La
2O
3–Al
2O
3 support. Thus, they reported that lanthana species were either highly dispersed on alumina surface as an amorphous phase or incorporated into the pore network of alumina. Iriondo et al. [
12] studied the addition of 10 wt.% ceria on Ni/Al
2O
3 catalyst and also detected peaks related to CeO
2 with fluorite structure at the same positions mentioned in this work. They also mentioned the ceria capacity to form aggregates, which could decrease its dispersion on alumina.
Ni crystallite size, calculated by Scherrer equation using the peak centered at 51.8°, and nickel dispersion, obtained by Anderson correlation, are shown in
Table 2. Considering the analysis error, it was observed that the addition of both promoters did not significantly change the nickel crystallite size compared with NiAl catalyst. However, NiLaAl presented a smaller crystallite size and higher dispersion than NiCeAl, attributed to a better interaction with nickel species provided by small lanthanum particles dispersed on the support. Big ceria crystals (8.8 ± 0.8 nm), calculated using the peak centered at 28.6° of the reduced sample, should provide a weaker interaction with nickel.
Iriondo et al. [
12] suggested that for a 20 wt.% CeO
2 promotion on Ni/Al
2O
3, ceria tends to interact with alumina instead of nickel, decreasing stability of the nickel phase and reducing its dispersion. They also reported that large ceria crystallites could hinder the interaction between ceria and nickel.
Dan et al. [
20] reported that 6 wt.% lanthana on nickel/alumina catalyst increased nickel dispersion measured by H
2 chemisorption approximately three times. In contrast, for 6 wt.% ceria addition, the dispersion was very similar compared to the nonpromoted catalyst. Furthermore, nickel crystallite size measured from diffractograms was decreased from 10 to 6.7 nm by lanthana addition, which agrees with the values presented in this work. However, this tendency of reducing was not confirmed in this work due to the analysis error.
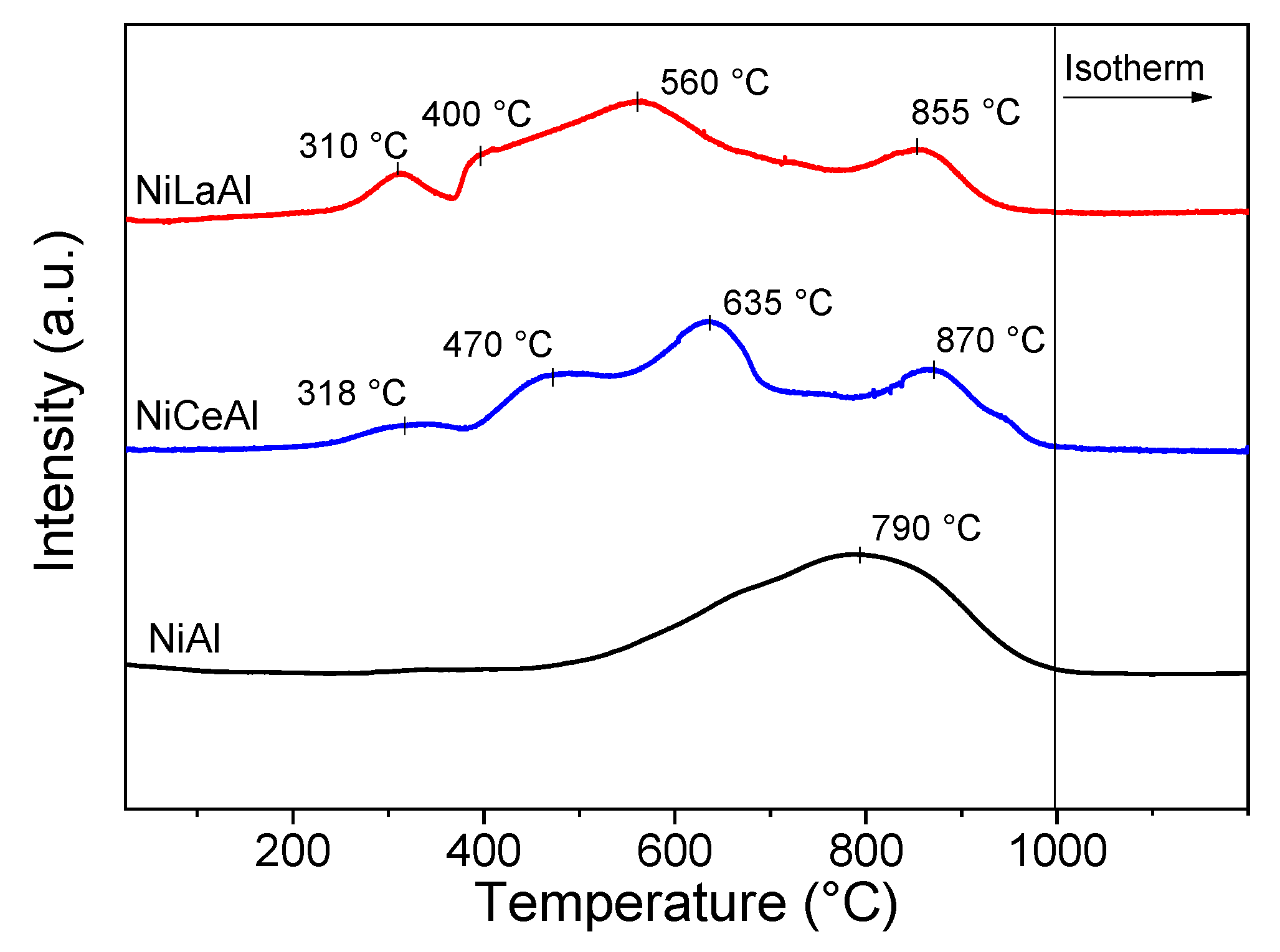
Figure 3 shows TPR profiles of the catalysts. NiAl presented only a broad peak centered at 790 °C, associated with the reduction of nickel aluminate species (NiAl
2O
4). According to Rynkowski et al. [
21], nickel aluminate species reduce at temperatures above 700 °C.
The addition of both promoters shifted the curves to lower temperatures, indicating that these promoters decrease the formation of aluminate species on calcined catalysts. This hypothesis is confirmed by the increase in reduction degree with lanthana and ceria addition reported in
Table 2, as nickel aluminate is difficult to reduce. NiCeAl and NiLaAl presented four reduction peaks. The first, centered at 310–318 °C, is related to NiO with poor interaction with the support. The peaks centered at 400–470 °C and 560–635 °C are associated with NiO species in the pores with intermediate and strong interaction with the promoted support, respectively, and the last peak, centered at 855–870 °C, is related to nickel aluminate reduction.
Mazumder et al. [
19] reported that lanthana addition (5 wt.%) on Ni/Al
2O
3 shifted TPR curves to lower temperatures, indicating that this promoter decreases Ni–Al
2O
3 interaction. Furthermore, by adding lanthana, they noticed a small peak at around 390 °C related to the reduction of dispersed NiO species on the support surface, in line with our findings. The reducibility was also increased from 90 to 94% by La
2O
3 incorporation, suggesting a higher proportion of easily reducible species and diminished formation of nickel aluminate species. Dan et al. [
20] also reported the same tendency on TPR curves by adding lanthana on nickel/alumina catalyst. This promoter extends the proportion of NiO
x species in weak interaction with the support.
Figure 4 shows the results of ammonia desorption by increasing temperature. All catalysts presented only one broad peak, centered at 240–250 °C, related to the majority presence of weak acid sites, according to the literature [
22]. However, the temperature range in which the desorption peak is centered is narrower for NiLaAl, indicating that lanthana addition decreased the strength of catalyst acid sites.
The acidity expressed by mass and per specific area is summarized in
Table 2. A significant decrease of catalyst acidity was observed by both promoter additions, especially by lanthana, which promoted a reduction from 3.1 to 0.5 μmol NH
3 m
−2. This result was expected due to the basic nature of lanthanide oxides. Furthermore, the greatest decrease observed for NiLaAl may be associated with the highest dispersion of lanthana on alumina support, and the highest coverage of alumina pores, titrating the acid sites. Mazunder et al. [
19] found that 5 wt.% La
2O
3 addition on γ-Al
2O
3 support decreased acidity from 370 to 251 µmol g
−1. They also reported that some of the lanthana basic sites are employed to neutralize the acid sites of γ-Al
2O
3. Montini et al. [
23] reported that lanthana and ceria incorporation on Pt/Al
2O
3 catalyst decreased the amount of desorbed NH
3, together with a shift to lower temperatures, indicating a reduction in acid strength. This behavior was more pronounced for lanthana incorporation due to its higher dispersion on alumina, which agrees with this work.
3.2. Catalytic Tests
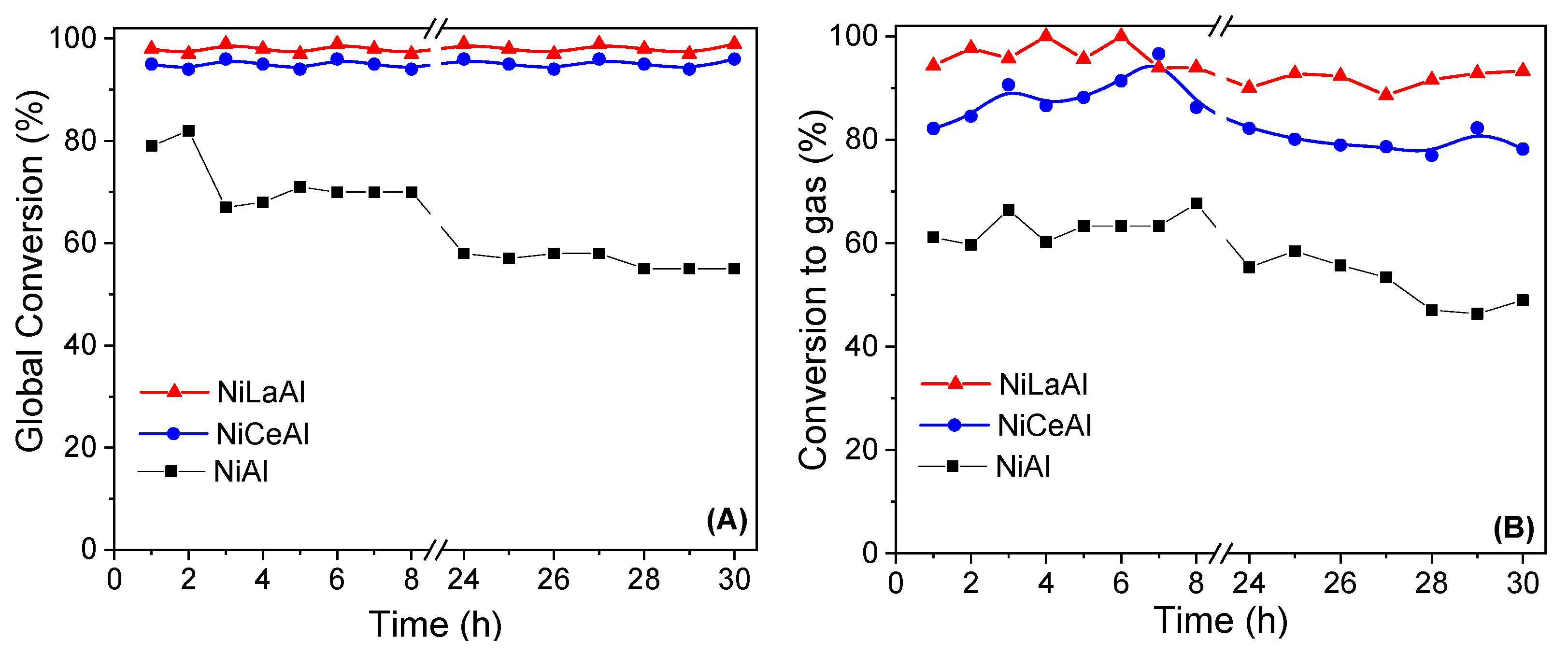
Figure 5 shows the results of global conversion and conversion to gas for butanol steam reforming reaction at 500 °C during 30 h of time on stream. NiAl presented the lowest activity in comparison with the promoted catalysts. Moreover, the nonpromoted catalyst suffered the most severe deactivation, as conversion decreased from 79 to 55% and conversion to gas dropped from 61 to 49%. This indicates that both promoters increased the stability of nickel active phase, especially the lanthana. Furthermore, the deactivation may be explained by coke deposition on nickel surface, favored by alumina acid sites [
10].
NiLaAl catalyst presented the best performance, as its global conversion remained practically constant at 100% and its conversion to gas suffered small variation in the range of 90–100%. Moreover, the discrepancy between global conversion and conversion to gas is low during all reactions, which indicates that parallel reactions of coke and liquid byproducts formation are less important for this catalyst, providing its best stability. These results agree with its highest nickel dispersion and reducibility, which increased the availability of nickel metallic active sites, and with its lowest acidity, decreasing the population of acid sites for parallel reactions.
On the other hand, the ceria promotion did not present the same benefits in terms of catalytic performance. Ceria was less dispersed on the support, and its interaction with nickel was poor, leading to the lowest nickel dispersion. For NiCeAl catalyst, the global conversion remained constant at approximately 95%; however, conversion to gas decreased from 95% at the seventh hour to 78% at the end of the reaction. It was observed that a reduction in conversion to gas did not accompany the drop in global conversion. These conversion profiles indicated that the activity for parallel reactions, mainly coke formation, increased throughout time on stream.
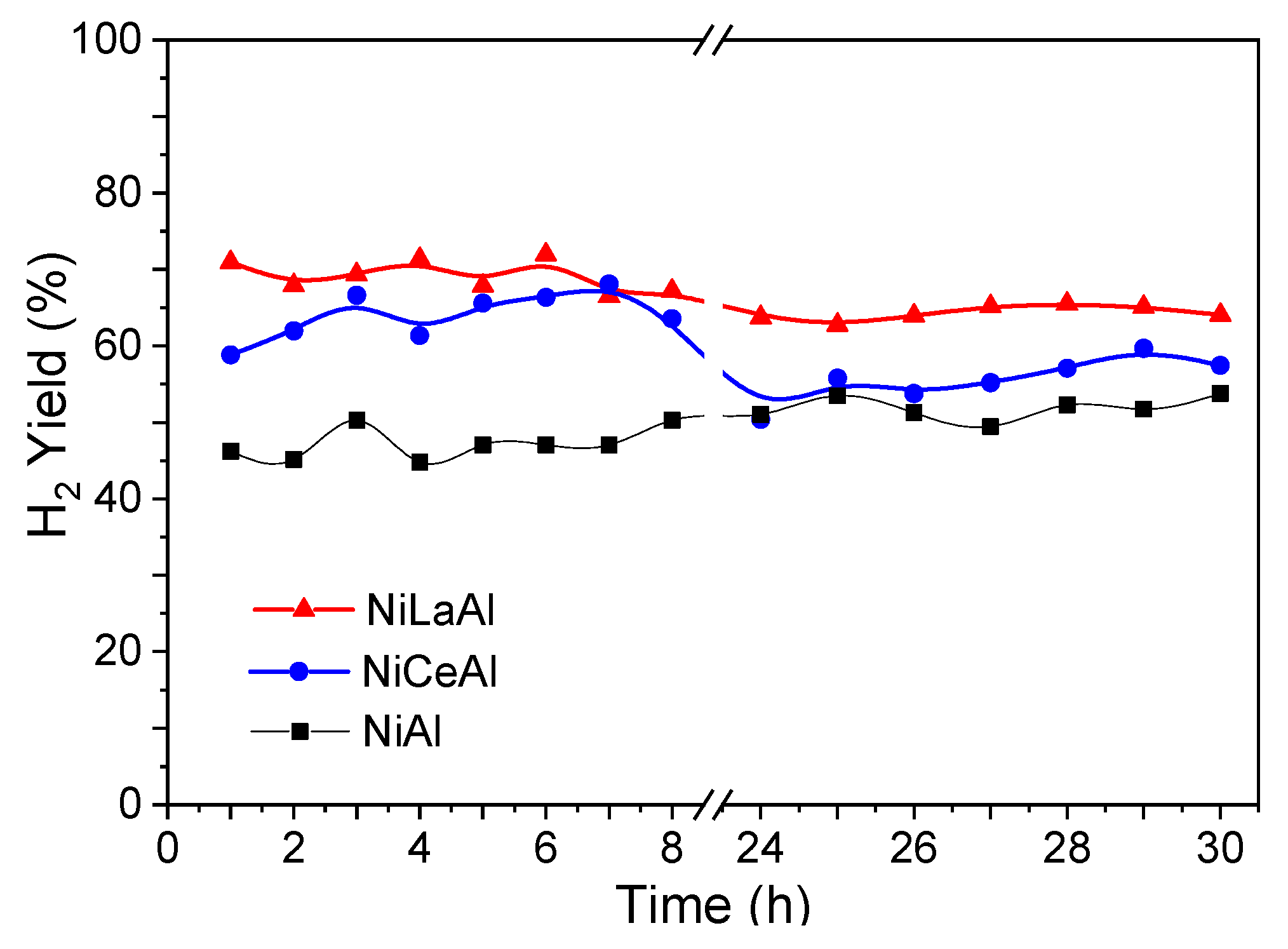
Figure 6 shows the hydrogen yield versus time on stream. Both promoters increased the average hydrogen yield, which presented the following order: NiLaAl (71.2%) > NiCeAl (63.8%) > NiAl (49.4%). These results indicate an enhancement of butanol steam reforming and shift reactions by adding basic promoters, whose basic sites promoted CO adsorption and water dissociation, leading to hydrogen formation. Wang et al. [
24] studied the effect of different supports on water-gas shift reaction over Pt–Ni bimetallic catalysts. They reported a higher activity for ceria support than alumina due to the ability of ceria to promote water dissociation to form labile OH/O/H species, surface oxygen vacancies, and defect sites.
Ashok et al. [
11] suggested that the surface properties of supports as availability of surface hydroxyl groups and ability to disperse the active metals are crucial for water gas-shift reaction. In this work, lanthana promoter matched both requirements, while ceria could not efficiently disperse the nickel phase. Thus, the NiLaAl catalyst provided the highest hydrogen yield. In contrast, NiCeAl presented a drop in hydrogen mean yield from 68 to 59%, comparing the first 8 h with the last 7 h of reaction.
Bizkarra et al. [
7] evaluated the addition of 10 wt.% CeO
2 and 6 wt.% La
2O
3 on 13 wt.% Ni/Al
2O
3 for butanol steam reforming at 600 °C, atmospheric pressure, and steam to carbon ratio of 5. They performed the reaction for 4 h and reported butanol conversion of 100% for all the catalysts, higher than the values reported in this work due to the higher temperature employed. Furthermore, they reported that ceria addition improved hydrogen yield from 58 to 65%, while lanthana addition decreased this parameter to 46%. Compared with the first 4 h of reaction, we also obtained a similar increase from 47 to 66% by ceria addition. On the other hand, NiLaAl presented a higher mean hydrogen yield (74%) in this work. This difference may be attributed to the higher lanthana content used since basic sites favor the activity for butanol reaction. Moreover, the lower temperature and higher steam to carbon ratio (11.4) employed in our work increase the shift reaction activity [
11], leading to a higher hydrogen production.
Figure 7 displays CO and CO
2 yields versus time on stream. It was observed that all the catalysts presented low CO yield, with values lower than 6% during all reaction time. NiLaAl presented the highest CO
2 and hydrogen yields, which corroborates its great activity for shift reaction. NiCeAl showed a high CO
2 yield at the beginning of the reaction. However, its reduction throughout the reaction indicates a loss in shift reaction activity, which agrees with the decrease in hydrogen yield. Furthermore, the increase in coke formation throughout time on stream by CO and CO
2 hydrogenation, presented in Equations (10) and (11), could explain this behavior. Bizkarra et al. [
7] also reported deactivation for a 13 wt.% Ni/10% CeO
2–Al
2O
3 catalyst in butanol steam reforming. They reported a decrease in carbon dioxide and hydrogen yield due to carbon formation.
The NiAl catalyst presented the lowest CO and CO
2 yield during all reaction time, associated with the consumption of these oxides for coke production by CO and CO
2 hydrogenation, harming hydrogen production for this catalyst. On the other hand, at the beginning of the reaction, the high CO
2 and low CO yields for NiCeAl and NiLaAl indicate that coke formation was mainly produced by disproportionation of CO, as presented in Equation (12). This reaction does not consume hydrogen and contributes to the higher hydrogen yield of the promoted catalysts.
Methane was produced in very low amounts, as its yield was lower than 5% for all the catalysts. Thus, the catalysts were not active for methanation reactions under the reaction conditions employed in this work.
Figure 8 displays the results of thermogravimetric and differential thermal analysis of the spent catalysts. The exothermic peaks on DTA, associated with the mass loss observed by TGA, are related to the burning of coke deposits. The addition of both promoters did not significantly modify the nature of coke deposits, as the burning peak is centered approximately at the same temperature, around 580 °C. However, lanthana addition slightly shifted DTA curves to lower temperatures, indicating the presence of a more active carbon, which is easier to remove. This result also corroborates the higher stability of the lanthana-promoted catalyst. Santamaria et al. [
15] also observed a shift towards lower temperatures on TPO curves of spent catalysts due to lanthana addition on the Ni/Al
2O
3 catalyst. They attributed this behavior to the inhibition of coke evolution due to lanthana basicity and water adsorption capacity.
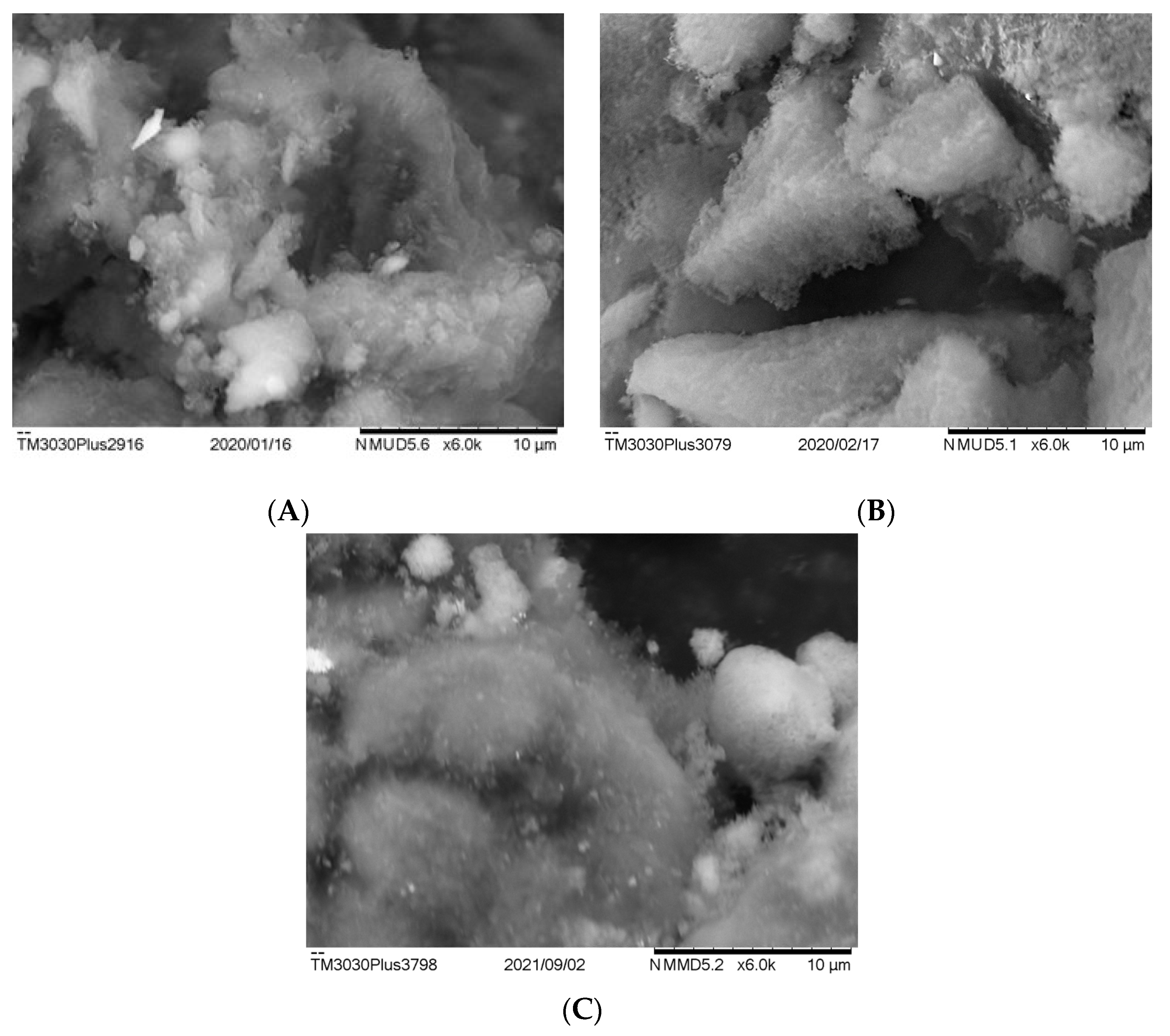
Figure 9 shows the SEM images of the spent catalysts. For all catalysts, only an irregular deposition of amorphous coke was observed, which contributes to nickel encapsulation and causes deactivation. Filamentous coke was not observed for all catalysts, agreeing with the only peak detected in TGA analysis. Santamaria et al. [
15] also reported only amorphous coke on both nonpromoted and lanthana-promoted nickel/alumina catalyst after biomass–pyrolysis reforming.
Table 3 shows the coke amount on spent catalysts. NiLaAl presented the lowest coke formation, associated with its lowest acidity, as coke formation by dehydration, cracking, and polymerization reactions are favored on alumina acid sites. This result corroborates the best performance of this catalyst in terms of higher stability and nondeactivation during 30 h of reaction.
Furthermore, the addition of La
2O
3 could play an important role in removing carbon from the nickel surface during the reaction, as reported in the literature. Thyssen et al. [
14] evaluated the addition of lanthana with different contents (10–30 wt.%) on Ni/SiO
2 catalyst for glycerol steam reforming. They observed that coke formation was lower for the promoted catalyst because the formation of a La carbonate removed C species deposited on the nickel sites.
On the other hand, NiCeAl and NiAl presented a higher amount of coke on spent catalysts, explaining the deactivation observed for these catalysts. NiCeAl presented the highest coke amount (77.4%), corroborating the result reported on XRD analysis of the spent catalyst. This result indicates that, although ceria addition has also decreased catalyst acidity, the poor interaction with nickel phase promoted a higher coke formation and a less effective coke removal from the surface. Furthermore, the coke formation is enhanced by big nickel crystallite sizes, as reported in the literature [
25], which agrees with this work. Silva et al. [
26] and Greluk et al. [
27] reported that nucleation of coke requires relatively large domains of flat terraces or large metal ensemble sizes. Thus, large ceria crystals on NiCeAl catalyst enhanced coke formation.