Abstract
The hydrocarbon compositions of shale oils, generated from two different lithological–facial Domanic deposits of the Tatarstan Republic (Russia), were studied under hydrothermal impact with 30% of water addition in a 350 °С and CO2 environment. The samples were extracted from carbonate–siliceous rocks of the Semiluky–Mendym deposits of the Berezovskaya area, and carbonate deposits of the Dankovo–Lebedyan horizon of the Zelenogorskaya area of the Romashkino oil field. The distinctive features of rocks are in the composition and content of organic matter (OM), its thermal stability, as well as the structural-group composition of the shale oil products. The hydrothermal treatment of the rock samples increased the content of saturates and decreased the content of aromatics, resins and asphaltenes in the composition of crude oil. The decomposition of the polymer-like kerogen structure and destruction processes of high-molecular compounds, such as resins and asphaltenes, are accompanied with the formation of substances highly rich in carbons—carbenes and carboids. The contents of n-alkanes and acyclic isoprenoids increase in the composition of saturated hydrocarbons. According to the chemical classification of Al. A. Petrov, the character of the molecular mass distribution of such substances corresponds to oil type A1, which is considered paraffinic. The contents of dibenzothiophene, naphthalene and phenanthrene are increased in the composition of aromatic hydrocarbons, while the contents of tri-methyl-alkyl-benzene and benzothiophene are decreased. The increase in the aryl isoprenoid ratio (AIR = С13–С17/С18–С22) and maturity parameter (4-MDBT/1-MDBT) under the influences of hydrothermal factors indicates the increasing thermal maturity degree of the hydrocarbon system. The differences in the distribution behavior of saturated and aromatic hydrocarbons—biomarkers in rocks of various lithological-facies types, which are reasoned by different conditions of initial organic matter transformation as well as under the impact of hydrothermal factors—were revealed.
1. Introduction
Domanic-type deposits are widely distributed within the territory of the Tatarstan Republic in the Upper Devonian sediments, which have a potential to generate liquid and gaseous hydrocarbons as well as to accumulate them in the unconventional reservoir types [1,2,3,4,5,6,7,8,9,10,11]. Currently, many international service and oil production companies are actively working on creating innovative methods, which lead to effectively developing unconventional oil reservoirs [6]. Public Joint Stock Company (PJSC) Tatneft also has certain expectations from the Domanic strata in terms of shale oil production [2,3,11]. PJSC Tatneft has been developing Domanic deposits or shale oil since 2012. According to the head of the geology department of the Tatarstan Republic Rais Khisamov, the shale oil resources of Tatarstan are estimated at about 4–16 billion tons [1,9]. The Semiluky (Domanic), Mendym and Sargaevsk horizons of Upper Devonian, with TOC 5–20%, correspond to typical Domanicites [1,2,3]. The rock samples are source rocks, which are introduced by siliceous–carbonate limestones, dolomites, marls with various degrees of caverns and fractures. Deposits with Corg 0.5–5% from the Tournaisian stage to the Mendym horizon correspond to Domanicoids. The industrial oil-bearing deposits are determined mainly by carbonate formations of the Dankovo–Lebedyan and Zavolzhsky horizons, where porous–cavernous limestones alternate with the denser carbonate rocks enriched with organic matters. PJSC Tatneft carries out industrial-scale works on the production of light shale oil from the dense carbonate rocks of the Dankovo–Lebedyan horizon, using hydrofracturing techniques in the Bavlinskoye oil field (East of Tatarstan) [11]. On the territory of Tatarstan, oil production is carried out from Domaniс deposits, discovered many years ago during prospecting operations for oil. The development of these deposits is carried out using the methods for conventional reservoirs since these deposits are associated with linear zones of increased fracturing of rocks. A thorough study is being carried out toward creating unconventional hydrocarbon production technology. The main parameters of quality and technological properties of Domanic rocks are determined by low permeability, high density, the content of organic matter, its nature and transformation degree [1,2,3]. The significant part of OM corresponds to the bituminous resins and asphaltenes, and kerogen, which is considered one of the petroleum sources. However, the transformation of kerogen into the hydrocarbons occurs at a temperature above 100 °С [12,13,14,15,16,17]. Thus, the thermal methods, which are applied for the development of Domanic deposits, provide catagenic transformation of organic matters in a certain degree [8]. Besides the in situ combustion, hydrofracturing and acidizing techniques, one of the promising ways of producing light hydrocarbons from kerogen is its transformation in aquatic medium in high-temperature or supercritical conditions [18,19,20]. Superheated water as well as supercritical water improve the transportation characteristics of heavy oil by increasing its solubility and decreasing its viscosity. However, water contributes to safer and more environmentally friendly technological processes. Recently, some enhanced recovery techniques of dense, kerogen-containing formations were proposed, where the oil recovery is accelerated or increased. In [21], the injection of catalytic substrates into the reservoir with further reduction, i.e., by hydrogen, and the heating of heavy oil by steam generating in situ is proposed. The authors also propose another interesting approach, which is based on the application of catalysts during the in situ combustion process. The heat is transferred to the kerogen-containing reservoir formations, and the temperature is increased up to 250 °С in certain parts of the reservoir. In such reservoir conditions, transformation of OM and kerogen can be industrially feasible. Moreover, the given approach seems to be very promising, as the content of trace elements, which are capable of exhibiting catalytic properties, is very high in kerogen-containing reservoir rocks [22]. It is significant to note that the interaction of supercritical water and hydrocarbons is always carried out by evolving gases, among which CO2 is very attractive, as it swells oil and lowers oil viscosity [23]. However, high temperatures and pressures lead to the destruction of organic matter with the formation of not only light hydrocarbons, but also heavier products, which can create additional problems in the development of tight rocks. In this regard, the study of the composition of Domanic rocks, establishment of the nature and forms of organic matter in them are crucial in evaluating their hydrocarbon potential and developing technologies for extracting hydrocarbons from them by thermal methods [24,25,26,27,28,29,30,31,32,33].
The aim of this study is to compare the composition of shale oil generated by the organic matter of Domanic rocks of different lithological–facial types in hydrothermal processes.
2. Experimental Procedures
The object of the given study were two samples of Domanic rocks from the different lithological–facies formations of the Romashkino oil field and the transformation products of organic matter in hydrothermal processes. One of the rock samples was isolated from the depth interval of 1379–1394 m of carbonate (Domanicoid) deposits of the Dankovo–Lebedyan horizon of the Zelenogorskaya area, while the other sample was isolated from the depth interval of 1705–1728 m of the Semiluky–Buregsky (Domanic) carbonate–siliceous deposits of the Berezovskaya area of the Romashkino field—one of the largest reservoirs located within the South Tatar arch on the territory of Tatarstan [33,34].
According to the XRD, the rock sample from the Berezovskaya area is composed of quartz = 88.87% and calcite = 11.13%. The Zelenogorskaya rock sample is mainly composed of calcite = 99.76%, while the content of quartz is only 0.24%.
The hydrothermal experiments were carried out in laboratory conditions in a Parr Instruments autoclave manufactured in the U.S.A. with a volume of 1 L at a temperature of 350 °С in a CO2 medium for 5 hours. The content of the water was 30 wt.% to the weight of the rock sample, which was 200 g. The initial pressure in the system CO2 was 2 MPa. As the temperature in the system increased, the pressure of the vapor–gas mixture increased to 17 MPa.
The total Corg content in the rock samples (TOC) before and after hydrothermal experiments was evaluated by the Rock–Eval pyrolysis method in HAWK (Wild Cat Technologies, USA). Moreover, other pyrolysis parameters that characterize oil generating potential of the given rock samples were also considered [35]: S1—content of free HC, mg HC/gr rock; S2—content of kerogen, mg HC/gr rock; S3—content of СО2, mg СО2 /gr rock; TOC—total Corg content %; РI = S1/(S1 + S2)—productivity index; Tmax—temperature of maximum HC yield during kerogen pyrolysis, °С; НI and OI—hydrogen and oxygen indices, mg HC/gr Сorg.; OSI—oil saturation index, mg HC/gr Сorg.; АI—adsorption index, wt.%; CaСО3—content of calcium carbonate in rocks, wt.%.
Extraction of the rock samples before and after the experiments was carried out in Soxhlet by the mixture of the following solvents: benzene, chloroform, and isopropyl alcohol (1:1:1 by volume) [33]. The yield of the extracts was evaluated with respect to the weight of the rock samples.
The group composition of oil was determined by separating them into four fractions: saturates, aromatics, resins and asphaltenes, according to the GOST 32269-2013–“Petroleum bitumens. Method of separation into four fractions”, which is an analogue of the SARA analysis. The asphaltenes were precipitated in 40-fold amount of aliphatic solvent (hexane). The precipitated asphaltenes were filtered and washed from the filter by toluene in a Soxhlet apparatus. If some black carbonaceous particles still remained on the filter, we referred to them as carbenes and carboids, which are not soluble in toluene [33]. Carbenes and carboids are polycondensed compounds with high carbon content; the carboids are more condensed and in some cases can be considered carbon particles. In light and medium fractions of oil, carbenes and carboids are practically absent, and in heavy and residual oil products, their concentration is usually significant. Also, carbenes and carboids are considered compaction products and oxidation products of asphaltenes [36]. In work [37], asphaltenes insoluble in toluene were named preasphaltenes.
The changes in the structural-group composition of asphaltenes, carbenes and carboids were evaluated by the FT-IR method. The measurements were carried out by a Tenor-27 (Bruker) operated at a resolution of 4 cm−1 in the range of 4000–400 cm−1. The spectral coefficients were proposed to evaluate the changes in their compositions: С1 = D1600/D720 (aromaticity); С2 = D1710/D1465 (oxidation); С3 = D1380/D1465 (branching); С4 = (D720 + D1380)/D1600 (aliphaticity); С5 = D1030/D1465 (sulfurization), where D is the optical density at the maximum absorption band of corresponding compounds [33,38].
The analysis of the group and individual hydrocarbon composition of saturated and aromatic fractions was carried out by GC/MS on a Thermo Fisher Scientific instrument with an ISQ LT Single Quadrupole mass selective detector based on a Chromatec-Crystal 5000 chromatograph with Xcalibur software. The energy of ionizing electrons was 70 eV. We used a CR-5ms quartz capillary column 30 m long and 0.25 mm in inner diameter with a deposited methylsiloxane phase (0.25 μm). The flow rate of carrier gas (helium) was 1 mL/min. The temperature of the injector was −310 °С and the thermostat temperature program was adjusted as follows: temperature rise from 100 to 150 °C at a rate of 12 °C/min, from 150 to 300 °C at a rate of 3 °C/min followed by an isotherm until the end of the analysis. The total analysis time of a sample was 70 min. All samples were diluted in carbon tetrachloride at a concentration of 10−3 g/μL before being introduced into the device. Chromatograms were recorded, according to the total ionic current (TIC), followed by reconstruction of the molecular weight distribution of various types of compounds by characteristic ions: n-alkanes and acyclic isoprenoids (m/z 57 + 113), alkyltrimethylbenzenes (m/z 133 + 134), triterpanes ( m/z 191), steranes (m/z 217 + 259) and monoaromatic steroids (m/z 253) in saturated fractions; naphthalenes (m/z 128 + 142 + 156 + 170), phenanthrenes (m/z 178 + 192 + 206), benzothiophenes (m/z 147 + 161 + 175) and dibenzothiophenes (m/z 184 + 198 + 212) in aromatic fractions [39,40,41,42,43,44,45,46,47,48,49]. The processing of mass spectral data was carried out, using the Xcalibur program. Compounds were identified, using the NIST 02 electronic mass spectra library and literature data. The relative content of various groups of compounds was estimated by calculating and comparing the areas of the peaks corresponding to individual compounds on mass chromatograms (Si) relative to the total area of all peaks (ΣSi) of the identified compounds.
3. Results and Discussion
Characterization of rock samples by the pyrolytic Rock–Eval method. According to the results obtained from the pyrolytic Rock–Eval method, the organic carbon content (Corg)¬ in the carbonate rock sample of Zelenogorskaya area was 3.03%, while in carbonate–siliceous rocks of Berezovskaya area, it was –17.44% (Table 1). According to the classification of Tisso and Welte (1984), the rocks are divided into two types: good (Corg > 3%) and very good productive deposits. The significant difference of the given rock samples is in the pyrolysis parameters. The S1 value, which shows the share of the initial genetic potential of OM transformed into the free hydrocarbons, is equal to 9.91 and 2.29 mg HC/gr of rock.

Table 1.
Characterization of rock samples according to the Rock–Eval pyrolysis data.
The residual oil generating potential S2 or the content of hydrocarbons pyrolyzed from the kerogen varied from 1.49 to 109.44 mg HC/gr of rock. The low value of S1 and high value of S2 parameters are specific for the kerogens of source carbonate–siliceous rocks of the Berezovskaya area. After the extraction of oil from the rock sample of Berezovskaya area by the mixture of solvents (chloroform, toluene, isopropyl alcohol), the value of S2 parameter remained very high. The S2 value was significantly reduced from 1.49 to 0.05 mg HC/gr of rock after the extraction of carbonate rocks of the Zelenogorskaya area. The low hydrogen and oxygen indices (HI and OI) indicate that the kerogen of carbonate rocks corresponds to type III kerogen. The significant contribution to the formation of this type of kerogen is carried out by the residue of terrestrial plants. This shows that the destruction products of kerogen do not affect the pyrolysis parameters. Instead, resins and asphaltene fractions, which are transformed in carbonate deposits, have more influence on the pyrolysis parameters. The migratory nature of hydrocarbons is justified by the low Tmax (398 versus 426 °C), which shows that kerogen in these rocks is immature and could not generate hydrocarbons. This indicates the ease of kerogen pyrolysis and its immaturity. According to the high value of S1 and productivity index (PI), the carbonate rock sample from the Dankovo–Lebedyan Domanic deposits is isolated from the productive formations. For the initial carbonate–siliceous rock sample of the Berezovskaya area, the hydrogen and oxygen indices that characterize the quality of kerogen is significantly high. The same is true for Tmax, but even this temperature indicates the zone of insufficient kerogen maturity, and it is in the range of temperature values corresponding to epigenetic organic matter of the sedimentary rock formations. The productivity index (PI), which stands for the degree of kerogen depletion and its catagenesis measure, is extremely low (0.09 vs. 0.61). On the one hand, the rock sample is characterized by low productivity value. On the other hand, the rock sample has high productive residual potential, which is specific for oil and gas source rocks. Thus, the oil generating potential of the given rocks can be realized by application of technologies that stimulate artificial maturity of kerogen in reservoir formations. The hydrothermal impact on the reservoir rocks at 350 °С contributes to the total destruction of kerogen in carbonate rocks of the Zelenogorskaya area, while kerogen in carbonate–siliceous rocks of the Domanic deposits of the Berezovskaya area is strongly stable to hydrothermal impacts. That is why the S2 value indicates the high oil-generating potential of the given rock sample. In [50], the structure of the kerogen residue undergoes certain changes with an increase in the temperature of rock treatment in an autoclave in the presence of water. It was established by the 13C NMR spectroscopy method that natural catagenesis as well as artificial catagenesis, lead to unidirectional changes in the structure of aromatic cores in kerogen. These changes are related to the transition of substituted aromatics in the condensed aromatic structure as a result of the thermal destruction of sulfide bridging bonds and C–C alkyl chain bonds.
The rock properties for both samples are comparative. For the Domanic rock sample, a low value of oil saturating index (OSI) and high value of AI (adsorption index) are specific. The oil saturation of carbonate rock sample from the Zelenogorskaya area is drastically reduced from 75 to 3 mg HC/gr Corg after hydrothermal treatment of the rock samples. The oil saturation of carbonate–siliceous Domanic rock is also reduced, but not drastically.
The relatively high and different concentration of CaCO3 in the given rock samples leads to the different amount of evolved CO2 (S3) during the pyrolysis process. Thus, the development of such reservoir rocks by steam techniques will increase the oil recovery factor to varying degrees [51,52].
The hydrocarbon group composition of shale oil. Hydrothermal treatment of rock samples increases the yield of shale oil and the content of saturated hydrocarbons in it, while the content of aromatic hydrocarbons, resins and asphaltenes decreases (Table 2).

Table 2.
Group composition (SARA analysis) of rock extracts before and after the experiments.
It was shown that polyaromatic hydrocarbons undergo destruction processes upon mechanical treatment at 180 °C [53]. Thermal destruction products enrich the composition of other studied groups of compounds: saturated and hydrocarbons with a smaller number of cycles. Based on the composition of the products, it can be assumed that changes in the quantitative composition are possible, due to the occurrence of not only isomerization reactions, but also cyclization and addition processes during the thermal processes.
It was shown in [54] that all oils after thermolysis of oil shale at 350 °С consist mainly of normal alkanes. In all samples, an increase in n-alkanes and a decrease in cyclic saturated hydrocarbons—hopanes—are detected, the content of which decreases 9 times in thermally activated samples compared to bitumen of the initial GS shale and 15 times compared to a sample that is not thermally treated, which obviously indicates the course of OM destruction processes.
Studying the forms of capture of free hydrocarbons by kerogen in work [55], it was shown that a significant part of the captured hydrocarbons remains in the structure of kerogen. It was determined that the group of saturated hydrocarbons С15–С35 is very significant among the captured components. It was shown that at the stage of destruction (peak S2, according to Rock–Eval), not only the products of destruction, but also the previously captured free hydrocarbons are released.
Carbenes and carboids were observed in the composition of shale oil extracted from the rock samples. They are probably the decomposition fragments of kerogen [25]. On the other hand, the stability of asphaltenes by the thermal destruction method was studied in [56]. It was revealed that the destruction of sulfurous structures that were in the composition of asphaltene molecules starts at 200 °С with the detachment of alkyl sulfide bonds. At 300 °С, the intensive destruction process of the weakest carbon–heteroatom bonds and relatively strong C–C bonds initiates, which leads to a reduction in molecular mass. The conversion degree of resins at the given temperature is higher than that of asphaltenes. This is due to the high content of alkyl sulfide bonds. In [56], it was shown that the hydrothermal impact at 360 °С leads to the destruction of aliphatic parts of asphaltene molecules and carbonization of their structures. Hence, they lose solubility in aromatic solvents and become like carbenes and carboids. It was shown that the formation of carbenes and carboids not only due to the hydrothermal destruction of kerogen, but also the destruction of high-molecular components of OM—resins and asphaltenes.
The differences in the content and composition of hydrothermal products were evaluated based on the content of OM in rock samples and their thermal stability. The yield of shale oil from carbonate rock samples of Zelenogorskaya area before and after the hydrothermal experiments do not prevail 1% and 56.4%, corresponding to the saturate fraction. The yield of oil extract from the initial Domanic rock of the Berezovskaya area is sharply low, –0.25%. However, the hydrothermal influence on the rock samples increases the yield of shale oil up to 4.56%. The content of saturates in extracts increases from 18.58 up to 35.69%, but their content is two times lower than the saturates of extracts from carbonate rocks of the Zelenegorskaya area. The composition of the last is rich in aromatic compounds, resins and asphaltenes.
Structural-group composition of asphaltenes, carbenes and carboids. Hydrothermal treatment of Domanic rock samples at 350 °С results in the carbonization of asphaltene structures and changes of their structural-group composition, which is concluded by the changes in the FT-IR intensity of absorption bands at 1600 cm−1, 952 cm−1 and 817 cm−1. The given absorption bands correspond to the bonds of C=C aromatic structures (Figure 1a,b). In the FT-IR spectra of carbenes and carboids, absorption bands of aromatic structures at 1600–1642 cm−1 are significant in contrast to the asphaltenes from the initial rock samples and after hydrothermal experiments (Figure 1c,d).
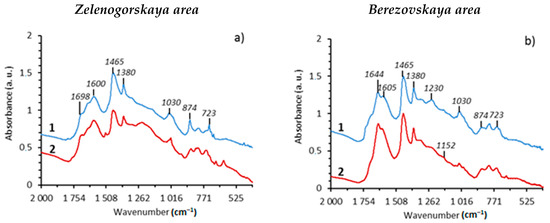
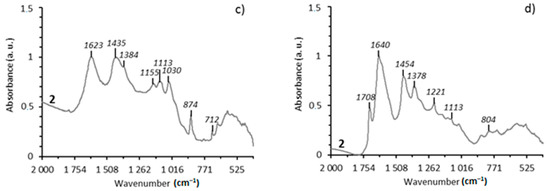
Figure 1.
IR specters of asphaltenes (a,b) and carbene-carboids (c,d) from the rocks of the Zelenogorskaya and Berezovskaya areas before and after the experiments: 1—initial rock, 2—experiment at 350 °С.
The aromaticity parameter C1 = D1600/D720 for carbenes/carboids is four times higher than the aromaticity of asphaltenes (Table 3). It should be noted that the aromaticity parameter (C1) for asphaltenes of the Berezovskaya area increases from 3.64 to 6.03, while that for the asphaltenes of the Zelenogorskaya area decreases from 3.9 to 3.52. It may be related with the more intensive destruction of OM components of carbonate rocks in hydrothermal processes. At 350 °С, the total destruction of kerogen with the formation of significant amount of carbenes and carboids, in the case of the carbonate rock sample from the Zelenogorskaya area, is observed (Table 1). However, the content of resins and asphaltenes in such shale oil is reduced due to the transformation into insoluble carbenes and carboids under the hydrothermal impact [46,47]. As the result, asphaltenes with more aliphatic structure remains in the oil system.

Table 3.
Spectral parameters of asphaltenes, carbenes and carboids for the asphaltenes of Domanic rock samples before and after the hydrothermal experiments.
The results of the FT-IR analysis of asphaltenes from Domanic rock samples of the Berezovskaya area after hydrothermal experiment show a decrease in intensity of the absorption band at 1030 cm−1, which indicates the destruction processes carried out in SO-containing groups.
This also reflects the sulfurization parameter C5 = D1030/D1465, which significantly decreases from 0.44 to 0.23. The given parameter is higher for the asphaltenes of shale oil from the Zelenogorskaya area and it is probably due to the transition into carbenes/carboids. In this regard, it is important to evaluate the changes in the content of SO-group in carbenes and carboids. Thus, carbenes and carboides form after the hydrothermal process. The carbenes and carboids formed after hydrothermal processes at 350 °С are quite similar in structural-group composition, independent from initial rock types (Table 3). However, the content of the SO group is two times higher in carbenes and carboids of rock samples from the Zelenogorskaya area rather than in asphaltenes. In the case of the Berezovskaya area samples, their content in carbenes and carboids is almost the same as in the initial rock sample.
Hydrocarbon composition of saturated and aromatic fractions of shale oil. The content of various group of hydrocarbons in saturated fractions of shale oil from Domanicoid rocks of Zelegosrk area and Domanic rocks of Berezovskaya area, as well as individual hydrocarbon composition of the given fractions, were determined by the GC/MS method, the results of which are presented in Table 4 and Figure 2 and Figure 3. The saturated fractions contain predominantly aliphatic hydrocarbons. The content of n-alkanes and isoprenoids is high in the composition of saturated fractions of shale oil from carbonate rocks of the Zelenogorskaya area. After hydrothermal treatment of rocks, their content increases from 83.46% to 91.72% in carbonate rocks, for the carbonate–siliceous rocks of the Berezovskaya area from 67.37 to 74.43%.

Table 4.
The relative content (%) of different type hydrocarbons in saturated fractions of shale oil from the rocks of Zelenogorskaya and Berezovskaya areas.
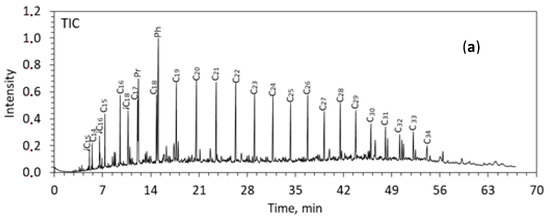
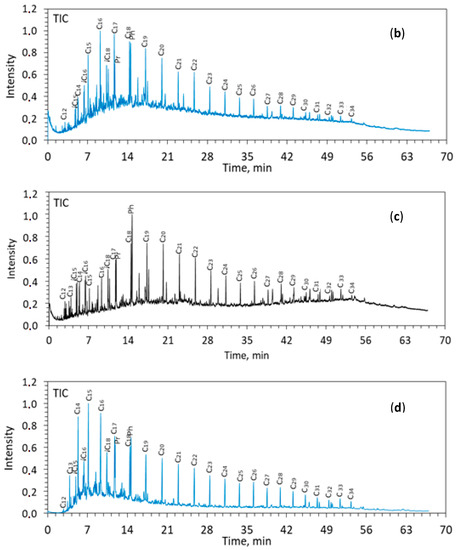
Figure 2.
TIC of saturated fractions from the rocks of Zelenogorskaya and Berezovskaya areas before (a,b) and after (c,d) the experiments. C12–C35—the number of carbon atoms in n-alkanes. Pr—pristane (C19), Ph—phytane (C20).
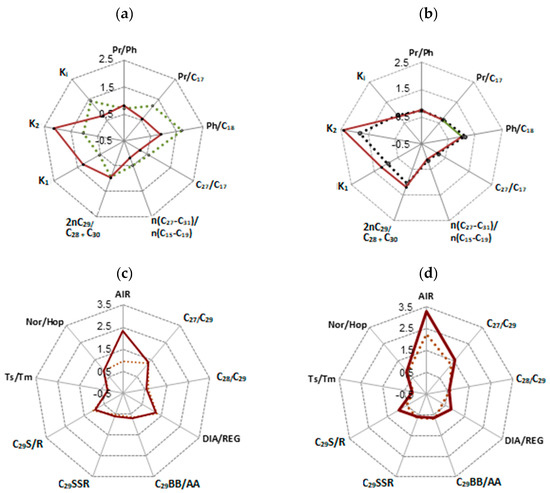
Figure 3.
Geochemical parameters of saturated fractions of the rocks from Zelenogorskaya and Berezovskaya areas before and after hydrothermal experiments: (a,b) alkanes; (c,d) steranes and hopanes, arylisoprenoids. AIR = (С13–С17)/(С18–С22); К1 = (C12–C14)/(C14–C15); К2 = (C14–C19)/(C19–C23); Ki = (П + Ф)/(С17 + С18); TS/TM = C27 18α Трисноргопан (TS)/C27 17α Трисноргопан (Тm); DIA/REG = C27 20S βα Диастеран/С29 20R ααα Cтеран: C29S/R = C29 20S ααα Стеран /20R ααα Стеран: C29BB/AA = C29 20R αββ Стеран/C29 20R ααα Стеран: C29SSR =С2920Sααα- sterane/С2920Sααα-sterane + С2920Rααα-sterane.
According to the chemical classification of Al. A. Petrov [39], which is based on the molecular-mass distribution of normal and isoprenoid alkanes, extracts from initial Domanic rock samples correspond to the highly paraffinic oil, type A. Further, depending on the Кi, which is the ratio of pristane (C19) and phytane (C20), to the C17 and C18 n-alkanes, we subdivide type A into two categories: A1 if Ki > 1 and A2 if Ki < 1 (Figure 3).
The specific feature of the hydrocarbon extracts from the original Domanic rocks is the predominance of phytane (C20) over the content of n-alkane C18. This difference is especially pronounced for the carbonate rocks of the Zelenogorskaya area (Figure 2a). The hydrothermal impact on the rock samples smooths out these differences. The difference in the molecular-mass distribution of normal and isoprenoid alkanes is little after hydrothermal treatment of shale oil (Figure 3a,b).
The results of the investigation justify the correspondence of hydrocarbon fluids to a single genetic series and its connection with sapropel matter of marine genesis [33,39,40]. The hydrocarbon composition of shale oil after hydrothermal treatment becomes similar to the shale oil produced by PJSC Tatneft from low-permeable carbonate rocks of the Dankovo–Lebedyan horizon of the Bavlinsky reservoir [14,57,58], which is located in the east of Tatarstan.
The content of polycyclic biomarkers, steranes and triterpenes, in saturated fractions of shale oil from the rocks of the Zelenogorskaya and Berezovskaya areas is not high and almost the same (Table 4). Their content is significantly reduced after hydrothermal treatment: steranes from 1.62 and 1.38 to 0.44 and 0.63%, triterpenes from 5.72 and 5.34 to 1.80 and 2.30, correspondingly. The values of sterane parameters: C29BB/AA (С2920R αββ-sterane/С2920Rααα-sterane), C29SSR (С2920Sααα- sterane/С2920Sααα-sterane + С2920Rααα-sterane) and C29S/R (С2920Sααα-sterane/С2920Rααα-sterane), as well as Ts/Tm parameter, which is the ratio of stable C2718α (H) to the less stable isomer—C2717α(H), reflect the close maturity degree of initial OM of shale oil from the rocks of the Zelenogorskaya and Berezovskaya areas of the Romashkino field (Figure 3c,d). However, the differences in their composition before and after the experiments are observed in the DIA/REG parameter (the ratio of regrouped C2720Sβα-diasterane to C2920Rααα-sterane; 1.29 and 0.36 versus 1.21 and 0.83, correspondingly). The oil from carbonate formations of the Dankovo–Lebedyan horizon of the Zelenogorskaya area has higher values of the given parameter, which indicates a relationship between the formation of its hydrocarbon composition with clay minerals [40,42,44]. This justifies the conclusions of some authors [32,33]; according to the pyrolysis data (see Table 1), hydrocarbons in the carbonate rocks of the Zelenogorskaya area are not syngenetic to the enclosing sediments, but are migration fluids. The DIA/REG ratio increases almost twice (from 0.36 to 0.83) after hydrothermal processes for the shale oil of rocks extracted from the Berezovskaya area. In the case of the Zelenogorskaya area, the given value is almost constant (1.29 and 1.21).
The distinguished feature of saturated fractions of rock samples is the content of alkyltrimethylbenzene (or aryl isoprenoid) and monoaromatic steroid (Table 4). The content of alkyltrimethylbenzene (C11–C22) in oil decreases after hydrothermal treatment by three times, while the content of relatively light homologues increases.
The significant difference between the rock samples is observed in the values of arylisoprenoid index (AIR), which stands for the ratio of the sum of arylisoprenoids (С13–С17) to their high-molecular homologues of С18–С22 (Figure 3 and Figure 4). The higher value of AIR is specific for the rock sample of Berezovskaya area (2.25 versus 0.96), which is probably due to different intensity of unstable photic zone of hydrogen sulfide contaminations on the territory of initial OM transformations. The destruction of OM of Domanic rocks in hydrothermal processes increases the AIR value from 0.96 to 2.32 for the crude oil from the Zelenogorskaya area, and from 2.25 to 3.30 for the rock sample from the Berezovskaya area. The differences in the composition of arylisoprenoids of initial extracts from the rock samples with different composition persist in the composition of shale oil generated in the course of hydrothermal experiments. The content of monoaromatic steroids is significantly higher in the composition of oil from the Domanic rocks of the Berezovskaya area in comparison to the oil from carbonate rocks of the Zelenogorskaya area: 3.46 vs. 19.16% and 5.75 vs. 10.74%, correspondingly (Table 3). The content of such hydrocarbons after hydrocarbon treatment of rocks decreases from 5.75% to 4.99% in the saturates fraction of the oil extracted from the Zelenogorskaya rock sample. Oppositely, the mentioned hydrocarbons significantly increase (from 10.74 to 18.18%) in the saturates fraction of oil extracted from the Berezovskaya rock sample.
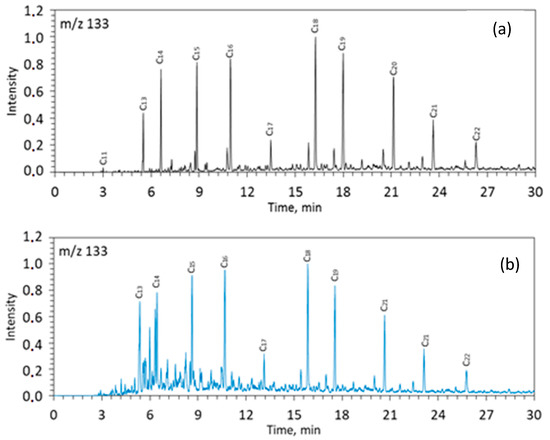
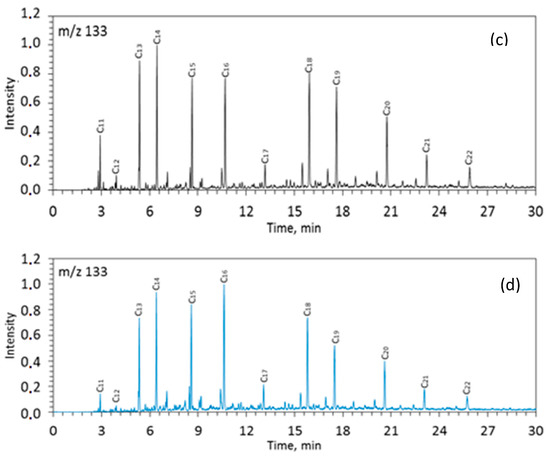
Figure 4.
Mass-fragmentogram at m/z 133 for saturated fractions of rock samples from the Zelenogorskaya and Berezovskaya areas before (a,c) and after (b,d) the experiments. C12–C22—the number of carbon atoms in aryl isoprenoids.
Hydrocarbon composition of aromatic fractions of shale oil. The relative content of different hydrocarbon groups considering trimethyl benzene, naphthalene, phenanthrene, benzthiophene, dibenzothiophene (DBT) in aromatic fractions of shale oil from Zelenogorskaya and Berezovskaya rock samples are presented in Table 5, and the TIC of aromatic fractions are presented in Figure 5 [43,46,47,48,49].

Table 5.
Relative content (%) of different type of compounds in aromatic fractions of shale oil before and after the hydrothermal experiments.
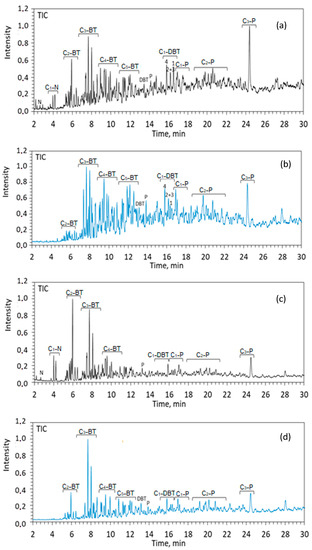
Figure 5.
TIC of aromatic fractions of shale oil from the rock samples of Zelenogorskaya and Berezovskaya areas before (a,c) and after (b,d) hydrothermal experiments. N—naphthalene, P—phenanthrene, DBT—dibenzothiophene and BT—benzothiophene, n—number of carbon atoms in alkyl substitutes of Cn.
The concentrations of aromatic sulfur organic compounds, benzothiophene (56.64 and 56.10%), are high in the composition of aromatic fractions of shale oil, independent from the rock type. After the hydrothermal treatment of rock samples, the content of such compounds decreases to 42.71% and 38.75%, correspondingly. This indicates that the destruction processes are carried out mainly on sulfur-containing bonds. The content of organosulfur compounds is very important in terms of shale oil production. The aromatic organosulfur compounds, such as benzothiophene and dibenzothiophene, and the alkyl-substituted isomers of them, present mainly in heavy oil and heavy fractions [43]. The hydrothermal transformation of the OM of Domanic rocks decreases the content of benzothiophene and increases the content of dibenzothiophene in shale oil by almost twice. It increases from 9.42 to 17.07% in the case of Zelenogorskaya rock samples and in the Berezovskaya rock samples, from 11.12% to 20.42%. In [59], a correlation was found between the values of the ratio of the benzothiophene isomers to dibenzothiophenes and the types of hydrocarbon reservoirs: with an increase in the degree of catagenesis of the hydrocarbon system and with a significant decrease in the content of benzothiophenes, the concentration of methyl-substituted dibenzothiophenes increases. Similar behavior was revealed in the composition of shale oil under hydrothermal processes. The correlation of benzothiophene/dibenzothiophene for the shale oil of Zelenogorskaya area reduces from 6.01 to 2.50, for the shale oil of Berezovskaya area from 5.04 to 1.89. The molecular-mass distribution of aromatic organosulfur compounds, benzothiophene and dibenzothiophene, in the composition of shale oil from Domanic rocks before and after hydrothermal experiments at 350 °С are presented in Figure 6.
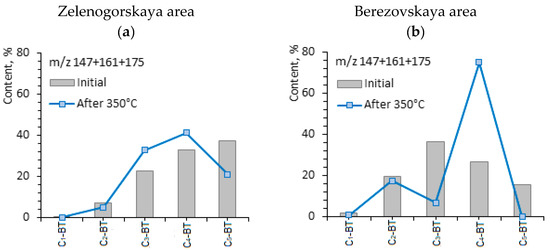
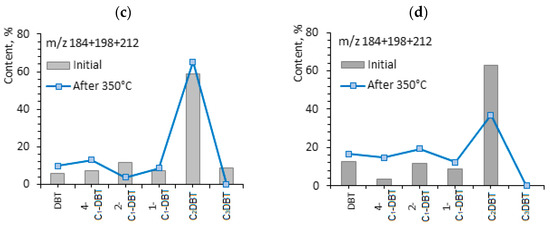
Figure 6.
Molecular-mass distribution of aromatic organosulfur compounds: benzothiophene (a,b) and dibenzothiophene (c,d) in shale oil before and after hydrothermal experiments at 350 °С.
According to the abovementioned authors [59] the ratio of 4-methyl-di-benzothiophene to 1-methyl-di-benzothiophene (4-MDBT/1-MDBT) is very sensitive to thermal maturity evolution system. In [60], the authors also proposed that the ratio of di-methyl-di-benzothiophene may reflect the maturity degree of naphtides. The 4-MDBT/1-MDBT ratio in shale oil of carbonate rocks from the Zelenogorskaya area after hydrothermal treatment increases from 1.01 to 1.53, and in the case of Berezovskaya shale oil, from 0.39 to 1.19. According to the changes in the given ratio, one may propose that hydrothermal impact on the given rock samples leads to an increase in thermal maturation, from which shale oil is produced.
Hence, it is attractive to interpret and apply the ratio of 4-MDBT/1-MDBT. In [60], 4-MDBT is less stable than 1-MDBT during the microbial decomposition processes and the ratio of 4-MDBT/1-MDBT is used in the evaluation of bacterial degradation degree of heavy oil in reservoir development processes. The higher value of the given parameter is specific for the original extracts from carbonate–siliceous rocks of the Berezovskaya area in contrast to the carbonate rocks of the Zelenogorskaya area (2.57 versus 0.99). This indicates not only the different thermal transformation degree of hydrocarbons in the given Domanic rock samples, but also the different biochemical degradation degree.
Some other authors investigated the stability of asphaltenes by the thermal destruction method [56]. They revealed that the main share of sulfur in asphaltenes is found in thiocyclan fragments and the least part in aromatic structures. The content of dibenzothiophene rather than benzothiophene and benzonaphthothiophene structures was higher in the destruction products of asphaltenes. Since the content of asphaltenes in the products of hydrothermal experiments decreases, this may serve as additional evidence for the increase in aromatic fractions of shale oil of thermally more stable methyl-substituted dibenzothiophenes and can be the result of both the decomposition of the kerogen structure with the formation of new heterocyclic sulfur-containing aromatic fragments and processes of destruction of high molecular weight compounds, such as resins and asphaltenes.
In this regard, data on the content of total sulfur in the composition of oil extracted from the studied Domanic rocks before and after the experiments are of interest. The sulfur content in the composition of oil from the original rock from the Zelenogorskaya area is 1.58%; after the experiment, its content increases to 3.60%. In the oil from the original rock of the Berezovskaya area, in comparison with the Zelenogorskaya area, the sulfur content is four times higher, -6.60%. After the experiment, the sulfur content does not increase so sharply, but remains quite high at 6.74%. The sulfur analysis data indicate a different type of kerogen in the studied rocks of different lithological–facies types, as well as an additional source of sulfur input into the oil composition as a result of kerogen decomposition and destruction of its high-molecular sulfur-containing fragments.
Besides aromatic organosulfur compounds, some naphthalenes, phenanthrenes and tri-methyl-alkyl-benzenes are identified in the aromatic fractions of shale oil (Table 5). The molecular mass distribution of the given hydrocarbon groups before and after hydrothermal treatment is presented in Figure 7. The content of naphthalene and phenanthrene increases in aromatic fractions after hydrothermal experiments. If the content of phenanthrene in the rock extracts before the experiments is almost the same (6.84 and 6.24%), then their content is different and increases up to 14.34% and 11.86% in aromatic fractions of shale oil after hydrothermal treatment. The content of naphthalene is higher in shale oil from the Domanic rocks of the Berezovskaya area, in contrast to the oil from carbonate rocks of the Zelenogorskaya area (25.14 vs. 18.01%). In [61], the possible ways of phenanthrene formation—biomarkers in OM of rocks and crude oils—are discussed. It is shown that such molecules can play a role of “secondary” biomarkers in shale oils generated by dispersed organic matter of different facies and ages.
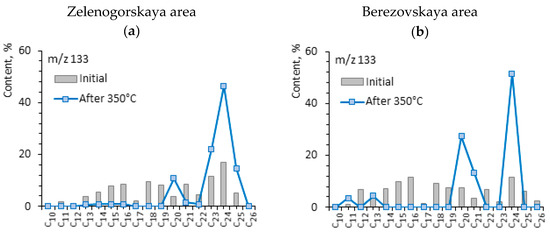
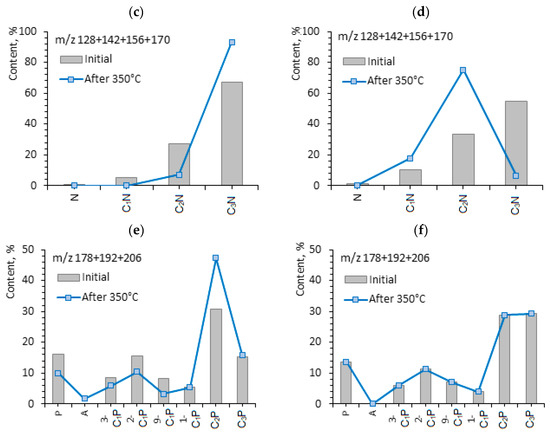
Figure 7.
Diagrams of the molecular weight distribution of (a,b) arylisoprenoids, (c,d) naphthalenes, and (e,f) phenanthrenes before and after hydrothermal experiments at 350 °C.
It is important to note the presence of alkyl-trimethylbenzenes (m/z 133 + 134) or arylisoprenoids—aromatic compounds characterized by the presence of long alkyl chains of isoprenoid structure in saturated fractions of the studied oils [20]. Due to the presence of long alkyl substitutes in aromatic benzene rings, aryl isoprenoids may concentrate both in saturates and aromatic fractions during SARA analysis. Aromatic and saturated fractions differ in the content and nature of the molecular weight distribution of these hydrocarbons. Thus, the content of aryl isoprenoids in saturate fractions of shale oil from Berezovskaya rock sample is very high, 19.16%, while their content in aromatic fraction is only 1.40%. Oppositely, in the shale oil of the Zelenogorskaya rock sample, saturates have only 3.46%, while aromatics contain about three times more (9.08%) aryl isoprenoids. The redistribution of aryl isoprenoids between saturates and aromatics depends on the difference in their molecular-mass composition of a certain shale oil. The aryl isoprenoid index AIR = С13–С17/С18–С22 increases in saturates after hydrothermal experiments (Figure 2 and Figure 3), due to the increase in low-molecular aryl isoprenoids composed of C13–C17, while AIR in aromatics decreases because of increasing high-molecular homologues (Figure 6a,b). Long alkyl chains of arylisoprenoids, apparently, undergo destruction with the formation of lower molecular weight homologues. The results of the study show that low-molecular homologues of aryl isoprenoids formed during hydrothermal destruction processes concentrate in saturated fractions.
4. Conclusions
The results of the carried out experiments reveal the influence of hydrothermal processes at a temperature of 350 °C in a carbon dioxide environment on the ability of OM of Domanic rock samples to generate shale oil. Moreover, the experimental results allow to justify the in situ dilution of heavy bituminous components of Domanic deposits by light hydrocarbons, which are generated in the given formations. The degree of heavy oil conversion is determined by their initial types and activation degree of destruction reactions on C–C, C–N, C–O, and C–S bonds of kerogen as well as high-molecular components, such as resins and asphaltenes. Hydrothermal impact on Domanic rock samples leads to more intensive and total extraction of shale oil, with an increased content of saturates and decreased content of aromatic hydrocarbons, reins and asphaltenes. The content of paraffinic hydrocarbons in the composition of saturates is significant. In the composition of aromatics, benzothiophene and dibenzothiophene are significant. It is revealed that asphaltenes from the Domanic and carbonate rock samples are different in their content and structural-group compositions. New insoluble fractions of coal-like matters, such as carbenes/carboids, are formed in the hydrothermal experimental products that can determine the various properties of the oil disperse system in a porous medium under hydrothermal impact.
The hydrothermal treatment of carbonate rocks with low content of OM contributes to the total destruction of kerogen, while in similar conditions, kerogen of Domanic rocks undergoes partial destruction. This shows the different oil-generating potential of the given rocks under hydrothermal influences. Thus, different stability of OM of the given rocks to the thermal influences and distinguished features of the composition and quality of the generated crude oil require special and probably different development approaches.
Author Contributions
Conceptualization, G.P.K.; methodology, Z.R.N. and I.P.K.; investigation, A.N.M.; writing—original draft preparation, F.A.A. and A.V.V. All authors have read and agreed to the published version of the manuscript.
Funding
The authors declare no competing financial interest.
Acknowledgments
This work was supported by the Ministry of Science and Higher Education of the Russian Federation under agreement No. 075-15-2020-931 within the framework of the development program for a world-class Research Center “Efficient development of the global liquid hydrocarbon reserves”.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Stoupakova, A.V.; Kalmykov, G.A.; Korobova, N.I.; Fadeeva, N.P.; Gatovskii, Y.A.; Suslova, A.A.; Sautkin, R.S.; Pronina, N.V.; Bolshakova, M.A.; Zavyalova, A.P.; et al. Domanic deposits of the Volga-Ural basin—Types of section, formation conditions and prospects of oil and gas potential. Georesursy 2017, 1, 112–124. [Google Scholar] [CrossRef]
- Khisamov, R.S.; Bazarevskaya, V.G.; Mikhailova, O.V.; Podavalov, V.B. Domanik pay zones in Tatarstan as analogs of shale plays in USA. Subsoil Use Xxi Century 2016, 3, 82–91. [Google Scholar]
- Khisamov, R. The field development strategy at a later stage, the prospects for extraction of hydrocarbon resources from unconventional sources of hydrocarbons in the Tatarstan Republic. Drill. Oil 2015, 1, 40–44. [Google Scholar]
- Khisamov, R.S.; Zakirov, I.S.; Zakharova, E.F.; Bazarevskaya, V.G.; Abusalimova, R.R.; Timirov, D.A. Experience of studying and development of Domanic deposits on the example of Bavlinskoye field of the Republic of Tatarstan (Russian). Neftyanoe Khozyaystvo-Oil Ind. 2018, 2018, 78–83. [Google Scholar] [CrossRef]
- Tissot, B.P.; Welte, D.H. Petroleum Formation and Occurrence, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 1984; p. 702. [Google Scholar]
- Prishchepa, O.M.; Averyanova, O.Y. The role of unconventional hydrocarbon sources in the mineral resource policy. Econ. Manag. 2013, 1, 21–24. [Google Scholar]
- Ananiev, V.V. Quality estimation of Semiluk and Rechitskiy source rocks potential of Tatarstan. Georesursy 2010, 3, 30–33. [Google Scholar]
- Kiselyova, I.A.; Mozhegova, S.V. Genetic groups and sources of oils in the central part of the Volga-Urals petroleum. Oil Gas Geol. Theory Pract. 2012, 7, 3. [Google Scholar]
- Kiryukhina, T.A.; Fadeyeva, N.P.; Stoupakova, A.V.; Poludetkina, E.N.; Sautkin, R.S. Domanik deposits of Timano-Pechora and Volga-Ural basins. Geol. Oil Gas 2013, 3, 76–87. [Google Scholar]
- Muslimov, R.; Plotnikova, I. About shale oil of the republic of Tatarstan. Oil Ind. 2014, 1, 12–15. [Google Scholar]
- Khisamov, R.S.; Bazarevskaya, V.G.; Tarasova, T.I.; Mikhailova, O.V.; Mikhailov, S.N. Geochemical evidence for petroleum potential of Domanic deposits in the Republic of Tatarstan. Oil Ind. 2016, 2016, 10–13. [Google Scholar]
- Vakhin, A.V.; Onishchenko, Y.V.; Chemodanov, A.E.; Sitnov, S.A.; Mukhamatdinov, I.I.; Nazimov, N.A.; Sharifullin, A.V. The composition of aromatic destruction products of Domanic shale kerogen after aquathermolysis. Pet. Sci. Technol. 2019, 37, 390–395. [Google Scholar] [CrossRef]
- Burdel'naya, N.S.; Bushev, D.A. Fragment of the chemical structure of type II and II-s kerogen in the Upper Jurassic and Upper Devonian formations of the east European Platform. Geochem. Int. 2010, 48, 492–504. [Google Scholar]
- Nasyrova, Z.R.; Kayukova, G.P.; Vakhin, A.V.; Djimasbe, R.; Chemodanov, A.E. Heavy oil hydrocarbons and kerogen destruction of carbonate-siliceous domanic shale rock in suband supercritical water. Processes 2020, 8, 800. [Google Scholar] [CrossRef]
- Wellington, S.L.; Madgavkar, A.M.; Ryan, R.C. Thermal Treatment of a Hydrocarbon-Bearing Formation at the Location and Improving the Quality of the Resulting Fluids before Further Processing. RU Patent No. 2004115632/03, 27 October 2005. [Google Scholar]
- Passi, K.R.; Thomas, M.M.; Bohaks, K.M. Mehtod for Obtaining Hydrocarbons from Rock Rich in Organic Compounds. RU Patent No. 2263774 C2, 1 November 2005. [Google Scholar]
- Kayukova, G.P.; Mikhailova, A.N.; Kosachev, I.P.; Musin, R.Z.; Nasyrova, Z.R.; Aliev, F.A.; Vakhin, A.V. Hydrothermal Impact on Hydrocarbon Generation from Low-Permeable Domanic Sedimentary Rocks with Different Lithofacies. Energy Fuels 2021, 35, 11223–11238. [Google Scholar] [CrossRef]
- Akiya, N.; Savage, P.E. Roles of water for chemical reactions in high-temperature water. Chem. Rev. ACS Publ. 2002, 102, 2725–2750. [Google Scholar] [CrossRef]
- Siskin, M.; Katritzky, A.R. Review of the reactivity of organic compounds with oxygen-containing functionality in superheated water. J. Anal. Appl. Pyrolysis 2000, 54, 193–214. [Google Scholar] [CrossRef]
- Nasyrova, Z.R.; Kayukova, G.P.; Onishchenko, Y.V.; Morozov, V.P.; Vakhin, A.V. Conversion of High-Carbon Domanic Shale in Sub- and Supercritical Waters. Energy Fuels 2020, 34, 1329–1336. [Google Scholar] [CrossRef]
- Kiryachek, V.G.; Kolomiychenko, O.V.; Klinkov, N.N.; Chernov, A.A.; Tsvetkov, D.B. Method for Combined Effect on Formations Containing Hydrocarbons and/or Solid Organic Substances and Device for Implementing Said Method. RU Patent No. 2576267, 15 May 2015. [Google Scholar]
- Punanova, S.A.; Nukenov, D. Estimation of the trace element composition of shale formations. Actual Probl. Oil Gas 2019, 1, 20. [Google Scholar] [CrossRef] [Green Version]
- Trukhina, O.S.; Sintsov, I.A. Experience of Carbone dioxide usage for enhanced oil recovery. Adv. Mod. Nat. Sci. 2016, 3, 205–209. [Google Scholar]
- Rokosova, N.N.; Rokosov, Y.V.; Uskov, S.I.; Bodoev, N.V. Simulation of transformations of organic matter into hydrothermal petroleum (a review). Pet. Chem. 2001, 41, 221–233. [Google Scholar]
- Kiyamova, A.M.; Kayukova, G.P.; Romanov, G.V. Composition of the high-molecular-mass components of oil- and bitumen-bearing rocks and their hydrothermal transformation products. Pet. Chem. 2011, 51, 231–242. [Google Scholar] [CrossRef]
- Gordadze, G.N. Thermolysis of organic matter in oil and gas exploration geochemistry. M IGiRGI 2002, 334. [Google Scholar]
- Melenevsky, V.N.; Kontorovich, A.E.; Huang, W.-L.; Larichev, A.I.; Bul'bak, T.A. Hydrothermal pyrolysis of organic matter in riphean mudstone. Geochem. Int. 2009, 47, 476–484. [Google Scholar] [CrossRef]
- Antipenko, V.R.; Golubina, O.A.; Goncharov, I.V.; Nosova, S.V.; Rokosov, Y.V. Composition of products of hydrothermal transformation of natural asphaltite. Bull. Tomsk Polytech. Univ. 2005, 308, 122–127. [Google Scholar]
- Kayukova, G.P.; Feoktistov, D.A.; Pronin, N.V.; Eskin, A.A. Oil-generating potential of bituminous rocks from Permian and Domanic deposits in Tatarstan by the data of the pyrolytic Rock-Eval method. Oil Ind. 2017, 12, 86–89. [Google Scholar] [CrossRef]
- Shock, V.L.; Canovas, P.; Yang, Z.; Boyer, G.; Johnson, K.; Robinson, K.; Fecteau, K.; Windman, T.; Cox, A. Thermodynamics of Organic Transformations in Hydrothermal Fluids. Rev. Mineral. Geochem. 2013, 76, 311–350. [Google Scholar] [CrossRef]
- Stennikov, A.V.; Bugaev, I.A.; Kalmykov, A.G.; Bychkov, Y.A.; Kozlova, E.V.; Kalmykov, G.A. Experimental study of gydrotermal production of oil from domanik formation rocks. Bull. Mosc. Univ. Ser. 4 Geol. 2017, 6, 64–69. [Google Scholar] [CrossRef]
- Plotnikova, I.N.; Ostroukhov, S.B.; Laptev, A.A.; Gazizov, I.G.; Emelyanov, V.V.; Pronin, N.V.; Nosova, F.F.; Salikhov, A.D. Migration aspect in the oil-bearing capacity of the Domanic formation in Tatarstan. Georesursy 2017, 19, 348–355. [Google Scholar] [CrossRef] [Green Version]
- Kayukova, G.P.; Mikhailova, A.N.; Morozov, V.P.; Musin, R.Z.; Vandyukova, I.I.; Sotnikov, O.S.; Remeev, M.M. Study of Changes in the Composition of Organic Matter of Rocks from Different Sampling-Depth Intervals of Domanik and Domankoid Deposits of the Romashkino Oilfield. Pet. Chem. 2019, 59, 1124–1137. [Google Scholar] [CrossRef]
- Bazarevskaya, V.G. The unique Romashkinskoye field in Tatarstan is an inexhaustible source of growth in oil reserves. Georesursy 2006, 2, 9–11. [Google Scholar]
- Disnar, J.R.; Guillet, B.; Keravis, D.; Giovanni, C.D.; Sebag, D. Soil organic matter (SOM) сharacterization by Rock-Eval pyrolysis: Scope and limitations. Organ. Geochem. 2003, 34, 327–343. [Google Scholar] [CrossRef] [Green Version]
- Nekozyreva, T.N.; Shalamberidze, O.V. Chemistry of Oil and Gas: Textbook; TyumGNGU: Tyumen, Russia, 2013; p. 76. [Google Scholar]
- Zhang, C.; Lee, C.W.; Keogh, R.A.; Demire, B.; Davis, B.H. Thermal and catalytic conversion of asphaltenes. Fuel 2001, 80, 1131–1146. [Google Scholar] [CrossRef]
- Ivanova, L.V.; Safieva, R.Z.; Koshelev, V.N. IR spectrometry in the analysis of oil and oil products. Bull. Bashkir Univ. 2008, 13, 869–874. [Google Scholar]
- Petrov, A.A. Hydrocarbons of Oil; Nauka: Moscow, Russia, 1984; p. 263. [Google Scholar]
- Gordadze, G.N.; Tikhomirov, V.I. Geochemical characteristics of oils and dispersed organic matter from the rocks of the central Volga-Ural basin: Hydrocarbon biomarker data. Geochem. Int. 2005, 43, 1108–1123. [Google Scholar]
- Schwark, L.; Frimmel, F. Chemostratigraphy of the Posidonia Black Shale, SW-Germany 11. Assessment of extent and persistence of photic-zone anoxia using arylisoprenoid distributions. Chem. Geol. 2004, 206, 231–248. [Google Scholar] [CrossRef]
- Kayukova, G.P.; Minnegalieva, A.M.; Romanov, A.G.; Kiyamov, A.M.; Sharipova, N.S.; Smelkov, V.M.; Dakhnova, M.V.; Nechitailo, G.S. Differentiation of Romashkino Crude Oils According to Biomarker Hydrocarbon Parameters. Pet. Chem. 2006, 46, 314–323. [Google Scholar] [CrossRef]
- Antipenko, V.R. Thermal Transformations of High-Sulfur Natural Asphaltite: Geochemical and Technological Aspects; Nauka: Novosibirsk, Russia, 2013; p. 184. [Google Scholar]
- Kiseleva, Y.A.; Zheglova, T.P.; Dakhnova, M.V.; Mozhegova, S.V.; Nazarova, E.S.; Nechitailo, G.S. The role of Domanik deposits in the formation of oil pools in the central areas of the Volga-Ural petroleum province (Buzuluk depression). Russ. Geol. Geophys. 2017, 58, 310–321. [Google Scholar] [CrossRef]
- Poludetkina, E.N.; Fadeeva, N.P.; Smirnov, M.B.; Kozlova, E.V. Proof of formation of organic matter in upper Devonian carbonate and carbonate-siliceous sediments of the South-Tatar uplift in constant photic layer anoxia. Geochem. Int. 2017, 55, 726–736. [Google Scholar] [CrossRef]
- Antipenko, V.R.; Kayukova, G.P.; Abdrafikova, I.M. Composition of hydrothermal–catalytic conversion products of asphaltite from the Spiridonovskoe oilfield. Pet. Chem. 2019, 59, 48–56. [Google Scholar] [CrossRef]
- Krasnoyarova, N.A.; Chirkova, D.Y.; Serebrennikova, O.V. Depositional environment and composition of dispersed organic matter of the lower Jurassic-Paleozoic rocks in the Archinskaya field (south-east of western Siberia). Bull. Tomsk State Univ. 2014, 388, 235–245. [Google Scholar]
- Antipenko, V.R. Change in composition of the oil fraction during nonisothermal aquathermolysis of natural asphaltite. Pet. Chem. 2012, 52, 171–178. [Google Scholar] [CrossRef]
- Antipenko, V.R.; Grin’ko, A.A.; Melenevskii, V.N. Composition of products of analytical pyrolysis of resin and asphaltene fractions of Usa oil. Pet. Chem. 2014, 54, 178–186. [Google Scholar] [CrossRef]
- Elkhoury, J.E.; Ameli, Р.; Detwiler, R.L. Dissolution and deformation in fractured carbonates caused by flow of CO2-rich brine under reservoir conditions. Int. J. Greenh. Gas. Control 2013, 16, 203–215. [Google Scholar] [CrossRef]
- Eisenlohr, L.; Meteva, К.; Gabrovšek, F.; Dreybrodt, W. The inhibiting action of intrinsic impurities in natural calcium carbonate minerals to their dissolution kinetics in aqueous H2O–CO2 solutions. Geochim. Cosmochim. Acta 1999, 63, 989–1001. [Google Scholar] [CrossRef]
- Surkov, V.G.; Golovko, A.K.; Mozhaiskaya, M.V. Influence of the conditions of mechanical action on the change in the composition of oil paraffins. Bull. Tomsk. Polytech. Univ. 2012, 321, 148–152. [Google Scholar]
- Saveliev, V.V.; Golovko, A.K.; Kamyanov, V.F. Influence of conditions for preliminary mechanical activation of oil shale on the yield and composition of products during thermolysis in water. Bull. Tomsk. Polytech. Univ. 2013, 323, 52–59. [Google Scholar]
- Batalin, O.Y.; Vafina, N.G. Forms of free-hydrocarbon capture by kerogen. Int. J. Appl. Basic Res. 2013, 10, 418–425. [Google Scholar]
- Burdelnaya, N.S.; Boushnev, D.A.; Mokeev, M.V. Structural evolution in the kerogen during artificial and natural maturation by solid-state 13c NMR spectroscopy. Bull. IG Komi Sci. Cent. Ural Branch RAS 2015, 6, 33–39. [Google Scholar]
- Grynko, A.A.; Golovko, A.K. Investigation of the stability of oil pyrobitumen by means of thermal destruction. Chem. Sustain. Dev. 2011, 19, 327–334. [Google Scholar]
- Kayukova, G.P.; Kiyamova, A.M.; Romanov, G.V. Hydrothermal transformations of asphaltenes. Pet. Chem. 2012, 52, 5–14. [Google Scholar] [CrossRef]
- Kayukova, G.P.; Mikhailova, A.N.; Kosachev, I.P.; Nazimov, N.A.; Sotnikov, O.S.; Evdokimov, A.E.; Khisamov, R.S. Composition of shale oil from poorly permeable carbonate rocks of domanikovian deposits of Dankov-Lebedyan horizon of Romashkino field. Chem. Technol. Fuels Oils 2018, 54, 173–186. [Google Scholar] [CrossRef]
- Chakhmakhchev, A.V.; Vinogradova, T.L.; Agafonova, Z.G.; Gordadze, G.N.; Chakhmakhchev, V.A. Benzothiophenes—Highly informative indicators of the catagenesis of hydrocarbon systems. Geol. Oil Gas 1995, 7, 32–37. [Google Scholar]
- Jawanarajah, S.R.; Kruge, M.A. Lacustrine shales from Stellarton Basin, Nova Scotia, Canada: Organofacies variations and use of polyaromatic hydrocarbons as maturity indicators. Org. Geochem. 1994, 21, 153–170. [Google Scholar] [CrossRef]
- Kashirtsev, V.A.; Parfenova, T.M.; Golovko, A.K.; Nikitenko, B.L.; Zueva, I.N.; Chalaya, O.N. Phenanthrene biomarkers in the organic matter of Precambrian and Phanerozoic deposits and in the oils of the Siberian platform. Russ. Geol. Geophys. 2018, 59, 1380–1388. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).