Photocatalysis for Heavy Metal Treatment: A Review
Abstract
1. Introduction
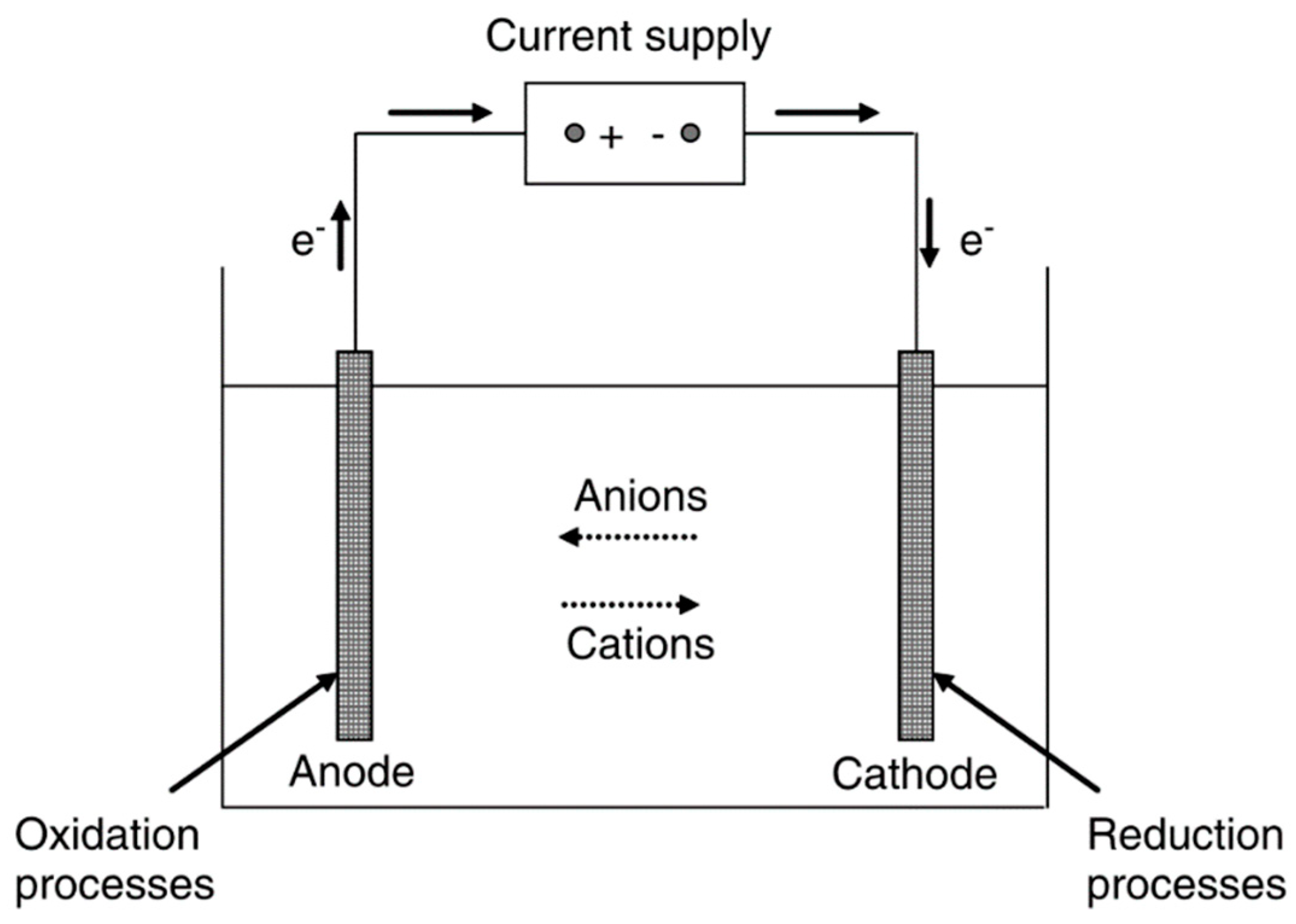
2. Traditional Heavy Metal Treatment
3. Photocatalytic Heavy Metal Treatment
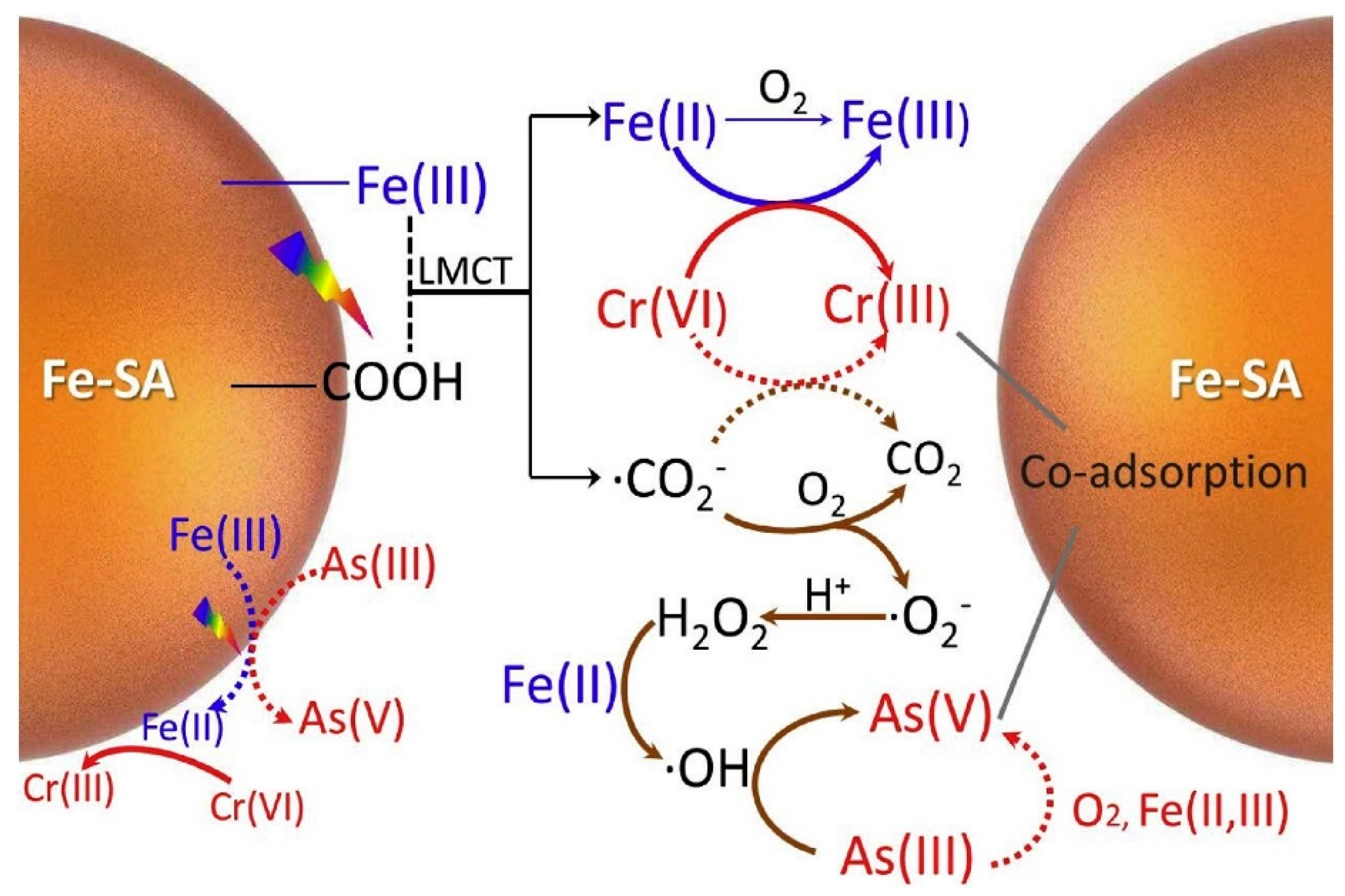
3.1. Chromium (Cr)
3.2. Arsenic (As)
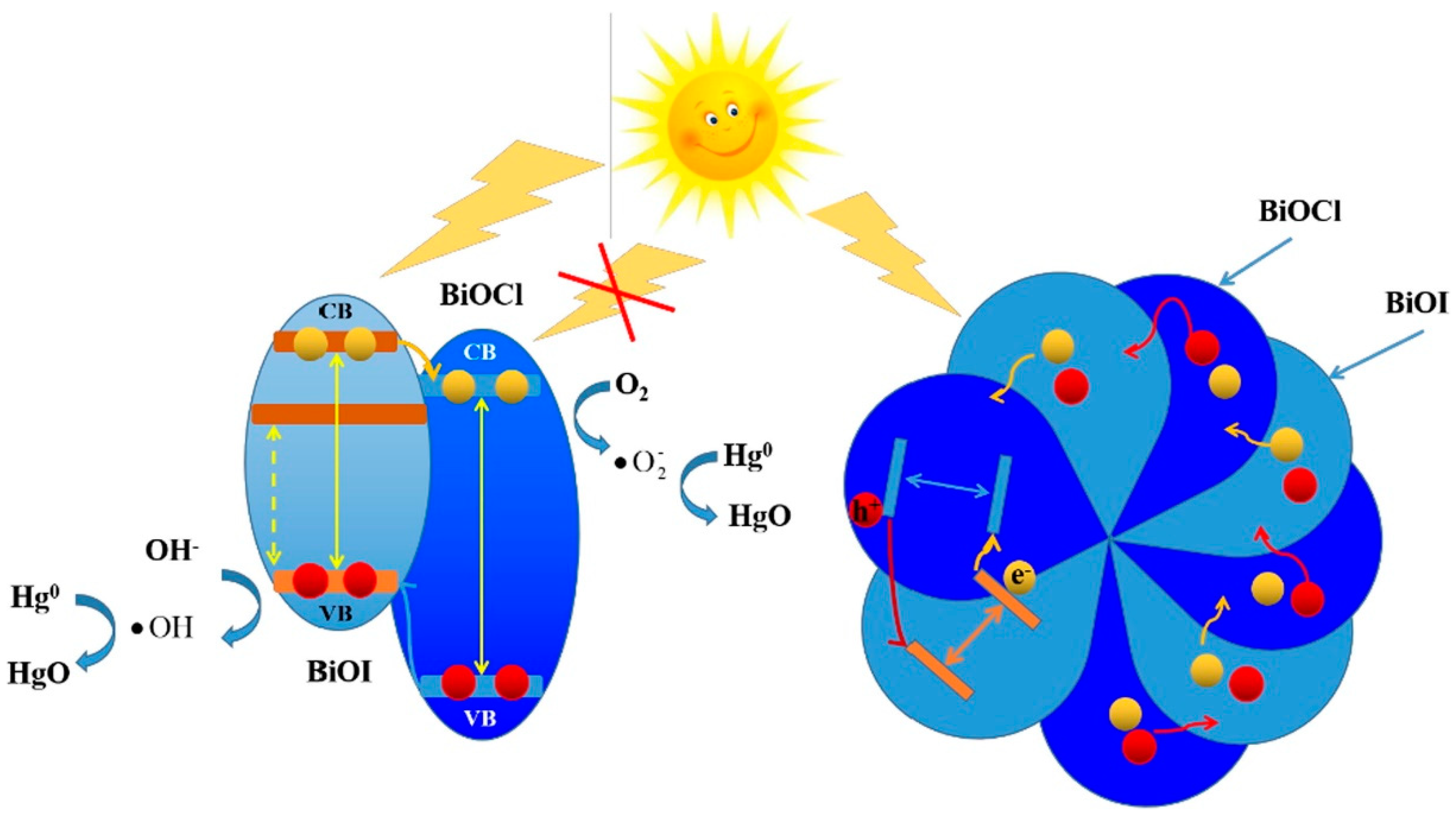
3.3. Mercury (Hg)
3.4. Other Heavy Metals
4. Discussion and Outlooks
- Low photocatalytic efficiency: At present, the efficiency of the photocatalytic reaction remains low. This is reflected not only in the removal of heavy metals but also in other photocatalytic processes. This situation is expected to be improved as more new materials presenting higher catalytic activity and higher stability efficiency or modification methods (such as doping and morphology control) that can improve the photocatalytic activity and stability of existing materials are proposed.
- Low light utilization efficiency: Although natural solar energy resources are extremely abundant, the current light energy that can be utilized by photocatalysis is still very low. From one perspective, this is caused by the poor response of the photocatalyst to visible light. From another perspective, it is related to the current photocatalytic system. The former can be improved through the improvement of materials while the latter may require breakthroughs in reactor design.
- Continuous operation method: It is difficult to remove heavy metals through simple oxidation or reduction. It is a common photocatalytic treatment method to convert difficult-to-treat heavy metal atoms or ions into a form that is easier to adsorb or settle and before removal. Its practical application would lie in the organic combination of the catalytic process and the adsorption/sedimentation process to ensure that the entire process has better overall continuity.
- No standard platform: Although there is a WHO standard for the removal of heavy metals, this standard has not been widely adopted, especially in photocatalysis research. Moreover, the existing research on the use of light sources and other aspects of the divergence is sufficient. To date, no standard platform can use a unified standard to evaluate the photoactivity of different photocatalysts in different laboratories. The establishment of this standard is necessary and urgent.
- Technology coupling: With the current efficiency of photocatalysis, it is significantly difficult to complete the task of water treatment on its own. Generally, the combination of different technologies is effective. It may be a good choice to organically combine the photocatalytic system with the existing water treatment technology.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Litter, M.I. Mechanisms of removal of heavy metals and arsenic from water by TiO2-heterogeneous photocatalysis. Pure Appl. Chem. 2015, 87, 557–567. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Gao, X.; Yao, Y.; Meng, X. Recent development on BN-based photocatalysis: A review. Mater. Sci. Semicond. Process. 2020, 120, 105256. [Google Scholar] [CrossRef]
- Yao, Y.; Gao, X.; Li, Z.; Meng, X. Photocatalytic Reforming for Hydrogen Evolution: A Review. Catalysts 2020, 10, 335. [Google Scholar] [CrossRef]
- Yao, Y.; Gao, X.; Meng, X. Recent advances on electrocatalytic and photocatalytic seawater splitting for hydrogen evolution. Int. J. Hydrogen Energy 2021, 46, 9087–9100. [Google Scholar] [CrossRef]
- Yao, Y.; Ren, G.; Li, Z.; Bai, H.; Hu, X.; Meng, X. Nitrogen Vacancy-Induced Deposition of Pd Nanoparticles onto g-C3N4 with Greatly Improved Photocatalytic Activity in H2 Evolution. Sol. RRL 2021, 5, 2100145. [Google Scholar] [CrossRef]
- Argurio, P.; Fontananova, E.; Molinari, R.; Drioli, E. Photocatalytic membranes in photocatalytic membrane reactors. Processes 2018, 6, 162. [Google Scholar] [CrossRef]
- Ullah, H.; Viglašová, E.; Galamboš, M. Visible Light-Driven Photocatalytic Rhodamine B Degradation Using CdS Nanorods. Processes 2021, 9, 263. [Google Scholar] [CrossRef]
- Ulyankina, A.; Mitchenko, S.; Smirnova, N. Selective photocatalytic oxidation of 5-HMF in water over electrochemically synthesized TiO2 nanoparticles. Processes 2020, 8, 647. [Google Scholar] [CrossRef]
- Wang, C.; Sun, X.; Shan, H.; Zhang, H.; Xi, B. Degradation of Landfill Leachate Using UV-TiO2 Photocatalysis Combination with Aged Waste Reactors. Processes 2021, 9, 946. [Google Scholar] [CrossRef]
- Li, Z.; Meng, X.; Zhang, Z. Recent development on MoS2-based photocatalysis: A review. J. Photochem. Photobiol. C Photochem. Rev. 2018, 35, 39–55. [Google Scholar] [CrossRef]
- Mukherjee, D.; Barghi, S.; Ray, A.K. Preparation and characterization of the TiO2 immobilized polymeric photocatalyst for degradation of aspirin under UV and solar light. Processes 2014, 2, 12–23. [Google Scholar] [CrossRef]
- Mukherjee, D.; Ray, A.K.; Barghi, S. Mechanism of acetyl salicylic acid (aspirin) degradation under solar light in presence of a TiO2-polymeric film photocatalyst. Processes 2016, 4, 13. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, Z. Bismuth-based photocatalytic semiconductors: Introduction, challenges and possible approaches. J. Mol. Catal. A Chem. 2016, 423, 533–549. [Google Scholar] [CrossRef]
- Safaei, J.; Ullah, H.; Mohamed, N.A.; Mohamad Noh, M.F.; Soh, M.F.; Tahir, A.A.; Ahmad Ludin, N.; Ibrahim, M.A.; Wan Isahak, W.N.R.; Mat Teridi, M.A. Enhanced photoelectrochemical performance of Z-scheme g-C3N4/BiVO4 photocatalyst. Appl. Catal. B Environ. 2018, 234, 296–310. [Google Scholar] [CrossRef]
- Liang, H.; Li, T.; Zhang, J.; Zhou, D.; Hu, C.; An, X.; Liu, R.; Liu, H. 3-D hierarchical Ag/ZnO@CF for synergistically removing phenol and Cr(VI): Heterogeneous vs. homogeneous photocatalysis. J. Colloid Interface Sci. 2020, 558, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, Z.; Wang, L.; Meng, X. Bismuth chromate (Bi2CrO6): A promising semiconductor in photocatalysis. J. Catal. 2020, 382, 40–48. [Google Scholar] [CrossRef]
- Zhu, C.; Liu, C.; Fu, Y.; Gao, J.; Huang, H.; Liu, Y.; Kang, Z. Construction of CDs/CdS photocatalysts for stable and efficient hydrogen production in water and seawater. Appl. Catal. B Environ. 2019, 242, 178–185. [Google Scholar] [CrossRef]
- Di, J.; Xia, J.; Chisholm, M.F.; Zhong, J.; Chen, C.; Cao, X.; Dong, F.; Chi, Z.; Chen, H.; Weng, Y.X.; et al. Defect-Tailoring Mediated Electron-Hole Separation in Single-Unit-Cell Bi3O4 Br Nanosheets for Boosting Photocatalytic Hydrogen Evolution and Nitrogen Fixation. Adv. Mater. 2019, 31, e1807576. [Google Scholar] [CrossRef]
- Li, Z.; Meng, X.; Zhang, Z. Fabrication of surface hydroxyl modified g-C3N4with enhanced photocatalytic oxidation activity. Catal. Sci. Technol. 2019, 9, 3979–3993. [Google Scholar] [CrossRef]
- Chowdhury, P.; Elkamel, A.; Ray, A.K. Photocatalytic Processes for the Removal of Toxic Metal Ions. In Heavy Metals in Water: Presence, Removal and Safety; Royal Society of Chemistry: London, UK, 2014; pp. 25–43. [Google Scholar]
- WHO Regional Office for Europe. Health Risks of Heavy Metals from Long-Range Transboundary Air Pollution; WHO Regional Office for Europe: Copenhagen, Denmark, 2007; Available online: https://www.euro.who.int/en/publications/abstracts/health-risks-of-heavy-metals-from-long-range-transboundary-air-pollution-2007 (accessed on 19 September 2021).
- Subramani, A.; Jacangelo, J.G. Emerging desalination technologies for water treatment: A critical review. Water Res. 2015, 75, 164–187. [Google Scholar] [CrossRef]
- Song, L.; Zhao, R.; Yun, D.; Lu, P.; He, J.; Wang, X. Influence of Co (II), Ni (II), tartrate, and ethylenediaminetetraacetic acid on Cu (II) adsorption onto a polyvinylidene fluoride-based chelating membrane. Toxicol. Environ. Chem. 2014, 96, 362–378. [Google Scholar] [CrossRef]
- Anglada, Á.; Urtiaga, A.; Ortiz, I. Contributions of electrochemical oxidation to waste-water treatment: Fundamentals and review of applications. J. Chem. Technol. Biotechnol. 2009, 84, 1747–1755. [Google Scholar] [CrossRef]
- Ramachandra, T.V.; Ahalya, N.; Kanamadi, R.D. Biosorption: Techniques and mechanisms. CES Tech. Rep. 2005, 110, 1–91. [Google Scholar]
- Trivunac, K.; Stevanovic, S. Removal of heavy metal ions from water by complexation-assisted ultrafiltration. Chemosphere 2006, 64, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Joshi, N.C. Heavy metals, conventional methods for heavy metal removal, biosorption and the development of low cost adsorbent. Eur. J. Pharm. Med. Res. 2017, 4, 388–393. [Google Scholar]
- Bakalár, T.; Búgel, M.; Gajdošová, L. Heavy metal removal using reverse osmosis. Acta Montanistica Slovaca 2009, 14, 250. [Google Scholar]
- U.S. Congress, Office of Technology Assessment. Copper: Technology and Competiveness; U.S. Government Printing Office: Washington, DC, USA, 1998. [Google Scholar]
- Fiyadh, S.S.; AlSaadi, M.A.; Jaafar, W.Z.; AlOmar, M.K.; Fayaed, S.S.; Mohd, N.S.; Hin, L.S.; El-Shafie, A. Review on heavy metal adsorption processes by carbon nanotubes. J. Clean. Prod. 2019, 230, 783–793. [Google Scholar] [CrossRef]
- Pulford, I.D.; Watson, C. Phytoremediation of heavy metal-contaminated land by trees—A review. Environ. Int. 2003, 29, 529–540. [Google Scholar] [CrossRef]
- Hasanpour, M.; Hatami, M. Photocatalytic performance of aerogels for organic dyes removal from wastewaters: Review study. J. Mol. Liq. 2020, 309, 113094. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, Z.; Li, X. Synergetic photoelectrocatalytic reactors for environmental remediation: A review. J. Photochem. Photobiol. C Photochem. Rev. 2015, 24, 83–101. [Google Scholar] [CrossRef]
- Kubota, Y.; Watanabe, K.; Tsuda, O.; Taniguchi, T. Deep Ultraviolet Light-Emitting Hexagonal Boron Nitride Synthesized at Atmospheric Pressure. Science 2007, 317, 932. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Zhang, M.; Liu, D.; Lei, W.; Sun, L.; Wang, X. Photocatalytic TiO2/porous BNNSs composites for simultaneous LR2B and Cr(VI) removal in wool dyeing bath. J. Photochem. Photobiol. A Chem. 2017, 333, 165–173. [Google Scholar] [CrossRef]
- Meng, X.; Yun, N.; Zhang, Z. Recent advances in computational photocatalysis: A review. Can. J. Chem. Eng. 2019, 97, 1982–1998. [Google Scholar] [CrossRef]
- Lazar, M.; Varghese, S.; Nair, S. Photocatalytic Water Treatment by Titanium Dioxide: Recent Updates. Catalysts 2012, 2, 572–601. [Google Scholar] [CrossRef]
- Mozia, S.; Morawski, A.W.; Toyoda, M.; Tsumura, T. Effect of process parameters on photodegradation of Acid Yellow 36 in a hybrid photocatalysis–membrane distillation system. Chem. Eng. J. 2009, 150, 152–159. [Google Scholar] [CrossRef]
- Chen, C.; Ma, W.; Zhao, J. Semiconductor-mediated photodegradation of pollutants under visible-light irradiation. Chem. Soc. Rev. 2010, 39, 4206–4219. [Google Scholar] [CrossRef]
- Qu, Y.; Duan, X. Progress, challenge and perspective of heterogeneous photocatalysts. Chem. Soc. Rev. 2013, 42, 2568–2580. [Google Scholar] [CrossRef]
- Ni, Y.; Wang, W.; Huang, W.; Lu, C.; Xu, Z. Graphene strongly wrapped TiO2 for high-reactive photocatalyst: A new sight for significant application of graphene. J. Colloid Interface Sci. 2014, 428, 162–169. [Google Scholar] [CrossRef]
- Chen, G.; Sun, M.; Wei, Q.; Zhang, Y.; Zhu, B.; Du, B. Ag3PO4/graphene-oxide composite with remarkably enhanced visible-light-driven photocatalytic activity toward dyes in water. J. Hazard. Mater. 2013, 244, 86–93. [Google Scholar] [CrossRef]
- Su, J.; Zhang, Y.; Xu, S.; Wang, S.; Ding, H.; Pan, S.; Wang, G.; Li, G.; Zhao, H. Highly efficient and recyclable triple-shelled Ag@ Fe3O4@ SiO2@ TiO2 photocatalysts for degradation of organic pollutants and reduction of hexavalent chromium ions. Nanoscale 2014, 6, 5181–5192. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Feng, J.; Fan, M.; Pi, Y.; Hu, L.; Han, X.; Liu, M.; Sun, J.; Sun, J. Recent developments in heterogeneous photocatalytic water treatment using visible light-responsive photocatalysts: A review. RSC Adv. 2015, 5, 14610–14630. [Google Scholar] [CrossRef]
- Casbeer, E.; Sharma, V.K.; Li, X.-Z. Synthesis and photocatalytic activity of ferrites under visible light: A review. Sep. Purif. Technol. 2012, 87, 1–14. [Google Scholar] [CrossRef]
- Bahnemann, D. Photocatalytic water treatment: Solar energy applications. Sol. Energy 2004, 77, 445–459. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Chow, C.W.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef]
- Li, F.-F.; Yang, D.-F.; Xia, M.-S.; Wang, Y.; Jiang, Y.-S. Visible-light photocatalytic activity of Sn (2+)-doped TiO (2) and its loading effect on mesoporous montmorillonite. Wuji Cailiao Xuebao J. Inorg. Mater. 2011, 26, 917–922. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, M.W.; Xie, W.J.; Sun, L.; Chen, Y.; Lei, W.W. Efficient photocatalytic reduction of aqueous Cr(VI) over porous BNNSs/TiO2 nanocomposites under visible light irradiation. Catal. Sci. Technol. 2016, 6, 8309–8313. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, G.; Deng, Q.; Wang, H.; Zhang, Y.; Ng, D.H.L.; Zhao, H. Enhanced photocatalytic activity of hierarchical structure TiO2 hollow spheres with reactive (001) facets for the removal of toxic heavy metal Cr(VI). RSC Adv. 2014, 4, 34577–34583. [Google Scholar] [CrossRef]
- Zhang, X.; Song, L.; Zeng, X.; Li, M. Effects of electron donors on the TiO2 photocatalytic reduction of heavy metal ions under visible light. Energy Procedia 2012, 17, 422–428. [Google Scholar] [CrossRef][Green Version]
- Cao, D.; Wang, Q.; Liu, Z.; Zhang, H.; Wang, Y.; Jin, R.; Gao, S. Enhanced the photoelectrocatalytic performance of TiO2 nanotube arrays by the synergistic sensitization of Ag-AgBr nanospheres. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 227, 117674. [Google Scholar] [CrossRef]
- Deng, Y.; Xiao, Y.; Zhou, Y.; Zeng, T.; Xing, M.; Zhang, J. A structural engineering-inspired CdS based composite for photocatalytic remediation of organic pollutant and hexavalent chromium. Catal. Today 2019, 335, 101–109. [Google Scholar] [CrossRef]
- Chen, F.; Yu, C.; Wei, L.; Fan, Q.; Ma, F.; Zeng, J.; Yi, J.; Yang, K.; Ji, H. Fabrication and characterization of ZnTiO3/Zn2Ti3O8/ZnO ternary photocatalyst for synergetic removal of aqueous organic pollutants and Cr(VI) ions. Sci. Total Environ. 2020, 706, 136026. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Pan, L.; Lv, T.; Lu, T.; Zhu, G.; Sun, Z.; Sun, C. Microwave-assisted synthesis of ZnO–graphene composite for photocatalytic reduction of Cr(VI). Catal. Sci. Technol. 2011, 1, 1189–1193. [Google Scholar] [CrossRef]
- Yang, R.; Zhong, S.; Zhang, L.; Liu, B. PW12/CN@Bi2WO6 composite photocatalyst prepared based on organic-inorganic hybrid system for removing pollutants in water. Sep. Purif. Technol. 2020, 235, 116270. [Google Scholar] [CrossRef]
- He, H.; Li, J.; Yu, C.; Luo, Z. Surface decoration of microdisk-like g-C3N4/diatomite with Ag/AgCl nanoparticles for application in Cr(VI) reduction. Sustain. Mater. Technol. 2019, 22, e00127. [Google Scholar] [CrossRef]
- Josue, T.G.; Almeida, L.N.B.; Lopes, M.F.; Santos, O.A.A.; Lenzi, G.G. Cr(VI) reduction by photocatalyic process: Nb2O5 an alternative catalyst. J. Environ. Manag. 2020, 268, 110711. [Google Scholar] [CrossRef]
- Jiang, W.; Liu, Q.; Tao, Y.; Mu, K.; Wang, Z.; Zhu, Y.; Yue, H.; Liang, B. An environment-friendly strategy for one-step turning Cr(VI) contaminant into a Cr-Loaded catalyst for CO2 utilization. Adv. Sustain. Syst. 2018, 2, 1700165. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, F.; Sun, Y.; Zhang, J.; Hao, Z. Simultaneous redox conversion and sequestration of chromate (VI) and arsenite (III) by iron (III)-alginate based photocatalysis. Appl. Catal. B Environ. 2019, 259, 118046. [Google Scholar] [CrossRef]
- ZabihiSahebi, A.; Koushkbaghi, S.; Pishnamazi, M.; Askari, A.; Khosravi, R.; Irani, M. Synthesis of cellulose acetate/chitosan/SWCNT/Fe3O4/TiO2 composite nanofibers for the removal of Cr(VI), As(V), methylene blue and congo red from aqueous solutions. Int. J. Biol. Macromol. 2019, 140, 1296–1304. [Google Scholar] [CrossRef]
- Hu, J.; Weng, S.; Zheng, Z.; Pei, Z.; Huang, M.; Liu, P. Solvents mediated-synthesis of BiOI photocatalysts with tunable morphologies and their visible-light driven photocatalytic performances in removing of arsenic from water. J. Hazard. Mater. 2014, 264, 293–302. [Google Scholar] [CrossRef]
- Dutta, P.K.; Pehkonen, S.O.; Sharma, V.K.; Ray, A.K. Photocatalytic oxidation of arsenic (III): Evidence of hydroxyl radicals. Environ. Sci. Technol. 2005, 39, 1827–1834. [Google Scholar] [CrossRef]
- Yan, R.; Luo, D.; Fu, C.; Wang, Y.; Zhang, H.; Wu, P.; Jiang, W. Harmless treatment and selective recovery of acidic Cu (II)-Cr(VI) hybrid wastewater via coupled photo-reduction and ion exchange. Sep. Purif. Technol. 2020, 234, 116130. [Google Scholar] [CrossRef]
- Kanakaraju, D.; Rusydah bt Mohamad Shahdad, N.; Lim, Y.-C.; Pace, A. Concurrent removal of Cr(III), Cu (II), and Pb (II) ions from water by multifunctional TiO2/Alg/FeNPs beads. Sustain. Chem. Pharm. 2019, 14, 100176. [Google Scholar] [CrossRef]
- Kadi, M.W.; Mohamed, R.M.; Ismail, A.A.; Bahnemann, D.W. Performance of mesoporous α-Fe2O3/g-C3N4 heterojunction for photoreduction of Hg (II) under visible light illumination. Ceram. Int. 2020, 46, 23098–23106. [Google Scholar] [CrossRef]
- Sun, X.; Lu, J.; Wu, J.; Guan, D.; Liu, Q.; Yan, N. Enhancing photocatalytic activity on gas-phase heavy metal oxidation with self-assembled BiOI/BiOCl microflowers. J. Colloid Interface Sci. 2019, 546, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Tan, S.; Wang, D.; Wu, J.; Jia, T.; Liu, Q.; Qi, Y.; Qi, X.; He, P.; Zhou, M. CeO2/BiOIO3 heterojunction with oxygen vacancies and Ce4+/Ce3+ redox centers synergistically enhanced photocatalytic removal heavy metal. Appl. Surf. Sci. 2020, 530, 147116. [Google Scholar] [CrossRef]
- Jia, T.; Xu, K.; Wu, J.; Liu, Q.; Lin, Y.; Gu, M.; Tian, F.; Pan, W.; Wu, J.; Xiao, Y. Constructing 2D BiOIO3/MoS2 Z-scheme heterojunction wrapped by C500 as charge carriers transfer channel: Enhanced photocatalytic activity on gas-phase heavy metal oxidation. J. Colloid Interface Sci. 2020, 562, 429–443. [Google Scholar] [CrossRef] [PubMed]
- Elleuch, L.; Messaoud, M.; Djebali, K.; Attafi, M.; Cherni, Y.; Kasmi, M.; Elaoud, A.; Trabelsi, I.; Chatti, A. A new insight into highly contaminated landfill leachate treatment using Kefir grains pre-treatment combined with Ag-doped TiO2 photocatalytic process. J. Hazard. Mater. 2020, 382, 121119. [Google Scholar] [CrossRef] [PubMed]
- Thomas, B.; Alexander, L.K. Removal of Pb2+ and Cd2+ toxic heavy metal ions driven by Fermi level modification in NiFe2O4–Pd nano hybrids. J. Solid State Chem. 2020, 288, 121417. [Google Scholar] [CrossRef]
- Al-Sherbini, A.A.; Ghannam, H.E.A.; El-Ghanam, G.M.A.; El-Ella, A.A.; Youssef, A.M. Utilization of chitosan/Ag bionanocomposites as eco-friendly photocatalytic reactor for Bactericidal effect and heavy metals removal. Heliyon 2019, 5, e01980. [Google Scholar] [CrossRef]
- Ebrahimian, J.; Mohsennia, M.; Khayatkashani, M. Photocatalytic-degradation of organic dye and removal of heavy metal ions using synthesized SnO2 nanoparticles by vitex agnus-castus fruit via a green route. Mater. Lett. 2020, 263, 127255. [Google Scholar] [CrossRef]
- McCarron, P.; Harvey, I.; Brogan, R.; Peters, T.J. Self reported health of people in an area contaminated by chromium waste: Interview study. BMJ 2000, 320, 11–15. [Google Scholar] [CrossRef][Green Version]
- Tuprakay, S.; Liengcharernsit, W. Lifetime and regeneration of immobilized titania for photocatalytic removal of aqueous hexavalent chromium. J. Hazard. Mater. 2005, 124, 53–58. [Google Scholar] [CrossRef]
- Idris, A.; Hassan, N.; Rashid, R.; Ngomsik, A.-F. Kinetic and regeneration studies of photocatalytic magnetic separable beads for chromium (VI) reduction under sunlight. J. Hazard. Mater. 2011, 186, 629–635. [Google Scholar] [CrossRef]
- Iorio, Y.D.; Román, E.S.; Litter, M.I.; Grela, M.A. Photoinduced reactivity of strongly coupled TiO2 ligands under visible irradiation: An examination of an alizarin red@TiO2 nanoparticulate system. J. Phys. Chem. C 2008, 112, 16532–16538. [Google Scholar] [CrossRef]
- Litter, M.I.; Quici, N. New advances of heterogeneous photocatalysis for treatment of toxic metals and arsenic. In Nanomaterials for Environmental Protection; Wiley: Hoboken, NJ, USA, 2014; pp. 143–167. [Google Scholar]
- Meichtry, J.M.; Rivera, V.; Iorio, Y.D.; Rodríguez, H.B.; Román, E.S.; Grela, M.A.; Litter, M.I. Photoreduction of Cr(VI) using hydroxoaluminiumtricarboxymonoamide phthalocyanine adsorbed on TiO2. Photochem. Photobiol. Sci. 2009, 8, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Sahel, K.; Elsellami, L.; Mirali, I.; Dappozze, F.; Bouhent, M.; Guillard, C. Hydrogen peroxide and photocatalysis. Appl. Catal. B Environ. 2016, 188, 106–112. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A.Y. Generation and Detection of Reactive Oxygen Species in Photocatalysis. Chem. Rev. 2017, 117, 11302–11336. [Google Scholar] [CrossRef] [PubMed]
- Lousada, C.M.; Johansson, A.J.; Brinck, T.; Jonsson, M. Mechanism of H2O2 Decomposition on Transition Metal Oxide Surfaces. J. Phys. Chem. C 2012, 116, 9533–9543. [Google Scholar] [CrossRef]
- Zhang, J.; Nosaka, Y. Photocatalytic oxidation mechanism of methanol and the other reactants in irradiated TiO2 aqueous suspension investigated by OH radical detection. Appl. Catal. B Environ. 2015, 166–167, 32–36. [Google Scholar] [CrossRef]
- Smedley, P.L.; Kinniburgh, D.G. A review of the source, behaviour and distribution of arsenic in natural waters. Appl. Geochem. 2002, 17, 517–568. [Google Scholar] [CrossRef]
- Rehman, K.; Fatima, F.; Waheed, I.; Akash, M.S.H. Prevalence of exposure of heavy metals and their impact on health consequences. J. Cell. Biochem. 2018, 119, 157–184. [Google Scholar] [CrossRef]
- Guan, X.; Du, J.; Meng, X.; Sun, Y.; Sun, B.; Hu, Q. Application of titanium dioxide in arsenic removal from water: A review. J. Hazard. Mater. 2012, 215–216, 1–16. [Google Scholar] [CrossRef]
- Mamindy-Pajany, Y.; Hurel, C.; Marmier, N.; Roméo, M. Arsenic (V) adsorption from aqueous solution onto goethite, hematite, magnetite and zero-valent iron: Effects of pH, concentration and reversibility. Desalination 2011, 281, 93–99. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U. Arsenic removal from water/wastewater using adsorbents—A critical review. J. Hazard. Mater. 2007, 142, 1–53. [Google Scholar] [CrossRef] [PubMed]
- Meichtry, J.M.; Lin, H.J.; Luciana, D.; Levy, I.K.; Gautier, E.A.; Blesa, M.A.; Litter, M.I. Low-cost TiO2 photocatalytic technology for water potabilization in plastic bottles for isolated regions photocatalyst fixation. J. Sol. Energy Eng. 2007, 129, 119–126. [Google Scholar] [CrossRef]
- Levy, I.K.; Brusa, M.A.; Aguirre, M.E.; Custo, G.; San Román, E.; Litter, M.I.; Grela, M.A. Exploiting electron storage in TiO2 nanoparticles for dark reduction of As(V) by accumulated electrons. Phys. Chem. Chem. Phys. 2013, 15, 10335–10338. [Google Scholar] [CrossRef] [PubMed]
- Levy, I.K.; Quici, N.; Custo, G.; Litter, M.I.; Brusa, M.; Aguirre, M.E.; Grela, M.A.; San Román, E. Dark reduction of As(V) by accumulated electrons stored in alcoholic TiO2 nanoparticles. In Proceedings of the 5th International Congress on Arsenic in the Environment, Buenos Aires, Argentina, 11–16 May 2014; pp. 802–804. [Google Scholar]
- Ynalvez, R.; Gutierrez, J.; Gonzalez-Cantu, H. Mini-review: Toxicity of mercury as a consequence of enzyme alteration. Biometals 2016, 29, 781–788. [Google Scholar] [CrossRef]
- Chen, D.; Ray, K.A. Removal of toxic metal ions from wastewater by semiconductor photocatalysis. Chem. Eng. Sci. 2001, 56, 1561–1570. [Google Scholar] [CrossRef]


| Heavy Metals | Use/Exposure | Health Effects | Maximum Contamination Level (WHO *) [22] |
|---|---|---|---|
| Chromium (Cr) | Electroplating/Lather tanning/paint industry | Respiratory cancers | 50 ppb |
| Zinc (Zn) | Mining/manufacturing | Metal fume fever/restlessness | / |
| Cadmium (Cd) | Electroplating/pigment/plastic/polymerization industry | Bone damage/nephrotoxic effects | 3 ppb |
| Mercury (Hg) | Pesticides/chlorine-alkali/paint/petrochemical industry | Dyslexia/neurobehavioral disorders/intellectual retardation/attention deficit hyperactivity disorder | 1 ppb |
| Nickel (Ni) | Electroplating/mining/paint industry | Chronic bronchitis/cancers of the lungs and nasal sinus/decreased lung function | / |
| Platinum (Pt) | Mining/catalytic converter | Platinosis/allergic reactions/respiratory hypersensitive reaction | / |
| Arsenic (As) | Mining/wood preservative/biocides | Skin cancers/liver tumours/acute poisoning/gastrointestinal issues | 10 ppb |
| Techniques | Drawbacks | Ref. |
|---|---|---|
| Direct adsorption | Inefficient in the presence of ligand | [24] |
| Chemical precipitation | Inefficient in the presence of ligand and potential pollution | [26] |
| Ozonation | Difficulties in separation and potential contamination | [23] |
| Ultrafiltration | Sludge generation | [27] |
| Ion-exchange | High cost and partial removal of some ions | [28] |
| Reverse osmosis | High cost | [29] |
| Electrowinning | Many equipment restrictions, large investment and continuous power input demand | [30] |
| Carbon adsorption | High cost and low adsorption rates of water-soluble components | [31] |
| Phytoremediation | Time-consuming and difficult to regenerate plants | [32] |
| Photocatalyst | Heavy Metal | Redox Products | Light Type | Efficiency | Irradiation Time | Ref. |
|---|---|---|---|---|---|---|
| Porous BNNSs/TiO2 | Cr(VI) | Cr(III) | Simulated solar light and visible light | 99% and 99% | 70 min and 80 min | [50] |
| TiO2 hollow sphere | Cr(VI) | Cr(III) | UV light | 0.0867 min−1 | 80 min | [51] |
| TiO2 | Cr(VI) | Cr(III) | Visible light | 100% (formic acid as electron donor) | 80 min | [52] |
| TiO2 nanotube arrays/Ag-AgBr | Cr(VI) | Cr(III) | Solar light | 58.63% | 180 min | [53] |
| CdS/TiO2 | Cr(VI) | Cr(III) | Visible light | 2.14 × 10−2 min−1 | 180 min | [54] |
| ZnTiO3/Zn2Ti3O8/ZnO | Cr(VI) | Cr(III) | Full spectrum light | 47% | 150 min | [55] |
| ZnO–graphene | Cr(VI) | Cr(III) | UV light | 98% | 240 min | [56] |
| Ag/ZnO@CF | Cr(VI) | Cr(III) | Full spectrum light | 71.82% | 210 min | [16] |
| PW12/CN@Bi2WO6 | Cr(VI) | Cr(III) | Visible light | 98.7% | 90 min | [57] |
| g-C3N4/diatomite composites/Ag/AgCl | Cr(VI) | Cr(III) | Visible light | 7.4 × 10−2 min−1 | 45 min | [58] |
| Nb2O5 | Cr(VI) | Cr(III) | Full spectrum light | 90% | 120 min | [59] |
| ZrO2 | Cr(VI) | Cr(III)/Cr | UV light | About 100% | 90 min | [60] |
| Iron(III) cross-linking alginate hydrogel beads | Cr(VI) and As(III) | Cr(III) and As(V) | Full spectrum light | 90% and 100% | 150 min | [61] |
| Cellulose acetate/chitosan/single walled carbon nanotubes/ferrite/titanium dioxide | Cr(VI) and As(V) | Cr(III) and As | UV light | 0.0925 and 0.0896 min−1 | 60 min | [62] |
| BiOI | As(III) | As(V) | Natural light | 1 mg/L to 10 μg/L | 3 h | [63] |
| TiO2 | As(III) | As(V) | UV light | About 100% | 30 min | [64] |
| TiO2-ZrO2 | Cu(II) and Cr(VI) | Cu and Cr(III)/Cr | UV light | 96.29 and 99.17% | 630 min | [65] |
| TiO2/Alg/FeNPs | Cr(III), Cu(II) and Pb(II) | Cr, Cu and Pb | UV light | 98.6%, 98.4% and 99.5% | 120 min | [66] |
| α-Fe2O3/g-C3N4 | Hg(II) | Hg | Visible light | 90% | 60 min | [67] |
| BiOI/BiOCl | Hg | HgO/Hg(II) | Visible light | 72.4% | 50 min | [68] |
| CeO2/BiOIO3 | Hg | HgO | Visible light | 86.53% | 30 min | [69] |
| BiOIO3/MoS2/C500 | Hg | HgO | UV light | 78.32% | 70 min | [70] |
| Ag/TiO2 | Cd(II), Ni(II), Zn(II), Mn(II) and Cu(II) | Cd, Ni, Zn, Mn and Cu | UV light | 100, 96, 65.13, 58.22 and 56.20% | 120 min | [71] |
| NiFe2O4-Pd | Pb(II) and Cd(II) | Pb and Cd | Full spectrum light | 1.4 × 10−1 and 0.86 × 10−1 min−1 | 60 min | [72] |
| Chitosan/Ag | Cu(II), Pb(II) and Cd(II) | Cu, Pb and Cd | Natural sunlight | 1.10 × 10−4, 1.4 × 10−4 and 1.5 × 10−4 mol dm−3s−1 | 240 min | [73] |
| SnO2 nanoparticles | Co(II) | Co | UV light | 94% | 60 min | [74] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, X.; Meng, X. Photocatalysis for Heavy Metal Treatment: A Review. Processes 2021, 9, 1729. https://doi.org/10.3390/pr9101729
Gao X, Meng X. Photocatalysis for Heavy Metal Treatment: A Review. Processes. 2021; 9(10):1729. https://doi.org/10.3390/pr9101729
Chicago/Turabian StyleGao, Xinyu, and Xiangchao Meng. 2021. "Photocatalysis for Heavy Metal Treatment: A Review" Processes 9, no. 10: 1729. https://doi.org/10.3390/pr9101729
APA StyleGao, X., & Meng, X. (2021). Photocatalysis for Heavy Metal Treatment: A Review. Processes, 9(10), 1729. https://doi.org/10.3390/pr9101729








