Cold Plasma Deposition of Polymeric Nanoprotrusion, Nanoparticles, and Nanofilm Structures on a Slide Glass Surface
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Instrumentation
2.3. Plasma Coating of the Slide Glass Surface
3. Results
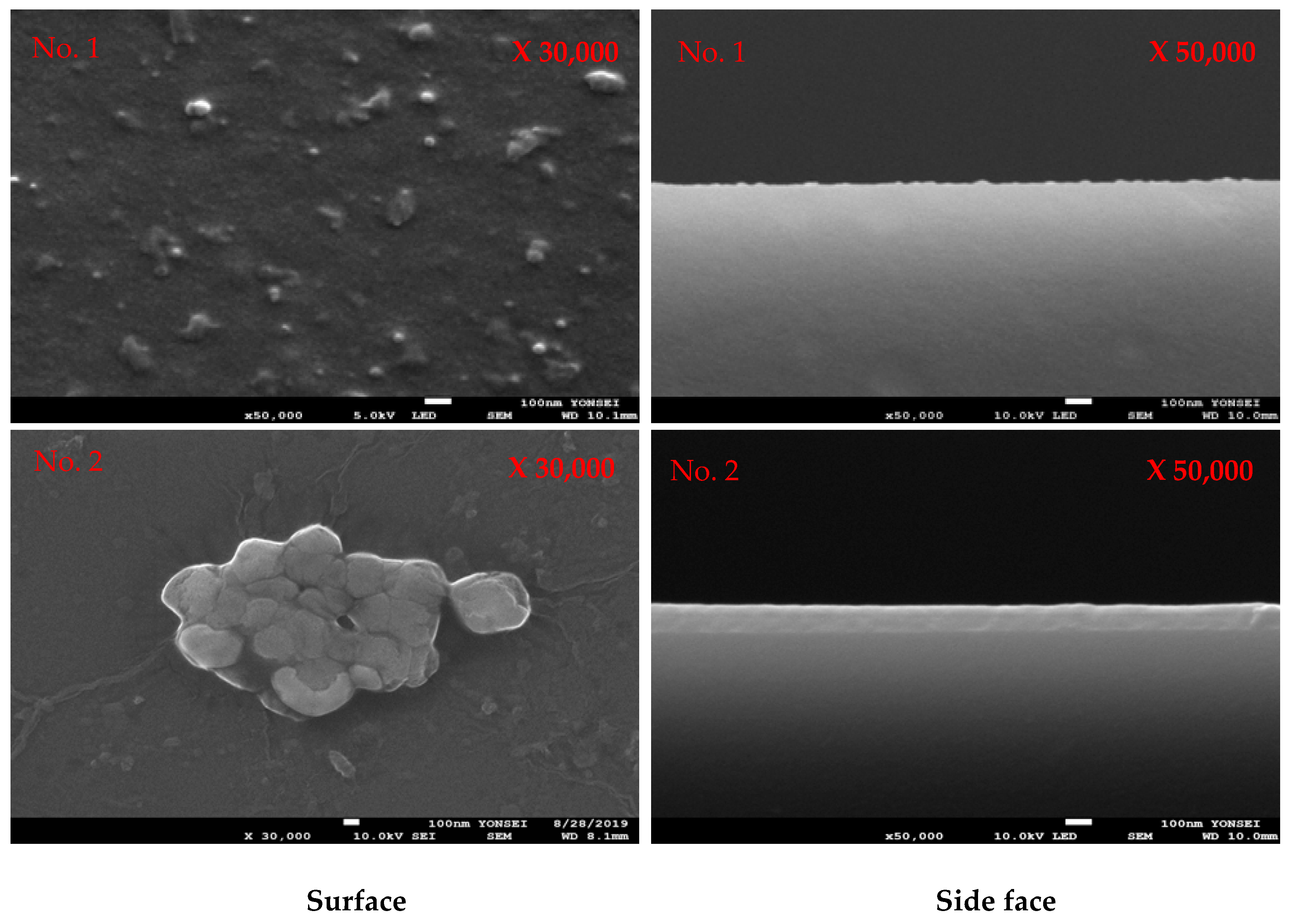
3.1. Plasma Polymerization of Poly(TFMA-co-MMA) on the Slide Glass Surface
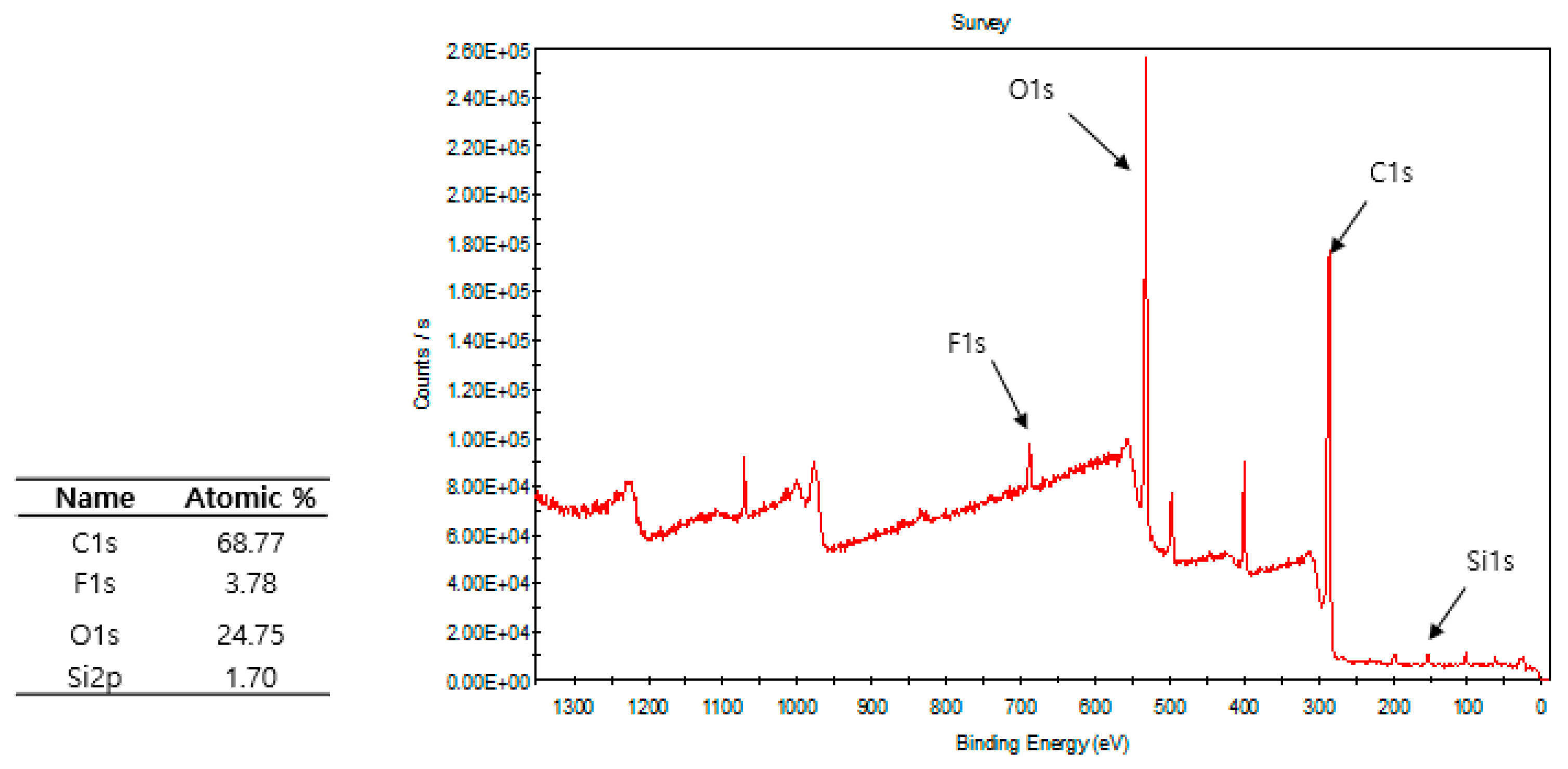
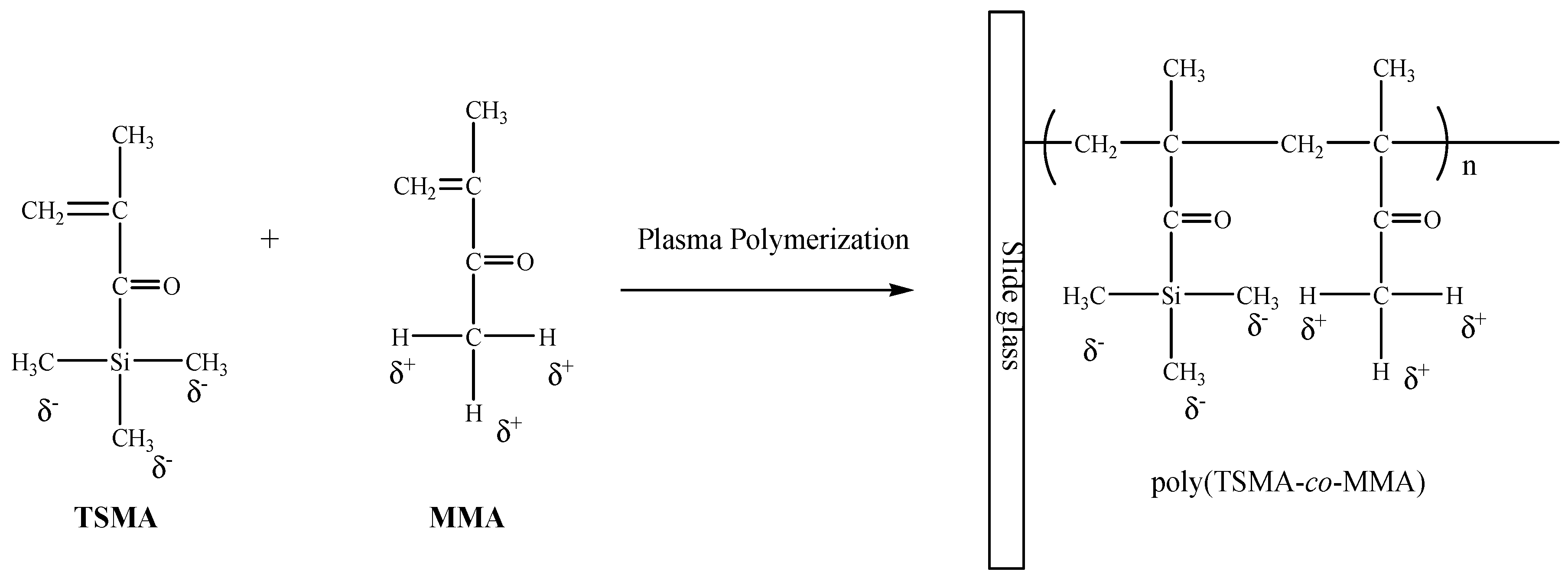
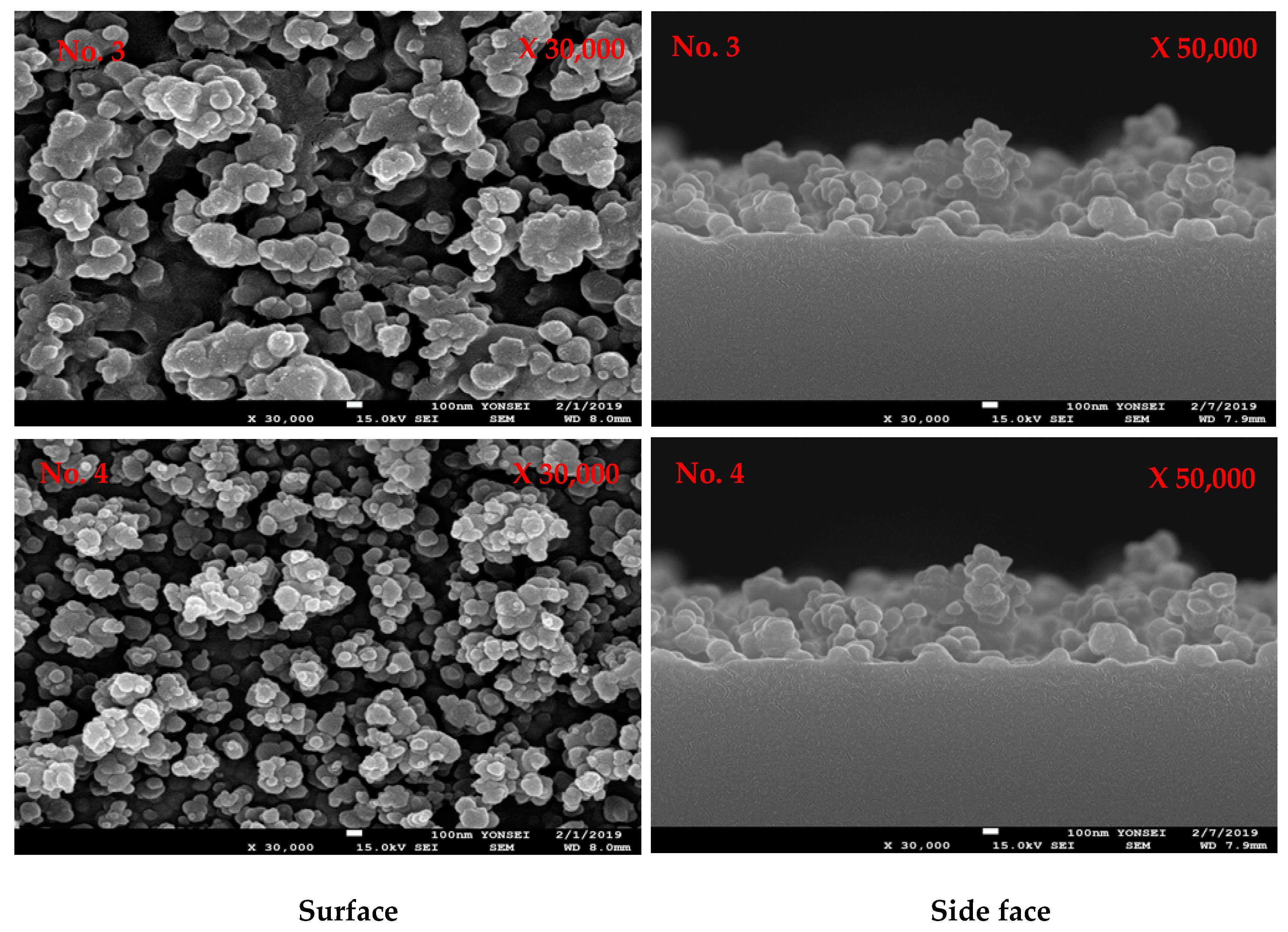
3.2. Plasma Polymerization of Poly(TSMA-co-MMA) on a Slide Glass Surface
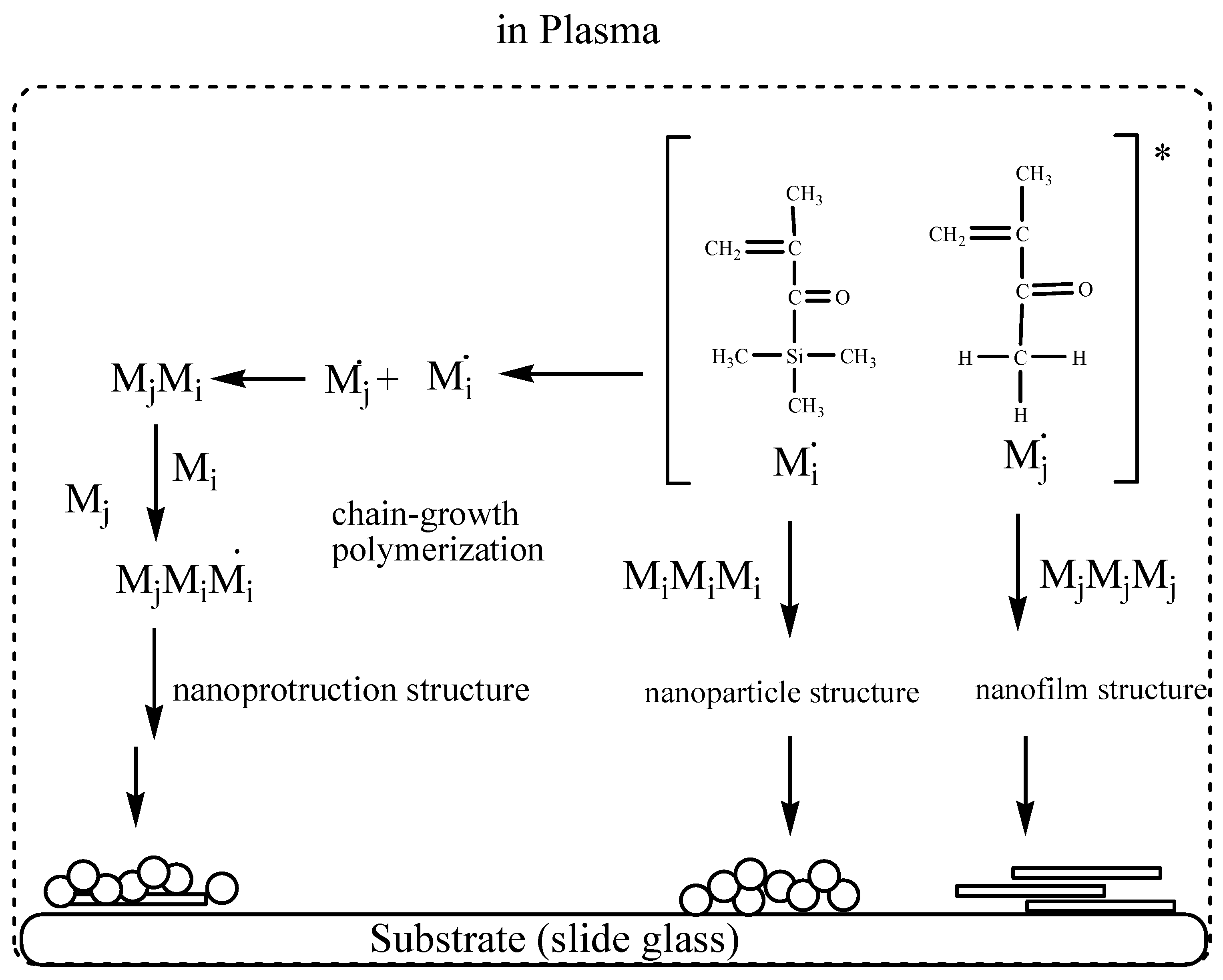
4. Discussion
5. Conclusions
- (1)
- Polymeric nanoparticles can be coated onto the surface of slide glass using TSMA through a plasma process.
- (2)
- Polymeric nanofilms can be coated onto the surface of slide glass using TFMA, MMA and a mixture of TFMA and MMA.
- (3)
- Polymeric nanoprotrusions can be deposited onto the surface of slide glass using a mixture of TSMA and MMA through a plasma process.
- (4)
- The relative magnitude of the hydrophobic properties produced are as follows. Polymeric nanoprotrusions > polymeric nanoparticles > polymeric nanofilm.
- (5)
- Superhydrophobicity (153° contact angle) was obtained on the poly(TSMA/MMA) (80/20, mol-%)-coated slide glass surface.
- (6)
- The transmittance (%) in the UV, IR, and VL regions was very high.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hou, J.; Zhang, H.; Yang, Q.; Li, M.; Song, Y.; Jiang, L. Bio-inspired photonic-crystal microchip for fluorescent ultratrace detection. Angew. Chem. Int. Ed. Engl. 2014, 53, 5791–5795. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhou, J.; Su, B.; Shi, L.; Wang, J.; Chen, S.; Wang, L.; Zi, J.; Song, Y.; Jiang, L. Colloidal photonic crystals with narrow stopbands assembled from low-adhesive superhydrophobic substrates. J. Am. Chem. Soc. 2012, 134, 17053–17058. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, J.; Yang, L.; Song, Y. Superoleophilic and superhydrophobic inverse opals for oil sensors. Adv. Funct. Mater. 2008, 18, 3258–3264. [Google Scholar] [CrossRef]
- Shen, W.; Li, M.; Ye, C.; Jiang, L.; Song, Y. Direct-writing colloidal photonic crystal microfluidic chips by inkjet printing for label-free protein detection. Lab Chip 2012, 12, 3089–3095. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Song, Y.; Jiang, L. Patterning of controllable surface wettability for printing techniques. Chem. Soc. Rev. 2013, 42, 5184–5209. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Y.; Wang, S.; Song, Y.; Jiang, L. Bioinspired colloidal photonic crystals with controllable wettability. Acc. Chem. Res. 2011, 44, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Wang, S.; Lin, L.; Chen, L.; Liu, M.; Feng, L.; Jiang, L. A novel superhydrophilic and underwater superoleophobic hydrogel-coated mesh for oil/water separation. Adv. Mater. 2011, 23, 4270–4273. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, X.; Xin, Z.; Deng, M.; Wen, Y.; Song, Y. Controlled inkjetting of a conductive pattern of silver nanoparticles based on the coffee-ring effect. Adv. Mater. 2013, 25, 6714–6718. [Google Scholar] [CrossRef] [PubMed]
- Ben Salem, D.; Carton, O.; Fakhouri, H.; Pulpytel, J.; Arefi-Khonsari, F. Deposition of water stable plasma polymerized acrylic acid/MBA organic coatings by atmospheric pressure air plasma jet. Plasma Process. Polym. 2014, 11, 269–278. [Google Scholar] [CrossRef]
- Biederman, H.; Slavínská, D. Plasma polymer films and their future prospects. Surf. Coat. Technol. 2000, 125, 371–376. [Google Scholar] [CrossRef]
- Friedrich, J. Mechanisms of plasma polymerization—Reviewed from a chemical point of view. Plasma Process. Polym. 2011, 8, 783–802. [Google Scholar] [CrossRef]
- Bui, Q.-T.; Yu, I.-K.; Gopalan, A.I.; Saianand, G.; Kim, W.; Choi, S.-H. Facile fabrication of metal oxide based catalytic electrodes by AC plasma deposition and electrochemical detection of hydrogen peroxide. Catalysts 2019, 9, 888. [Google Scholar] [CrossRef]
- Wang, X.; Cho, Y.H.; Kawakam, Y. Improvement of the performance of transmission holographic grating by hydrolysis-induced change of the property of polymer matrix. Polym. J. 2008, 40, 601–606. [Google Scholar] [CrossRef]
- Jeon, J.-S.; Yu, I.-K.; Kim, W.; Choi, S.-H. Electrocatalytic oxidation of methanol by a polymeric Ni complex-modified electrode prepared by a one-step cold-plasma process. Front. Chem. 2020, 8, 595616. [Google Scholar] [CrossRef] [PubMed]






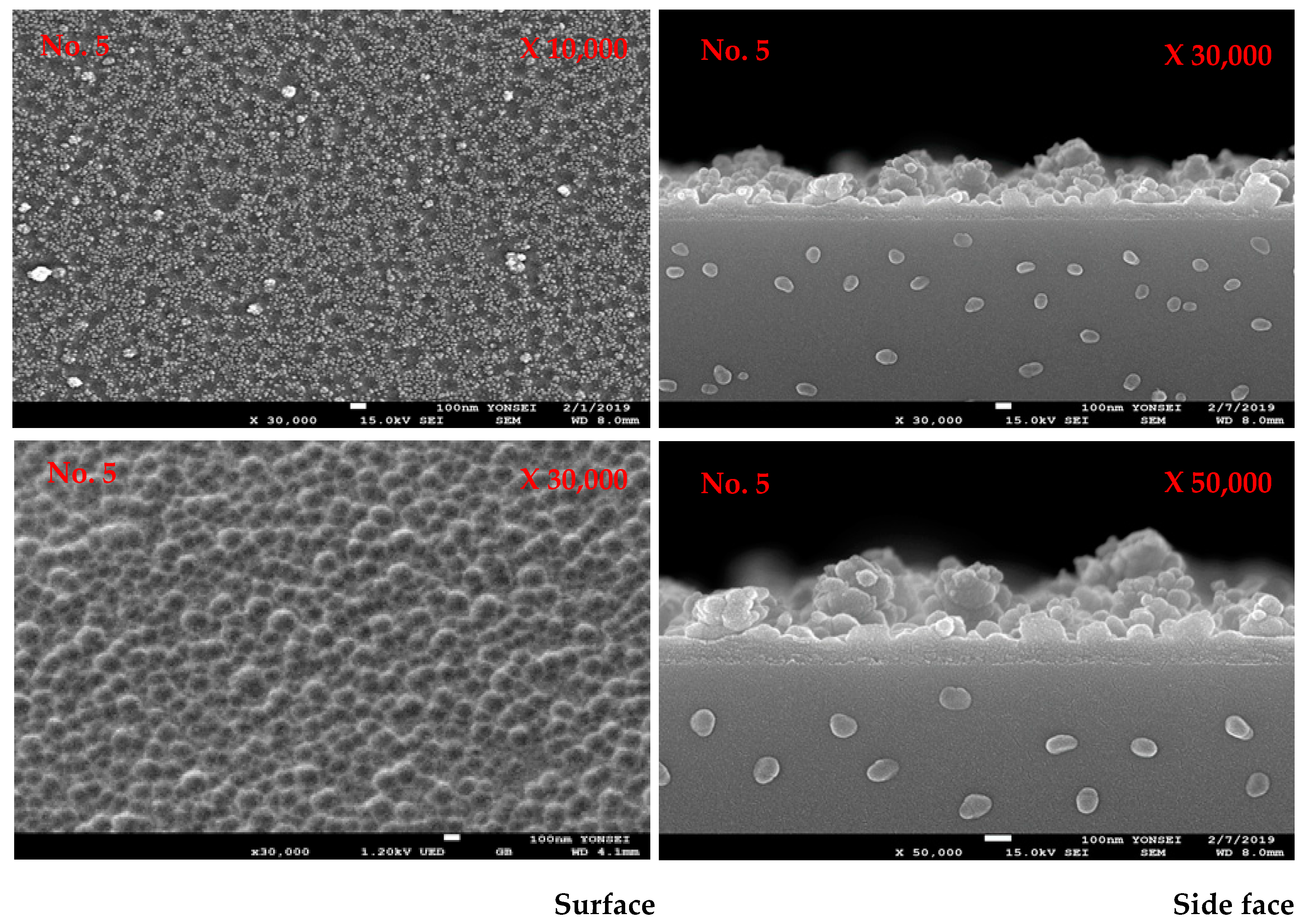
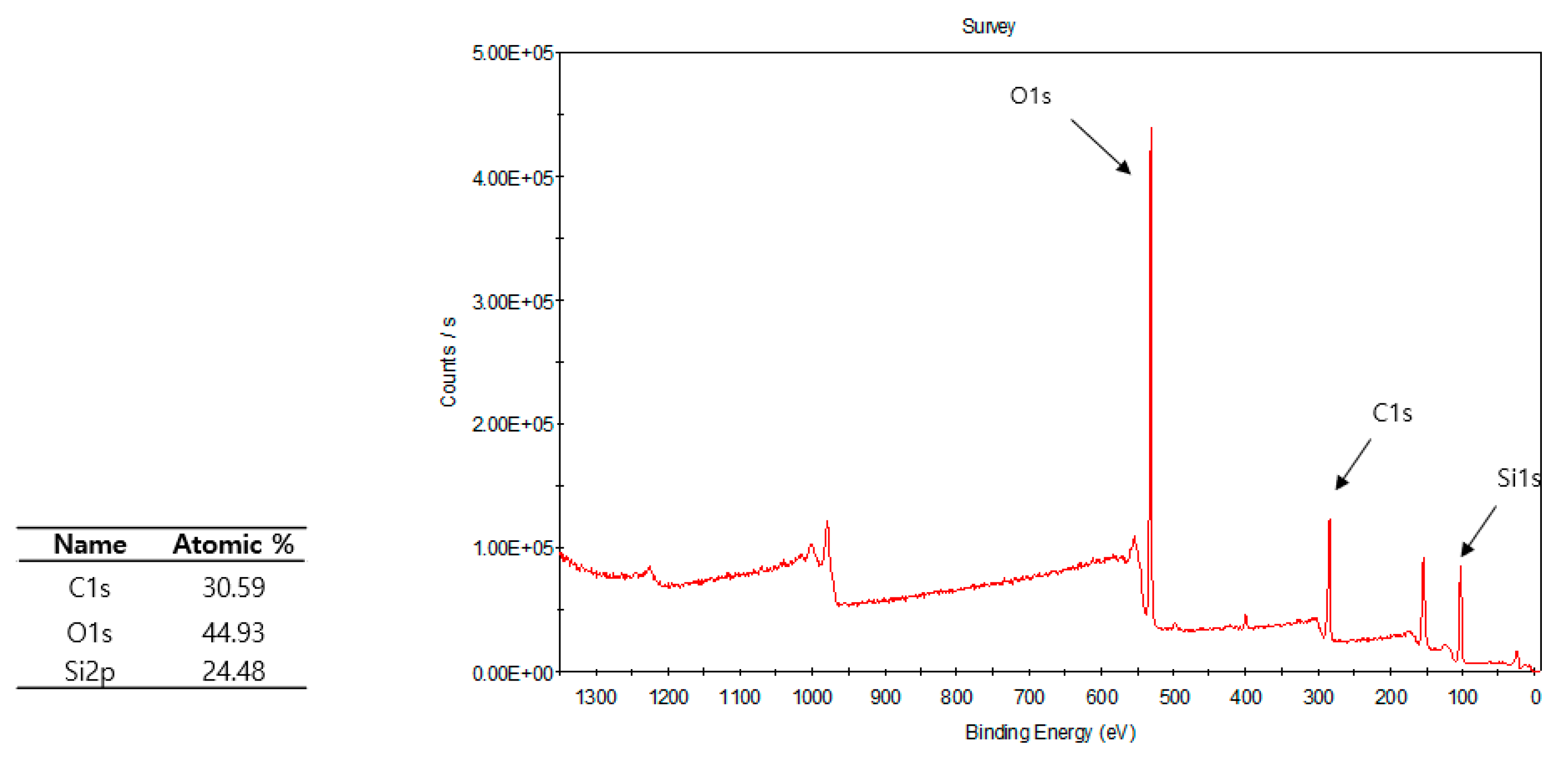
| Exp. No | TFMA/MMA (mol-%) | Contact Angle (°) | Transmittance (%) | ||
|---|---|---|---|---|---|
| UV (365 nm) | IR (940 nm) | VL (380 nm~700 nm) | |||
| 1 | 100/0 | 51.0° | 91.0 | 89.1 | 92.3 |
| 2 | 80/20 | 56.6° | 91.3 | 88.6 | 92.8 |
| 3 | 60/40 | 78.2° | 91.4 | 88.7 | 92.8 |
| 4 | 40/60 | 40.1° | 93.4 | 89.3 | 92.7 |
| 5 | 20/80 | 34.7° | 93.0 | 89.1 | 92.5 |
| 6 | 0/100 | 42.6° | 91.3 | 88.4 | 90.6 |
| 7 | Glass surface | 18.5° | 99.0 | 99.0 | 99.0 |
| Exp. No. | TSMA/MMA (mol-%) | Contact Angle (°) | Transmittance (%) | ||
|---|---|---|---|---|---|
| UV (365 nm) | IR (940 nm) | VL (380~700 nm) | |||
| 1 | 100/0 | 110 | 58.4 | 87.9 | 84.1 |
| 2 | 80/20 | 153 | 77.1 | 90.0 | 85.0 |
| 3 | 60/40 | 123 | 69.3 | 88.7 | 88.2 |
| 4 | 40/60 | 115 | 55.5 | 89.7 | 84.2 |
| 5 | 20/80 | 95.0 | 54.4 | 86.9 | 84.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yi, S.-W.; Yu, I.-K.; Kim, W.-J.; Choi, S.-H. Cold Plasma Deposition of Polymeric Nanoprotrusion, Nanoparticles, and Nanofilm Structures on a Slide Glass Surface. Processes 2021, 9, 99. https://doi.org/10.3390/pr9010099
Yi S-W, Yu I-K, Kim W-J, Choi S-H. Cold Plasma Deposition of Polymeric Nanoprotrusion, Nanoparticles, and Nanofilm Structures on a Slide Glass Surface. Processes. 2021; 9(1):99. https://doi.org/10.3390/pr9010099
Chicago/Turabian StyleYi, Sun-Woo, In-Keun Yu, Woon-Jung Kim, and Seong-Ho Choi. 2021. "Cold Plasma Deposition of Polymeric Nanoprotrusion, Nanoparticles, and Nanofilm Structures on a Slide Glass Surface" Processes 9, no. 1: 99. https://doi.org/10.3390/pr9010099
APA StyleYi, S.-W., Yu, I.-K., Kim, W.-J., & Choi, S.-H. (2021). Cold Plasma Deposition of Polymeric Nanoprotrusion, Nanoparticles, and Nanofilm Structures on a Slide Glass Surface. Processes, 9(1), 99. https://doi.org/10.3390/pr9010099






