Investigation of Ni–Fe–Cu-Layered Double Hydroxide Catalysts in Steam Reforming of Toluene as a Model Compound of Biomass Tar
Abstract
1. Introduction
2. Materials and Methods
2.1. Catalyst Preparation
2.2. Catalyst Characterization
2.2.1. Chemical Element Analysis
2.2.2. Characterization of the Calcination Process
2.2.3. Temperature-Programmed Reduction (TPR) Analysis
2.2.4. X-Ray Diffraction (XRD) Analysis
2.2.5. Textural Properties
2.2.6. Scanning Electron Microscopy (SEM)
2.2.7. Type and Amount of Carbonaceous Species Deposited on the Catalyst
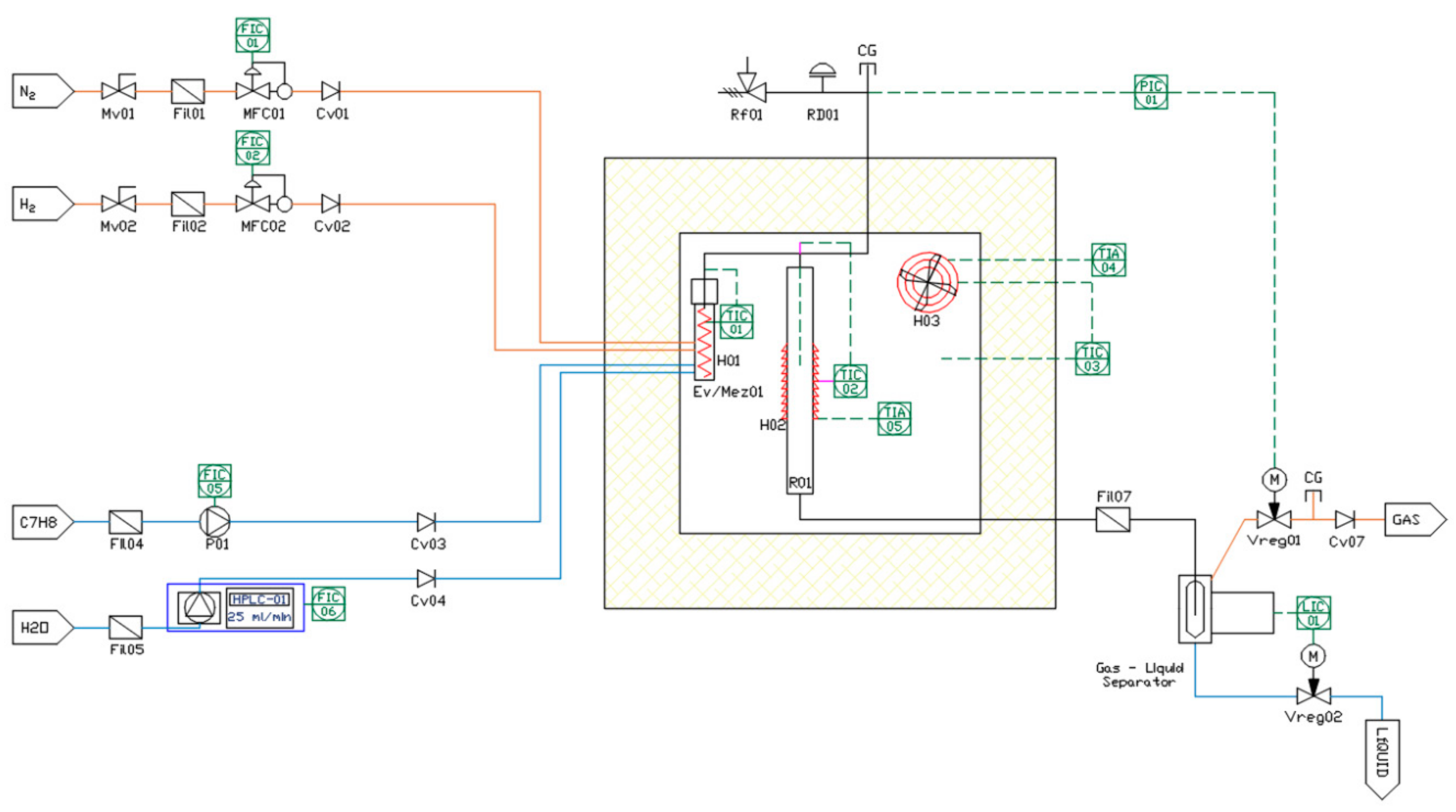
2.3. Catalytic Tests
3. Results and Discussion
3.1. Catalyst Characterization
3.1.1. Chemical Element Analysis Results
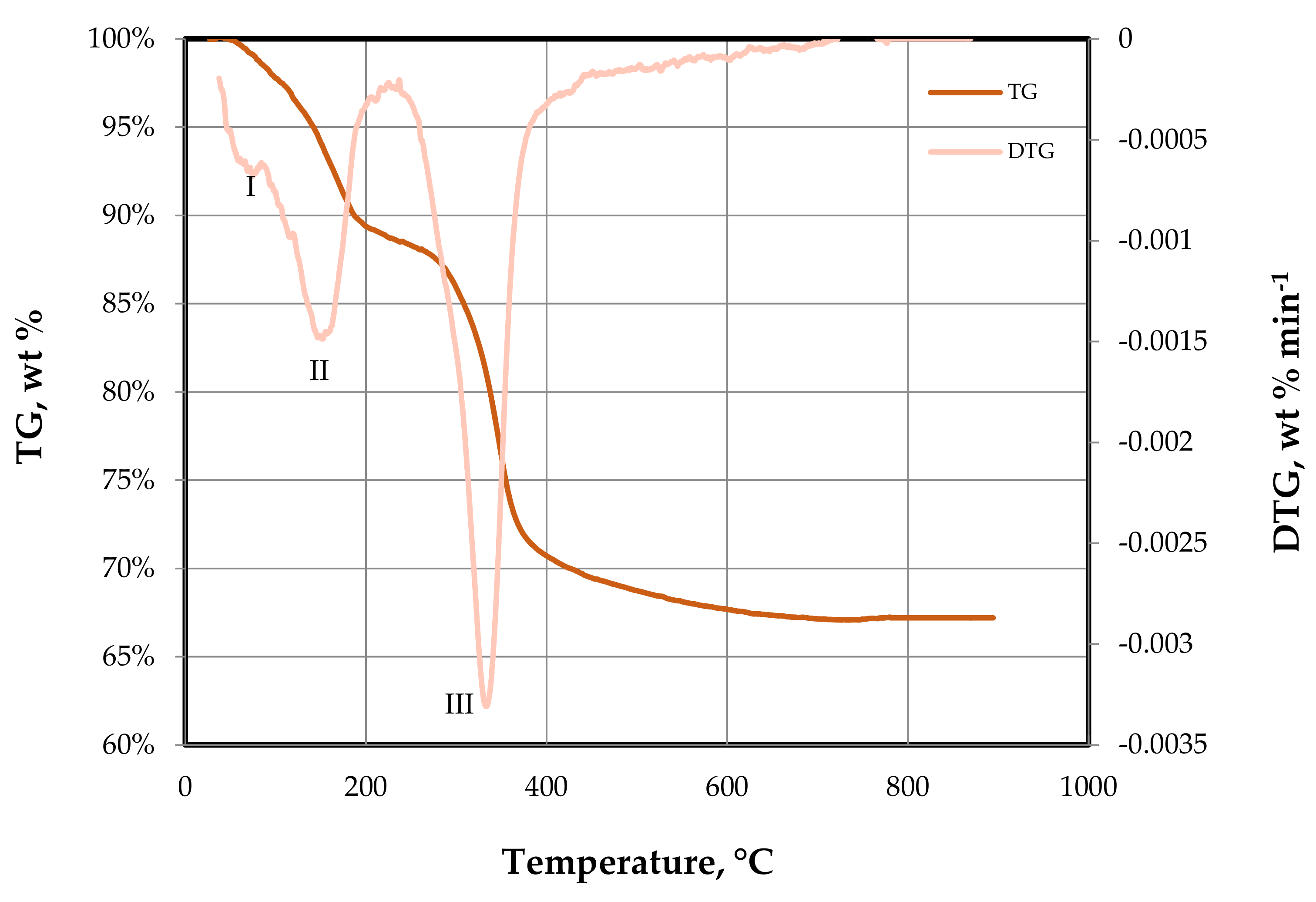
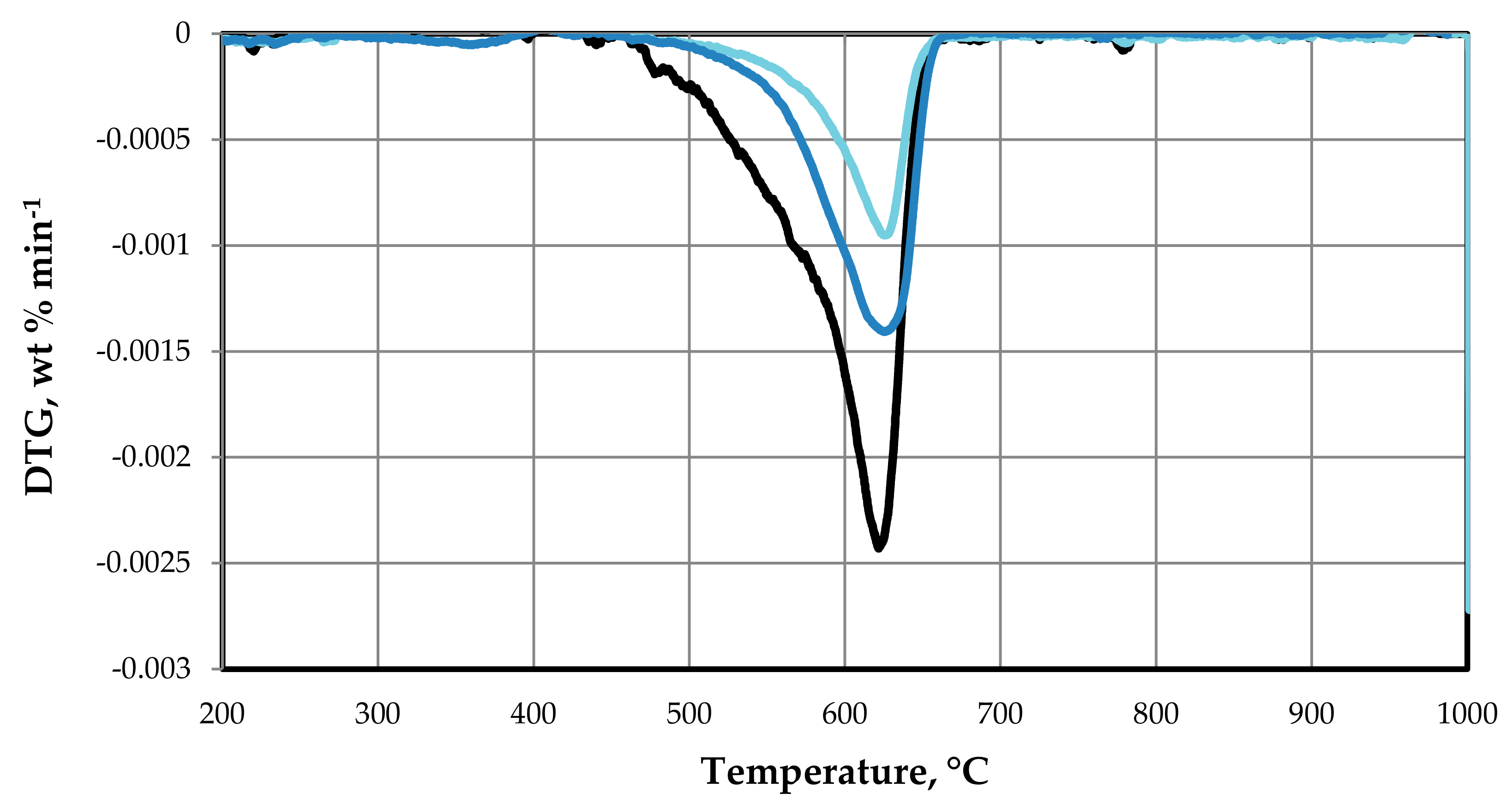
3.1.2. TGA of the As-Synthesized Ni–Fe–Cu/LDH
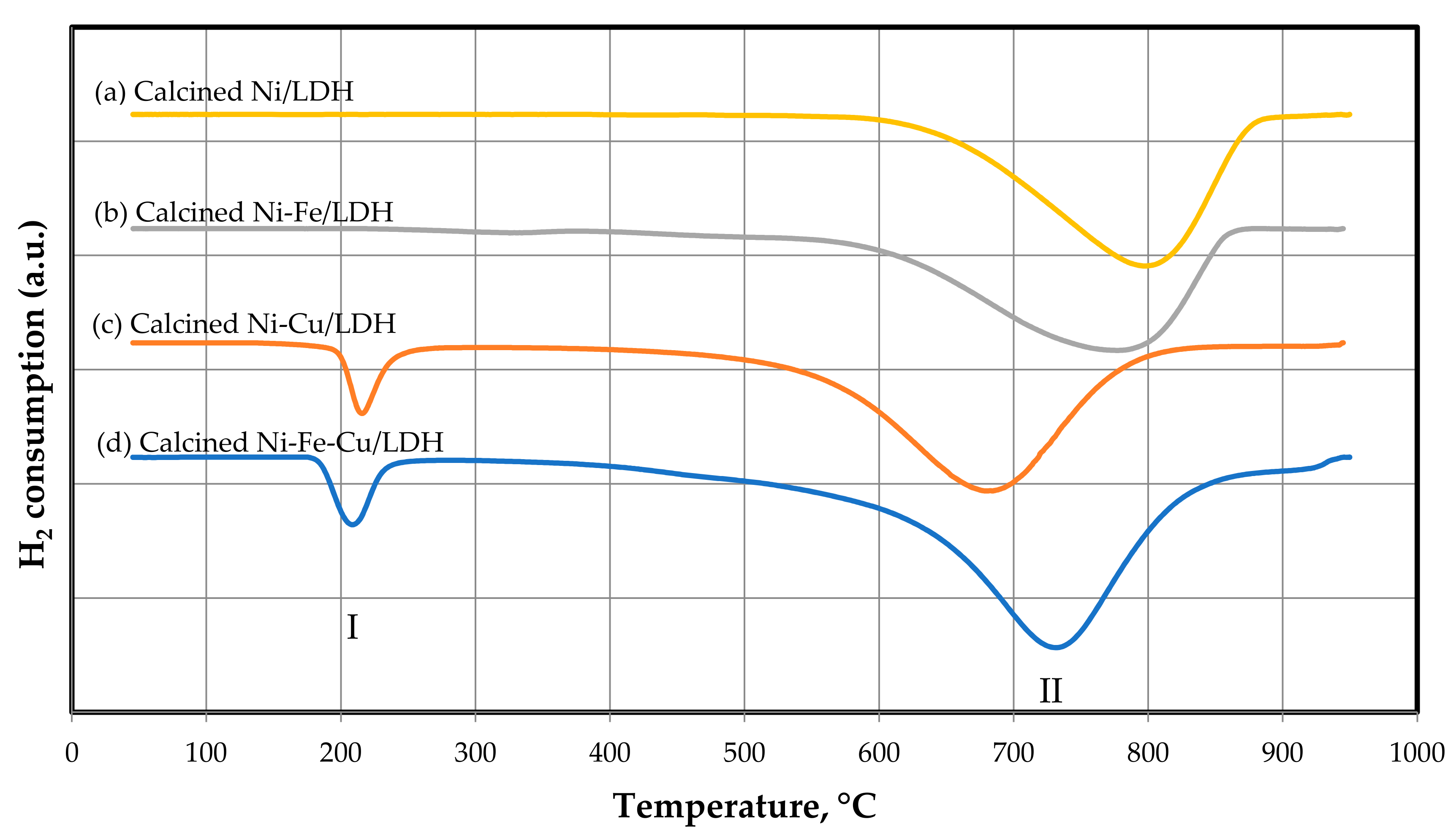
3.1.3. TPR Analysis Results
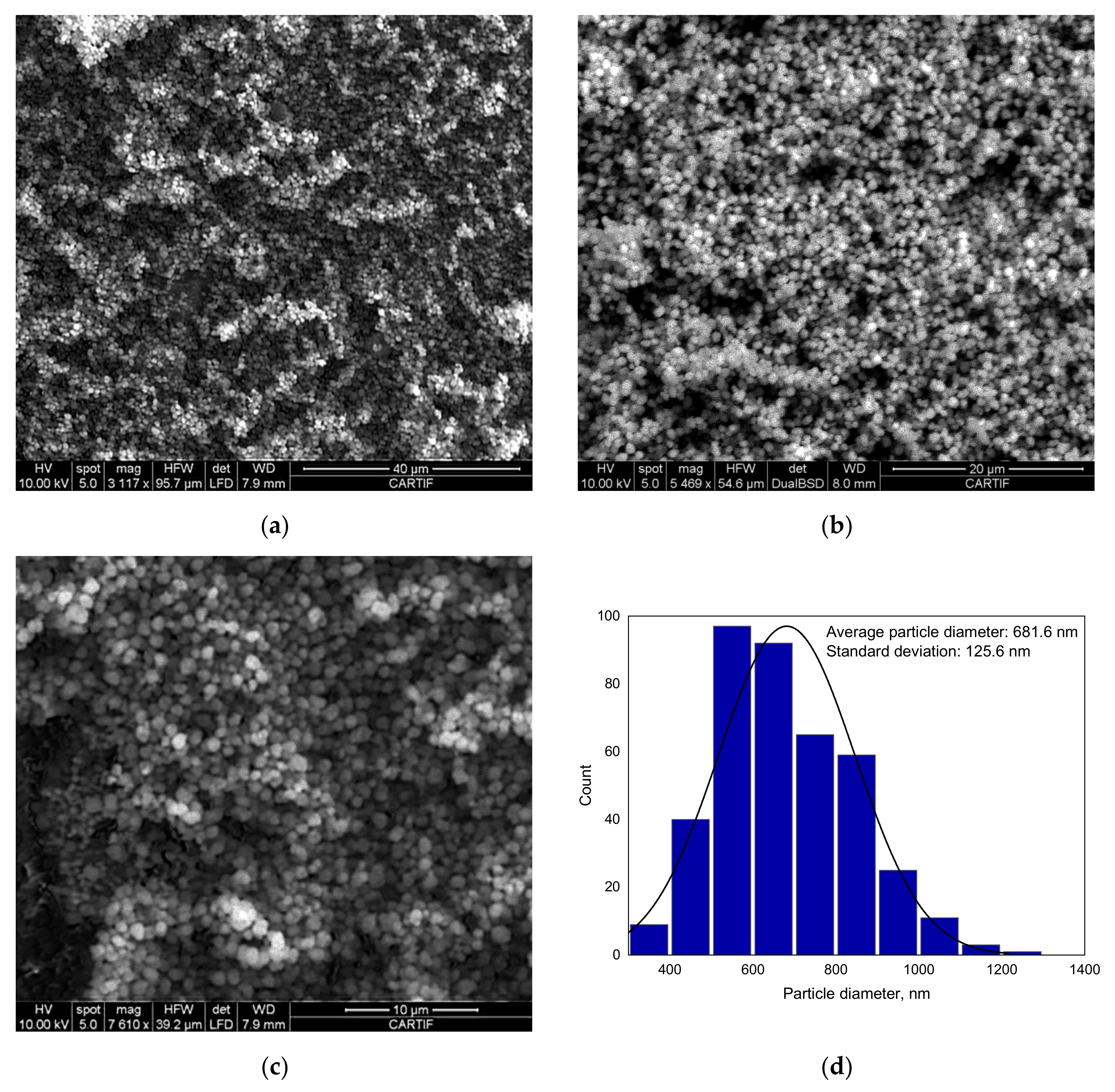
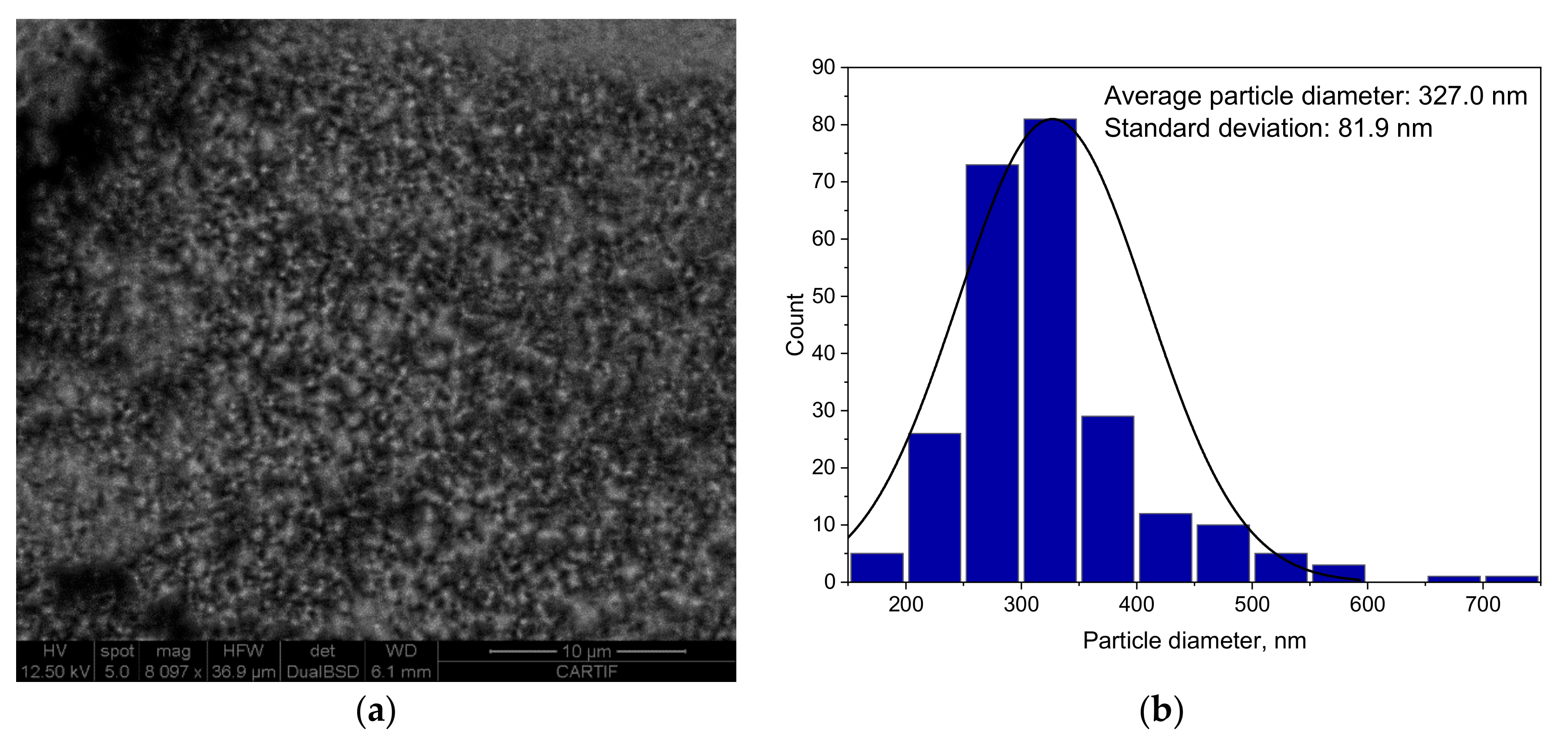
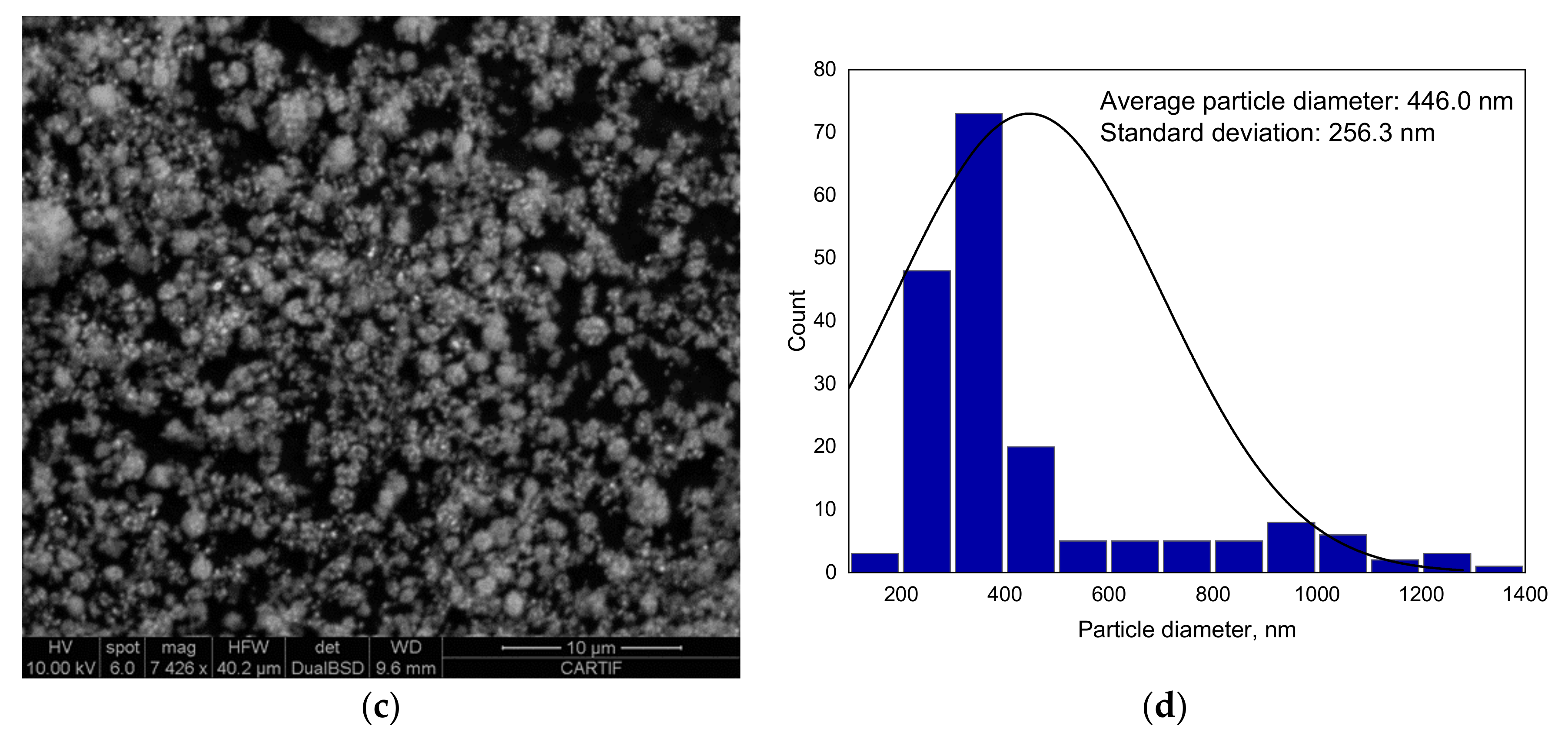
3.1.4. SEM Analysis Results
3.1.5. XRD Analysis Results
3.1.6. Textural Properties Analysis Results
3.2. Effect of Temperature and S/C Ratio on Catalytic Performance of Ni–Fe–Cu/LDH Catalyst over Steam Reforming of Toluene
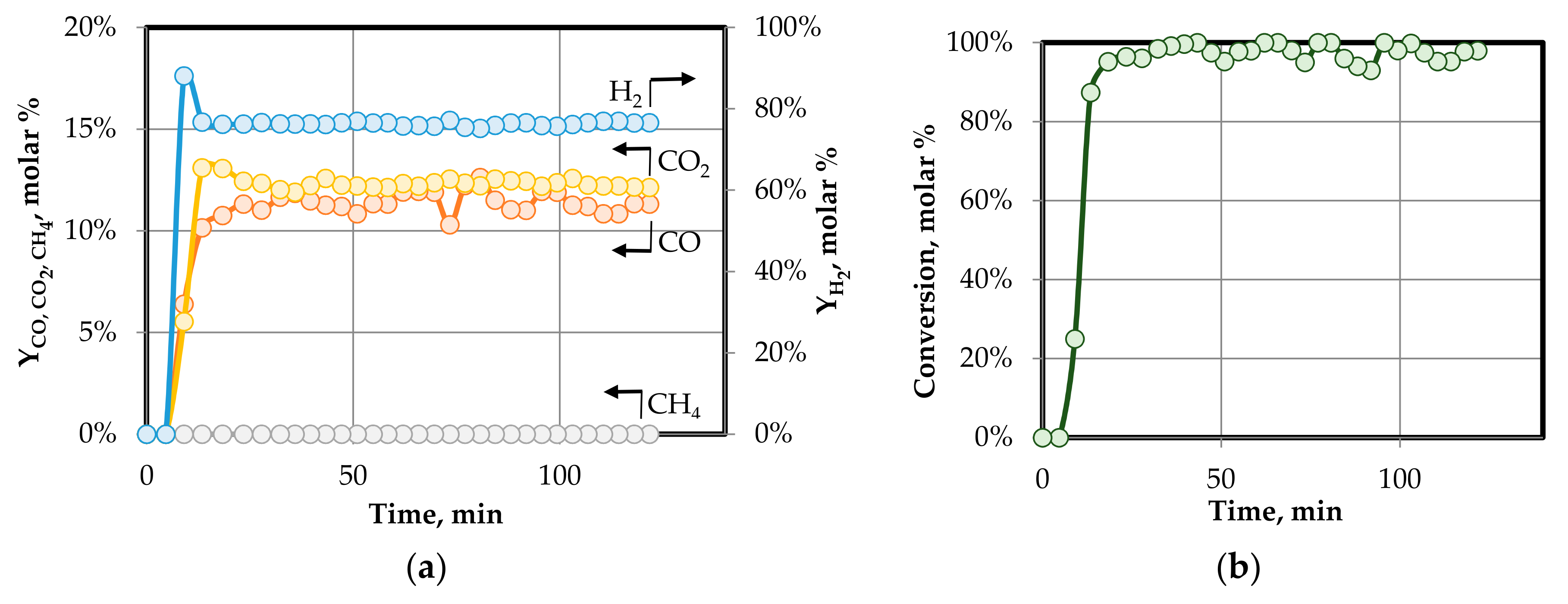
3.2.1. Effect of Temperature on Gas Yield and Gas Composition
3.2.2. Effect of S/C Ratio on Gas Yield and Gas Composition
3.3. Catalyst Characterization after Reaction
3.3.1. XRD Analysis Results
3.3.2. Amount and Type of Carbon Deposited on the Surface of Catalyst
3.3.3. SEM Analysis of the Catalyst after Reaction
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, J.; Tamura, M.; Nakagawa, Y.; Okumura, K.; Tomishige, K. Promoting effect of trace Pd on hydrotalcite-derived Ni/Mg/Al catalyst in oxidative steam reforming of biomass tar. Appl. Catal. B Environ. 2015, 179, 412–421. [Google Scholar] [CrossRef]
- Artetxe, M.; Alvarez, J.; Nahil, M.A.; Olazar, M.; Williams, P.T. Steam reforming of different biomass tar model compounds over Ni/Al2O3 catalysts. Energy Convers. Manag. 2017, 136, 119–126. [Google Scholar] [CrossRef]
- Chen, Y.H.; Schmid, M.; Kertthong, T.; Scheffknecht, G. Reforming of toluene as a tar model compound over straw char containing fly ash. Biomass Bioenergy 2020, 141, 105657. [Google Scholar] [CrossRef]
- Satyanarayana, K.G. Clay Surfaces: Fundamentals and Applications; Elsevier: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Theiss, F.L.; Ayoko, G.A.; Frost, R.L. Synthesis of layered double hydroxides containing Mg2+, Zn2+, Ca2+ and Al3+ layer cations by co-precipitation methods—A review. Appl. Surf. Sci. 2016, 383, 200–213. [Google Scholar] [CrossRef]
- Mishra, G.; Dash, B.; Pandey, S. Layered double hydroxides: A brief review from fundamentals to application as evolving biomaterials. Appl. Clay Sci. 2018, 153, 172–186. [Google Scholar] [CrossRef]
- Duan, X.; Evans, D.G. (Eds.) Layered Double Hydroxides; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2006; Volume 119. [Google Scholar]
- Liu, H.; Wierzbicki, D.; Debek, R.; Motak, M.; Grzybek, T.; da Costa, P.; Gálvez, M.E. La-promoted Ni-hydrotalcite-derived catalysts for dry reforming of methane at low temperatures. Fuel 2016, 182, 8–16. [Google Scholar] [CrossRef]
- Świrk, K.; Galvez, M.E.; Motak, M.; Grzybek, T.; Rønning, M.; da Costa, P. Yttrium promoted Ni-based double-layered hydroxides for dry methane reforming. J. Co2 Util. 2018, 27, 247–258. [Google Scholar] [CrossRef]
- Shen, Y.; Yoshikawa, K. Recent progresses in catalytic tar elimination during biomass gasification or pyrolysis—A review. Renew. Sustain. Energy Rev. 2013, 21, 371–392. [Google Scholar] [CrossRef]
- Mette, K.; Ressler, T.; Muhler, M. Development of Hydrotalcite-Derived Ni Catalysts for the Dry Reforming of Methane at High Temperatures. Ph.D. Thesis, Technische Universität, Berlin, Germany, 2015. [Google Scholar]
- Rached, A.J.; Dahdah, E.; Gennequin, C.; Tidahy, H.L.; Aboukais, A.; Abi-Aad, E.; Nsouli, B. Steam reforming of toluene for hydrogen production over NiMgALCe catalysts prepared via hydrotalcite route. In Proceedings of the 2016 7th International Renewable Energy Congress (IREC), Hammamet, Tunisia, 22–24 March 2016; IEEE: New York, NY, USA, 2016; pp. 1–6. [Google Scholar]
- Yu, X.; Wang, N.; Chu, W.; Liu, M. Carbon dioxide reforming of methane for syngas production over La-promoted NiMgAl catalysts derived from hydrotalcites. Chem. Eng. J. 2012, 209, 623–632. [Google Scholar] [CrossRef]
- Choi, S.C.; Lee, D.K.; Sohn, S.H. Morphological and Optical Properties of Cobalt Ion-Modified ZnO Nanowires. Catalysts 2020, 10, 614. [Google Scholar] [CrossRef]
- Koike, M.; Li, D.; Watanabe, H.; Nakagawa, Y.; Tomishige, K. Comparative study on steam reforming of model aromatic compounds of biomass tar over Ni and Ni–Fe alloy nanoparticles. Appl. Catal. A Gen. 2015, 506, 151–162. [Google Scholar] [CrossRef]
- Li, D.; Koike, M.; Wang, L.; Nakagawa, Y.; Xu, Y.; Tomishige, K. Regenerability of hydrotalcite-derived nickel–iron alloy nanoparticles for syngas production from biomass tar. ChemSusChem 2014, 7, 510–522. [Google Scholar] [CrossRef] [PubMed]
- Touahra, F.; Sehailia, M.; Ketir, W.; Bachari, K.; Chebout, R.; Trari, M.; Halliche, D. Effect of the Ni/Al ratio of hydrotalcite-type catalysts on their performance in the methane dry reforming process. Appl. Petrochem. Res. 2016, 6, 1–13. [Google Scholar] [CrossRef]
- Ferreira, R.A.R.; Ávila-Neto, C.N.; Noronha, F.B.; Hori, C.E. Study of LPG steam reform using Ni/Mg/Al hydrotalcite-type precursors. Int. J. Hydrog. Energy 2019, 44, 24471–24484. [Google Scholar] [CrossRef]
- Lu, P.; Huang, Q.; Bourtsalas, A.C.; Chi, Y.; Yan, J. Effect of operating conditions on the carbon formation and nickel catalyst performance during cracking of tar. Waste Biomass Valorization 2019, 10, 155–165. [Google Scholar] [CrossRef]
- Labaki, M.; Lamonier, J.F.; Siffert, S.; Aboukaïs, A. Thermal analysis and temperature-programmed reduction studies of copper–zirconium and copper–zirconium–yttrium compounds. Thermochim. Acta 2005, 427, 193–200. [Google Scholar] [CrossRef]
- Li, D.; Wang, L.; Koike, M.; Nakagawa, Y.; Tomishige, K. Steam reforming of tar from pyrolysis of biomass over Ni/Mg/Al catalysts prepared from hydrotalcite-like precursors. Appl. Catal. B Environ. 2011, 102, 528–538. [Google Scholar] [CrossRef]
- Cavani, F.; Trifiro, F.; Vaccari, A. Hydrotalcite-type anionic clays: Preparation, properties and applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Casenave, S.; Martinez, H.; Guimon, C.; Auroux, A.; Hulea, V.; Cordoneanu, A.; Dumitriu, E. Acid–base properties of Mg–Ni–Al mixed oxides using LDH as precursors. Thermochim. Acta 2001, 379, 85–93. [Google Scholar] [CrossRef]
- Silva, C.C.C.; Ribeiro, N.F.; Souza, M.M.; Aranda, D.A. Biodiesel production from soybean oil and methanol using hydrotalcites as catalyst. Fuel Process. Technol. 2010, 91, 205–210. [Google Scholar] [CrossRef]
- Serrano-Lotina, A.M. Obtención de Hidrógeno a Partir de Biogás Mediante Catalizadores Derivados de Hidrotalcita; Universidad Autónoma de Madrid: Madrid, Spain, 2012. [Google Scholar]
- Morlanés, N.; Melo, F. Obtención de Hidrógeno Mediante Reformado Catalítico de Nafta con Vapor de Agua; Universidad Politécnica de Valencia: Valencia, Spain, 2007. [Google Scholar]
- Zhou, F.; Pan, N.; Chen, H.; Xu, X.; Wang, C.; Du, Y.; Li, L. Hydrogen production through steam reforming of toluene over Ce, Zr or Fe promoted Ni-Mg-Al hydrotalcite-derived catalysts at low temperature. Energy Convers. Manag. 2019, 196, 677–687. [Google Scholar] [CrossRef]
- Bîrjega, R.; Pavel, O.D.; Costentin, G.; Che, M.; Angelescu, E. Rare-earth elements modified hydrotalcites and corresponding mesoporous mixed oxides as basic solid catalysts. Appl. Catal. A Gen. 2005, 288, 185–193. [Google Scholar] [CrossRef]
- Tsyganok, A.I.; Inaba, M.; Tsunoda, T.; Uchida, K.; Suzuki, K.; Takehira, K.; Hayakawa, T. Rational design of Mg–Al mixed oxide-supported bimetallic catalysts for dry reforming of methane. Appl. Catal. A Gen. 2005, 292, 328–343. [Google Scholar] [CrossRef]
- Ertl, G.; Knözinger, H.; Weitkamp, J. (Eds.) Preparation of Solid Catalysts; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Josuinkas, F.M.; Quitete, C.P.; Ribeiro, N.F.; Souza, M.M. Steam reforming of model gasification tar compounds over nickel catalysts prepared from hydrotalcite precursors. Fuel Process. Technol. 2014, 121, 76–82. [Google Scholar] [CrossRef]
- Qian, K.; Kumar, A. Catalytic reforming of toluene and naphthalene (model tar) by char supported nickel catalyst. Fuel 2017, 187, 128–136. [Google Scholar] [CrossRef]
- RJ, B.S.; Loganathan, M.; Shantha, M.S. A review of the water gas shift reaction kinetics. Int. J. Chem. React. Eng. 2010, 8. [Google Scholar] [CrossRef]
- Demirel, E.; Azcan, N. Thermodynamic modeling of water-gas shift reaction in supercritical water. In Proceedings of the World Congress on Engineering and Computer Science, San Francisco, CA, USA, 24–26 October 2012; Volume 2012. [Google Scholar]
- Zhu, H.L.; Pastor-Pérez, L.; Millan, M. Catalytic Steam Reforming of Toluene: Understanding the Influence of the Main Reaction Parameters over a Reference Catalyst. Energies 2020, 13, 813. [Google Scholar] [CrossRef]
- Ahmed, T.; Xiu, S.; Wang, L.; Shahbazi, A. Investigation of Ni/Fe/Mg zeolite-supported catalysts in steam reforming of tar using simulated-toluene as model compound. Fuel 2018, 211, 566–571. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, H.; Hou, X. Catalytic reforming of toluene as tar model compound: Effect of Ce and Ce–Mg promoter using Ni/olivine catalyst. Chemosphere 2014, 97, 40–46. [Google Scholar] [CrossRef]
- Bona, S.; Guillén, P.; Alcalde, J.G.; García, L.; Bilbao, R. Toluene steam reforming using coprecipitated Ni/Al catalysts modified with lanthanum or cobalt. Chem. Eng. J. 2008, 137, 587–597. [Google Scholar] [CrossRef]
- Świerczyński, D.; Libs, S.; Courson, C.; Kiennemann, A. Steam reforming of tar from a biomass gasification process over Ni/olivine catalyst using toluene as a model compound. Appl. Catal. B Environ. 2007, 74, 211–222. [Google Scholar] [CrossRef]
- Soongprasit, K.; Aht-Ong, D.; Sricharoenchaikul, V.; Atong, D. Synthesis and catalytic activity of sol-gel derived La–Ce–Ni perovskite mixed oxide on steam reforming of toluene. Curr. Appl. Phys. 2012, 12, S80–S88. [Google Scholar] [CrossRef]
- Nafday, D.; Sarkar, S.; Ayyub, P.; Saha-Dasgupta, T. A reduction in particle size generally causes body-centered-cubic metals to expand but face-centered-cubic metals to contract. ACS Nano 2018, 12, 7246–7252. [Google Scholar] [CrossRef] [PubMed]

 ) hydrotalcite, (
) hydrotalcite, (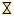 ) MgO-like phase, (•) NiO, (
) MgO-like phase, (•) NiO, (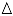 ) Ni(Fe)Ox, (
) Ni(Fe)Ox, ( ) Ni metal, (
) Ni metal, (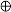 ) Ni–Fe alloy.
) Ni–Fe alloy.
 ) hydrotalcite, (
) hydrotalcite, (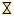 ) MgO-like phase, (•) NiO, (
) MgO-like phase, (•) NiO, (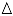 ) Ni(Fe)Ox, (
) Ni(Fe)Ox, ( ) Ni metal, (
) Ni metal, (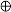 ) Ni–Fe alloy.
) Ni–Fe alloy.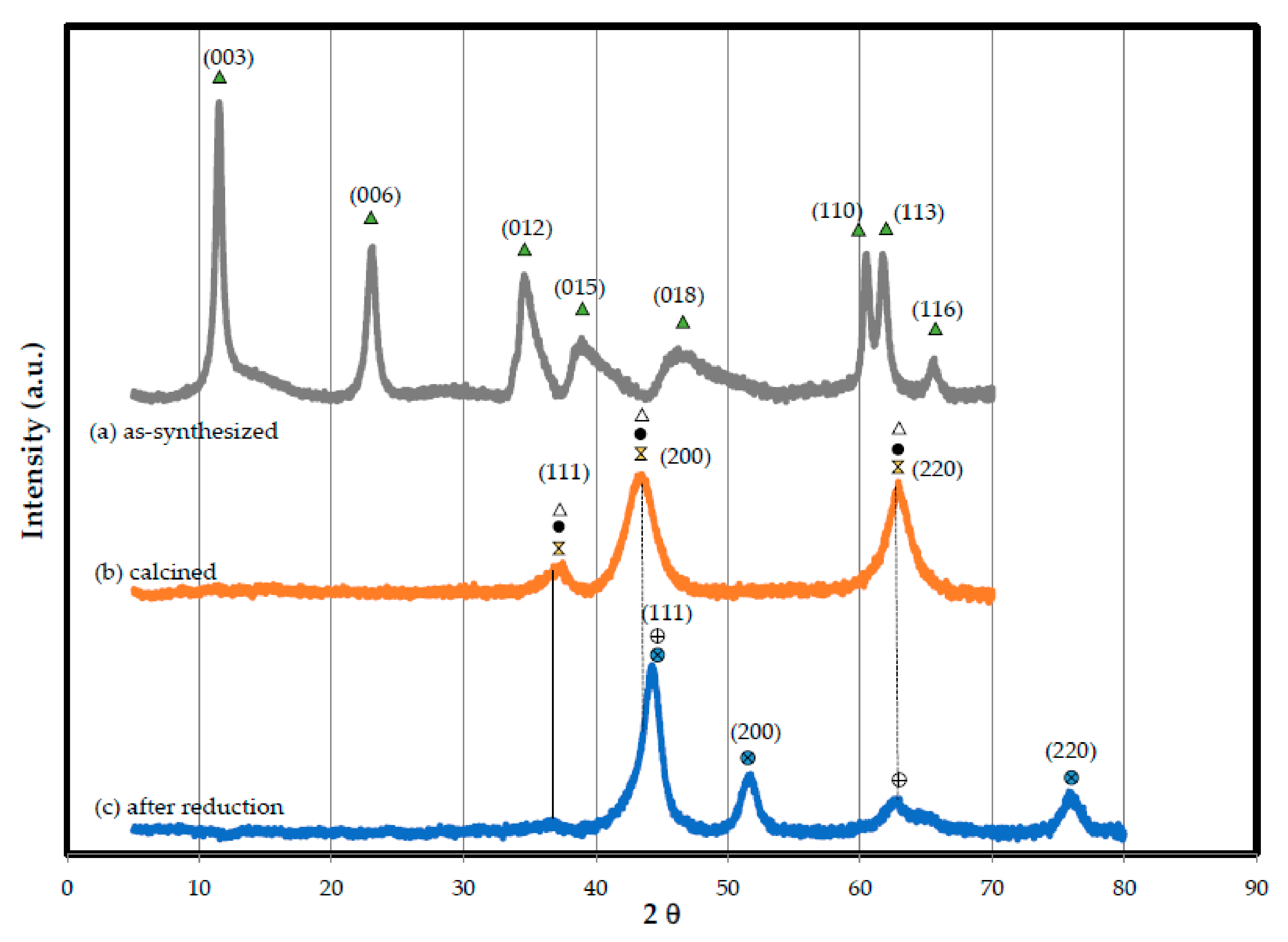
 ) as-synthesized; (
) as-synthesized; ( ) calcined.
) calcined.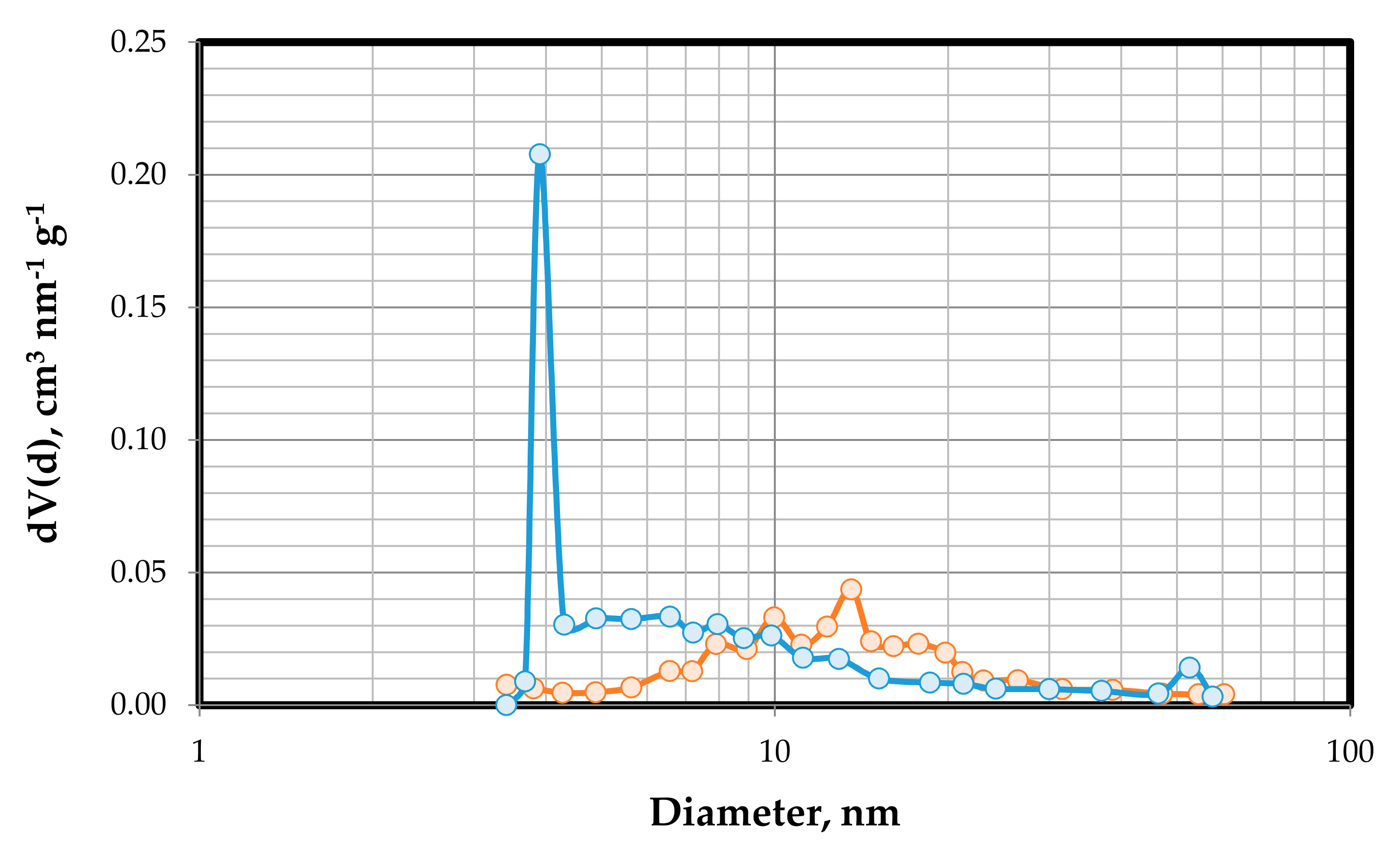
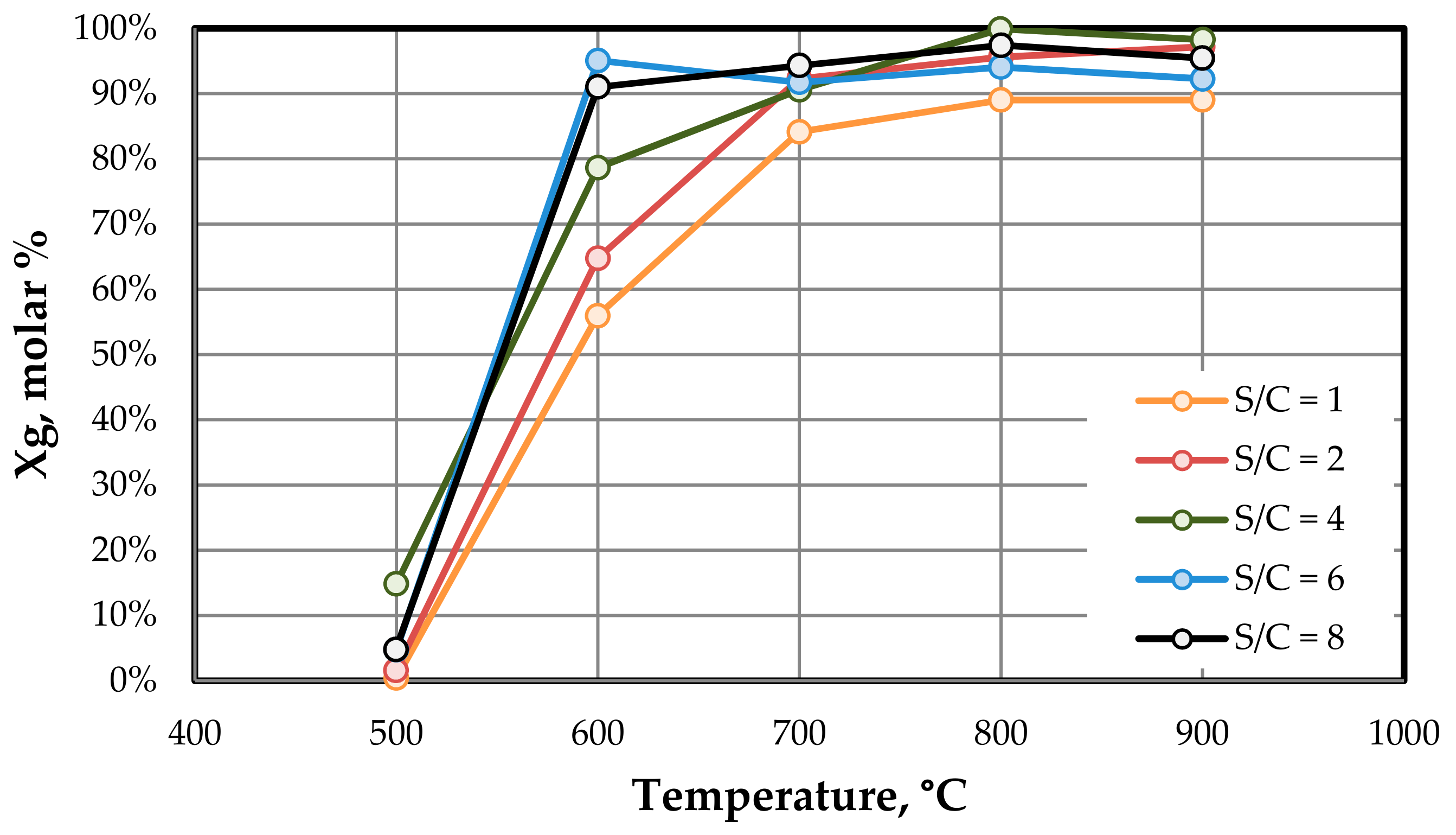
 ), S/C = 2 (
), S/C = 2 ( ), S/C = 4(
), S/C = 4( ), S/C = 6 (
), S/C = 6 ( ), S/C = 8 (
), S/C = 8 ( ).
).
 ), S/C = 2 (
), S/C = 2 ( ), S/C = 4(
), S/C = 4( ), S/C = 6 (
), S/C = 6 ( ), S/C = 8 (
), S/C = 8 ( ).
).

 ) Ni metal, (
) Ni metal, ( ) Ni–Fe alloy, (
) Ni–Fe alloy, ( ) MgO-like phase, (•) NiO.
) MgO-like phase, (•) NiO.
 ) Ni metal, (
) Ni metal, ( ) Ni–Fe alloy, (
) Ni–Fe alloy, ( ) MgO-like phase, (•) NiO.
) MgO-like phase, (•) NiO.

 ), 4 (
), 4 ( ), and 6 (
), and 6 ( ).
).
| LDH | Molar Cation Concentration Ni/Fe/Cu/Mg/Al |
|---|---|
| Ni−Fe−Cu/LDH | 30/5/5/40/20 |
| Ni−Fe/LDH | 35/5/0/40/20 |
| Ni−Cu/LDH | 35/0/5/40/20 |
| Ni/LDH | 40/0/0/40/20 |
| S/C | Reactant | Flow Rate, mmol min−1 | Feedstock Concentration, vol % |
|---|---|---|---|
| Toluene | 0.20 | 1.5% | |
| 1 | Water | 1.38 | 10.5% |
| Nitrogen | 11.59 | 88.0% | |
| Toluene | 0.20 | 1.5% | |
| 2 | Water | 2.77 | 21.0% |
| Nitrogen | 10.21 | 77.5% | |
| Toluene | 0.20 | 1.5% | |
| 4 | Water | 5.53 | 42.0% |
| Nitrogen | 7.45 | 56.5% | |
| Toluene | 0.20 | 1.5% | |
| 6 | Water | 8.30 | 63.0% |
| Nitrogen | 4.68 | 35.5% | |
| Toluene | 0.20 | 1.5% | |
| 8 | Water | 11.07 | 84.0% |
| Nitrogen | 1.91 | 14.5% |
| LDH | Al | Cu | Mg | Fe | Ni |
|---|---|---|---|---|---|
| As-synthesized Ni–Fe–Cu/LDH | 18.86% | 5.87% | 38.70% | 4.75% | 31.82% |
| Calcined Ni–Fe–Cu/LDH | 18.90% | 5.92% | 38.54% | 4.70% | 31.94% |
| LDH Calcined | Mmol H2/g |
|---|---|
| Ni/LDH | 6.68 |
| Ni–Fe/LDH | 7.30 |
| Ni–Cu/LDH | 7.22 |
| Ni–Fe–Cu/LDH | 7.84 |
| LDH | Crystallite Size | Lattice Parameters | |
|---|---|---|---|
| D, nm | a, Å | c, Å | |
| As-synthesized Ni–Fe–Cu/LDH | 12.83 | 3.06 | 23.06 |
| Calcined Ni–Fe–Cu/LDH | 4.32 | 4.20 | |
| Samples | SBET | Vpore | Average Pore Diameter |
|---|---|---|---|
| m2 g−1 | cm3 g−1 | nm | |
| As-synthesized Ni-Fe-Cu/LDH | 131.10 | 0.509 | 3.90 |
| Calcined Ni-Fe-Cu/LDH | 136.70 | 0.556 | 13.57 |
| Reference | Catalyst | Toluene Conversion, % | Temperature, °C | S/C |
|---|---|---|---|---|
| [37] | Ni–Ce–Mg/olivine | 93 | 790 | 3.5 |
| [2] | Ni/Al2O3 | 64 | 750 | 3 |
| [38] | Ni/Al/La | 94.53 | 650 | 5.7 |
| [39] | Ni/olivine | 100 | 650–850 | 2.3 |
| [40] | La0.6Ce0.4NiO3 | 80 | 800 | 2 |
| Samples | Crystallite Size | Lattice Parameter |
|---|---|---|
| D, nm | a, Å | |
| Ni–Fe–Cu/LDH after reduction | 6.11 | 3.53 |
| Ni–Fe–Cu/LDH after reaction | 15.44 | 3.54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díez, D.; Urueña, A.; Antolín, G. Investigation of Ni–Fe–Cu-Layered Double Hydroxide Catalysts in Steam Reforming of Toluene as a Model Compound of Biomass Tar. Processes 2021, 9, 76. https://doi.org/10.3390/pr9010076
Díez D, Urueña A, Antolín G. Investigation of Ni–Fe–Cu-Layered Double Hydroxide Catalysts in Steam Reforming of Toluene as a Model Compound of Biomass Tar. Processes. 2021; 9(1):76. https://doi.org/10.3390/pr9010076
Chicago/Turabian StyleDíez, David, Ana Urueña, and Gregorio Antolín. 2021. "Investigation of Ni–Fe–Cu-Layered Double Hydroxide Catalysts in Steam Reforming of Toluene as a Model Compound of Biomass Tar" Processes 9, no. 1: 76. https://doi.org/10.3390/pr9010076
APA StyleDíez, D., Urueña, A., & Antolín, G. (2021). Investigation of Ni–Fe–Cu-Layered Double Hydroxide Catalysts in Steam Reforming of Toluene as a Model Compound of Biomass Tar. Processes, 9(1), 76. https://doi.org/10.3390/pr9010076






