Olive Tree Leaves—A Source of Valuable Active Compounds
Abstract
1. Introduction
2. Bioactive Compounds in Olive Leaves
2.1. Polyphenolic Compounds
2.2. Triterpenoids
2.3. Tocopherols
2.4. Pigments
3. Technological Aspects for Extraction of Bioactive Compounds from Olive Leaves
3.1. Stabilisation of Raw Biomass to Promote Physicochemical Quality of Olive Leaves
3.2. Conventional Extraction Techniques
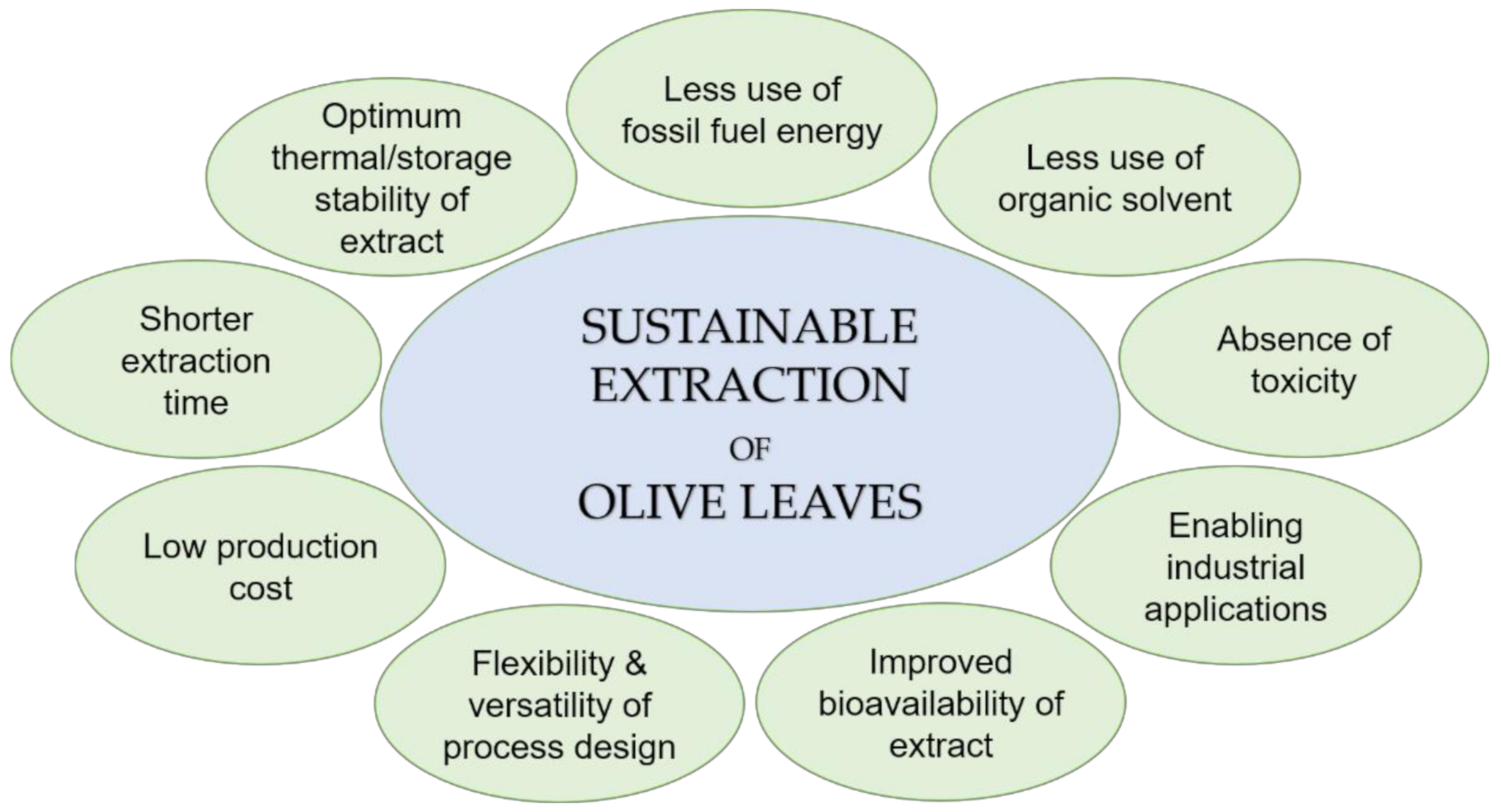
3.3. Alternative Strategies to Improve Extraction Performance
4. Significance of Stability of Olive Leaf Extract
5. Potential Industrial Applications of Olive Leaf Extracts
6. Future Trends
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gilani, A.H.; Khan, A.U. Medicinal Value of Combination of Cholinergic and Calcium Antagonist Constituents in Olives. In Olives and Olive Oil in Health and Disease Prevention; Preedy, V.R., Watson, R.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2010; pp. 835–843. [Google Scholar]
- Mannina, L.; Segre, A.L. NMR and olive oils: A geographical characterization. In Olives and Olive Oil in Health and Disease Prevention; Preedy, V.R., Watson, R.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2010; pp. 117–124. [Google Scholar]
- Ray, N.B.; Lam, N.T.; Luc, R.; Bonvino, N.P.; Karagiannis, T.C. Cellular and molecular effects of bioactive phenolic compounds in olives and olive oil. In Olive and Olive Oil Bioactive Constituents; Boskou, D., Ed.; AOCS Press, Elsevier: Amsterdam, The Netherlands, 2015; pp. 53–91. [Google Scholar]
- Caballero, B.; Trugo, L.C.; Finglas, P.M. Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Academic Press, Elsevier: Amsterdam, The Netherlands, 2003. [Google Scholar]
- Zipori, I.; Erel, R.; Yermiyahu, U.; Ben-Gal, A.; Dag, A. Sustainable Management of Olive Orchard Nutrition: A Review. Agriculture 2020, 10, 1–21. [Google Scholar] [CrossRef]
- Rahmanian, N.; Jafari, S.M.; Wani, T.A. Bioactive profile, dehydration, extraction and application of the bioactive components of olive leaves. Trends Food Sci. Technol. 2015, 42, 150–172. [Google Scholar] [CrossRef]
- Del Mar Contreras, M.; Lama-Muñoz, A.; Espínola, F.; Moya, M.; Romero, I.; Castro, E. Valorization of olive mill leaves through ultrasound-assisted extraction. Food Chem. 2020, 314, 1–8. [Google Scholar]
- Abaza, L.; Taamalli, A.; Nsir, H.; Zarrouk, M. Olive tree (Olea europeae L.) leaves: Importance and advances in the analysis of phenolic compounds. Antioxidants 2015, 4, 682–698. [Google Scholar] [CrossRef] [PubMed]
- Lama-Muñoz, A.; del Mar Contreras, M.; Espínola, F.; Moya, M.; Romero, I.; Castro, E. Content of phenolic compounds and mannitol in olive leaves extracts from six Spanish cultivars: Extraction with the Soxhlet method and pressurized liquids. Food Chem. 2020, 320, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Romero-García, J.M.; Niño, L.; Martínez-Patiño, C.; Álvarez, C.; Castro, E.; Negro, M.J. Biorefinery based on olive biomass. State of the art and future trends. Bioresour. Technol. 2014, 159, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Talhaoui, N.; Taamalli, A.; Gómez-Caravaca, A.M.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Phenolic compounds in olive leaves: Analytical determination, biotic and abiotic influence, and health benefits. Food Res. Int. 2015, 77, 92–108. [Google Scholar] [CrossRef]
- Bulotta, S.; Corradino, R.; Celano, M.; D’Agostino, M.; Maiuolo, J.; Oliverio, M.; Procopio, A.; Iannone, M.; Rotiroti, D.; Russo, D. Antiproliferative and antioxidant effects on breast cancer cells of oleuropein and its semisynthetic peracetylated derivatives. Food Chem. 2011, 127, 1609–1614. [Google Scholar] [CrossRef]
- Cavaca, L.A.; López-Coca, I.M.; Silvero, G.; Afonso, C.A. The olive-tree leaves as a source of high-added value molecules: Oleuropein. In Studies in Natural Products Chemistry; Atta-Ur-Rahman, Ed.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 64, pp. 131–180. [Google Scholar]
- Benavente-García, O.; Castillo, J.; Lorente, J.; Ortuño, A.D.R.J.; Del Rio, J.A. Antioxidant activity of phenolics extracted from Olea europaea L. leaves. Food Chem. 2000, 68, 457–462. [Google Scholar] [CrossRef]
- Jemai, H.; Fki, I.; Bouaziz, M.; Bouallagui, Z.; El Feki, A.; Isoda, H.; Sayadi, S. Lipid-lowering and antioxidant effects of hydroxytyrosol and its triacetylated derivative recovered from olive tree leaves in cholesterol-fed rats. J. Agric. Food Chem. 2008, 56, 2630–2636. [Google Scholar] [CrossRef]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Erbay, Z.; Icier, F. The importance and potential uses of olive leaves. Food Rev. Int. 2010, 26, 319–334. [Google Scholar] [CrossRef]
- Acar-Tek, N.; Ağagündüz, D. Olive Leaf (Olea europaea L. folium): Potential Effects on Glycemia and Lipidemia. Ann. Nutr. Metab. 2020, 76, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Tsimidou, M.Z.; Papoti, V.T. Bioactive ingredients in olive leaves. In Olives and Olive Oil in Health and Disease Prevention; Preedy, V.R., Watson, R.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2010; pp. 349–356. [Google Scholar]
- Souilem, S.; Fki, I.; Kobayashi, I.; Khalid, N.; Neves, M.A.; Isoda, H.; Sayadi, S.; Nakajima, M. Emerging technologies for recovery of value-added components from olive leaves and their applications in food/feed industries. Food Bioprocess Technol. 2017, 10, 229–248. [Google Scholar] [CrossRef]
- Hamzalıoğlu, A.; Gökmen, V. Interaction between Bioactive Carbonyl Compounds and Asparagine and Impact on Acrylamide. In Acrylamide in Food: Analysis, Content and Potential Health Effects; Gökmen, V., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 355–376. [Google Scholar]
- Bernhoft, A. A. A brief review on bioactive compounds in plants. In Bioactive Compounds in Plants-Benefits and Risks for Man and Animals; The Norwegian Academy of Science and Letters: Oslo, Norway, 2010; Volume 50, pp. 11–17. [Google Scholar]
- Ortega, A.M.M.; Campos, M.R.S. Bioactive Compounds as Therapeutic Alternatives. In Bioactive Compounds: Health Benefits and Potential Applications; Campos, M.R.S., Ed.; Woodhead Publishing: Cambridge, UK, 2019; pp. 247–264. [Google Scholar]
- Šaponjac, V.T.; Čanadanović-Brunet, J.; Ćetković, G.; Djilas, S. Detection of bioactive compounds in plants and food products. In Emerging and Traditional Technologies for Safe, Healthy and Quality Food; Nedović, V., Peter Raspor, P., Jovanka Lević, J., Šaponjac, V.T., Barbosa-Cánovas, G.V., Eds.; Springer: Cham, Switzerland, 2016; pp. 81–109. [Google Scholar]
- Galanakis, C.M. Introduction. In Nutraceutical and Functional Food Components: Effects of Innovative Processing Techniques; Elsevier: Oxford, UK, 2017; pp. 1–10. [Google Scholar]
- Moreno, J.; Peinado, R. Polyphenols. In Enological Chemistry; Elsevier, Academic Press: Cambridge, MA, USA, 2012; pp. 53–76. [Google Scholar]
- Souto, E.B.; Sampaio, A.C.; Campos, J.R.; Martins-Gomes, C.; Aires, A.; Silva, A.M. Polyphenols for skin cancer: Chemical properties, structure-related mechanisms of action and new delivery systems. In Studies in Natural Products Chemistry; Rahman, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 63, pp. 21–42. [Google Scholar]
- Petti, S.; Scully, C. Polyphenols, oral health and disease: A review. J. Dent. 2009, 37, 413–423. [Google Scholar] [CrossRef]
- Belitz, H.D.; Grosch, W.; Schieberle, P. Food Chemistry, 4th ed.; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Oniszczuk, A.; Widelska, G.; Wójtowicz, A.; Oniszczuk, T.; Wojtunik-Kulesza, K.; Dib, A.; Matwijczuk, A. Content of phenolic compounds and antioxidant activity of new gluten-free pasta with the addition of chestnut flour. Molecules 2019, 24, 1–14. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Wojtunik-Kulesza, K.; Oniszczuk, A.; Oniszczuk, T.; Combrzyński, M.; Nowakowska, D.; Matwijczuk, A. Influence of In Vitro Digestion on Composition, Bioaccessibility and Antioxidant Activity of Food Polyphenols—A Non-Systematic Review. Nutrients 2020, 12, 1401. [Google Scholar] [CrossRef]
- Oniszczuk, A.; Wójtowicz, A.; Oniszczuk, T.; Matwijczuk, A.; Dib, A.; Markut-Miotła, E. Opuntia Fruits as Food Enriching Ingredient, the First Step towards New Functional Food Products. Molecules 2020, 25, 916. [Google Scholar] [CrossRef]
- Segura-Carretero, A.; Menéndez-Menéndez, J.; Fernández-Gutiérrez, A. Polyphenols in olive oil: The importance of phenolic compounds in the chemical composition of olive oil. In Olives and Olive Oil in Health and Disease Prevention; Preedy, V.R., Watson, R.R., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2010; pp. 167–175. [Google Scholar]
- Huang, Y.L.; Oppong, M.B.; Guo, Y.; Wang, L.Z.; Fang, S.M.; Deng, Y.R.; Gao, X.M. The Oleaceae family: A source of secoiridoids with multiple biological activities. Fitoterapia 2019, 136, 1–18. [Google Scholar] [CrossRef]
- Mander, L.; Liu, H.W. Comprehensive Natural Products II: Chemistry and Biology; Townsend, C.A., Ebizuka, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2010; Volume 1. [Google Scholar]
- Rodriguez, S.; Marston, A.; Wolfender, J.L.; Hostettmann, K. Iridoids and secoiridoids in the Gentianaceae. Curr. Org. Chem. 1998, 2, 627–648. [Google Scholar]
- Nediani, C.; Ruzzolini, J.; Romani, A.; Calorini, L. Oleuropein, a Bioactive Compound from Olea europaea L., as a Potential Preventive and Therapeutic Agent in Non-Communicable Diseases. Antioxidants 2019, 8, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Romani, A.; Mulas, S.; Heimler, D. Polyphenols and secoiridoids in raw material (Olea europaea L. leaves) and commercial food supplements. Eur. Food Res. Technol. 2017, 243, 429–435. [Google Scholar] [CrossRef]
- Bouaziz, M.; Sayadi, S. Isolation and evaluation of antioxidants from leaves of a Tunisian cultivar olive tree. Eur. J. Lipid Sci. Technol. 2005, 107, 497–504. [Google Scholar] [CrossRef]
- Visioli, F.; Bellosta, S.; Galli, C. Oleuropein, the bitter principle of olives, enhances nitric oxide production by mouse macrophages. Life Sci. 1998, 62, 541–546. [Google Scholar] [CrossRef]
- Geyikoglu, F.; Isikgoz, H.; Onalan, H.; Colak, S.; Cerig, S.; Bakir, M.; Hosseinigouzdagani, M.; Koc, K.; Erol, H.S.; Saglam, Y.S.; et al. Impact of high-dose oleuropein on cisplatin-induced oxidative stress, genotoxicity and pathological changes in rat stomach and lung. J. Asian Nat. Prod. Res. 2017, 19, 1214–1231. [Google Scholar] [CrossRef]
- Paiva-Martins, F.; Pinto, M. Isolation and characterization of a new hydroxytyrosol derivative from olive (Olea europaea) leaves. J. Agric. Food Chem. 2008, 56, 5582–5588. [Google Scholar] [CrossRef]
- Zoidou, E.; Melliou, E.; Moatsou, G.; Magiatis, Ρ. Preparation of Functional Yogurt Enriched With Olive-Derived Products. In Yogurt in Health and Disease Prevention; Shah, N.P., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 203–220. [Google Scholar]
- Xu, F.; Li, Y.; Zheng, M.; Xi, X.; Zhang, X.; Han, C. Structure properties, acquisition protocols, and biological activities of oleuropein aglycone. Front. Chem. 2018, 6, 1–10. [Google Scholar] [CrossRef]
- Siliani, S.; Mattei, A.; Innocenti, L.B.; Zanoni, B. Bitter taste and phenolic compounds in extra virgin olive oil: An empirical relationship. J. Food Qual. 2006, 29, 431–441. [Google Scholar] [CrossRef]
- Leri, M.; Natalello, A.; Bruzzone, E.; Stefani, M.; Bucciantini, M. Oleuropein aglycone and hydroxytyrosol interfere differently with toxic Aβ1-42 aggregation. Food Chem. Toxicol. 2019, 129, 1–12. [Google Scholar] [CrossRef]
- Babu, P.V.A.; Liu, D. Flavonoids and cardiovascular health. In Complementary and Alternative Therapies and the Aging Population; Watson, R.R., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2009; pp. 371–392. [Google Scholar]
- Havsteen, B.H. The biochemistry and medical significance of the flavonoids. Pharmacol. Ther. 2002, 96, 67–202. [Google Scholar] [CrossRef]
- Ghomari, O.; Sounni, F.; Massaoudi, Y.; Ghanam, J.; Kaitouni, L.B.D.; Merzouki, M.; Benlemlih, M. Phenolic profile (HPLC-UV) of olive leaves according to extraction procedure and assessment of antibacterial activity. Biotechnol. Rep. 2019, 23, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Tamasi, G.; Bonechi, C.; Belyakova, A.; Pardini, A.; Rossi, C. The olive tree, a source of antioxidant compounds. J. Siena Acad. Sci. 2016, 8, 10–29. [Google Scholar] [CrossRef][Green Version]
- Martínez, L.; Ros, G.; Nieto, G. Hydroxytyrosol: Health benefits and use as functional ingredient in meat. Medicines 2018, 5, 1–12. [Google Scholar] [CrossRef]
- Fki, I.; Sayadi, S.; Mahmoudi, A.; Daoued, I.; Marrekchi, R.; Ghorbel, H. Comparative Study on Beneficial Effects of Hydroxytyrosol-and Oleuropein-Rich Olive Leaf Extracts on High-Fat Diet-Induced Lipid Metabolism Disturbance and Liver Injury in Rats. Biomed Res. Int. 2020, 2020, 1–15. [Google Scholar] [CrossRef]
- Karković Marković, A.; Torić, J.; Barbarić, M.; Jakobušić Brala, C. Hydroxytyrosol, tyrosol and derivatives and their potential effects on human health. Molecules 2019, 24, 1–39. [Google Scholar] [CrossRef]
- Bilgin, M.; Şahin, S. Effects of geographical origin and extraction methods on total phenolic yield of olive tree (Olea europaea) leaves. J. Taiwan Inst. of Chem. Eng. 2013, 44, 8–12. [Google Scholar] [CrossRef]
- Wang, B.; Qu, J.; Feng, S.; Chen, T.; Yuan, M.; Huang, Y.; Liao, J.; Yang, R.; Ding, C. Seasonal Variations in the Chemical Composition of Liangshan Olive Leaves and Their Antioxidant and Anticancer Activities. Foods 2019, 8, 1–17. [Google Scholar] [CrossRef]
- Ranalli, A.; Contento, S.; Lucera, L.; Di Febo, M.; Marchegiani, D.; Di Fonzo, V. Factors affecting the contents of iridoid oleuropein in olive leaves (Olea europaea L.). J. Agric. Food Chem. 2006, 54, 434–440. [Google Scholar] [CrossRef]
- Di Donna, L.; Mazzotti, F.; Naccarato, A.; Salerno, R.; Tagarelli, A.; Taverna, D.; Sindona, G. Secondary metabolites of Olea europaea leaves as markers for the discrimination of cultivars and cultivation zones by multivariate analysis. Food Chem. 2010, 121, 492–496. [Google Scholar] [CrossRef]
- Howes, M.J.R. Phytochemicals as anti-inflammatory nutraceuticals and phytopharmaceuticals. In Immunity and Inflammation in Health and Disease: Emerging Roles of Nutraceuticals and Functional Foods in Immune Support; Chatterjee, S., Jungraithmayr, W., Bagchi, D., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 363–388. [Google Scholar]
- Jäger, S.; Trojan, H.; Kopp, T.; Laszczyk, M.N.; Scheffler, A. Pentacyclic triterpene distribution in various plants–rich sources for a new group of multi-potent plant extracts. Molecules 2009, 14, 2016–2031. [Google Scholar] [CrossRef] [PubMed]
- Stiti, N.; Hartmann, M.A. Nonsterol triterpenoids as major constituents of Olea europaea. J. Lipids 2012, 2012, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Du, J.R.; Long, F.Y.; Chen, C. Research progress on natural triterpenoid saponins in the chemoprevention and chemotherapy of cancer. In The Enzymes; Bathaie, Z.S., Tamanoi, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 36, pp. 95–130. [Google Scholar]
- Sánchez-Quesada, C.; López-Biedma, A.; Warleta, F.; Campos, M.; Beltrán, G.; Gaforio, J.J. Bioactive properties of the main triterpenes found in olives, virgin olive oil, and leaves of Olea europaea. J. Agric. Food Chem. 2013, 61, 12173–12182. [Google Scholar] [CrossRef] [PubMed]
- Martín-García, B.; Verardo, V.; León, L.; De la Rosa, R.; Arráez-Román, D.; Segura-Carretero, A.; Gómez-Caravaca, A.M. GC-QTOF-MS as valuable tool to evaluate the influence of cultivar and sample time on olive leaves triterpenic components. Food Res. Int. 2019, 115, 219–226. [Google Scholar] [CrossRef]
- Hsu, C.L.; Yen, G.C. Ganoderic acid and lucidenic acid (triterpenoid). In The Enzymes; Bathaie, S.Z., Tamanoi, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 36, pp. 33–56. [Google Scholar]
- Guinda, A.; Rada, M.; Delgado, T.; Gutiérrez-Adánez, P.; Castellano, J.M. Pentacyclic triterpenoids from olive fruit and leaf. J. Agric. Food Chem. 2010, 58, 9685–9691. [Google Scholar] [CrossRef]
- Guinda, Á.; Castellano, J.M.; Santos-Lozano, J.M.; Delgado-Hervás, T.; Gutiérrez-Adánez, P.; Rada, M. Determination of major bioactive compounds from olive leaf. LWT-Food Sci. Technol. 2015, 64, 431–438. [Google Scholar] [CrossRef]
- Canabarro, N.I.; Mazutti, M.A.; do Carmo Ferreira, M. Drying of olive (Olea europaea L.) leaves on a conveyor belt for supercritical extraction of bioactive compounds: Mathematical modeling of drying/extraction operations and analysis of extracts. Ind. Crop. Prod. 2019, 136, 140–151. [Google Scholar] [CrossRef]
- Tsoumani, M.; Kontogianni, V.; Kellici, T.; Mavromoustakos, T.; Gerothanassis, I.; Tzakos, A.; Tselepis, A. Antiplatelet effect of the main triterpenoids of an olive leaf extract. Atherosclerosis 2016, 252, e1–e196. [Google Scholar] [CrossRef]
- Duncan, S.E.; Chang, H.H. Implications of light energy on food quality and packaging selection. In Advances in Food and Nutrition Research; Henry, J., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2012; Volume 67, pp. 25–73. [Google Scholar]
- Mokrosnop, V.M. Functions of tocopherols in the cells of plants and other photosynthetic organisms. Ukr. Biochem. J. 2014, 86, 26–36. [Google Scholar] [CrossRef]
- Colville, L. Seed storage. In Encyclopedia of Applied Plant Sciences, 2nd ed.; Thomas, B., Murray, B.G., Murray, D.J., Eds.; Elsevier: Oxford, UK, 2017; Volume 1, pp. 335–339. [Google Scholar]
- Tena, N.; Lobo-Prieto, A.; Aparicio, R.; García-González, D.L. Storage and preservation of fats and oils. In Encyclopedia of Food Security and Sustainability; Ferranti, P., Berry, E.M., Anderson, J.R., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; Volume 2, pp. 605–618. [Google Scholar]
- De Lucas, A.D.; de la Ossa, E.M.; Rincón, J.; Blanco, M.A.; Gracia, I. Supercritical fluid extraction of tocopherol concentrates from olive tree leaves. J. Supercrit. Fluid. 2002, 22, 221–228. [Google Scholar] [CrossRef]
- Botsoglou, E.; Govaris, A.; Ambrosiadis, I.; Fletouris, D. Lipid and protein oxidation of α-linolenic acid-enriched pork during refrigerated storage as influenced by diet supplementation with olive leaves (Olea europea L.) or α-tocopheryl acetate. Meat Sci. 2012, 92, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Tarchoune, I.; Sgherri, C.; Eddouzi, J.; Zinnai, A.; Quartacci, M.F.; Zarrouk, M. Olive leaf addition increases olive oil nutraceutical properties. Molecules 2019, 24, 545. [Google Scholar] [CrossRef] [PubMed]
- Sudhakar, P.; Latha, P.; Reddy, P.V. Phenotyping Crop Plants for Physiological and Biochemical Traits; BS Publications: Hyderabad, India; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Schoefs, B. Chlorophyll and carotenoid analysis in food products. A practical case-by-case view. TrAc Trend. Anal. Chem. 2003, 22, 335–339. [Google Scholar] [CrossRef]
- Aadil, R.M.; Roobab, U.; Sahar, A.; ur Rahman, U.; Khalil, A.A. Functionality of Bioactive Nutrients in Beverages. In Nutrients in Beverages; Grumezescu, A.M., Holban, A.M., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; Volume 12, pp. 237–276. [Google Scholar]
- Garcia-Vaquero, M.; Rajauria, G. Analytical techniques for phytochemical estimation in fruit juices. In Fruit Juices: Extraction, Composition, Quality and Analysis; Rajauria, G., Tiwari, B.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 669–692. [Google Scholar]
- Domonkos, I.; Kis, M.; Gombos, Z.; Ughy, B. Carotenoids, versatile components of oxygenic photosynthesis. Prog. Lipid Res. 2013, 52, 539–561. [Google Scholar] [CrossRef] [PubMed]
- Solymosi, K.; Latruffe, N.; Morant-Manceau, A.; Schoefs, B. Food colour additives of natural origin. In Colour Additives for Foods and Beverages; Scotter, M.J., Ed.; Woodhead Publishing: Cambridge, UK, 2015; pp. 3–34. [Google Scholar]
- Roca, M.; Chen, K.; Pérez-Gálvez, A. Chlorophylls. In Handbook on Natural Pigments in Food and Beverages; Carle, R., Schweiggert, R.M., Eds.; Woodhead Publishing: Cambridge, UK, 2016; pp. 125–158. [Google Scholar]
- Casida, J.E. Pest toxicology: The primary mechanisms of pesticide action. Chem. Res. Toxicol. 2009, 22, 609–619. [Google Scholar] [CrossRef]
- Indrasti, D.; Andarwulan, N.; Purnomo, E.H.; Wulandari, N. Stability of Chlorophyll as Natural Colorant: A Review for Suji (Dracaena Angustifolia Roxb.) Leaves’ Case. Curr. Res. Nutr. Food Sci. J. 2018, 6, 609–625. [Google Scholar] [CrossRef]
- Yilmaz, C.; Gökmen, V. Chlorophyll. In Reference Module in Food Science. Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 37–41. [Google Scholar]
- Aramrueang, N.; Asavasanti, S.; Khanunthong, A. Leafy Vegetables. In Integrated Processing Technologies for Food and Agricultural By-Products; Pan, Z., Zhang, R., Zicari, S., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 245–272. [Google Scholar]
- Trees, C.C.; Clark, D.K.; Bidigare, R.R.; Ondrusek, M.E.; Mueller, J.L. Accessory pigments versus chlorophyll a concentrations within the euphotic zone: A ubiquitous relationship. Limnol. Oceanogr. 2000, 45, 1130–1143. [Google Scholar] [CrossRef]
- Bahloul, N.; Kechaou, N.; Mihoubi, N.B. Comparative investigation of minerals, chlorophylls contents, fatty acid composition and thermal profiles of olive leaves (Olea europeae L.) as by-product. Grasas y Aceites 2014, 65, e035. [Google Scholar] [CrossRef]
- Brahmi, F.; Mechri, B.; Dabbou, S.; Dhibi, M.; Hammami, M. The efficacy of phenolics compounds with different polarities as antioxidants from olive leaves depending on seasonal variations. Ind. Crop Prod. 2012, 38, 146–152. [Google Scholar] [CrossRef]
- Pokorný, J.; Korczak, J. Preparation of natural antioxidants. In Antioxidants in Food: Practical Applications, 1st ed.; Pokorný, J., Yanishlieva, N., Gordon, M., Eds.; Woodhead Publishing: Cambridge, UK, 2001; pp. 311–330. [Google Scholar]
- Duh, P.D.; Yen, G.C. Antioxidant efficacy of methanolic extracts of peanut hulls in soybean and peanut oils. J. Am. Oil Chem. Soc. 1997, 74, 745. [Google Scholar]
- Brglez Mojzer, E.; Knez Hrnčič, M.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction methods, antioxidative action, bioavailability and anticarcinogenic effects. Molecules 2016, 21, 1–38. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Erbay, Z.; Icier, F. Optimization of hot air drying of olive leaves using response surface methodology. J. Food Eng. 2009, 91, 533–541. [Google Scholar] [CrossRef]
- Erbay, Z.; Icier, F. Optimization of drying of olive leaves in a pilot-scale heat pump dryer. Dry. Technol. 2009, 27, 416–427. [Google Scholar] [CrossRef]
- Nourhène, B.; Mohammed, K.; Nabil, K. Experimental and mathematical investigations of convective solar drying of four varieties of olive leaves. Food Bioprod. Process. 2008, 86, 176–184. [Google Scholar] [CrossRef]
- Martinho, D.; Karmali, A.; Rosa, E. Extraction of Phenolic Compounds from Olive Leaf Extracts and Their Effect on Proliferation of Human Carcinoma Cell Lines. J. Agric. Sci. 2019, 10, 1271–1285. [Google Scholar] [CrossRef]
- Babu, A.K.; Kumaresan, G.; Raj, V.A.A.; Velraj, R. Review of leaf drying: Mechanism and influencing parameters, drying methods, nutrient preservation, and mathematical models. Renew. Sustain. Energy Rev. 2018, 90, 536–556. [Google Scholar] [CrossRef]
- Malik, N.S.; Bradford, J.M. Recovery and stability of oleuropein and other phenolic compounds during extraction and processing of olive (Olea europaea L.) leaves. J. Food Agric. Environ. 2008, 6, 8–13. [Google Scholar]
- Ahmad-Qasem, M.H.; Barrajón-Catalán, E.; Micol, V.; Mulet, A.; García-Pérez, J.V. Influence of freezing and dehydration of olive leaves (var. Serrana) on extract composition and antioxidant potential. Food Res. Int. 2013, 50, 189–196. [Google Scholar] [CrossRef]
- Romero-García, J.M.; Lama-Muñoz, A.; Rodríguez-Gutiérrez, G.; Moya, M.; Ruiz, E.; Fernández-Bolaños, J.; Castro, E. Obtaining sugars and natural antioxidants from olive leaves by steam-explosion. Food Chem. 2016, 210, 457–465. [Google Scholar] [CrossRef]
- Žuntar, I.; Putnik, P.; Bursać Kovačević, D.; Nutrizio, M.; Šupljika, F.; Poljanec, A.; Dubrović, I.; Barba, F.J.; Režek Jambrak, A. Phenolic and Antioxidant Analysis of Olive Leaves Extracts (Olea europaea L.) Obtained by High Voltage Electrical Discharges (HVED). Foods 2019, 8, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Lama-Muñoz, A.; del Mar Contreras, M.; Espínola, F.; Moya, M.; de Torres, A.; Romero, I.; Castro, E. Extraction of oleuropein and luteolin-7-O-glucoside from olive leaves: Optimization of technique and operating conditions. Food Chem. 2019, 293, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Varelis, P.; Melton, L.; Shahidi, F. Encyclopedia of Food Chemistry; Elsevier: Amsterdam, The Netherlands, 2018; Volume 1. [Google Scholar]
- Panzella, L.; Moccia, F.; Nasti, R.; Marzorati, S.; Verotta, L.; Napolitano, A. Bioactive phenolic compounds from agri-food wastes: An update on green and sustainable extraction methodologies. Front. Nutr. 2020, 7, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Da Rosa, G.S.; Vanga, S.K.; Gariepy, Y.; Raghavan, V. Comparison of microwave, ultrasonic and conventional techniques for extraction of bioactive compounds from olive leaves (Olea europaea L.). Innov. Food Sci. Emerg. Technol. 2019, 58, 102234. [Google Scholar] [CrossRef]
- Sánchez-Ávila, N.; Priego-Capote, F.; Ruiz-Jiménez, J.; de Castro, M.L. Fast and selective determination of triterpenic compounds in olive leaves by liquid chromatography–tandem mass spectrometry with multiple reaction monitoring after microwave-assisted extraction. Talanta 2009, 78, 40–48. [Google Scholar] [CrossRef]
- Mandal, S.C.; Mandal, V.; Das, A.K. Essentials of Botanical Extraction: Principles and Applications; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Baldino, L.; Della Porta, G.; Osseo, L.S.; Reverchon, E.; Adami, R. Concentrated oleuropein powder from olive leaves using alcoholic extraction and supercritical CO2 assisted extraction. J. Supercrit. Fluid. 2018, 133, 65–69. [Google Scholar] [CrossRef]
- Le Floch, F.; Tena, M.T.; Rıos, A.; Valcarcel, M. Supercritical fluid extraction of phenol compounds from olive leaves. Talanta 1998, 46, 1123–1130. [Google Scholar] [CrossRef]
- Paulo, F.; Santos, L. Deriving valorization of phenolic compounds from olive oil by-products for food applications through microencapsulation approaches: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2020, 1–26. [Google Scholar] [CrossRef]
- Xynos, N.; Papaefstathiou, G.; Psychis, M.; Argyropoulou, A.; Aligiannis, N.; Skaltsounis, A.L. Development of a green extraction procedure with super/subcritical fluids to produce extracts enriched in oleuropein from olive leaves. J. Supercrit. Fluid. 2012, 67, 89–93. [Google Scholar] [CrossRef]
- Pereira, R.N.; Rodrigues, R.M.; Genisheva, Z.; Oliveira, H.; de Freitas, V.; Teixeira, J.A.; Vicente, A.A. Effects of ohmic heating on extraction of food-grade phytochemicals from colored potato. LWT-Food Sci. Technol. 2016, 74, 493–503. [Google Scholar] [CrossRef]
- Parisi, O.I.; Puoci, F.; Restuccia, D.; Farina, G.; Iemma, F.; Picci, N. Polyphenols and their formulations: Different strategies to overcome the drawbacks associated with their poor stability and bioavailability. In Polyphenols in Human Health and Disease; Watson, R.R., Preedy, V.R., Zibadi., S., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 1, pp. 29–45. [Google Scholar]
- Bishayee, A.; Ahmed, S.; Brankov, N.; Perloff, M. Triterpenoids as potential agents for the chemoprevention and therapy of breast cancer. Front. Biosci. 2011, 16, 980–996. [Google Scholar] [CrossRef] [PubMed]
- Mourtzinos, I.; Salta, F.; Yannakopoulou, K.; Chiou, A.; Karathanos, V.T. Encapsulation of olive leaf extract in β-cyclodextrin. J. Agric. Food Chem. 2007, 55, 8088–8094. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.; Jafari, S.M.; Esfanjani, A.F.; Akhavan, S. Application of nano-encapsulated olive leaf extract in controlling the oxidative stability of soybean oil. Food Chem. 2016, 190, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Urzúa, C.; González, E.; Dueik, V.; Bouchon, P.; Giménez, B.; Robert, P. Olive leaves extract encapsulated by spray-drying in vacuum fried starch–gluten doughs. Food Bioprod. Process. 2017, 106, 171–180. [Google Scholar] [CrossRef]
- Soleimanifar, M.; Jafari, S.M.; Assadpour, E. Encapsulation of olive leaf phenolics within electrosprayed whey protein nanoparticles; production and characterization. Food Hydrocol. 2020, 101, 105572. [Google Scholar] [CrossRef]
- Sanchez de Medina, V.; Priego-Capote, F.; Jiménez-Ot, C.; Luque de Castro, M.D. Quality and stability of edible oils enriched with hydrophilic antioxidants from the olive tree: The role of enrichment extracts and lipid composition. J. Agric. Food Chem. 2011, 59, 11432–11441. [Google Scholar] [CrossRef]
- Ammar, S.; Kelebek, H.; Zribi, A.; Abichou, M.; Selli, S.; Bouaziz, M. LC-DAD/ESI-MS/MS characterization of phenolic constituents in Tunisian extra-virgin olive oils: Effect of olive leaves addition on chemical composition. Food Res. Int. 2017, 100, 477–485. [Google Scholar] [CrossRef]
- Sevim, D.; Tuncay, O.; Koseoglu, O. The effect of olive leaf addition on antioxidant content and antioxidant activity of “Memecik” olive oils at two maturity stages. J. Am. Oil Chem. Soc. 2013, 90, 1359–1369. [Google Scholar] [CrossRef]
- Nunes, M.A.; Pimentel, F.B.; Costa, A.S.; Alves, R.C.; Oliveira, M.B.P. Olive by-products for functional and food applications: Challenging opportunities to face environmental constraints. Innov. Food Sci. Emerg. Technol. 2016, 35, 139–148. [Google Scholar] [CrossRef]
- Chiou, A.; Salta, F.N.; Kalogeropoulos, N.; Mylona, A.; Ntalla, I.; Andrikopoulos, N.K. Retention and distribution of polyphenols after pan-frying of French fries in oils enriched with olive leaf extract. J. Food Sci. 2007, 72, S574–S584. [Google Scholar] [CrossRef]
- Paiva-Martins, F.; Correia, R.; Félix, S.; Ferreira, P.; Gordon, M.H. Effects of enrichment of refined olive oil with phenolic compounds from olive leaves. J. Agric. Food Chem. 2007, 55, 4139–4143. [Google Scholar] [CrossRef] [PubMed]
| Harvest Stage | Determinant | Reference |
|---|---|---|
| Pre-harvest | Geographical origin | [55] |
| Maturity stage | [19] | |
| Climate | [56] | |
| Cultivar | [55,57] | |
| Tree/leaf lifetime | [57] | |
| Post-harvest | Storage (time/temperature) | [19] |
| Harvest time/period | [58] | |
| Extraction technique | [19,55] |
| Nature of Study | Extraction Method | Advantage | Reference |
|---|---|---|---|
| Optimising extraction of total phenolic compounds (including oleuropein, flavonoids, luteoline-7-O-glucoside) | Pressurised liquid-extraction (optimised by Response Surface Methodology) Solvent: ethanol/water | Efficient method for isolation of phytochemicals; low operation/input cost; high speed; greater recovery | [104] |
| Study of various extraction methods and assess the influence of designed parameters on recovery of total phenolics and antioxidant activity | Microwave-assisted extraction (MAE) Ultrasonic assisted extraction (UAE) Conventional (maceration) | MAE: Most efficient method; less time consuming UAE: Lower temperature provides better quality and greater yield | [107] |
| Determination of increasing oleuropein isolation | Hybrid extraction technique: aqueous ethanolic extract (from liquid–solid extraction) was subjected to supercritical antisolvent extraction, to obtain precipitated/concentrated oleuropein | Enables precipitation and formation of oleuropein powder; efficient method. Only traces of ethanol remain in final extract | [110] |
| Investigation of total phenolic compounds through developing supercritical fluid extraction | Supercritical fluid extraction | High-speed; automated means; requires less solvent; selective isolation | [111] |
| Effective development of phenolic extraction using high voltage electrical discharges compared to conventional method | High voltage electric discharges (characterisation analysis performed by ultra-performance liquid chromatography-tandem mass spectrometry) | Use of organic solvents; effective extraction method (high extractability) | [103] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Markhali, F.S.; Teixeira, J.A.; Rocha, C.M.R. Olive Tree Leaves—A Source of Valuable Active Compounds. Processes 2020, 8, 1177. https://doi.org/10.3390/pr8091177
Markhali FS, Teixeira JA, Rocha CMR. Olive Tree Leaves—A Source of Valuable Active Compounds. Processes. 2020; 8(9):1177. https://doi.org/10.3390/pr8091177
Chicago/Turabian StyleMarkhali, Fereshteh Safarzadeh, José A. Teixeira, and Cristina M. R. Rocha. 2020. "Olive Tree Leaves—A Source of Valuable Active Compounds" Processes 8, no. 9: 1177. https://doi.org/10.3390/pr8091177
APA StyleMarkhali, F. S., Teixeira, J. A., & Rocha, C. M. R. (2020). Olive Tree Leaves—A Source of Valuable Active Compounds. Processes, 8(9), 1177. https://doi.org/10.3390/pr8091177







