Coupling of Water Activity and Colour Development of Roast Duck Skin under Forced Convection Drying
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Standards
2.2. Sample Preparation
2.3. Oven Condition and Description of the Drying Process
2.3.1. Description of the Drying Process and Monitoring of the Internal Temperature
2.3.2. Instrumental Measurement of Water Activity and Surface
2.3.3. The BaP and Heterocyclic Aromatic Amines Determination
Analysis of 3,4-benzo(a)pyrene Content
Analysis of Heterocyclic Aromatic Amines Content
2.3.4. Statistical Analysis
3. Result and Discussion
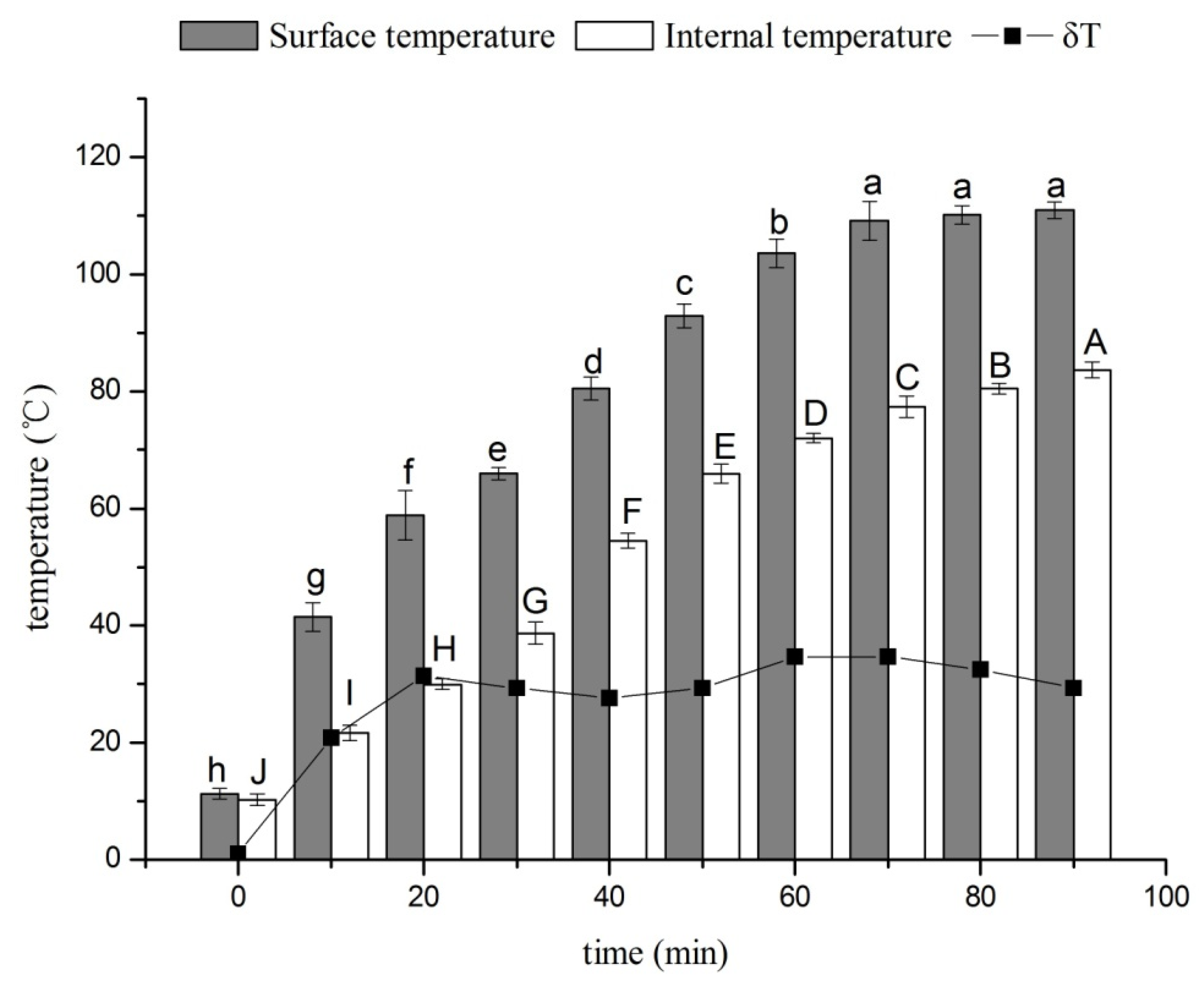
3.1. Effects of Drying Time on the Surface and Internal Temperatures of Ducks
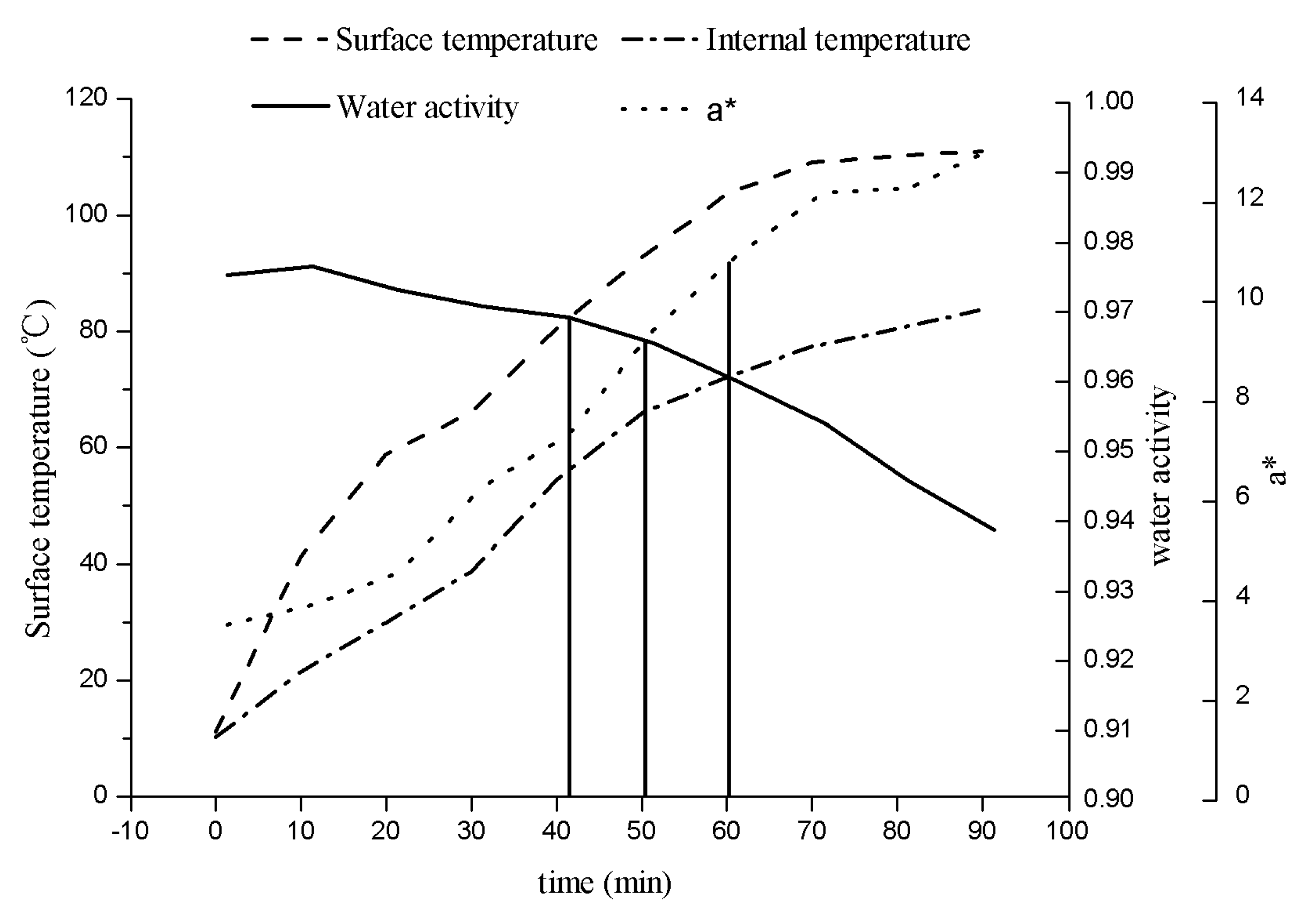
3.2. Coupling of Water Activity and a* of Roast Duck Skin under Simultaneous Heat and Mass Transfer
3.3. Prediction of Finished Duck Skin Colour under Simultaneous Heat and Mass Transfer
3.4. BaP and Heterocyclic Aromatic Amine Contents in Hot-Air Drying Ducks
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chen, B.H.; Lin, Y.S. Formation of Polycyclic Aromatic Hydrocarbons during Processingof Duck Meat. J. Agric. Food Chem. 1997, 45, 1394–1403. [Google Scholar] [CrossRef]
- Felix, R.; Hailu, F.A. Modelling the transport phenomena and texture changes of chicken breast meat during the roasting in a convective oven. J. Food Eng. 2018, 237, 60–68. [Google Scholar]
- Feyissa, A.H.; Gernaey, K.V.; Adler-Nissen, J. 3D modelling of coupled mass and heat transfer of a convection-oven roasting process. Meat Sci. 2013, 93, 810–820. [Google Scholar] [CrossRef]
- Knize, M.G.; Felton, J.S. Formation and human risk of carcinogenic heterocyclic amines formed from natural precursors in meat. Nutr. Rev. 2005, 63, 158–165. [Google Scholar] [CrossRef] [PubMed]
- IARC, International Agency for Research on Cancer. Monographs on the Evaluation of Carcinogenic Risks to Humans: Some Non-Heterocyclic Polycyclic Aromatic Hydrocarbons and Some Related Exposures; IARC: Lyon, France, 2010; Volume 92, pp. 1–868. [Google Scholar]
- Skog, K.I.; Johansson, M.A.E.; Jägerstad, M.I. Carcinogenic heterocyclic amines in model systems and cooked foods: A review on formation, occurrence and intake. Food Chem. Toxicol. 1998, 36, 879–896. [Google Scholar] [CrossRef]
- Matsumoto, T.; Yoshida, D.; Tomita, H. Determination of mutagens, amino-alpha-carbolines in grilled foods and cigarette smoke condensate. Cancer Lett. 1981, 12, 105–110. [Google Scholar] [CrossRef]
- Lin, G.F.; Weigel, S.; Tang, B. The occurrence of polycyclic aromatic hydrocarbons in Peking duck: Relevance to food safety assessment. Food Chem. 2011, 129, 524–527. [Google Scholar] [CrossRef]
- Chung, S.Y.; Yettella, R.R.; Kim, J.S. Effects of grilling and roasting on the levels of polycyclic aromatic hydrocarbons in beef and pork. Food Chem. 2011, 129, 1420–1426. [Google Scholar] [CrossRef]
- Aaslyng, M.D.; Duedahl-Olesen, L.; Jensen, K. Content of heterocyclic amines and polycyclic aromatic hydrocarbons in pork, beef and chicken barbecued at home by Danish consumers. Meat Sci. 2013, 93, 85–91. [Google Scholar] [CrossRef]
- Yao, Y.; Peng, Z.Q.; Shao, B. Effects of frying and boiling on the formation of heterocyclic amines in braised chicken. Poult. Sci. 2013, 92, 3017–3025. [Google Scholar] [CrossRef]
- Rahman, U.U.; Sahar, A.; Khan, M.I. Production of heterocyclic aromatic amines in meat: Chemistry, health risks and inhibition. A review. LWT-Food Sci. Technol. 2014, 59, 229–233. [Google Scholar] [CrossRef]
- Bouvard, V.; Loomis, D.; Guyton, K. Carcinogenicity of consumption of red and processed meat. Lancet Oncol. 2015, 16, 1599–1600. [Google Scholar] [CrossRef]
- Lu, F.; Kuhnle, G.K.; Cheng, Q. Vegetable oil as fat replacer inhibits formation of heterocyclic amines and polycyclic aromatic hydrocarbons in reduced fat pork patties. Food Control 2017, 81, 113–125. [Google Scholar] [CrossRef]
- Min, S.; Patra, J.K.; Shin, H.S. Factors influencing inhibition of eight polycyclic aromatic hydrocarbons in heated meat model system. Food Chem. 2018, 239, 993–1000. [Google Scholar] [CrossRef]
- European Commission (EC). Council Regulation (EC) 835/2011/EC of 19 August 2011 amending Regulation (EC) No 1881/2006 as regards maximum levels for polycyclic aromatic hydrocarbons in foodstuffs. Off. J. Eur. Union 2011, L215, 4–8. [Google Scholar]
- Tolerance Limit of Benzo(a)pyrene in Foods, GB7104-1994. Available online: http://www.bzywb.com/bzdetail/487/E8E66E06.shtml (accessed on 10 September 2020).
- Belitz, H.D.; Grosch, W.; Schieberle, P. Food Chemistry, 4th ed.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 3–5. [Google Scholar]
- Ames, J.M. Control of the Maillard reaction in food systems. Trends Food Sci. Technol. 1990, 1, 150–154. [Google Scholar] [CrossRef]
- Warriss, P.D.; Ebrary, I. Meat science: An introductory text. Int. J. Food Sci. Technol. 1999, 36, 439–449. [Google Scholar]
- Lewicki, P.P.; Arboix, J.A.; Boto, P.G.; Beringues, J.C.; Moreno, I.M. Drying. In Encyclopedia of Meat Sciences, 2nd ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 471–479. [Google Scholar]
- Enriqueza, E.J.; Olivasa, G.I.; Ortega-Rivasb, E.; Zamudio-Floresa, P.B.; Perez-Vegab, S.; Sepulveda, D.R. Water activity, not moisture content, explains the influence of water on powder flowability. LWT-Food Sci. Technol. 2019, 100, 35–39. [Google Scholar]
- Labuza, T.P.; Saltmarch, M. The nonenzymatic browning reaction as affected by water in foods [Shelf life of food products]. Water Act. Influ. Food Qual. 1981, 605–650. [Google Scholar]
- Mundt, S.; Wedzicha, B.L. A kinetic model for browning in the baking of biscuits: Effects of water activity and temperature. LWT-Food Sci. Technol. 2007, 40, 1078–1082. [Google Scholar] [CrossRef]
- Jiménez-Castanõ, L.; Villamiel, M.; Martıń-Álvarez, P.J. Effect of the dry-heating conditions on the glycosylation of β-lactoglobulin with dextran through the Maillard reaction. Food Hydrocoll. 2005, 19, 831–837. [Google Scholar] [CrossRef]
- Singh, L.; Varshney, J.G.; Agarwal, T. Polycyclic aromatic hydrocarbons’ formation and occurrence in processed food. Food Chem. 2016, 199, 768–781. [Google Scholar] [CrossRef] [PubMed]
- Alomirah, H.; Al-Zenki, S.; Al-Hooti, S. Concentrations and dietary exposure to polycyclic aromatic hydrocarbons (PAHs) from grilled and smoked foods. Food Control 2011, 22, 2028–2035. [Google Scholar] [CrossRef]

| Samples | Contents | Samples | Contents | Samples | Contents | Samples | Contents | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| OWBF A * | 1 | 0.81 ± 0.1 | OWB B * | 1 | ND | OGF C * | 1 | 0.16 ± 0.02 | HAD D * | 1 | ND |
| 2 | ND | 2 | 0.91 ± 0.1 | 2 | ND | 2 | ND | ||||
| 3 | 2.9 ± 1 | 3 | ND | 3 | 0.32 ± 0.05 | 3 | ND | ||||
| Cooking Methods | ||||
|---|---|---|---|---|
| Samples | Open Wood Burning Fire A * | Open Wood Burning Fire B * | Open Gas Flame C * | Hot-Air Drying |
| IQ | ND | ND | 11 ± 2 | ND |
| MeIQx | 2.1 ± 0.8 | ND | ND | ND |
| 4,8-DiMeIQx | ND | 1.4 ± 0.5 b | 6.3 ± 2 a | ND |
| Norharman | 8.2 ± 1 b | 6.7 ± 1 b | 14 ± 2 a | 0.62 ± 0.1 |
| Harman | 5.2 ± 1 b | 3.8 ± 1 b | 12 ± 2 a | 0.31 ± 0.05 |
| Trp-P-2 | ND | 1.1 ± 0.3 b | 1.7 ± 0.4 a | ND |
| PhIP | 1.3 ± 0.4 b | ND | 4.3 ± 0.6 a | ND |
| AαC | ND | ND | 0.78 ± 0.2 | ND |
| Total | 16.82 | 13.01 | 49.95 | 0.93 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, Y.; Guo, X.; Ahmed Jamali, M.; Zhang, Y. Coupling of Water Activity and Colour Development of Roast Duck Skin under Forced Convection Drying. Processes 2020, 8, 1165. https://doi.org/10.3390/pr8091165
Peng Y, Guo X, Ahmed Jamali M, Zhang Y. Coupling of Water Activity and Colour Development of Roast Duck Skin under Forced Convection Drying. Processes. 2020; 8(9):1165. https://doi.org/10.3390/pr8091165
Chicago/Turabian StylePeng, Yingbo, Xiuyun Guo, Muneer Ahmed Jamali, and Yawei Zhang. 2020. "Coupling of Water Activity and Colour Development of Roast Duck Skin under Forced Convection Drying" Processes 8, no. 9: 1165. https://doi.org/10.3390/pr8091165
APA StylePeng, Y., Guo, X., Ahmed Jamali, M., & Zhang, Y. (2020). Coupling of Water Activity and Colour Development of Roast Duck Skin under Forced Convection Drying. Processes, 8(9), 1165. https://doi.org/10.3390/pr8091165




