Influences of Water Content in Feedstock Oil on Burning Characteristics of Fatty Acid Methyl Esters
Abstract
1. Introduction
2. Experimental Details
2.1. Preparation of Biodiesel from Palm Oil with Various Water Contents Added
2.2. Analysis of Burning Characteristics of Fatty Acid Methyl Esters from Palm Oil with Various Water Contents
3. Results and Discussion
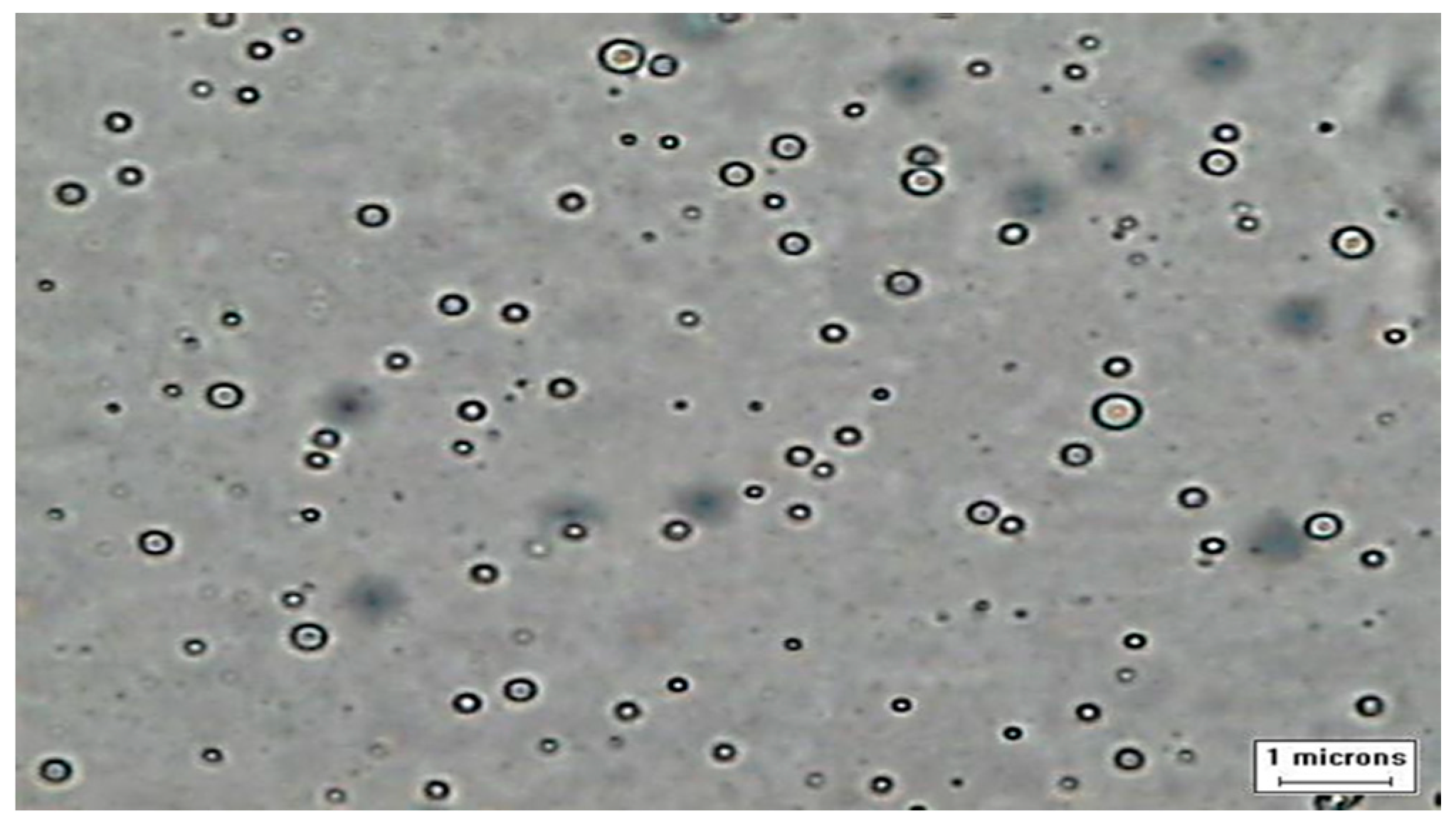
3.1. Micrograph of Water in Palm Oil and Fatty Acid Methyl Esters (FAME)
3.2. Heating Value
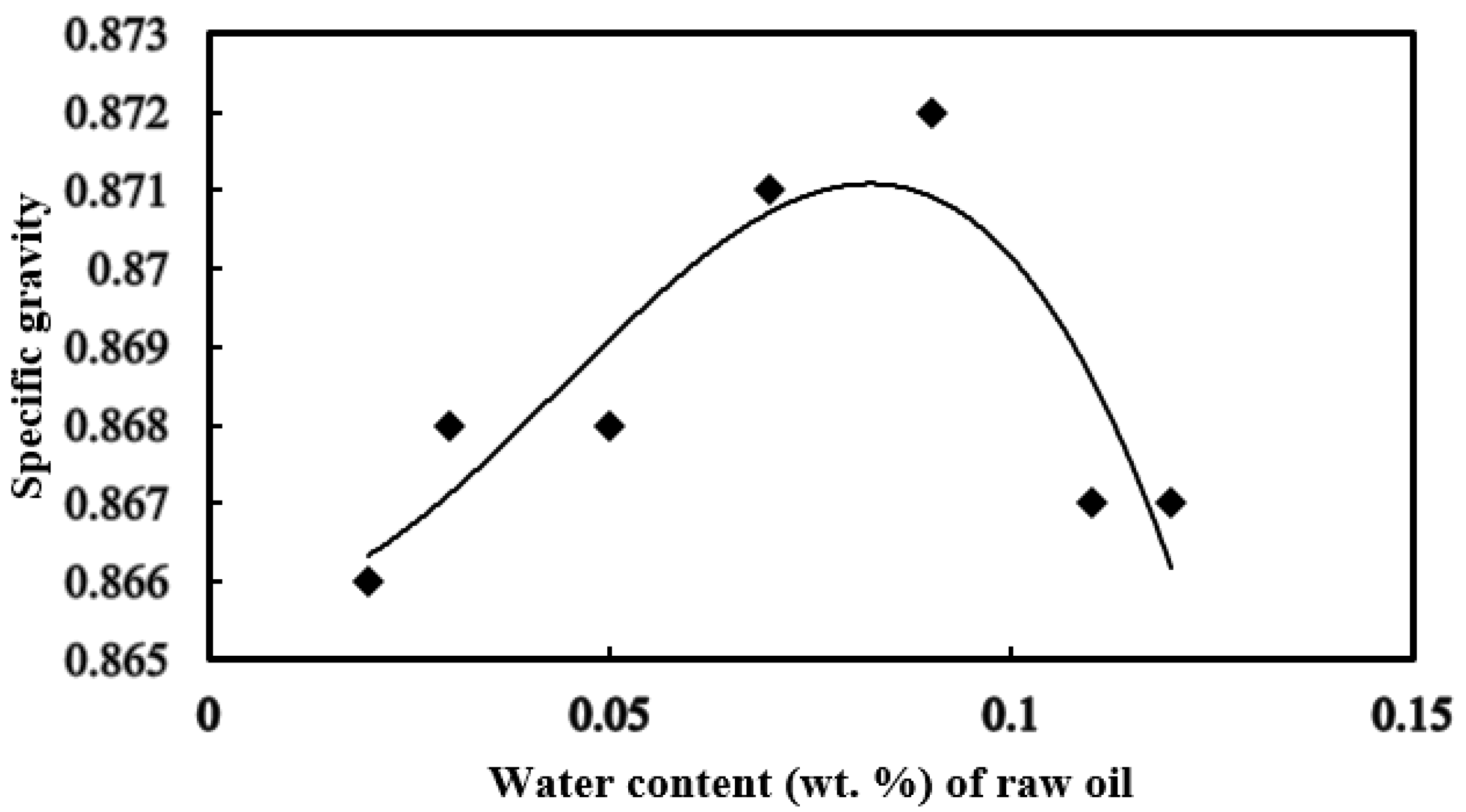
3.3. Specific Gravity
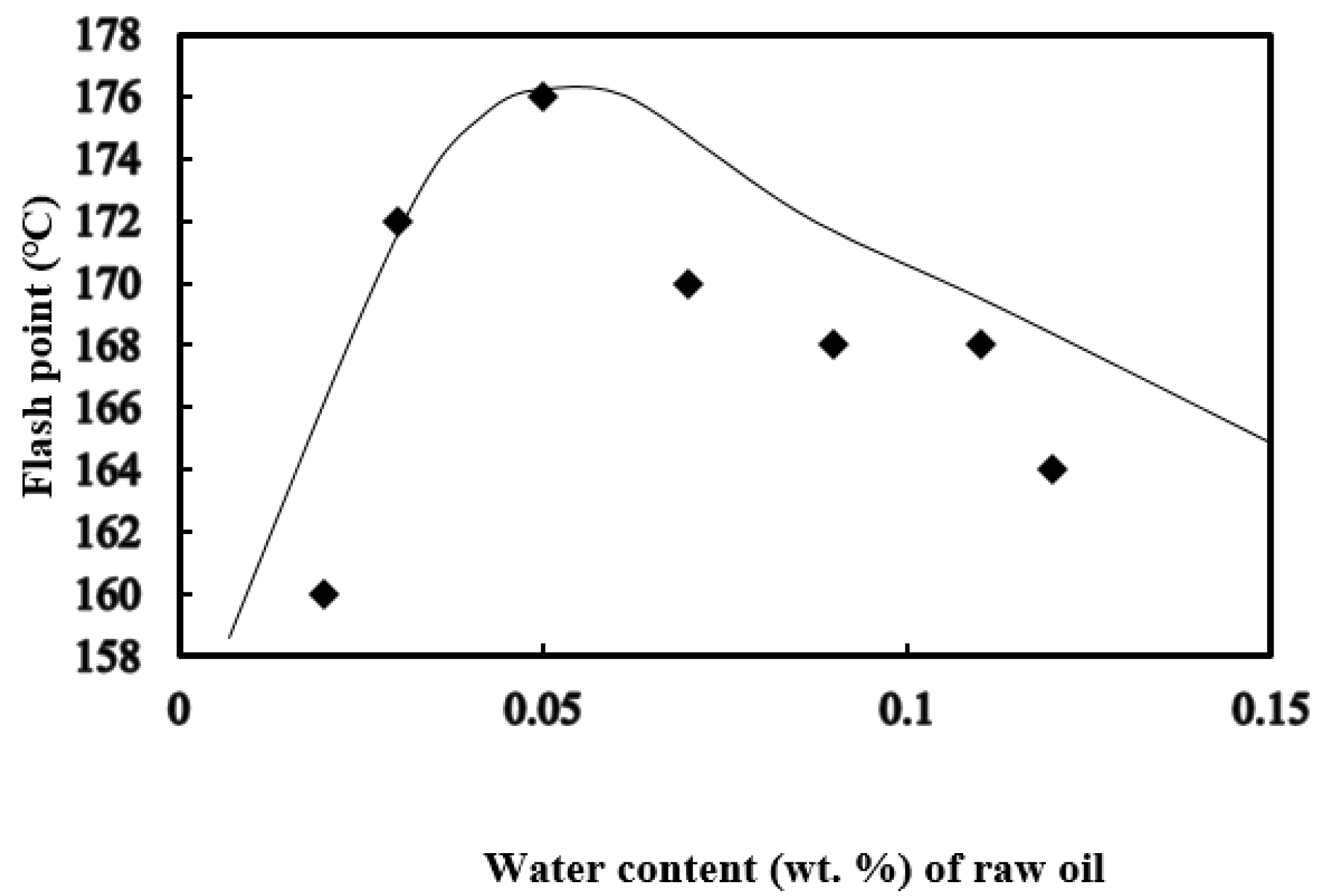
3.4. Flash Point and Ignition Point
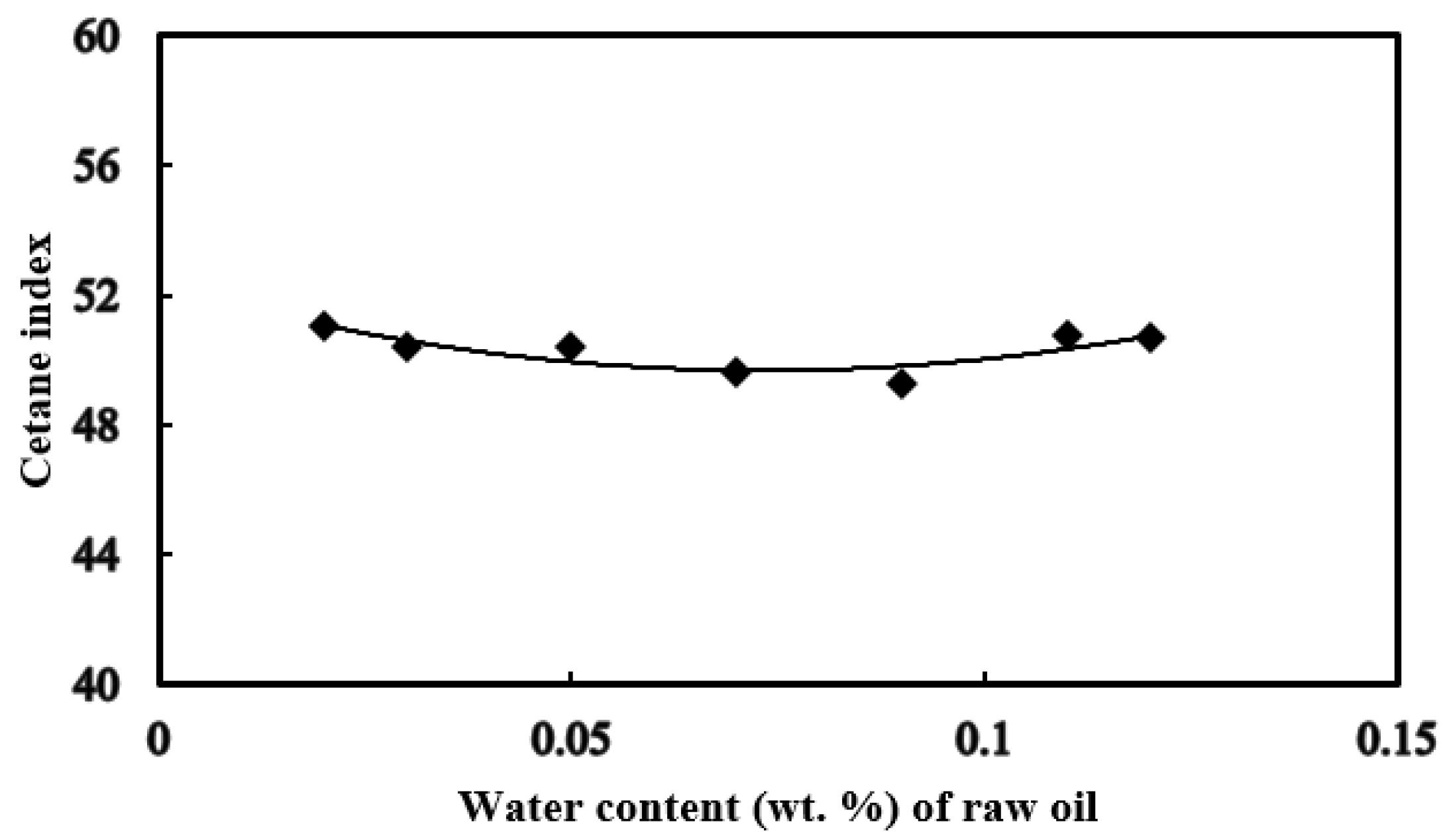
3.5. Distillation Temperature and Cetane Index
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Østerstrøm, F.F.; Anderson, J.E.; Mueller, S.A.; Collings, T.; Ball, J.C.; Wallington, T.J. Oxidation stability of rapeseed biodiesel/petroleum diesel blends. Energy Fuels 2016, 30, 344–351. [Google Scholar] [CrossRef]
- Othman, M.F.; Adam, A.; Najafi, G.; Mamat, R. Green fuel as alternative fuel for diesel engine: A review. Renew. Sustain. Energy Rev. 2017, 80, 694–709. [Google Scholar] [CrossRef]
- Kandasamy, S.; Samudrala, S.P.; Bhattacharya, S. The route towards sustainable production of ethylene glycol from a renewable resource, biodiesel waste: A review. Cat. Sci. Tec. 2019, 9, 567–577. [Google Scholar] [CrossRef]
- Ahmmad, M.S.; Haji Hassan, M.B.; Kalam, M.A. Comparative corrosion characteristics of automotive materials in Jatropha biodiesel. Int. J. Green Energy 2018, 15, 393–399. [Google Scholar] [CrossRef]
- Santos, D.; da Rocha, E.C.; Santos, R.L.; Cancelas, A.J.; Franceschi, E.; Santos, A.F.; Fortuny, M.; Dariva, C. Demulsification of water-in-crude oil emulsions using single mode and multimode microwave irradiation. Sep. Purif. Technol. 2017, 189, 347–356. [Google Scholar] [CrossRef]
- di Bitonto, L.; Pastore, C. Metal hydrated-salts as efficient and reusable catalysts for pre-treating waste cooking oils and animal fats for an effective production of biodiesel. Renew. Energy 2019, 143, 1193–1200. [Google Scholar] [CrossRef]
- Hakimi, M.I.; Goembira, F.; Ilham, Z. Engine-compatible biodiesel from Leucaena leucocephala seed oil. J. Soc. Automot. Eng. Malays. 2017, 1, 86–93. [Google Scholar]
- Yaşar, F. Comparision of fuel properties of biodiesel fuels produced from different oils to determine the most suitable feedstock type. Fuel 2020, 264, 116817. [Google Scholar] [CrossRef]
- Chen, J.; Li, J.; Dong, W.; Zhang, X.; Tyagi, R.D.; Drogui, P.; Surampalli, R.Y. The potential of microalgae in biodiesel production. Renew. Sustain. Energy Rev. 2018, 90, 336–346. [Google Scholar] [CrossRef]
- Shi, W.J.; Liu, H.Q.; Feng, S.B.; Zheng, E.L. Study on preparation of biodiesel with low acid value rapeseed oil. Renew. Energy Resour. 2009, 27, 37–39. [Google Scholar]
- Jia, W.; Xu, G.; Liu, X.; Zhou, F.; Ma, H.; Zhang, Y.; Fu, Y. Direct Selective Hydrogenation of Fatty Acids and Jatropha Oil to Fatty Alcohols over Cobalt-Based Catalysts in Water. Energy Fuels 2018, 32, 8438–8446. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiaqiang, E.; Chen, J.; Zhu, H.; Zhao, X.; Han, D.; Yin, Z. Effects of low-level water addition on spray, combustion and emission characteristics of a medium speed diesel engine fueled with biodiesel fuel. Fuel 2019, 239, 245–262. [Google Scholar] [CrossRef]
- Rao, M.S.; Anand, R.B. Performance and emission characteristics improvement studies on a biodiesel fuelled DICI engine using water and AlO (OH) nanoparticles. Appl. Therm. Eng. 2016, 98, 636–645. [Google Scholar]
- Sudalaimuthu, G.; Rathinam, S.; Munuswamy, D.B.; Thirugnanasambandam, A.; Devarajan, Y. Testing and evaluation of performance and emissions characteristics of water-biodiesel aspirated research engine. J. Test. Eval. 2020, 48, 20180306. [Google Scholar] [CrossRef]
- Zakaria, H.; Khalid, A.; Sies, M.F.; Mustaffa, N.; Manshoor, B. Effect of storage temperature and storage duration on biodiesel properties and characteristics. Appl. Mech. Mater. 2014, 465, 316–321. [Google Scholar] [CrossRef]
- Lawan, I.; Zhou, W.; Idris, A.L.; Jiang, Y.; Zhang, M.; Wang, L.; Yuan, Z. Synthesis, properties and effects of a multi-functional biodiesel fuel additive. Fuel Process. Technol. 2020, 198, 106228. [Google Scholar] [CrossRef]
- Delfino, J.R.; Pereira, T.C.; Viegas, H.D.C.; Marques, E.P.; Ferreira, A.A.P.; Zhang, L.; Marques, A.L.B. A simple and fast method to determine water content in biodiesel by electrochemical impedance spectroscopy. Talanta 2018, 179, 753–759. [Google Scholar] [CrossRef]
- Kusdiana, D.; Saka, S. Effects of water on biodiesel fuel production by supercritical methanol treatment. Bioresour. Technol. 2004, 91, 289–295. [Google Scholar] [CrossRef]
- Tan, K.T.; Lee, K.T.; Mohamed, A.R. Effects of free fatty acids, water content and co-solvent on biodiesel production by supercritical methanol reaction. J. Supercrit. Fluids 2010, 53, 88–91. [Google Scholar] [CrossRef]
- Srimhan, P.; Kongnum, K.; Taweerodjanakarn, S.; Hongpattarakere, T. Selection of lipase producing yeasts for methanol-tolerant biocatalyst as whole cell application for palm-oil transesterification. Enzyme Microb. Technol. 2011, 48, 293–298. [Google Scholar] [CrossRef]
- Yan, S.; Salley, S.O.; Ng, K.Y.S. Simultaneous transesterification and esterification of unrefined or waste oils over ZnO-La2O3 catalysts. Appl. Catal. A. 2009, 353, 203–212. [Google Scholar] [CrossRef]
- Shimadzu Corporation. GC-2014 Gas Chromatograph Instruction Manual; Shimadzu Corporation: Kyoto, Japan, 2014. [Google Scholar]
- International Organization for Standardization. Determination of Flash Point-Rapid Equilibrium Closed Cup Method; American National Standard Institute: Washington, DC, USA, 2015; ISO 3679:2015. [Google Scholar]
- Kalargaris, I.; Tian, G.; Gu, S. Experimental evaluation of a diesel engine fuelled by pyrolysis oils produced from low-density polyethylene and ethylene–vinyl acetate plastics. Fuel Process. Technol. 2017, 161, 125–131. [Google Scholar] [CrossRef]
- Holman, J.P. Analysis of experimental data. In Experimental Methods for Engineers, 8th ed.; McGraw Hill Inc.: Singapore, 2012; pp. 60–77. [Google Scholar]
- Moussa, O.; Tarlet, D.; Massoli, P.; Bellettre, J. Parametric study of the micro-explosion occurrence of W/O emulsions. Int. J. Therm. Sci. 2018, 133, 90–97. [Google Scholar] [CrossRef]
- Pangestu, T.; Kurniawan, Y.; Soetaredjo, F.E.; Santoso, S.P.; Irawaty, W.; Yuliana, M.; Ismadji, S. The synthesis of biodiesel using copper based metal-organic framework as a catalyst. J. Environ. Chem. Eng. 2019, 7, 103277. [Google Scholar] [CrossRef]
- Hoekman, S.K.; Broch, A.; Robbins, C.; Ceniceros, E.; Natarajan, M. Review of biodiesel composition, properties, and specifications. Renew. Sustain. Energy Rev. 2012, 16, 143–169. [Google Scholar] [CrossRef]
- Singh, D.; Sharma, D.; Soni, S.L.; Sharma, S.; Kumari, D. Chemical compositions, properties, and standards for different generation biodiesels: A review. Fuel 2019, 253, 60–71. [Google Scholar] [CrossRef]
- Pinzi, S.; Leiva, D.; Arzamendi, G.; Gandia, L.M.; Dorado, M.P. Multiple response optimization of vegetable oils fatty acid composition to improve biodiesel physical properties. Bioresour. Technol. 2011, 102, 7280–7288. [Google Scholar] [CrossRef]
- Wu, L.; Wei, T.Y.; Tong, Z.F.; Zou, Y.; Lin, Z.J.; Sun, J.H. Bentonite-enhanced biodiesel production by NaOH-catalyzed transesterification of soybean oil with methanol. Fuel Process. Technol. 2016, 144, 334–340. [Google Scholar] [CrossRef]
- Nguyen, H.C.; Nguyen, M.L.; Wang, F.M.; Juan, H.Y.; Su, C.H. Biodiesel production by direct transesterification of wet spent coffee grounds using switchable solvent as a catalyst and solvent. Bioresour. Technol. 2020, 296, 122334. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, C.; Igou, T.; Liu, P.; Hu, Z.; Van Ginkel, S.W.; Chen, Y. Effect of water content on [Bmim][HSO4] assisted in-situ transesterification of wet Nannochloropsis oceanica. Appl. Energy 2018, 226, 461–468. [Google Scholar] [CrossRef]
- Arumugam, A.; Ponnusami, V. Production of biodiesel by enzymatic transesterification of waste sardine oil and evaluation of its engine performance. Heliyon 2017, 3, e00486. [Google Scholar] [CrossRef]
- Oliverira, E.D.C.; Silva, P.R.D.; Ramos, A.P.; Aranda, D.A.G.; Freire, D.M.G. Study of soybean oil hydrolysis catalyzed by Thermomyces Ianuginosus lipase and its application to biodiesel production via hydroesterification. Enzyme Res. 2011, 2011, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Edeh, I.; Overton, T.; Bowra, S. Optimization of subcritical water-mediated lipid extraction from activated sludge for biodiesel production. Biofuels 2019, 1–7. [Google Scholar] [CrossRef]
- Nejad, A.S.; Zahedi, A.R. Optimization of biodiesel production as a clean fuel for thermal power plants using renewable energy source. Renew. Energy 2018, 119, 365–374. [Google Scholar] [CrossRef]
- Yamin, J.A.; Sheet, E.A.E.; Hdaib, I. Exergy analysis of biodiesel fueled direct injection CI engines. Energy Sources Part A 2018, 40, 1351–1358. [Google Scholar] [CrossRef]
- He, Y.; Wu, T.; Wang, X.; Chen, B.; Chen, F. Cost-effective biodiesel production from wet microalgal biomass by a novel two-step enzymatic process. Bioresour. Technol. 2018, 268, 583–591. [Google Scholar] [CrossRef]
- Caetano, N.S.; Caldeira, D.; Martins, A.A.; Mata, T.M. Valorisation of spent coffee grounds: Production of biodiesel via enzymatic catalysis with ethanol and a co-solvent. Waste Biomass Valori. 2017, 8, 1981–1994. [Google Scholar] [CrossRef]
- Elsanusi, O.A.; Roy, M.M.; Sidhu, M.S. Experimental investigation on a diesel engine fueled by diesel-biodiesel blends and their emulsions at various engine operating conditions. Appl. Energy 2017, 203, 582–593. [Google Scholar] [CrossRef]
- Hajilar, S.; Shafei, B. Thermal transport properties at interface of fatty acid esters enhanced with carbon-based nanoadditives. Int. J. Heat Mass Tran. 2019, 145, 118762. [Google Scholar] [CrossRef]
- Ramírez-Verduzco, L.F.; Rodríguez-Rodríguez, J.E.; del Rayo Jaramillo-Jacob, A. Predicting cetane number, kinematic viscosity, density and higher heating value of biodiesel from its fatty acid methyl ester composition. Fuel 2012, 91, 102–111. [Google Scholar] [CrossRef]
- Refaat, A.A. Correlation between the chemical structure of biodiesel and its physical properties. Int. J. Environ. Sci. Technol. 2009, 6, 677–694. [Google Scholar]
- Folayan, A.J.; Anawe, P.A.L.; Aladejare, A.E.; Ayeni, A.O. Experimental investigation of the effect of fatty acids configuration, chain length, branching and degree of unsaturation on biodiesel fuel properties obtained from lauric oils, high-oleic and high-linoleic vegetable oil biomass. Energy Rep. 2019, 5, 793–806. [Google Scholar] [CrossRef]
- Marlina, E.; Wijayanti, W.; Yuliati, L.; Wardana, I.N.G. The role of pole and molecular geometry of fatty acids in vegetable oils droplet on ignition and boiling characteristics. Renew. Energ. 2020, 145, 596–603. [Google Scholar] [CrossRef]
- Paula, A.J.; Stéfani, D.; Filho, A.G.S.; Kim, Y.A.; Endo, M.; Alves, O.L. Surface chemistry in the process of coating mesoporous SiO2 onto carbon nanotubes driven by the formation of Si-O-C Bonds. Chem.-Eur. J. 2011, 17, 3228–3237. [Google Scholar] [CrossRef] [PubMed]
- Ayoola, A.A.; Anawe, P.A.L.; Ojewumi, M.E.; Amaraibi, R.J. Comparison of the properties of palm oil and palm kerneloil biodiesel in relation to the degree of unsaturation of their oil feedstocks. Int. J. App. Nat. Sci. 2016, 5, 1–8. [Google Scholar]
- Su, Y.C.; Liu, Y.A.; Diaz Tovar, C.A.; Gani, R. Selection of prediction methods for thermophysical properties for process modeling and product design of biodiesel manufacturing. Ind. Eng. Chem. Res. 2011, 50, 6809. [Google Scholar] [CrossRef]
- Rao, G.L.N.; Ramadhas, A.S.; Nallusamy, N.; Sakthivel, P. Relationships among the physical properties of biodiesel and engine fuel system design requirement. Int. J. Energ. Environ. 2010, 1, 919–926. [Google Scholar]
- Sivaramakrishnan, K.; Ravikumar, P. Determination of cetane number of biodiesel and its influence on physical properties. ARPN J. Eng. Appl. Sci. 2012, 7, 205–211. [Google Scholar]
- Kumar, J.; Bansal, A. Application of artificial neural network to predict properties of diesel—biodiesel blends. Kathman Univ. J. Sci. Eng. Technol. 2010, 6, 98–103. [Google Scholar] [CrossRef]
- Bukkarapu, K.R.; Rahul, T.S.; Kundla, S.; Vardhan, G.V. Effects of blending on the properties of diesel and palm biodiesel. In IOP Conference Series: Materials Science and Engineering; IOP Publishing Ltd.: Hyderabad, India, 2018; Volume 330, pp. 1–15. [Google Scholar] [CrossRef]
- Yao, C.; Dou, Z.; Wang, B.; Liu, M.; Lu, H.; Feng, J.; Feng, L. Experimental study of the effect of heavy aromatics on the characteristics of combustion and ultrafine particle in DISI engine. Fuel 2017, 203, 290–297. [Google Scholar] [CrossRef]
- Phan, A.N.; Phan, T.M. Biodiesel production from waste cooking oils. Fuel 2008, 87, 3490–3496. [Google Scholar] [CrossRef]
- Chen, H.; Xie, B.; Ma, J.; Chen, Y. NOx emission of biodiesel compared to diesel: Higher or lower? Appl. Therm. Eng. 2018, 137, 584–593. [Google Scholar] [CrossRef]
- Giakoumis, E.G.; Sarakatsanis, C.K. A comparative assessment of biodiesel cetane number predictive correlations based on fatty acid composition. Energies 2019, 12, 422. [Google Scholar] [CrossRef]
- de Oliveira, F.M.; de Carvalho, L.S.; Teixeira, L.S.; Fontes, C.H.; Lima, K.M.; Câmara, A.B.; Sales, R.V. Predicting cetane index, flash point, and content sulfur of diesel–biodiesel blend using an artificial neural network model. Energy Fuels 2017, 31, 3913–3920. [Google Scholar] [CrossRef]
- Bemani, A.; Xiong, Q.; Baghban, A.; Habibzadeh, S.; Mohammadi, A.H.; Doranehgard, M.H. Modeling of cetane number of biodiesel from fatty acid methyl ester (FAME) information using GA-, PSO-, and HGAPSO-LSSVM models. Renew. Energy 2020, 150, 924–934. [Google Scholar] [CrossRef]
- Mishra, S.; Anand, K.; Mehta, P.S. Predicting the cetane number of biodiesel fuels from their fatty acid methyl ester composition. Energy Fuels 2016, 30, 10425–10434. [Google Scholar] [CrossRef]
- Moser, B.R. Biodiesel production, Properties and Feedstocks. In Biofuels; Springer: Singapore, 2011; pp. 285–347. [Google Scholar]
- Giakoumis, E.G.; Sarakatsanis, C.K. Estimation of biodiesel cetane number, density, kinematic viscosity and heating values from its fatty acid weight composition. Fuel 2018, 222, 574–585. [Google Scholar] [CrossRef]




| Item | Property |
|---|---|
| Water content (wt.%) | 0.029 |
| Acid value (mg KOH/g) | 0.16 |
| Peroxide value (meq/kg) | 0.53 |
| Lovibond Tintometer | R1.5 Y15 |
| Melting point (°C) | 23.01 |
| Specific gravity | 0.907 |
| Cold filter plugging Point (°C) | 16 |
| Types of Fatty Acids | Added Water Contents (wt.%) | ||||||
|---|---|---|---|---|---|---|---|
| 0.02 | 0.03 | 0.05 | 0.07 | 0.09 | 0.11 | 0.12 | |
| C14:0 | 0.8 | 0.6 | 0.8 | 0.6 | 0.6 | 0.6 | 0.8 |
| C16:0 | 33.3 | 31.1 | 34.2 | 27.6 | 27.3 | 28.3 | 34.1 |
| C18:0 | 43.4 | 14.1 | 11.2 | 14.6 | 15.5 | 48.9 | 43.2 |
| C18:1 | 32.4 | 31.7 | 35.4 | 34.4 | |||
| C18:2 | 3.6 | 3.9 | 3.8 | 3.4 | 3.4 | 3.3 | 3.2 |
| C18:3 | 0.4 | 0.1 | 0.1 | ||||
| C24:0 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| C24:1 | 0.3 | 0.3 | 0.2 | 0.3 | 0.3 | 0.3 | 0.3 |
| Saturated fatty acids | - | 45.9 | 46.3 | 42.9 | 43.5 | - | - |
| Longer carbon-chain fatty acids than C16 | 81.1 | 81.9 | 81.2 | 81.5 | 81.1 | 80.9 | 80.9 |
| Total FAME | 93.5 | 95 | 97.3 | 95.3 | 94.1 | 93.3 | 93.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-Y.; Ma, L. Influences of Water Content in Feedstock Oil on Burning Characteristics of Fatty Acid Methyl Esters. Processes 2020, 8, 1130. https://doi.org/10.3390/pr8091130
Lin C-Y, Ma L. Influences of Water Content in Feedstock Oil on Burning Characteristics of Fatty Acid Methyl Esters. Processes. 2020; 8(9):1130. https://doi.org/10.3390/pr8091130
Chicago/Turabian StyleLin, Cherng-Yuan, and Lei Ma. 2020. "Influences of Water Content in Feedstock Oil on Burning Characteristics of Fatty Acid Methyl Esters" Processes 8, no. 9: 1130. https://doi.org/10.3390/pr8091130
APA StyleLin, C.-Y., & Ma, L. (2020). Influences of Water Content in Feedstock Oil on Burning Characteristics of Fatty Acid Methyl Esters. Processes, 8(9), 1130. https://doi.org/10.3390/pr8091130





